Abstract
Introduction
Nonsmokers with chronic obstructive pulmonary disease (COPD) are neglected despite constituting half of all cases in studies from the developed world. Herein, we systematically reviewed the prevalence of COPD among nonsmokers in India.
Content
We searched Embase, Scopus, and PubMed databases for studies examining the prevalence of COPD among nonsmokers in India. We used the Joanna Briggs Institute (JBI) checklist to assess included studies’ quality. Meta-analysis was performed using random-effects model.
Summary
Seven studies comprising 6,903 subjects were included. The quality of the studies ranged from 5/9 to 8/9. The prevalence of COPD varied between 1.6 and 26.6 %. Studies differed considerably in demographics and biomass exposure profiles of subjects. Among the four studies that enrolled both middle-aged and elderly Indian nonsmokers not screened based on biomass fuel exposure, the pooled prevalence of COPD was 3 % (95 % CI, 2–3 %; I2=50.52 %, p=0.11). The pooled prevalence of COPD among biomass fuel-exposed individuals was 10 % (95 % CI, 2–18 %; I2=98.8 %, p<0.001).
Outlook
Limited evidence suggests a sizable burden of COPD among nonsmokers and biomass fuel-exposed individuals in India. More epidemiological studies of COPD in nonsmokers are needed from low and middle-income countries.
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by prolonged exposure to noxious stimuli and is characterized by a combination of respiratory symptoms and fixed spirometric airway obstruction. It affects about 10.3 % of the global population aged between 30 and 79 [1]. It has emerged as a major public health challenge, becoming the world’s third leading cause of death according to the World Health Organization [2]. In 2019, about 200 million people suffered from COPD worldwide, and 3.2 million people died due to it, with most of the deaths occurring in low- and middle-income countries (LMICs) [3]. Of the global COPD burden, about 55 million patients reside in India alone [4].
Smoking has long been recognized as the leading exposure responsible for the development of COPD. However, it has been found that about 40–56% of all COPD patients are nonsmokers [5]. Furthermore, among nonsmokers, women are disproportionately affected [6]. Some risk factors for COPD among nonsmokers include biomass fuel use for cooking and heating, environmental tobacco smoke exposure, and childhood respiratory infections [7]. Notably, these risk factors predominantly affect individuals of lower socio-economic status in developing countries. Biomass fuel has long been the dominant energy source for cooking in rural Indian households. The use of biomass fuel for cooking is associated with higher levels of indoor particulate matter (PM2.5 and PM10) compared with liquified petroleum gas (LPG) use [8]. The higher indoor air pollution with biomass fuel usage has also been linked with spirometric abnormalities and increased risk of developing COPD [8], 9]. Despite recent efforts of the Indian government to increase the adoption of cleaner fuels, the lingering effects of biomass fuel exposure will continue to affect the populace for the coming decades [10].
Further, evidence has shown that COPD may have different clinical and pathological characteristics among smokers and nonsmokers. For instance, smokers are more likely to suffer from emphysema, whereas nonsmokers are more likely to have a small airway disease phenotype [11]. Hence, it is concerning that most studies of therapeutic interventions in COPD have predominantly recruited only smokers with COPD.
Large cohorts from developed countries have estimated the prevalence of COPD among adult nonsmokers to be around 4.9–7.1 % [7], [12], [13], [14]. However, there are sparse data regarding the same from LMICs like India. With over 1.4 billion individuals, India has about one-sixth of the world’s population but contributes almost one-third of the disability-adjusted life years due to chronic respiratory diseases [4]. Existing studies have investigated the prevalence of COPD in India and neighboring countries but have not focussed on the nonsmoker population [4], 15]. Given that COPD is a heterogenous disease with distinct pathophysiological mechanisms in nonsmokers, the lack of systematic epidemiological data regarding COPD in nonsmokers from LMICs like India is a major impediment to investment in research and management of this neglected patient group. Hence, in this systematic review, we aim to establish the prevalence of COPD among nonsmokers in India.
Methods
This systematic review was prospectively registered in PROSPERO (CRD42022358489). It was conducted using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Search strategy
The electronic searches were performed for English language studies in PubMed, Embase, and Scopus for publications between 1st January 2000 and 31st August 2022. The main search terms included “prevalence”, “burden”, “epidemiology”, “chronic obstructive pulmonary disease”, “COPD”, and “India”. The specific search strategies for each database are outlined in Supplementary Table 1. In addition, we hand-searched the reference list of included studies.
Study selection
The search results were imported to CADIMA software, and duplicate records were removed. The study selection was performed in two sequential steps, i.e., screening titles and abstracts followed by reviewing the full-texts, independently by two investigators (TMS & TG). Disagreements were resolved mutually or with the help of a third reviewer (AM).
We included community-based studies which utilized a cross-sectional design to assess the prevalence of spirometrically-confirmed COPD among nonsmokers in the Indian adult population or age- and sex-specific subgroups of the same. We excluded studies which were based on specific disease or occupational subgroups. However, we included studies that exclusively enrolled biomass fuel-exposed nonsmokers, given the importance of this risk factor among nonsmokers. Previous systematic reviews, randomized control trials, and case-control studies were excluded.
Data extraction
Relevant information from the included studies was extracted into a Microsoft Excel spreadsheet, viz., the first author’s surname, the year of publication, the geographic region of the study, the population characteristics (including age and sex distribution), and the spirometric definition used for diagnosing COPD. For each study, the total number of nonsmoker subjects and the number of nonsmokers with spirometrically-confirmed COPD were recorded. We also noted the number of individuals exposed to biomass fuel and the prevalence of spirometrically-confirmed COPD in this subgroup. Wherever additional information was required to calculate the prevalence of COPD, we attempted to contact the corresponding author via email.
Quality assessment
To assess the quality of the included studies, two authors (TMS & TG) independently used the Joanna Briggs Institute (JBI) critical appraisal tool for systematic reviews of prevalence studies [16]. The JBI tool is a nine-item checklist, each component carrying one point. To ensure uniform scoring, we adapted the JBI checklist for application to our systematic review, as elaborated in the Supplementary Table 2. Any discrepancies in scoring between the two reviewers were resolved through mutual discussion or the involvement of a third reviewer (AM).
Statistical analysis
The prevalence of spirometrically-confirmed COPD among nonsmokers and the subgroup of biomass fuel-exposed individuals was calculated based on crude numerators and denominators provided by individual studies. To overcome the heterogenous age distributions in the included studies, we performed a meta-analysis of prevalence using only the subset of studies that reported the prevalence of COPD among all middle-aged (44–60 years) and older nonsmokers in the community [17], 18], not limited by any specific exposures. Hence, for this meta-analysis, we included studies that used a lower (or minimum) age cutoff below 44 years and no upper age cutoff in their inclusion criteria. Additionally, we performed a meta-analysis of the prevalence of COPD among the biomass fuel-exposed subjects of all included studies. Pooled prevalence and 95 % confidence intervals (95 % CI) were calculated using random-effects model, weighted by the inverse of variance. The random-effects model was preferred to account for clinical heterogeneity in the included studies in terms of age distributions, biomass fuel exposure, and other variables [19]. Heterogeneity was assessed by the I2 values, wherein 25 , 50, and 75 % represent low, medium, and high heterogeneity. Publication bias could not be assessed due to the paucity of studies and the unsuitability of forest plots in meta-analysis of prevalence studies [20]. A meta-regression analysis was performed to assess the association between the lower age cutoff and the reported prevalence of nonsmoker COPD in the included studies. Statistical analysis was performed using Stata version 14.
Results
Characteristics of included studies
After removing duplicates, 1,441 results were obtained on the literature search. Following the title, abstract, and full-text screening, we finally included seven studies in the review (see PRISMA flow diagram, Figure 1) [21], [22], [23], [24], [25], [26], [27]. The characteristics of the included studies are summarized in Table 1.

PRISMA diagram of the study selection process.
Characteristics of the studies included in the systematic review.
| Author, year | Study location | Study population | Sample size | Age distribution | Diagnostic criteria for COPD | Prevalence of COPD in nonsmokers | Prevalence of COPD in biomass-exposed subjects |
|---|---|---|---|---|---|---|---|
| Johnson et al., 2011 | Tamil Nadu (rural) | Female nonsmokers | 900 | Above 30 years | Symptoms and post-BD FEV1/FVC<0.7 without BDR | 2.44 % | 2.50 % |
| Dutta et al., 2013 | West Bengal (rural) | Premenopausal female nonsmoker with biomass fuel exposure | 244 | 22–41 years | Symptoms and FEV1/FVC<0.7 | 6.6 % | 6.6 % |
| Parasuramalu et al., 2014 | Karnataka (rural) | Nonsmokers and smokers of either sex | 1,400 (1,053 nonsmokers)a | Above 35 years | FEV1/FVC<0.7b | 3.04 % | – |
| Kalagouda et al., 2016 | Karnataka (urban & rural) | Nonsmoker females with biomass fuel exposure>10 years | 2,868 | Above 40 years | Post-BD FEV1/FVC<0.7 | 18.4 % | 18.4 % |
| Sinha et al., 2017 | Delhi (urban) | Nonsmokers and smokers of either sex | 1,203 (744 nonsmokers) | Above 30 years | Post-BD FEV1/FVC<0.7b | 1.6 % | 17.7 % |
| Christopher et al., 2019 | Tamil Nadu (rural) | Nonsmokers and smokers of either sex | 1,015 (899 nonsmokers)c | Above 30 years | Post-BD FEV1/FVC<0.7 | 3.3 % | 3.6 % |
| Kumar et al., 2021 | Haryana (rural) | Elderly nonsmokers and smokers of either sex | 449 (195 nonsmokers) | Above 60 years | Post-BD FEV1/FVC<0.7 | 26.6 % | – |
-
BDR, bronchodilator reversibility; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1st second; FVC, forced vital capacity; GOLD, global initiative for obstructive lung disease. aThe number of nonsmokers was not reported and was derived using the reported prevalence of COPD in the overall population, smokers and nonsmokers. bIn this study, spirometry was only performed in those subjects with symptoms suggestive of COPD. cA total of 787 participants performed technically adequate spirometries, of whom 700 were nonsmokers.
Of the seven studies, three included only females [21], 22], 24]. There was a wide variation in the age distributions of the studies. Dutta et al. enrolled only young, premenopausal females aged 22–41 years [22]. In contrast, Kumar et al. included elderly individuals from a rural community aged 60 and above [27]. All other studies included middle-aged and elderly subjects with a lower age cutoff between 30 and 40 years without any upper age cutoff [21], [23], [24], [25], [26]. Geographically, four studies were conducted in South India [21], 23], 24], 26], two in North India [25], 27], and one in East India [22]. Two studies enrolled urban subjects [24], 25], while the remaining studies examined rural populations. All studies used spirometric definitions to diagnose COPD (Table 1).
Five studies provided an estimate of the prevalence of COPD among nonsmokers in the community, not limited by any specific exposures [21], 23], [25], [26], [27]. The remaining two studies reported the prevalence of COPD only among female nonsmokers exposed to biomass fumes [22], 24].
Quality assessment
On the JBI critical appraisal tool, the scores achieved by the included studies ranged from five to eight out of nine points (Table 2). The sample frame was considered inappropriate in studies that exclusively enrolled female patients or elderly patients because our objective was to estimate the prevalence of COPD among nonsmoking adults of both sexes [21], 22], 24], 27]. Two studies were considered to have suboptimal sampling for using a two-step methodology, wherein spirometry was only conducted amongst those screened to be symptomatic for COPD [23], 25]. One study included subjects only if they exclusively used biomass or LPG for cooking, which was considered an unsuitable sampling method for an overall prevalence assessment in all nonsmokers [22]. In some studies that recruited smokers and nonsmokers, the mean ages were not separately described for the subgroup of nonsmoker subjects [23], [25], [26], [27]. Additionally, two studies that exclusively recruited nonsmokers did not report the mean age of the study population [21], 24]. In two studies, the diagnostic method was considered inappropriate because they did not perform post-bronchodilator testing for detecting airflow obstruction [22], 23]. Further, two studies did not diagnose fixed spirometric obstruction as COPD in the absence of respiratory symptoms [21], 22]. All studies except one reported the response rate, which was above 80 % in all cases [22].
Quality assessment of the studies included in the systematic review.
| Johnson et al. 2011 | Dutta et al. 2013 | Parasuramalu et al. 2014 | Kalagouda et al. 2016 | Sinha et al. 2017 | Kumar et al. 2019 | Christopher et al. 2020 | |
|---|---|---|---|---|---|---|---|
| Was the sample frame appropriate to address the target population? | No | No | Yes | No | Yes | No | Yes |
| Were study participants sampled in an appropriate way? | Yes | No | No | Yes | No | Yes | Yes |
| Was the sample size adequate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the study subjects and the setting described in detail? | No | Yes | No | No | No | No | No |
| Was the data analysis conducted with sufficient coverage of the identified sample? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were valid methods used for the identification of the condition? | No | No | No | Yes | Yes | Yes | Yes |
| Was the condition measured in a standard, reliable way for all participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there appropriate statistical analysis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the response rate adequate, and if not, was the low response rate managed appropriately? | Yes | Uncertain | Yes | Yes | Yes | Yes | Yes |
Study outcomes
Prevalence of COPD in nonsmokers in India
The prevalence of COPD among nonsmokers varied between 1.6 and 26.6 % in the included studies. As the mean age was not reported for the nonsmoker subgroup in a few included studies, we explored the association between the minimum age cutoffs and prevalence of COPD among nonsmokers in individual studies (Figure 2). There was a significant association between a higher minimum age cutoff and higher prevalence of COPD among included studies (coefficient=0.69, 95 % CI: 0.23–1.15; p=0.012; R2=74.7 %).
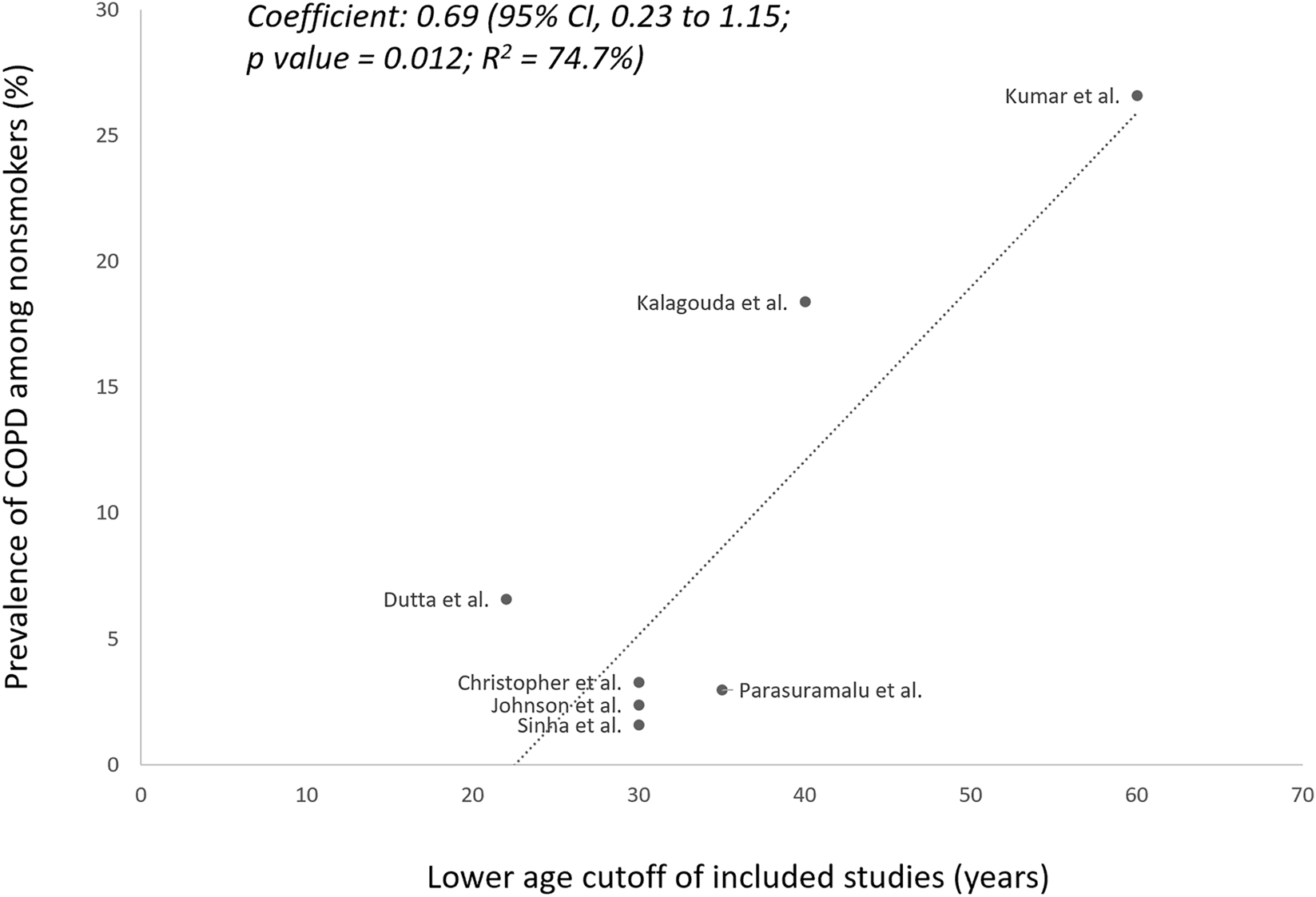
Meta-regression analysis of the association between the lower age cutoff and the prevalence of nonsmoker COPD in the included studies.
Among included studies, those which exclusively enrolled biomass fuel-exposed subjects reported higher prevalences (Table 1), specifically 6.6 % by Dutta et al. and 18.4 % by Kalagouda et al. [22], 24]. The prevalence was highest (26.6 %) in the study by Kumar et al., which included only elderly subjects, of whom 97 % were exposed to biomass fuel [27]. Among the studies which enrolled both nonsmokers and smokers, the prevalence of COPD was generally higher among the smoker subgroups (Supplementary Table 3). However, the proportion of nonsmokers who constituted the overall COPD patients in these studies varied widely between 10 and 72 % (Supplementary Figure 1) [23], [25], [26], [27].
Prevalence of COPD among middle-aged and elderly nonsmokers in India
Among the four studies that reported the prevalence of COPD among middle-aged and elderly nonsmokers not selected based on biomass fuel exposure, the prevalence varied between 1.6 and 3.3 %. Upon meta-analysis, the pooled prevalence of COPD was 3 % (95 % CI, 2–3 %) with medium heterogeneity (I2=50.52 %, p=0.11; Figure 3).

Forest plot of the meta-analysis of the prevalence of chronic obstructive pulmonary disease (COPD) among middle-aged and older nonsmokers in India.
Prevalence of COPD in biomass-exposed population in India
The prevalence of COPD, specifically in the biomass fuel-exposed population, was reported in five included studies and ranged from 2.5 to 18.4 %. The pooled prevalence of COPD among the biomass-exposed population was 10 % (95 % CI, 2–18 %) with high heterogeneity (I2=98.8 %, p<0.001; Figure 4).

Forest plot of the meta-analysis of the prevalence of chronic obstructive pulmonary disease (COPD) among biomass fuel-exposed subjects of the included studies.
Discussion
We performed the first systematic review of the prevalence of COPD among nonsmokers from India. We included seven studies comprising 6,903 nonsmoking adults and found that the prevalence of COPD varied between 1.6 and 26.6 %. This wide variation in the prevalence resulted from considerable heterogeneity in the included studies regarding the age and sex distribution and biomass fuel exposure profile of the subjects. Among the studies which included middle-aged and elderly Indian nonsmokers not selected for biomass fuel exposure, we found the pooled prevalence of COPD to be 3 % with medium heterogeneity. In contrast, among those exposed to biomass fuel, we found the pooled prevalence to be 10 % with high heterogeneity.
Although a systematic review of COPD prevalence in India had been conducted by Jarhyan et al., it had not studied the nonsmoking population separately [15]. Jahyran et al. included all South Asian countries in the review. Among the studies from India, the pooled prevalence of COPD was 11.1 %. The authors determined that besides smoking, additional risk factors included exposures to biomass fuel, occupational dust and environmental tobacco smoke, and a previous diagnosis of tuberculosis or asthma. Additionally, the Global Burden of Disease study found that only one-fourth of the COPD burden in India is attributable to tobacco use. They observed that about half of the COPD cases in India are primarily caused by outdoor and indoor air pollution [4]. Various single-center observational studies from India have also shown that about 40–69 % of COPD patients were non-smokers [28], [29], [30]. In the present systematic review, the studies which reported on both smokers and nonsmokers found that the proportion of COPD belonging to the nonsmoker subgroup varied between 10 and 72 % [23], [25], [26], [27]. The wide range probably reflected differences in the proportion of smokers in the overall sample and the rates of other exposures like biomass fuel usage. Nonetheless, smokers had a higher prevalence rate of COPD than nonsmokers in all of these studies [23], [25], [26], [27].
It is hardly surprising that the bulk of COPD in India is unrelated to smoking because the Global Adult Tobacco Survey found that only 10.4 % of the adult Indian population uses a smoked form of tobacco [31]. Smokeless tobacco use is more prevalent in India; however, this is not an established risk factor for COPD. In contrast, about 55 % of the Indian population used biomass fuel for cooking in 2017, according to the Global Burden of Disease study [32]. In 2016, the Government of India launched the “Ujjwala Yojana” to provide LPG connections to families below the poverty line [33]. However, those who have already experienced prolonged biomass fuel exposure will be at risk for COPD for years to come. Our finding of the high prevalence of COPD in the biomass-exposed population re-emphasizes its important contribution to the burden of nonsmoker COPD.
The choice of including middle-aged and elderly nonsmokers in the meta-analysis was done as these are the age groups for which COPD is a public health menace. The definition of the middle-aged population is contentious; while the lower age cutoff varies between 35 and 45 years, the upper age cutoff varies between 60 and 65 years [17], 18], 34]. We increased the inclusivity of our meta-analysis by using a lower age cutoff of 44 years or below as the eligibility criterion. Hence, no study using even a restrictive definition of middle-age (e.g., 44–60 years) got excluded. As per the United Nations Statistics Department, about one-fourth of India’s population in 2021 was aged 45 years old or above, amounting to around 340.8 million people [35]. Assuming that 89.6 % of these individuals are nonsmokers [31], approximately 9.2 million middle-aged and elderly nonsmokers may be suffering from COPD in India. This number is likely to grow in the coming decades owing to India’s gradually ageing population, and the lifelong exposure to indoor air pollution from biomass fuel usage in a substantial proportion of the populace.
Our findings are also a significant addition to the meager data from LMICs on the prevalence of nonsmoker COPD. Our estimate that 3 % of middle-aged and older Indian nonsmokers have COPD is comparable to results from other LMICs. Waked et al. reported that 3.4 % of Lebanese nonsmokers had COPD using spirometric definitions [36]. Denguezli et al. found that 4.7 % of Tunisian nonsmokers had COPD, which accounted for 45 % of all COPD cases [37]. The risk factors for nonsmoking COPD, which remained consistent among these LMIC studies, included advanced age and biomass fuel usage [36], [37], [38]. In addition to our finding of higher COPD prevalence among biomass fuel-exposed subjects, we also observed a disproportionately higher prevalence of COPD in the Indian study that enrolled only elderly subjects [27].
Although we have stressed upon the role of biomass fuel exposure and air pollution in the development of nonsmoker COPD in LMICs, a significant confounding factor may involve the concept of a “false start” in childhood lung development [39]. While many COPD patients experience accelerated lung function decline due to noxious exposures, a sizable proportion may have normal rates of decline of lung function. In these patients, COPD develops because they have a lower value of peak lung function achieved in early adulthood due to impaired lung development. Underserved populations of LMICs are particularly susceptible to impaired lung growth due to various maternal (e.g., anemia, smoking etc.), perinatal (low birth weight or prematurity), or childhood (e.g., malnutrition, biomass fuel exposure, frequent respiratory infections etc.) factors [39], 40]. Hence, despite normal rates of lung function decline, they may develop COPD in later life. The present review is unable to determine what proportion of patients may have acquired COPD in this manner.
Strengths and limitations
An important strength of our study was the inclusion of studies which used a spirometric definition of COPD. In the past, epidemiological studies in LMICs like India have used questionnaires to estimate the prevalence of COPD because spirometry has been considered too resource-intensive to apply to the field setting [41], 42]. However, clinical questionnaires often miss milder cases of COPD that can be diagnosed on spirometry [43]. The omission of milder cases not only underestimates the burden of COPD but also represents a missed opportunity to intervene early in the natural history of the disease.
The main limitations of our review were the small number of included studies and substantial heterogeneity in their enrolled subjects. In view of the significant heterogeneity, we were unable to perform a meta-analysis of all included studies. However, we partially overcame this limitation by including only those studies in our meta-analysis which enrolled middle-aged and elderly nonsmokers not screened based on biomass fuel exposure. The paucity of studies precluded further exploration of heterogeneity using additional subgroup analyses of age, gender, and place of residence on the prevalence. These results obtained from limited evidence are suboptimal for extrapolation to the whole of India, which is a vast and diverse country. Simultaneously, this is a wake-up call regarding the lack of epidemiological data about COPD among nonsmokers in India.
Conclusions and future directions
In conclusion, few studies have examined the prevalence of COPD among nonsmokers in India. While the estimated prevalence is 3 % among middle-aged and older nonsmokers in the community, it may be about 10 % among biomass fuel-exposed individuals. Given India’s sizeable nonsmoking population and its widespread usage of biomass fuel for cooking, this implies that more than 9 million nonsmoker adults in India already have COPD. Furthermore, India’s increasing outdoor air pollution levels and rising median age will further expose tens of millions of nonsmokers to developing COPD in the coming years.
Considering that nonsmokers with COPD represent a different phenotype associated with female preponderance, lesser emphysema, and higher small airway disease, it is concerning that measly research has been devoted to therapies for nonsmokers with COPD. Even lesser attention has been paid to the most vulnerable sufferers of this neglected condition; i.e., those residing in LMICs. Future studies need to examine not just the epidemiological footprint of COPD among Indian nonsmokers but also its pathophysiology, morbidity, economic impact, and unique management aspects in LMICs worldwide. Since the current estimate of nonsmokers suffering from COPD in LMICs is based on a few small studies and likely imprecise, large multinational studies can shed further light on the true burden of nonsmoker COPD. Such information would help prioritize resource-allocation for the research and management of this grossly underappreciated condition. Long-term cohorts in LMICs can help unravel patterns of spirometric declines and the natural history of nonsmoker COPD in association with impaired childhood lung development and environmental exposures like air pollution and biomass fuel usage. A better understanding of pathophysiology of nonsmoker COPD will enable clinical trials enrolling nonsmokers with COPD for tailored pharmacological and non-pharmacological interventions including exposure mitigation. Finally, economic evaluation of these management strategies would help optimize treatment of nonsmoker COPD in LMICs.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Adeloye, D, Song, P, Zhu, Y, Campbell, H, Sheikh, A, Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022;10:447–58. https://doi.org/10.1016/s2213-2600(21)00511-7.Search in Google Scholar PubMed PubMed Central
2. The top 10 causes of death [Internet]. [cited 2023 Oct 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.Search in Google Scholar
3. Safiri, S, Carson-Chahhoud, K, Noori, M, Nejadghaderi, SA, Sullman, MJM, Ahmadian Heris, J, et al.. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ 2022;378:e069679. https://doi.org/10.1136/bmj-2021-069679.Search in Google Scholar PubMed PubMed Central
4. Salvi, S, Kumar, GA, Dhaliwal, RS, Paulson, K, Agrawal, A, Koul, PA, et al.. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health 2018;6:e1363–74. https://doi.org/10.1016/s2214-109x(18)30409-1.Search in Google Scholar PubMed PubMed Central
5. Cunalata-Paredes, AV, Gea-Izquierdo, E. COPD in the major nonsmoking adult: a systematic review and meta-analysis. Arch Environ Occup Health 2021;76:319–29. https://doi.org/10.1080/19338244.2020.1828243.Search in Google Scholar PubMed
6. Fuller-Thomson, E, Chisholm, RS, Brennenstuhl, S. COPD in a population-based sample of never-smokers: interactions among sex, gender, and race. Int J Chron Dis 2016;2016:1–7. https://doi.org/10.1155/2016/5862026.Search in Google Scholar PubMed PubMed Central
7. Terzikhan, N, Verhamme, KMC, Hofman, A, Stricker, BH, Brusselle, GG, Lahousse, L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol 2016;31:785–92. https://doi.org/10.1007/s10654-016-0132-z.Search in Google Scholar PubMed PubMed Central
8. Pathak, U, Kumar, R, Suri, TM, Suri, JC, Gupta, NC, Pathak, S. Impact of biomass fuel exposure from traditional stoves on lung functions in adult women of a rural Indian village. Lung India 2019;36:376–83. https://doi.org/10.4103/lungindia.lungindia_477_18.Search in Google Scholar PubMed PubMed Central
9. Zhang, X, Zhu, X, Wang, X, Wang, L, Sun, H, Yuan, P, et al.. Association of exposure to biomass fuels with occurrence of chronic obstructive pulmonary disease in rural Western China: a Real-World Nested Case-Control Study. COPD 2023;18:2207–24. https://doi.org/10.2147/copd.s417600.Search in Google Scholar
10. James, BS, Shetty, RS, Kamath, A, Shetty, A. Household cooking fuel use and its health effects among rural women in southern India—a cross-sectional study. PLoS One 2020;15:e0231757. https://doi.org/10.1371/journal.pone.0231757.Search in Google Scholar PubMed PubMed Central
11. Salvi, SS, Brashier, BB, Londhe, J, Pyasi, K, Vincent, V, Kajale, SS, et al.. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res 2020;21:50. https://doi.org/10.1186/s12931-020-1310-9.Search in Google Scholar PubMed PubMed Central
12. Tan, WC, Sin, DD, Bourbeau, J, Hernandez, P, Chapman, KR, Cowie, R, et al.. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax 2015;70:822–9. https://doi.org/10.1136/thoraxjnl-2015-206938.Search in Google Scholar PubMed
13. Hagstad, S, Backman, H, Bjerg, A, Ekerljung, L, Ye, X, Hedman, L, et al.. Prevalence and risk factors of COPD among never-smokers in two areas of Sweden – occupational exposure to gas, dust or fumes is an important risk factor. Respir Med 2015;109:1439–45. https://doi.org/10.1016/j.rmed.2015.09.012.Search in Google Scholar PubMed
14. Lee, SH, Hwang, ED, Lim, JE, Moon, S, Kang, YA, Jung, JY, et al.. The risk factors and characteristics of COPD among nonsmokers in Korea: an analysis of KNHANES IV and V. Lung 2016;194:353–61. https://doi.org/10.1007/s00408-016-9871-6.Search in Google Scholar PubMed
15. Jarhyan, P, Hutchinson, A, Khaw, D, Prabhakaran, D, Mohan, S. Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: a systematic review and meta-analysis. Bull World Health Organ 2022;100:216–30. https://doi.org/10.2471/blt.21.286870.Search in Google Scholar PubMed PubMed Central
16. Munn, Z, Moola, S, Lisy, K, Riitano, D, Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Base Healthc 2015;13:147–53. https://doi.org/10.1097/xeb.0000000000000054.Search in Google Scholar PubMed
17. Dyussenbayev, A. Age periods of human life. Adv Soc Sci Res J 2017;4:258-63. https://doi.org/10.14738/assrj.46.2924. [Internet] [cited 2023 Jul 12] Available from: https://journals.scholarpublishing.org/index.php/ASSRJ/article/view/2924.Search in Google Scholar
18. Shinan-Altman, S, Werner, P. Subjective age and its correlates among middle-aged and older adults. Int J Aging Hum Dev 2019;88:3–21. https://doi.org/10.1177/0091415017752941.Search in Google Scholar PubMed
19. Dettori, JR, Norvell, DC, Chapman, JR. Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine J 2022;12:1624–6. https://doi.org/10.1177/21925682221110527.Search in Google Scholar PubMed PubMed Central
20. Hunter, JP, Saratzis, A, Sutton, AJ, Boucher, RH, Sayers, RD, Bown, MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014;67:897–903. https://doi.org/10.1016/j.jclinepi.2014.03.003.Search in Google Scholar PubMed
21. Johnson, P, Balakrishnan, K, Ramaswamy, P, Ghosh, S, Sadhasivam, M, Abirami, O, et al.. Prevalence of chronic obstructive pulmonary disease in rural women of Tamilnadu: implications for refining disease burden assessments attributable to household biomass combustion. Glob Health Action 2011;4:7226. https://doi.org/10.3402/gha.v4i0.7226.Search in Google Scholar PubMed PubMed Central
22. Dutta, A, Ray, MR. Hypertension and respiratory health in biomass smoke-exposed premenopausal Indian women. Air Qual Atmos Health 2014;7:229–38. https://doi.org/10.1007/s11869-013-0228-5.Search in Google Scholar
23. Parasuramalu, B, Huliraj, N, Prashanth Kumar, S, Ramesh Masthi, N, Srinivasa Babu, C, Gangaboraiah. Prevalence of chronic obstructive pulmonary disease and its association with tobacco smoking and environmental tobacco smoke exposure among rural population. Indian J Publ Health 2014;58:45. https://doi.org/10.4103/0019-557x.128166.Search in Google Scholar
24. KalagoudaMahishale, V, Angadi, N, Metgudmath, V, Lolly, M, Eti, A, Khan, S. The prevalence of chronic obstructive pulmonary disease and the determinants of underdiagnosis in women exposed to biomass fuel in India – a cross section study. Chonnam Med J 2016;52:117. https://doi.org/10.4068/cmj.2016.52.2.117.Search in Google Scholar PubMed PubMed Central
25. Sinha, B, Vibha, SR, Chowdhury, R. An epidemiological profile of chronic obstructive pulmonary disease: a community-based study in Delhi. J Postgrad Med 2017;63:29. https://doi.org/10.4103/0022-3859.194200.Search in Google Scholar PubMed PubMed Central
26. Christopher, DJ, Oommen, AM, George, K, Shankar, D, Agrawal, A, Thangakunam, B. Prevalence of airflow obstruction as measured by spirometry, in rural southern Indian adults. COPD 2020;17:128–35. https://doi.org/10.1080/15412555.2020.1723074.Search in Google Scholar PubMed
27. Kumar, A, Kaur, R, Hadda, V, Mani, K, Nongkynrih, B, Gupta, S, et al.. Spirometry-based prevalence of chronic obstructive pulmonary disease & associated factors among community-dwelling rural elderly. Indian J Med Res 2021;154:707. https://doi.org/10.4103/ijmr.ijmr_358_19.Search in Google Scholar PubMed PubMed Central
28. Bajpai, J, Kant, S, Bajaj, D, Pradhan, A, Srivastava, K, Pandey, A. Clinical, demographic and radiological profile of smoker COPD versus nonsmoker COPD patients at a tertiary care center in North India. J Fam Med Prim Care 2019;8:2364. https://doi.org/10.4103/jfmpc.jfmpc_347_19.Search in Google Scholar PubMed PubMed Central
29. Mahmood, T, Singh, R, Kant, S, Shukla, A, Chandra, A, Srivastava, R. Prevalence and etiological profile of chronic obstructive pulmonary disease in nonsmokers. Lung India 2017;34:122. https://doi.org/10.4103/0970-2113.201298.Search in Google Scholar PubMed PubMed Central
30. Brashier, B, Gangavane, S, Valsa, S, Gaikwad, S, Ghorpade, S, Mandrekar, S. Almost half the patients treated for pulmonary tuberculosis (TB) show evidence of obstructive airways disease (OAD). Sweden: Stockholm; 2007:15–9 pp.Search in Google Scholar
31. Rai, B, Bramhankar, M. Tobacco use among Indian states: key findings from the latest demographic health survey 2019–2020. Tob Prev Cessat 2021;7:19. https://doi.org/10.18332/tpc/132466.Search in Google Scholar PubMed PubMed Central
32. Balakrishnan, K, Dey, S, Gupta, T, Dhaliwal, RS, Brauer, M, Cohen, AJ, et al.. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet Health 2019;3:e26–39. https://doi.org/10.1016/s2542-5196(18)30261-4.Search in Google Scholar PubMed PubMed Central
33. Brahmanandam, N, Nagarajan, R. Impact of change in household environment condition on morbidity in India: evidence from longitudinal data. PLoS One 2021;16:e0247465. https://doi.org/10.1371/journal.pone.0247465.Search in Google Scholar PubMed PubMed Central
34. APA Dictionary of Psychology [Internet]. [cited 2023 Oct 25]. Available from: https://dictionary.apa.org/.Search in Google Scholar
35. Demographic Yearbook 72nd Issue [Internet]. United Nations Department of Economic and Social Affairs; 2021 [cited 2023 Oct 25]. Available from: https://unstats.un.org/unsd/demographic-social/products/dyb/dybsets/2021.pdf.Search in Google Scholar
36. Waked, M, Salameh, K, Salameh, P. Correlates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional study. COPD 2012;7:577. https://doi.org/10.2147/copd.s35044.Search in Google Scholar
37. Denguezli, M, Daldoul, H, Harrabi, I, Gnatiuc, L, Coton, S, Burney, P, et al.. COPD in nonsmokers: reports from the Tunisian population-based burden of obstructive lung disease study. In: Behrens, T, editor. PLoS One 2016;11:e0151981.10.1371/journal.pone.0151981Search in Google Scholar PubMed PubMed Central
38. Bakr, RM, Elmahallawy, II. Prevalence characteristics of COPD in never smokers. Egypt J Chest Dis Tuberc 2012;61:59–65. https://doi.org/10.1016/j.ejcdt.2012.10.035.Search in Google Scholar
39. Brakema, EA, van Gemert, FA, van der Kleij, RMJJ, Salvi, S, Puhan, M, Chavannes, NH, et al.. COPD’s early origins in low-and-middle income countries: what are the implications of a false start? NPJ Prim Care Respir Med 2019;29:6. https://doi.org/10.1038/s41533-019-0117-y.Search in Google Scholar PubMed PubMed Central
40. Pleasants, RA, Riley, IL, Mannino, DM. Defining and targeting health disparities in chronic obstructive pulmonary disease. COPD 2016;11:2475–96. https://doi.org/10.2147/copd.s79077.Search in Google Scholar
41. Jindal, SK, Aggarwal, AN, Gupta, D, Agarwal, R, Kumar, R, Kaur, T, et al.. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tubercul Lung Dis 2012;16:1270–7. https://doi.org/10.5588/ijtld.12.0005.Search in Google Scholar PubMed
42. Mahesh, PA, Jayaraj, BS, Prahlad, ST, Chaya, SK, Prabhakar, AK, Agarwal, AN, et al.. Validation of a structured questionnaire for COPD and prevalence of COPD in rural area of Mysore: a pilot study. Lung India 2009;26:63–9. https://doi.org/10.4103/0970-2113.53226.Search in Google Scholar PubMed PubMed Central
43. Borlée, F, Yzermans, CJ, Krop, E, Aalders, B, Rooijackers, J, Zock, JP, et al.. Spirometry, questionnaire and electronic medical record based COPD in a population survey: comparing prevalence, level of agreement and associations with potential risk factors. PLoS One 2017;12:e0171494. https://doi.org/10.1371/journal.pone.0171494.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2023-0135).
© 2023 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Reviews
- Mercury and cadmium-induced inflammatory cytokines activation and its effect on the risk of preeclampsia: a review
- Prevalence of chronic obstructive pulmonary disease in Indian nonsmokers: a systematic review & meta-analysis
- Beyond the outdoors: indoor air quality guidelines and standards – challenges, inequalities, and the path forward
- Cadmium exposure and thyroid hormone disruption: a systematic review and meta-analysis
- New generation sequencing: molecular approaches for the detection and monitoring of bioaerosols in an indoor environment: a systematic review
- Concentration of Tetrabromobisphenol-A in fish: systematic review and meta-analysis and probabilistic health risk assessment
- The association between indoor air pollution from solid fuels and cognitive impairment: a systematic review and meta-analysis
- Phthalates and uterine disorders
- Effectiveness of educational interventions for the prevention of lead poisoning in children: a systematic review
- Association between exposure to per- and polyfluoroalkyl substances and levels of lipid profile based on human studies
- Summary of seven Swedish case reports on the microwave syndrome associated with 5G radiofrequency radiation
- Expanding the focus of the One Health concept: links between the Earth-system processes of the planetary boundaries framework and antibiotic resistance
- Exploring the link between ambient PM2.5 concentrations and respiratory diseases in the elderly: a study in the Muang district of Khon Kaen, Thailand
- Standards for levels of lead in soil and dust around the world
- Tributyltin induces apoptosis in mammalian cells in vivo: a scoping review
- The influence of geology on the quality of groundwater for domestic use: a Kenyan review
- Biological concentrations of DDT metabolites and breast cancer risk: an updated systematic review and meta-analysis
- Letter to the Editor
- Ancient medicine and famous iranian physicians
Articles in the same Issue
- Frontmatter
- Reviews
- Mercury and cadmium-induced inflammatory cytokines activation and its effect on the risk of preeclampsia: a review
- Prevalence of chronic obstructive pulmonary disease in Indian nonsmokers: a systematic review & meta-analysis
- Beyond the outdoors: indoor air quality guidelines and standards – challenges, inequalities, and the path forward
- Cadmium exposure and thyroid hormone disruption: a systematic review and meta-analysis
- New generation sequencing: molecular approaches for the detection and monitoring of bioaerosols in an indoor environment: a systematic review
- Concentration of Tetrabromobisphenol-A in fish: systematic review and meta-analysis and probabilistic health risk assessment
- The association between indoor air pollution from solid fuels and cognitive impairment: a systematic review and meta-analysis
- Phthalates and uterine disorders
- Effectiveness of educational interventions for the prevention of lead poisoning in children: a systematic review
- Association between exposure to per- and polyfluoroalkyl substances and levels of lipid profile based on human studies
- Summary of seven Swedish case reports on the microwave syndrome associated with 5G radiofrequency radiation
- Expanding the focus of the One Health concept: links between the Earth-system processes of the planetary boundaries framework and antibiotic resistance
- Exploring the link between ambient PM2.5 concentrations and respiratory diseases in the elderly: a study in the Muang district of Khon Kaen, Thailand
- Standards for levels of lead in soil and dust around the world
- Tributyltin induces apoptosis in mammalian cells in vivo: a scoping review
- The influence of geology on the quality of groundwater for domestic use: a Kenyan review
- Biological concentrations of DDT metabolites and breast cancer risk: an updated systematic review and meta-analysis
- Letter to the Editor
- Ancient medicine and famous iranian physicians

