Abstract
Cloud point extraction is a green alternative for separation and preconcentration, whose advantages are magnified by coupling with flow analysis. This results in fast extractions, with improved precision and lower reagent consumption and waste generation. Mechanization has been carried out mainly by flow injection analysis, but feasibility of innovative approaches including sequential injection analysis, multipumping flow systems and lab-in-syringe approaches have also been demonstrated. The approaches for flow-based cloud point extraction are critically revised by taking system designs and operational aspects into account. Applications in environmental, agronomic and food samples as well as biological fluids are also discussed.
Introduction
Cloud point extraction (CPE) is a green alternative for separation and preconcentration with the use of surfactants, which was proposed to circumvent the drawbacks of classical liquid-liquid extraction (LLE). These drawbacks include laborious and time-consuming procedures with the use of flammable, toxic and sometimes carcinogenic solvents. On the other hand, the surfactants usually employed in CPE show low toxicity, are non-flammable, non-volatile and some of them are biodegradable. The analytical application of CPE was first presented by Watanabe and Tanaka (1978) based on the cloud point phenomenon exhibited by non-ionic surfactants above the critical micelle concentration (CMC). By increasing the temperature or by electrolyte addition (salting-out effect) in solutions of non-ionic or zwitterionic surfactants, two isotropic phases are formed, one concentrated in surfactant (named as surfactant-rich phase, SRP) and a diluted aqueous phase containing the surfactant in a concentration close to the CMC, referred to as the surfactant-poor phase (Paleologos et al. 2005). Phase separation is reversible by, for example, cooling the mixture below the cloud point (Silva et al. 2006, Xie et al. 2010). The phenomenon can be exploited for the extraction of hydrophobic species entrapped inside the micelles (Pharr 2011) and hydrophilic ones, which interact with the external part of the aggregates. CPE has been combined with analytical techniques such as flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ETAAS), inductively coupled plasma optical emission spectrometry (ICP OES), inductively coupled plasma mass spectrometry (ICP-MS) and UV-Vis spectrophotometry, mainly for extraction of metal ions complexed with organic ligands (Bezerra et al. 2005). In this sense, CPE stands out because complex formation and extraction in the micellar aggregates occur in a single phase before separation (Stalikas 2002, Ojeda and Rojas 2012), thus yielding high extraction efficiencies. Furthermore, CPE has been employed for preconcentration of hydrophobic persistent organic pollutants before separation by high performance liquid chromatography (HPLC), gas chromatography or capillary zone electrophoresis (CZE) (Xie et al. 2010).
Another advantageous applicability of CPE is for sample clean-up, but only a few studies have been reported for such purposes, for example, removal of fats before melamine determination in milk (Nascimento et al. 2015), removal of organic matter on carbaryl determination in natural waters (Melchert and Rocha 2009) and separation of organic species containing phosphorous on nickel determination in plant materials (Silva et al. 2009). Analyte extraction by CPE and elution with a diluted acid solution has also potential to minimize background signals and then to improve the detection limits in spectrometric techniques.
Recent studies have explored CPE for metal speciation. For example, determination of arsenic species was based on CPE of the ternary complex of As(V) with acridine orange and tartaric acid using Triton X-114. The determination of total As was performed after oxidation of As(III) to As(V) with hydrogen peroxide in an alkaline medium (Gurkan et al. 2015). The other application for trace inorganic As determination in water samples was based on CPE combined with hydride generation atomic fluorescence spectrometry. As(III) and As(V) were complexed with ammonium pyrrolidinedithiocarbamate and molybdate, respectively, and extracted with Triton X-114. The fluorescence signal was lower for the As(V) because the AsH3 generation is slower; then As(V) was reduced to As(III) with a thiourea-ascorbic acid solution, before detection (Li et al. 2015). Chromium speciation at sub-ppb levels was based on CPE of Cr(III) bounded to silver nanoparticles (AgNPs) before determination by ETAAS. Without addition of ethylenediaminetetraacetic acid (EDTA), the total amount is measured because Cr(VI) is converted to Cr(III) by the excess of reductor present in the AgNPs suspension. As the Cr(III)-EDTA complex is not bounded to the nanoparticles, this species is not extracted to the SRP. Due to the slow kinetics of Cr(III) complexation by the EDTA compared with the rate of the retention of this species on AgNPs, the Cr(III) produced by the reduction of Cr(VI) is extracted, allowing differentiation from Cr(III) in the solution. The Cr(III) concentration was then determined by difference from the signal obtained without and with EDTA (López-García et al. 2015).
Flow-based CPE has been exploited to circumvent the limitations of the batch procedures, which are often time-consuming and susceptible to systematic errors due to losses of the SRP in the separation process (Fang et al. 2001, Yamini et al. 2008, Frizzarin and Rocha 2014). The general features of this coupling are highlighted in Figure 1. Flow analysis improves the sample throughput and makes CPE more reproducible by mechanizing the different steps involved in the process. As a consequence, precision is better than the usually achieved in batch procedures. However, enrichment factors (EF) are often lessened because of the lower sample volumes, although the consumptive indexes (CI) are comparable to or better than the achieved in batch. The first hyphenation of CPE to flow injection analysis (FIA) was reported by Fang et al. (2001). Solvent consumption and preconcentration time were drastically minimized due to the inherent characteristics of FIA. Additionally, a low volume of wastes was generated and both sample contamination risks and dilution of the SRP were reduced. Further, the analytical performance of batch and flow-based CPE was compared for extraction of polycyclic aromatic hydrocarbons (PAHs) by Song et al. (2006). The flow-based approach yielded better EF (40.2 vs. 19.8), even with a 7.4-fold lower sample volume. Advantages in relation to precision and sample throughput were also highlighted.

Advantages of coupling flow analysis with cloud point extraction.
A literature survey in the Web of Science database (December 2015) returned 1669 articles about CPE with applications in chemistry. These articles received 31,075 citations, reaching more than 4200 citations per year since 2013 (h=74). In addition, 123 review articles were published on the subject. On the other hand, the number of works on flow-based CPE is scarce, but the average number of citations is twice those for batch CPE (32.4 vs. 18.6 citations per article). These indicators characterize batch CPE as a well-established extraction technique, whereas flow-based CPE is yet an emerging research field.
The aim of this review is to highlight the potential, limitations and applications of coupling CPE and flow analysis, including discussion of operational aspects and system designs.
Operational aspects
Batch CPE usually involves a time-consuming phase separation step (e.g. by centrifugation), and dilution of the SRP is often required to diminish its viscosity and to match the volume with the required in the measurement technique. After these steps, the analytical measurement can be carried out in a flow-based system. As the main steps involved in CPE are carried out in batch, these works are out of the scope of this review. The first attempt for mechanization is the use of a flow-based system for separation of SRP produced in a batch procedure, followed by online elution toward detection (Ortega et al. 2002, Bai and Fan 2007, Gil et al. 2008, Yamini et al. 2008, Silva and Roldan 2009, Şahin et al. 2010, Durukan et al. 2011, Bakircioglu 2012, Baliza et al. 2012). This general approach (Figure 2A) usually involves relatively high sample volumes and surfactant consumption (as high as 500 mg per determination) (Silva and Roldan 2009); high flow-rates are often exploited to avoid hindering the sample throughput, but this can limit by the efficiency of retention of SRP (Durukan et al. 2011).

Schematic representations of the strategies for separation and elution of the SRP after batch (A) and flow-based (B–D) CPE.
S, Sample; R, reagent/surfactant solution; E, eluent; C, mini-column for SRP retention; B, coiled reactor (dashed lines indicated that this device can be heated); D, detection system; W, waste vessel.
The general strategy for flow-based CPE is essentially the same as used in the pioneer application (Fang et al. 2001). By exploiting time-based sampling, the sample, the derivatizing reagents and the surfactant are mixed by confluence and the cloud point is induced either by heating of the sample zone (Ortega et al. 2003, 2004, Dalali et al. 2008, Zahedi et al. 2009, Lemos and David 2010, Bakircioglu 2012, Baliza et al. 2012, Serenjeh et al. 2014), by the salting-out effect (Fang et al. 2001, Paleologos et al. 2003, Song et al. 2006, Lemos et al. 2008, Li et al. 2008, Kara 2009, Silva and Roldan 2009, Javadi and Dalali 2011) or both (Garrido et al. 2004). After separation, the SRP is eluted to the detection system. This general strategy is illustrated in Figure 2B. Variations include the intermittent addition of the surfactant in the multipumping (Frizzarin and Rocha 2014) and sequential injection analysis (SIA) (Lemos et al. 2008) approaches. This allows a significant reduction of the surfactant consumption, for example, from 150 (Li et al. 2006) to 0.2 mg (Lemos et al. 2008) per determination. Other variants of the process include the eluent injection for removal of the SRP from the retention mini-column (Figure 2C) (Kara 2009) or its retention at the measurement system to avoid the effect of dilution by the eluent before detection (Figure 2D) (Lu et al. 2007, Frizzarin and Rocha 2014).
General information about the surfactants used in flow-based CPE is shown in Table 1 (Hinze and Pramauro 1993), whereas Tables 2 and 3 show the main operational aspects. Most applications (ca. 64%) are based on Triton X-114 because of the convenient cloud point, close to the ambient temperature (from 22 to 25°C, depending on the surfactant concentration). Other advantages include low toxicity and low cost. Polyethyleneglycol-mono-p-nonylphenylether (PONPE 7.5) is also usual due to its cloud point below the ambient temperature (Ortega et al. 2002, 2003, 2004, Gil et al. 2008, 2010). Other surfactants for flow-based CPE were Triton X-100 (Garrido et al. 2004, Cao et al. 2013, Serenjeh et al. 2014) and Tergitol 15-S-7 (Li et al. 2008), which exhibit cloud point close to 65 and 38°C, respectively. Sodium dodecyl sulfate (SDS) has also been exploited in micelle-mediated extractions (Kara 2009, Acosta et al. 2014), although the CPE is characteristic of non-ionic and zwitterionic surfactants. The Triton X-114 concentration in the sample zone varied from 0.04 (Lemos et al. 2008) to 50 g l-1 (Paleologos et al. 2003) (Tables 2 and 3), which corresponds to the excess of up to 266-fold in relation to the CMC. This amount affects the extraction efficiency and the volume of SRP (and thus the efficiency of phase separation). Excess of surfactant can also cause undesirable dilution, thus hindering the EF.
Structure and micellar characteristics of non-ionic surfactants used in flow-based CPE (Hinze and Pramauro 1993).
| Surfactant | Abbreviation | Structure | CMC (mmol l-1) | Cloud point (°C) | CAS number |
|---|---|---|---|---|---|
| t-Octylphenoxy polyoxyethylene ethers | Triton X-100 |  | 0.17–0.99 | 64–65 | 9002-93-1 |
| Triton X-114 | 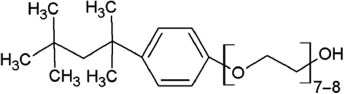 | 0.20–0.35 | 22–25 | 9036-19-5 | |
| Polyoxyethylene nonylphenyl ethers | PONPE 7.5 | 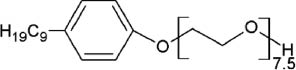 | 0.085 | 5 | 26264-02-8 |
| Alkyloxy polyethylene oxyethanol | Tergitol 15-S-7 | 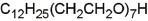 | 0.076 | 37.8 | 68131-40-8 |
CMC, Critical micelle concentration.
Operational parameters of flow-based CPE with Triton X-114.
| Flow system | Cloud point induction | Eluenta | Consumption per extraction (mg)b | Concentration in sample zone (g l-1)b | References |
|---|---|---|---|---|---|
| FIA (CPE offline) | Heating | H2SO4 (0.05)+ethanol | 50 | 0.9 | Durukan et al. 2011 |
| Heating | H2SO4 (0.05) | 25 | 0.9 | Şahin et al. 2010 | |
| Heating | HCl (0.5) | 2.64 | 0.3 | Baliza et al. 2012 | |
| Salting-out (NaCl) | HCl (3.0) | 500 | 0.5 | Silva and Roldan 2009 | |
| Ambient temperature | Methanol | 1.9 | 0.3 | Bai and Fan 2007 | |
| Heating – water bath | HNO3 (1.0)+methanol | 5.3 | 0.2 | Bakircioglu 2012 | |
| FIA | Salting-out (Na2SO4) | HNO3 (0.14)+ethanol | 12 | 0.3 | Javadi and Dalali 2011 |
| Ambient temperature | Acetonitrile | 1.35 | 0.4 | Nan et al. 2003 | |
| Heating – water bath | HNO3 (0.2)+ethanol | 3.5 | 0.7 | Zahedi et al. 2009 | |
| Salting-out (Na2SO4) | 90 g l-1 Triton X-100 | 20 | 50.0 | Paleologos et al. 2003 | |
| Ambient temperature | Acetonitrile | 150 | 1.5 | Li et al. 2006 | |
| Heating – water bath | Tetrahydrofuran | 2.1 | 0.5 | Dalali et al. 2008 | |
| Heating – water bath | HNO3 (0.25) | 13.2 | 0.9 | Lemos and David 2010 | |
| Ambient temperature | HCl (0.5) | – | 1.0 | Li and Hu 2010 | |
| Salting-out (Na2SO4) | Acetonitrile | 39.7 | 5.3 | Song et al. 2006 | |
| Salting-out (NH4)2SO4) | Acetonitrile/water | 2.7 | 0.5 | Fang et al. 2001 | |
| Salting-out (Na2SO4) | Acetonitrile:water 1:10 (v/v) | 7.6 | 2.0 | Lu et al. 2007 | |
| Heating | HNO3 (0.5)+75% propanol | 25 | 1.0 | Yamini et al. 2008 | |
| SIA | Salting-out (NaCl) | H2SO4 (0.05) | 0.2 | 0.04 | Lemos et al. 2008 |
| MPFS | Heating – chemical reaction | Water | 1.95 | 4.8 | Frizzarin and Rocha 2014 |
| Lab-in-syringe | Heating – dilution of H2SO4 | Not required | 2.0 | 0.5 | Frizzarin et al. 2016 |
FIA, Flow injection analysis; MPFS, multipumping flow system; SIA, sequential injection analysis.
aNumbers between parentheses are concentrations in mol l-1.
bData refers to Triton X-114 surfactant.
Operational parameters of flow injection systems with less usual surfactants.
| Surfactant | Cloud point induction | Eluenta | References | ||
|---|---|---|---|---|---|
| Consumption per extraction (mg) | Concentration in sample zone (g l-1) | ||||
| Triton X-100 | 13.5 | 0.5 | Electromagnetic induction assisted heating | Borax (0.5)+10% ethanol | Cao et al. 2013 |
| 54 | 7.7 | Heating+salting-out (Na2SO4) | Hot water | Garrido et al. 2004 | |
| 21.4 | 4.3 | Resistive heating | Ethanol | Serenjeh et al. 2014 | |
| PONPE 7.5 | 396 | 13.3 | Ambient temperature | HNO3 (1.4) | Gil et al. 2010 |
| 20 | 1.7 | HNO3 (4.0) | Ortega et al. 2002 | ||
| 40 | 8.0 | HNO3 (0.8)+methanol | Gil et al. 2008 | ||
| 125 | 2.0 | Heating – mixing coil at 30°C | HNO3 (4.0) | Ortega et al. 2003 | |
| 25 | 2.0 | Acetonitrile | Ortega et al. 2004 | ||
| Tergitol 15-S-7 | 9.9 | 4.4 | Salting-out ((NH4)2SO4) | Acetonitrile:water 7:3 (v/v) | Li et al. 2008 |
aNumbers between parentheses are concentrations in mol l-1.
Induction of cloud point has been usually based on external heating (Ortega et al. 2003, 2004, Dalali et al. 2008, Zahedi et al. 2009, Lemos and David 2010, Serenjeh et al. 2014), usually in a temperature-controlled water bath. Innovative approaches have exploited the heat released in an online neutralization reaction (Frizzarin and Rocha 2014) or the heat of dilution of an H2SO4 solution (Frizzarin et al. 2016). Both strategies provided temperatures higher than 30°C, which was enough for phase separation using Triton X-114. The former approach also increased the salt concentration due to neutralization of the acid in the sample digests. Alternatively, Na2SO4 has been the preferred salt to exploit the salting-out effect (Paleologos et al. 2003, Garrido et al. 2004, Song et al. 2006, Dalali et al. 2008, Javadi and Dalali 2011), but (NH4)2SO4 (Fang et al. 2001, Li et al. 2008) and NaCl (Lemos et al. 2008, Kara 2009, Silva and Roldan 2009) have also been used. The aim is decreasing the surfactant cloud point for phase separation at ambient temperature. The exploitation of the salting-out effect is advantageous by avoiding the use of an additional device (e.g. a temperature-controlled water bath). On the other hand, it can be critical when detection is based on spectrometric techniques with sample introduction as an aerosol, especially in plasma techniques, such as ICP OES and ICP-MS, whose introduction of samples with high salt contents can be a hindrance. The joint effect of salting-out and heating was exploited to minimize bubble generation in CPE with Triton X-100 (Garrido et al. 2004). However, several works have carried out CPE at ambient temperature even without salt addition in view of the characteristics of the surfactants, that is, Triton X-114 (Li et al. 2006, Bai and Fan 2007, Li and Hu 2010) or PONPE 7.5 (Ortega et al. 2002, Gil et al. 2008, 2010). An ingenious approach is the electromagnetic induction-assisted heating, which exploits coupling of an alternating magnetic field with iron particles inside a mini-column (Cao et al. 2013). The magnetic field is produced by applying an alternating current to a copper wire coiled around a quartz tube, yielding temperatures as high as 80°C when the power was set at 140 W. Advantages such as fast heating rate, good reproducibility and low energy consumption were highlighted. An interesting device that simulates the experimental conditions adopted for batch CPE was proposed for determination of phenazopyridine in human serum (Serenjeh et al. 2014). It comprises a heating device for induction of the cloud point and a cooling mode for retention of the SRP, which can be eluted with a solvent heated in the same device.
CPE is usually exploited for extraction of hydrophobic species, which are entrapped inside the surfactant micelles. Thus, extraction of hydrophilic species generally requires previous chemical derivatization, such as formation of hydrophobic complexes with metal ions. A diversity of ligands for CPE of metal ions was highlighted in a review article (Pytlakowska et al. 2013). The involved reactions need to be thermodynamically favorable and relatively fast for application for flow-based extractions. As this process can be affected by variables such as pH, temperature and ionic strength, the effect of these parameters needs to be carefully evaluated. A possible hindrance is that the hydrophobic reagent can also be extracted, thus yielding high blank signals in spectrophotometric determinations.
The critical aspect in flow-based CPE is the separation of the SRP. Alternatively to the centrifugation used in batch procedures, a mini-column filled with a filtering material [usually cotton, but also glass wool (Acosta et al. 2014), animal wool (Bakircioglu 2012), polyester (Baliza et al. 2012), polytetrafluorethylene particles (Gil et al. 2010), silica-gel (Nan et al. 2003, Bai and Fan 2007, Li et al. 2008, Li and Hu 2010) and C18-bonded silica (Serenjeh et al. 2014)] is used for retention. The separation efficiency depends on the flow rate, the type and amount of filtering material (or the geometry and length of the knotted reactor) and volume of the SRP. In relation to the latter, the extraction efficiency gradually decreased from 97% to 39%, when the sample volume increased from 0.4 to 5.4 ml (Song et al. 2006). This was due to the restricted capability of the filtering mini-column (filled with 0.03 g cotton) to retain the SRP. The efficiency of phase separation is also hindered in batch CPE with large sample volumes and a centrifuge with temperature control may be needed. Knotted reactors (Gil et al. 2008) or phase separation by density (Frizzarin et al. 2016) has replaced the packed mini-columns in some applications. Usually, the SRP needs to be eluted before measurement, which is carried out with organic solvents, such as methanol (Bai and Fan 2007), ethanol (Serenjeh et al. 2014), tetrahydrofuran (Dalali et al. 2008), acetonitrile (Nan et al. 2003, Ortega et al. 2004, Li et al. 2006, Song et al. 2006) or even hot water (Garrido et al. 2004) (Tables 2 and 3). As most of the retained species are metal complexes with weakly acid ligands, elution is usually carried out with diluted acids in aqueous media (Ortega et al. 2002, 2003, Lemos et al. 2008, Silva and Roldan 2009, Gil et al. 2010, Lemos and David 2010, Li and Hu 2010, Şahin et al. 2010, Baliza et al. 2012, Acosta et al. 2014) or aqueous organic solutions (Gil et al. 2008, Yamini et al. 2008, Kara 2009, Zahedi et al. 2009, Durukan et al. 2011, Javadi and Dalali 2011, Bakircioglu 2012, Cao et al. 2013). Elution needs to be highly efficient in order to constrain the analyte in an eluent amount significantly lower than the original sample volume. Anyway, elution inherently causes dilution, which is a contradiction in relation to the main aim of CPE, that is, analyte preconcentration. To avoid this drawback, the SRP has been directly retained in the observation area for chemiluminometric (Lu et al. 2007) or spectrophotometric (Frizzarin and Rocha 2014) detection.
Detection has been usually based on atomic spectrometry (FAAS, ETAAS and ICP OES), with the predominance of FAAS. This avoids the drawbacks usually observed in molecular spectrometric techniques, such as light scattering (including the Schlieren effect) and excessive attenuation of the radiation beam caused by excess of the surfactant. In addition, techniques based on sample introduction as an aerosol (e.g. FAAS and ICPs) take advantage of better nebulization efficiency provided by lowering the surface tension in a medium containing surfactant (Stalikas 2002, Silva et al. 2006). However, applications involving UV-Vis spectrophotometry (Garrido et al. 2004, Lemos et al. 2008, Frizzarin and Rocha 2014, Frizzarin et al. 2016), chemiluminescence (Fang et al. 2001, Paleologos et al. 2003, Song et al. 2006, Lu et al. 2007) or fluorescence (Li et al. 2008, Acosta et al. 2014) are also feasible, as well as CPE before separation by CZE (Ortega et al. 2004) or HPLC (Li et al. 2008).
Flow systems
Figures 3 and 4 illustrate the general approaches for flow-based CPE. Figure 3 shows a typical FIA manifold used in most of the applications, in which a sample and reagent are mixed by confluence, the surfactant being added to some of these solutions. In the load position (Figure 3A), the mixture flows through a coiled reactor usually inserted in a heated water bath. The SRP is retained in a mini-column packed with a filtering material and the surfactant poor phase is discarded. In the alternative position of the injection valve (Figure 3B), the mini-column is inserted in a suitable eluent and the retained species are carried toward detection. This approach stands out by its simplicity, but it wastes reagent and surfactant because of their continuous addition. Moreover, a relatively long coiled reactor is needed to assure suitable heat transfer to the sample zone. SIA is an alternative approach, in which discrete aliquots of sample and reagent plus surfactant are aspirated to a holding coil (Figure 4A), which can be heated for formation of the SRP or the reagent can contain an electrolyte to exploit the salting-out effect. In the simplest approach, an eluent aliquot could be first inserted in the holding coil, and an air bubble would be used to minimize mixing with the sample zone. In this way, when the syringe pump is reversed, the SRP is retained in the mini-column for analyte preconcentration, before elution to the detection system. This approach minimizes the reagent and surfactant consumption, an advantageous characteristic inherent to SIA. On the other hand, as the sample to reagent mixing is provided only by dispersion, the sample volume and thus the EF are limited. More recently, the lab-in-syringe (Figure 4B) and the multipumping (Figure 4C) approaches were applied to CPE (Frizzarin and Rocha 2014, Frizzarin et al. 2016). The former approach has been exploited for other kinds of LLE, such as dispersive liquid-liquid microextraction (Maya et al. 2014). For CPE, it is based on the sequential introduction of the sample, reagent and surfactant inside a syringe, in which the solutions are efficiently mixed. If necessary, the syringe could be heated by an external device to induce cloud point. In the simplest approach, the SRP is separated by differences in density (i.e. without filtering) and further carried out toward detection. Thus, the routine for CPE is significantly simplified without hindering the analytical performance and higher EF can be attained because high sample volumes can be used. The multipumping approach (Frizzarin and Rocha 2014), Figure 4C, is characterized by improved mixing conditions and better heat transference provided by pulsed flows (González et al. 2015). These characteristics are attractive for flow-based CPE. Sample, reagent and surfactant solutions are mixed and carried toward a coiled reactor, which can also be heated by an external device. After induction of the cloud point, the SRP is retained in a filtering device. This approach was also exploited for retention of the SRP directly in the detection cell to avoid dilution by the eluent before the spectrophotometric measurement (Frizzarin and Rocha 2014). After measurement, the flow rate was increased and the combined effect of pulsed flow and the absence of the surfactant were effective to remove the SRP without an additional eluent solution (Frizzarin and Rocha 2014).

General manifold for flow-injection CPE in the load (A) and elution (B) positions.
V, Injection valve. Other symbols are defined in the legend of Figure 2.

Alternative manifolds for flow-based CPE.
(A) Sequential injection analysis; (B) lab-in-syringe and (C) multipumping flow system. SV, Selection valve; HC, holding coil; SP, syringe pump; P1–P3, solenoid pumps; SS, surfactant solution; WS, washing solution. Dashed lines indicate devices that can be heated. Other symbols are defined in the legend of Figure 2.
Applications
In spite of the potential for sample clean-up and chemical speciation/fractionation, applications of flow-based CPE have focused essentially on analyte preconcentration. Most applications aimed at extraction of metal ions and detection by FAAS (Table 4). Applications based on other detection techniques are listed in Table 5. The determination of organic species includes benzo[a]pyrene (Song et al. 2006), phenazopyridine (Serenjeh et al. 2014), and PAHs (Li et al. 2008), as well as the extraction of the organometallic coproporphyrin (Fang et al. 2001).
Analytical features of flow-based CPE with FAAS (a) or ETAAS (b) detection.
| Analyte | Sample | EF | CE (min-1) | CI (ml-1) | SR (h-1) | References |
|---|---|---|---|---|---|---|
| Fe and Cua | Food | 141, 99 | – | 0.35, 0.50 | – | Durukan et al. 2011 |
| Fe and Cua | Spice | 98, 69 | – | 0.25, 0.36 | – | Şahin et al. 2010 |
| Cua | Water and hair | 45 | 11.3 | 0.22 | 15 | Javadi and Dalali 2011 |
| Cda | Biological | 27 | – | 0.22 | – | Baliza et al. 2012 |
| Cd and Pba | Water | 20.3, 16.2 | 12.5, 10.0 | 0.74, 0.93 | 37 | Silva and Roldan 2009 |
| Pbb | Water | 21.6 | 7.2 | 0.28 | 20 | Bai and Fan 2007 |
| Pbb | Biological | 22.5 | 11.2 | 0.098 | 30 | Nan et al. 2003 |
| Pba | Water and wastewater | 31 | 5.68 | 0.32 | 11 | Zahedi et al. 2009 |
| Pb and Pda | Environmental | 51, 44 | – | 0.49, 0.57 | – | Bakircioglu 2012 |
| Aga | Water | 38 | 6.3 | 0.11 | 10 | Dalali et al. 2008 |
| Mna | Food | 14 | 11.2 | 0.20 | 48 | Lemos et al. 2008 |
| Mna | Food | 17 | 8.0 | 0.19 | 30 | Lemos and David 2010 |
| Cob | Water | 15 | 6.0 | 0.33 | 24 | Gil et al. 2008 |
| Cd, Co, Cu, Mn, Ni, Pb and Zna | Water and plant | 8.4 | 4.2 | 2.0 | 30 | Kara 2009 |
CE, Concentration efficiency; CI, consumptive index; EF, enrichment factor; SR, sampling rate.
Analytical features of flow-based CPE exploiting other kinds of detection.
| Analyte | Detection | Sample | EF | CE (min-1) | CI (ml-1) | SR (h-1) | References |
|---|---|---|---|---|---|---|---|
| Fe | Spectrophotometry | Freshwater, food, biological samples | 8.9 | 3.9 | 0.022 | 26 | Frizzarin and Rocha 2014 |
| Cd | EMIH | Water | 76 | 6.3 | 0.26 | 5 | Cao et al. 2013 |
| Pb | USN-ICP OES | Water | 150 | 37.5 | 0.067 | 15 | Gil et al. 2010 |
| Sb | Spectrophotometry | Freshwater | 25 | 6.7 | 0.08 | 16 | Frizzarin et al. 2016 |
| Cr | Chemiluminescence | Sea and wastewater | 6.0 | 1.2 | 2.1 | 12 | Paleologos et al. 2003 |
| Hg | Spectrophotometry | Water | 6.0 | 3.0/0.4a | 0.37 | 30/4a | Garrido et al. 2004 |
| Sb | ETV-ICP OES | Urine and water | 872 | 21.8 | 0.11 | 1.5 | Li et al. 2006 |
| Gd | ICP OES | Urine | 20 | 7.3 | 0.50 | 22 | Ortega et al. 2002 |
| V, Cr, Mn, Co, Ni and Cu | ICP OES | Seawater | 14.9, 20.1, 16.2, 17.5, 18.8, 15.9 | 5.0, 6.7, 5.4, 5.8, 6.3, 5.3 | 0.20, 0.15, 0.18, 0.17, 0.16, 0.19 | 20 | Li and Hu 2009 |
| Rare earth elements | ICP OES | Biological | 8.5b | 2.4b | – | 17 | Li and Hu 2010 |
| Dy | ICP OES | Urine | 50 | 6.67 | 1.0 | 8 | Ortega et al. 2003 |
| Dy and Fe | CE-UV | Urine | 200 | 23 | 0.05 | 7 | Ortega et al. 2004 |
| Benzo[a]pyrene | Chemiluminescence | – | 5.2 | 1.73 | 0.66 | 20 | Song et al. 2006 |
| Coproporphyrin | Chemiluminescence | Urine | 9.6 | 4.8 | 0.42 | 30 | Fang et al. 2001 |
| Phenazopyridine | UV | Human serum | 13.3 | 1.33 | 0.30 | 6 | Serenjeh et al. 2014 |
| PAHs | HPLC-fluorescence | Soils | 6.0c | 1.6 | 0.17 | 16c | Li et al. 2008 |
CE, Concentration efficiency; CI, consumptive index; EF, enrichment factor; SR, sampling rate.
aValues estimated considering the online SPE for removing iron interference.
bMean of the values obtained for different elements.
cValues estimated from loading and elution times.
Besides the predominance of procedures for metal ions determination, most of the applications have been focused on water analysis (44.5%), in which these species need to be quantified in low concentrations. Other applications include food and condiments (19.4%), biological fluids (19.4%), samples of agronomical interest (11.1%) and wastewaters (5.6%). High EF were achieved in procedures exploiting the strategy described in Figure 2A because of the relatively higher sample volumes (Ortega et al. 2004, Li et al. 2006). On the other hand, EF can also be maximized by minimizing the eluent volumes, which is feasible when detection is carried out by ETAAS (Li et al. 2006) or CZE (Ortega et al. 2004), in which elution was carried out with 100 or 50 μl of acetonitrile, respectively. Because the sample volumes and flow rates vary significantly, a more realistic comparison of the preconcentration efficiency is possible from the concentration efficiency (CE) and CI. The former corresponds to the EF achieved in 1 min, whereas the second indicates the sample volume (in milliliters) required to achieve a unit EF. From Tables 4 and 5, it can be concluded that both parameters indicate that flow-based CPE yielded results comparable with those attained by the batch analogous, being also competitive with conventional LLE and solid-phase extraction in relation to preconcentration efficiency. This can be exemplified by considering the procedures for iron and manganese preconcentration. For the former, flow-based CPE yielded the lowest CI (0.022 ml) and a CE comparable with those achieved by solid-phase extraction and solid-phase spectrophotometry (Frizzarin and Rocha 2014). Both CI and CE were better than those achieved by LLE (2.3 ml and 2.8 min-1, respectively). The values achieved for flow-based CPE of manganese (Tables 4 and 5) are comparable with those attained by batch CPE (CI: 0.19–1.31 ml and CE: 8.0–12 min-1) and SPE (CI: 0.25–4.0 ml and CE: 0.5–20 min-1) (Oliveira et al. 2013).
Some applications have aimed at simultaneous determinations. Iron and copper were determined in water and food samples (Şahin et al. 2010, Durukan et al. 2011). After batch CPE, the SRP was retained in a cotton mini-column and further eluted directly to the FAAS nebulizer. The same mini-column can be used for up to 50 preconcentration cycles. A similar strategy was employed for the palladium and lead determination in environmental samples (Bakircioglu 2012). CPE provided low detection limits (in order 1.0 μg l-1) and avoided matrix effects. Lead and cadmium determination by FAAS has been proposed (Silva and Roldan 2009). The cloud point was induced by the salting-out effect (NaCl) and the preconcentration and elution steps were carried out in a flow system with four mini-columns placed in parallel aiming to improve the sampling rate (36.5 samples per hour). Seven analytes [Cd(II), Co(II), Cu(II), Mn(II), Ni(II), Pb(II) and Zn(II)] were sequentially determined by FAAS after complexation with N,N′-bis(2-hydroxy-5-bromobenzyl)1,2-diaminopropane in borate buffer (pH 8.5) and online CPE (Kara 2009). Detection limits from 0.4 (Cd) to 17.9 μg l-1 (Pb) were achieved with a 30-s preconcentration time.
Coupling CPE with ETAAS allows achieving higher EF because of the lower eluent volumes. On the other hand, coupling CPE with flow systems is more complex in comparison to FAAS. Whereas detection by FAAS allows continuous introduction, a discrete aliquot of the eluent (a few microliters) is required for ETAAS. In view of this difficulty, the eluate of CPE was manually inserted into the atomizer (10 μl selected with a micro-syringe) (Bai and Fan 2007). Online coupling of flow-based CPE and ETAAS was exploited for the trace analysis of lead (Nan et al. 2003) and cobalt (Gil et al. 2008). After separation, an air flow passed through the SRP to remove residual solution before elution to the atomizer. This avoids dilution of the eluent and improves the efficiency of removal of the SRP with 50 (Nan et al. 2003) or 75 μl (Gil et al. 2008) of the eluent.
Online coupling of FIA-CPE with ICP OES (Table 5) was proposed for gadolinium determination in urine samples (Ortega et al. 2002). After retention of the SRP in a mini-column, it was directly eluted into the nebulizer without further dilution, favoring a low detection limit (order ng l-1), even without quantitative retention on the resin (90% of the metal chelate was retained). This coupling has also been used for simultaneous determination of trace rare earth elements in biological samples (Li and Hu 2010). The study compared results obtained with and without chelating agents for the simultaneous determination of 17 elements. Searching for a higher sensitivity, the use of the chelating agent was recommended.
Spectrophotometric detection has also been exploited in flow-based CPE for metal determination (Garrido et al. 2004, Frizzarin and Rocha 2014, Frizzarin et al. 2016). The temporary retention of the SRP in the flow cell was exploited for determination of iron in freshwaters and digests of food and biological materials (Frizzarin and Rocha 2014). Dual-wavelength spectrophotometry and adjustment of the integration time of the multi-channel spectrophotometer were evaluated to minimize blank signals, which tended to be higher with the proposed strategy. The latter was preferred in view of the dependence of light scattering with the wavelength that could hinder the dual-wavelength strategy. The pioneering exploitation of CPE in the lab-in-syringe system was proposed for antimony determination in antileishmanial drugs, human serum and seawater samples (Frizzarin et al. 2016). The analytical procedure was based on formation of an ion pair between the antimony-iodide complex and H+. The coupling has several advantages inherent to lab-in-syringe systems (i.e. mechanization, improved mixing and reduced risks of contamination). In relation to the CPE, direct measurement in SRP without requiring filters or an elution solution was the great advantage. In the other application, Triton X-100 was used for both partial solubilization of the reagent (dithizone) from a solid-phase reactor and CPE aiming at mercury determination (Garrido et al. 2004). The achieved EF (6.0) did not yield a detection limit (14 μg l-1) suitable for application to determination of mercury in water samples.
Speciation of inorganic antimony in water and urine samples was feasible with online CPE combined with electrothermal vaporization ICP OES (Li et al. 2006). The method was based on hydrophobic complex formation of Sb(III) and ammonium pyrrolidine dithiocarbamate. The SRP was eluted with 100 μl acetonitrile, a volume 10-fold higher than that inserted in the electrothermal vaporizer. However, in view of the high EF (872) and the quantitative introduction of the eluate in the ICP OES, a low detection limit (90 ng l-1) was achieved. The Sb(V) reduction to Sb(III) was carried out batchwise with l-cysteine by heating in a boiling water bath for 25 min, thus hindering the sample throughput.
Dysprosium was determined in urine by flow-based CPE with ICP OES (Ortega et al. 2003) and CZE with UV detection (Ortega et al. 2004). Both exploited complex formation with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol and CPE with PONPE 7.5. The retained SRP was directly eluted to the ICP OES nebulizer or collected in a vial aiming at determination by CE. The latter allowed achieving higher EF (4-fold higher), lower detection limits (500-fold lower) and a more efficient use of sample (a 20-fold lower CI). Moreover, it allowed the simultaneous determination of iron.
Formation of hydrophobic species capable to be entrapped inside the micelles is often required in CPE (Pytlakowska et al. 2013). In addition to the usual approach, some procedures avoid derivatizing reagents, as demonstrated by extraction of lead (Gil et al. 2010) or cobalt (Gil et al. 2008) from drinking waters with PONPE 7.5 and trace metals from seawater with Triton X-114 (Li and Hu 2009). These works exploited complex formation of the metal ions with the polyoxyethylene groups of the surfactants. Complex formation with the ether group also allowed extraction of cobalt (Gil et al. 2008). In CPE, the product of the chemical derivatizations are often suitable for direct quantification (e.g. by spectrophotometry and fluorimetry) or their formation do not hinder the analyte quantification (e.g. by atomic spectrometry). However, as a different situation is observed in detection by chemiluminescence, an ingenious approach was proposed for CPE of chromium. It was based on the electrostatic interaction of Cr(III) with the outer hydrophobic sphere of the micelles of Triton X-114 (Paleologos et al. 2003). The process is affected by the ionic strength and addition of other surfactants such as SDS. The approach would also be useful with electrochemical detection or analytical separation (e.g. by CZE or HPLC).
Other applications involving CPE without chemical derivatization and detection by chemiluminescence involve the usual extraction of hydrophobic species inside the micelles, for example, coproporphyrin (Fang et al. 2001), benzo[a]pyrene (Song et al. 2006), PAHs (Li et al. 2008), and phenazopyridine (Serenjeh et al. 2014). Benzo[a]pyrene was used as a model compound to demonstrate the potential of the flow-based approach in relation to batch CPE exploiting peroxylate chemiluminescence detection (Song et al. 2006). The potential of coupling the flow extraction with a separation technique for the determination of PAHs was highlighted. This approach was further exploited for PAH determination by HPLC with fluorescence detection (Li et al. 2008). The procedure comprised two practical advantages: (i) the analytes were extracted from soil samples with the same surfactant used in the CPE (Tergitol 15-S-7) and (ii) the mobile phase used for the chromatographic separation of PAHs is used for elution of the SRP. Because of the higher sensitivity provided by CPE, the detection limits were one order of magnitude lower than that obtained by HPLC, which is highly relevant for determination of these organic pollutants.
Conclusions and perspectives
The potential of flow analysis to improve performance of CPE has been demonstrated, yielding better sampling rates and precision, with CI and CE similar to or better than those achieved batchwise. The extraction efficiency has been better than that achieved in conventional LLE in flow systems, because the analyte and the extractant are in the same medium before induction of the cloud point. Indeed, analyte preconcentration is hard to achieve in flow-based LLE because of the low extraction efficiencies. Moreover, CPE is an environmentally friendly alternative to conventional LLE. On the other hand, critical aspects are the efficiency of retention and elution of the SRP, aiming to minimize losses and the inherent dilution before detection.
The number of applications and innovative approaches for mechanization of CPE is relatively limited. For instance, there is a lack of procedures aiming at determination of organic species (including biomolecules and emerging pollutants), as well as chemical speciation and sample clean-up, which are common in batch procedures but yet scarce in flow analysis. This is also true in relation to different flow modalities, because most applications have exploited FIA with confluent streams. In this sense, other modalities, such as SIA, multipumping flow systems and lab-in-syringe, have allowed achieving better performance, especially in relation to surfactant consumption, mixing conditions and EF. This also holds for innovative strategies for induction of the phase separation (e.g. heat of dilution or reaction and electromagnetic assisted heating), and elution without using toxic organic solvents. Applications to analytical techniques that allow measurements with low eluate volumes (thus potential to achieve higher EF), such as ETAAS and electrochemical detection, are also underexplored. In this context, flow-based CPE has potential for online sample preparation (e.g. clean-up or analyte preconcentration) when coupled with CZE and HPLC techniques.
Acknowledgments
The authors acknowledge the fellowships and financial support from the Brazilian agencies CAPES, CNPq and FAPESP (process 2011/23498-9).
References
Acosta, G.; Talio, M. C.; Luconi, M. O.; Hinze, W. L.; Fernández, L. P. Fluorescence method using on-line sodium cholate coacervate surfactant mediated extraction for the flow injections analysis of Rhodamine B. Talanta2014, 129, 516–522.10.1016/j.talanta.2014.06.014Suche in Google Scholar PubMed
Bai, F.; Fan, Z. Flow injection micelle-mediated methodology for determination of lead by electrothermal atomic absorption spectrometry. Microchim. Acta2007, 159, 235–240.10.1007/s00604-007-0786-zSuche in Google Scholar
Bakircioglu, D. Cloud point extraction for the preconcentration of palladium and lead in environmental samples and determination by flow injection flame atomic absorption spectrometry. Environ. Sci. Pollut. Res. 2012, 19, 2428–2437.10.1007/s11356-012-0755-xSuche in Google Scholar PubMed
Baliza, P. X.; Cardoso, L. A. M.; Lemos, V. A. A preconcentration procedure for the determination of cadmium in biological material after on-line cloud point extraction. Environ. Monit. Assess.2012, 184, 4455–4460.10.1007/s10661-011-2277-2Suche in Google Scholar PubMed
Bezerra, M. A.; Arruda, M. A. Z.; Ferreira, S. L. C. Cloud point extraction as a procedure of separation and pre-concentration for metal determination using spectroanalytical techniques: a review. Appl. Spectrosc. Rev.2005, 40, 269–299.10.1080/05704920500230880Suche in Google Scholar
Cao, F.; Gan, W.; Sun, H. Electromagnetic induction-assisted heating as a new method for on-line cloud point extraction of cadmium from water samples. Microchim. Acta2013, 180, 469–475.10.1007/s00604-013-0953-3Suche in Google Scholar
Dalali, N.; Javadi, N.; Agrawal, Y. K. On-line incorporation of cloud point extraction in flame atomic absorption spectrometric determination of silver. Turk. J. Chem. 2008, 32, 561–570.Suche in Google Scholar
Durukan, İ.; Şahin, Ç. A.; Şatıroğlu, N.; Bektaş, S. Determination of iron and copper in food samples by flow injection cloud point extraction flame atomic absorption spectrometry. Microchem. J.2011, 99, 159–163.10.1016/j.microc.2011.04.016Suche in Google Scholar
Fang, Q.; Du, M.; Huie, C. W. On-line incorporation of cloud point extraction to flow injection analysis. Anal. Chem.2001, 73, 3502–3505.10.1021/ac010103fSuche in Google Scholar PubMed
Frizzarin, R. M.; Rocha, F. R. P. An improved approach for flow-based cloud point extraction. Anal. Chim. Acta2014, 820, 69–75.10.1016/j.aca.2014.02.035Suche in Google Scholar PubMed
Frizzarin, R. M.; Portugal, L. A.; Estela, J. M.; Rocha, F. R. P.; Cerdà, V. On-line lab-in-syringe cloud point extraction for the spectrophotometric determination of antimony. Talanta2016, 148, 694–699.10.1016/j.talanta.2015.04.076Suche in Google Scholar PubMed
Garrido, M.; Di Nezio, M. S.; Lista, A. G.; Palomeque, M.; Fernández-Band, B. S. Cloud-point extraction/preconcentration on-line flow injection method for mercury determination. Anal. Chim. Acta2004, 502, 173–177.10.1016/j.aca.2003.09.070Suche in Google Scholar
Gil, R. A.; Gásquez, J. A.; Olsina, R.; Martinez, L. D.; Cerutti, S. Cloud point extraction for cobalt preconcentration with on-line phase separation in a knotted reactor followed by ETAAS determination in drinking waters. Talanta2008, 76, 669–673.10.1016/j.talanta.2008.04.004Suche in Google Scholar PubMed
Gil, R. A.; Salonia, J. A.; Gásquez, J. A.; Olivieri, A. C.; Olsina, R.; Martinez, L. D. Flow injection system for the on-line preconcentration of Pb by cloud point extraction coupled to USN–ICP OES. Microchem. J.2010, 95, 306–310.10.1016/j.microc.2010.01.005Suche in Google Scholar
González, P.; Knochen, M.; Sasaki, M. K.; Zagatto, E. A. G. Pulsed flows in flow analysis: potentialities, limitations and applications. Talanta2015, 143, 419–430.10.1016/j.talanta.2015.05.018Suche in Google Scholar PubMed
Gurkan, R.; Kir, U.; Altunay, N. Development of a simple, sensitive and inexpensive ion-pairing cloud point extraction approach for the determination of trace inorganic arsenic species in spring water, beverage and rice samples by UV-Vis spectrophotometry. Food Chem.2015, 180, 32–41.10.1016/j.foodchem.2015.01.142Suche in Google Scholar PubMed
Hinze, W. L.; Pramauro, E. A critical review of surfactant-mediated phase separations (cloud-point extractions): theory and applications. Crit. Rev. Anal. Chem.1993, 24, 133–177.10.1080/10408349308048821Suche in Google Scholar
Javadi, N.; Dalali, N. Cloud-point extraction for on-line trace determination of copper(II) by flame atomic absorption spectrometry. J. Iran. Chem. Soc.2011, 8, 231–239.10.1007/BF03246220Suche in Google Scholar
Kara, D. Preconcentration and determination of trace metals by flow injection micelle-mediated extraction using flame atomic absorption spectrometry. Talanta2009, 79, 429–435.10.1016/j.talanta.2009.04.004Suche in Google Scholar PubMed
Lemos, V. A.; Baliza, P. X.; Carvalho, A. L.; Oliveira, R. V.; Teixeira, L. S. G.; Bezerra, M. A. Development of a new sequential injection in-line cloud point extraction system for flame atomic absorption spectrometric determination of manganese in food samples. Talanta2008, 77, 388–393.10.1016/j.talanta.2008.06.046Suche in Google Scholar PubMed
Lemos, V. A.; David, G. T. An on-line cloud point extraction system for flame atomic absorption spectrometric determination of trace manganese in food samples. Microchem. J.2010, 94, 42–47.10.1016/j.microc.2009.08.008Suche in Google Scholar
Li, Y. J.; Hu, B. Flow Injection on-line cloud point extraction without a chelating reagent for the determination of trace metals in seawater by inductively coupled plasma optical emission spectrometry. At. Spectrosc.2009, 30, 104–111.Suche in Google Scholar
Li, Y. J.; Hu, B. Cloud point extraction with/without chelating agent on-line coupled with inductively coupled plasma optical emission spectrometry for the determination of trace rare earth elements in biological samples. J. Hazard. Mater.2010, 174, 534–540.10.1016/j.jhazmat.2009.09.084Suche in Google Scholar PubMed
Li, Y.; Hu, B.; Jiang, Z. On-line cloud point extraction combined with electrothermal vaporization inductively coupled plasma atomic emission spectrometry for the speciation of inorganic antimony in environmental and biological samples. Anal. Chim. Acta2006, 576, 207–214.10.1016/j.aca.2006.06.018Suche in Google Scholar PubMed
Li, C. F.; Wong, J. W. C.; Huie, C. H.; Choi, M. M. F. On-line flow injection-cloud point preconcentration of polycyclic aromatic hydrocarbons coupled with high-performance liquid chromatography. J. Chromatogr. A. 2008, 1214, 11–16.10.1016/j.chroma.2008.10.062Suche in Google Scholar PubMed
Li, S.; Wang, M.; Zhong, Y.; Zhang, Z.; Yang, B. Cloud point extraction for trace inorganic arsenic speciation analysis in water samples by hydride generation atomic fluorescence spectrometry. Spectrochim. Acta Part B2015, 111, 74–79.10.1016/j.sab.2015.07.003Suche in Google Scholar
López-García, I.; Vicente-Martínez, Y.; Hernández-Córdoba, M. Non-chromatographic speciation of chromium at sub-ppb levels using cloud point extraction in the presence of unmodified silver nanoparticles. Talanta2015, 132, 23–28.10.1016/j.talanta.2014.08.036Suche in Google Scholar PubMed
Lu, C.; Song, G.; Lin, J.; Huie, C. W. Enhancement in sample preconcentration by the on-line incorporation of cloud point extraction to flow injection analysis inside the chemiluminescence cell and the determination of total serum bilirubin. Anal. Chim. Acta2007, 590, 159–165.10.1016/j.aca.2007.03.028Suche in Google Scholar PubMed
Maya, F.; Horstkotte, B.; Estela, J. M.; Cerdà, V. Automated in-syringe dispersive liquid-liquid microextraction. Trends Anal. Chem.2014, 59, 1–8.10.1016/j.trac.2014.03.009Suche in Google Scholar
Melchert, W. R.; Rocha, F. R. P. Cloud point extraction and concentration of carbaryl from natural waters. Inter. J. Environ. Anal. Chem.2009, 89, 969–979.10.1080/03067310902719134Suche in Google Scholar
Nan, J.; Jiang, Y.; Yan, X. A flow injection online micelle-mediated preconcentration and separation procedure without phase separation coupled with electrothermal atomic absorption spectrometry for determination of trace lead in biological samples. J. Anal. At. Spectrom.2003, 18, 946–950.10.1039/b304552hSuche in Google Scholar
Nascimento, C. F.; Rocha, D. L.; Rocha, F. R. P. A fast environmental friendly analytical procedure for determination of melamine in milk exploiting fluorescence quenching. Food Chem.2015, 169, 314–319.10.1016/j.foodchem.2014.07.144Suche in Google Scholar PubMed
Ojeda, C. B.; Rojas, F. S. Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim. Acta2012, 177, 1–21.10.1007/s00604-011-0717-xSuche in Google Scholar
Oliveira, T. F.; Oliveira, F. M.; Segatelli, M. G.; Tarley, C. R. T. Evaluation of poly(vinylpyridine)-supported protoporphyrin resin for the sampling/separation of manganese(II) using a hyphenated FIA-FAAS system. Anal. Methods2013, 5, 3264–3271.10.1039/c3ay40232kSuche in Google Scholar
Ortega, C.; Gomez, M. R.; Olsina, R. A.; Silva, M. F.; Martinez, L. D. On-line cloud point preconcentration and determination of gadolinium in urine using flow injection inductively coupled plasma optical emission spectrometry. J. Anal. At. Spectrom.2002, 17, 530–533.10.1039/b111633aSuche in Google Scholar
Ortega, C.; Cerutti, S.; Olsina, R. A.; Silva, M. F.; Martinez, L. D. On-line complexation/cloud point preconcentration for the sensitive determination of dysprosium in urine by flow injection inductively coupled plasma-optical emission spectrometry. Anal. Bioanal. Chem.2003, 375, 270–274.10.1007/s00216-002-1677-0Suche in Google Scholar
Ortega, C.; Cerutti, S.; Olsina, R. A.; Martinez, L. D.; Silva, M. F. Simultaneous determination of dysprosium and iron in urine by capillary zone electrophoresis coupled to cloud point extraction. J. Pharm. Biomed. Anal.2004, 36, 721–727.10.1016/j.jpba.2004.08.027Suche in Google Scholar
Paleologos, E. K.; Vlessidis, A. G.; Karayanis, M. I.; Evmiridis, N. P. On-line sorption preconcentration of metals based on mixed micelle cloud point extraction prior to their determination with micellar chemiluminescence. Application to the determination of chromium at ng l-1 levels. Anal. Chim. Acta2003, 477, 223–231.10.1016/S0003-2670(02)01429-0Suche in Google Scholar
Paleologos, E. K.; Giokas, D. L.; Karayannis, M. I. Micelle-mediated separation and cloud-point extraction. Trends Anal. Chem.2005, 24, 426–436.10.1016/j.trac.2005.01.013Suche in Google Scholar
Pharr, D. Y. A review of the use of surfactants in flow injection analysis. Anal. Lett.2011, 44, 2287–2311.10.1080/00032719.2010.551689Suche in Google Scholar
Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-forming organic ligands in cloud-point extraction of metal ions: a review. Talanta2013, 110, 202–228.10.1016/j.talanta.2013.02.037Suche in Google Scholar PubMed
Şahin, Ç. A.; Tokgoz, I.; Bektaş, S. Preconcentration and determination of iron and copper in spice samples by cloud point extraction and flow injection flame atomic absorption spectrometry. J. Hazard. Mater.2010, 181, 359–365.10.1016/j.jhazmat.2010.05.018Suche in Google Scholar PubMed
Serenjeh, F. N.; Hashemi, P.; Safdarian, M.; Kheirollahi, Z. Semi-automated cloud point extraction with cold column trapping of surfactant-rich phase for phenazopyridine determination in human serum. J. Iran. Chem. Soc.2014, 11, 733–739.10.1007/s13738-013-0346-xSuche in Google Scholar
Silva, E. L.; Roldan, P. S. Simultaneous flow injection preconcentration of lead and cadmium using cloud point extraction and determination by atomic absorption spectrometry. J. Hazard. Mater.2009, 161, 142–147.10.1016/j.jhazmat.2008.03.100Suche in Google Scholar
Silva, M. F.; Cerutti, E. S.; Martinez, L. D. Clouding cloud point extraction to instrumental detection systems for metal analysis. Microchim. Acta2006, 155, 349–364.10.1007/s00604-006-0511-3Suche in Google Scholar
Silva, S. G.; Oliveira, P. V.; Nóbrega, J. A.; Rocha, F. R. P. Cloud point extraction to avoid interferences by structured background on nickel determination in plant materials by FAAS. Anal. Methods2009, 1, 68–70.10.1039/b9ay00010kSuche in Google Scholar
Song, G. Q.; Lu, C.; Hayakawa, K. Comparison of traditional cloud-point extraction and on-line flow-injection cloud-point extraction with a chemiluminescence method using benzo[a]pyrene as a marker. Anal. Bioanal. Chem.2006, 384, 1007–1012.10.1007/s00216-005-0224-1Suche in Google Scholar
Stalikas, C. D. Micelle-mediated extraction as a tool for separation and preconcentration in metal analysis. Trends Anal. Chem.2002, 21, 343–355.10.1016/S0165-9936(02)00502-2Suche in Google Scholar
Watanabe, H.; Tanaka, H. A non-ionic surfactant as a new solvent for liquid-liquid extraction of zinc(II) with 1-(2-pyridylazo)-2-naphthol. Talanta1978, 52, 585–589.10.1016/0039-9140(78)80151-9Suche in Google Scholar
Xie, S.; Paau, M. C.; Li, C. F.; Xiao, D.; Choi, M. M. F. Separation and preconcentration of persistente organic pollutants by cloud point extraction. J. Chromatogr. A. 2010, 1217, 2306–2317.10.1016/j.chroma.2009.11.075Suche in Google Scholar PubMed
Yamini, Y.; Faraji, M.; Shariati, S.; Hassani, R.; Ghambarian, M. On-line metals preconcentration and simultaneous determination using cloud point extraction and inductively coupled plasma optical emission spectrometry in water samples. Anal. Chim. Acta2008, 612, 144–151.10.1016/j.aca.2008.02.034Suche in Google Scholar PubMed
Zahedi, M. M.; Dalali, N.; Yamini, Y. Cloud point extraction flow injection–atomic absorption spectrometry for determination of lead in water samples. Can. J. Anal. Sci. Spectrosc. 2009, 54, 23–30.Suche in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Cloud point extraction in flow-based systems
- Developing enhanced magnetoimmunosensors based on low-cost screen-printed electrode devices
- Molecularly imprinting: a tool of modern chemistry for analysis and monitoring of phenolic environmental estrogens
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Cloud point extraction in flow-based systems
- Developing enhanced magnetoimmunosensors based on low-cost screen-printed electrode devices
- Molecularly imprinting: a tool of modern chemistry for analysis and monitoring of phenolic environmental estrogens

