Thermoluminescence response of Ce doped CaTiO3 nanophosphor synthesized by hydrothermal method for gamma dosimetry
-
Taqmeem Hussain
, Rahila Kousar
Abstract
Nanocrystalline calcium titanate (CaTiO3) doped with cerium ions (0.02 mol%) was synthesized via hydrothermal method and its luminescent properties were studied for gamma dosimetry. For the synthesized samples, the best luminescent response was achieved after annealing at 700 °C for 8 h. The synthesis was confirmed via XRD technique yielding the cyrstallite size of 22 nm for the most intense peak (121). The surface morphology was studied via scanning electron microscopy (SEM) yielding the grain size of 12 µm. The strong IR absorptions appeared at 700 cm−1 and 712 cm−1 attributed to bending mode of vibrations changing angle between Ti–O–Ti for CaTiO3 and CaTiO3:Ce respectively, as confirmed via Fourier-Transform Infrared (FTIR) Spectroscopy. Thermoluminescent (TL) response of undoped and doped samples was studied by Mikrolab RA94 TLD Reader-Analyzer having heating rate 10 °C/s, for absorbed doses 0–20 mGy from 137Cs γ-source having a dose rate 100 mSv/h. The found values at 662 keV for the photon-sensing parameters, i.e., mass attenuation coefficients (μ/ρ), mass-energy attenuation coefficient (μ en/ρ), effective atomic number for photon interaction (Z eff,PI) and effective atomic number for total photon energy absorption (Z eff,PEA) were 0.07030882 cm2/g, 0.0280245 cm2/g, 13.9 and 13.4 respectively. Thus, synthesized nanophosphor has shown excellent thermoluminescent dosimetric response for gamma radiation sensing in the selected dose range.
1 Introduction
Luminescent dosimetry is an active research area nowadays, spanning over photoluminescence, cathodoluminescence, chemiluminescence, electroluminescence, mechanoluminescence, sonoluminescence, radioluminescence, bioluminescence, triboluminescence, fractoluminescence, piezoluminescence, thermoluminescence and optically stimulated luminescence. 1 Among these luminescence phenomena, thermoluminescent dosimetry is one of the most practical and widely used techniques owing to its different advantages and feasibility. TL is known as the best solid state dosimetry. 2 It can be used for radiation dosimetry. The intensity of TL is proportional to the amount of the absorbed radiation. The property of TLD detector is an exhibit of a linear relationship between TL intensity and absorbed dose. LiF used for radiation dosimetry was observed in 1953. Because of its high sensitivity, small pellets of this material are used in dosimetry of cancer treatment. 3
Many phosphors have been studied for their thermoluminescent response, e.g., fluorides, borates, titanates, orthosilicates, sulphates, oxides, etc. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Titanates family is a nice addition in thermoluminescent dosimetric research area owing to their dielectric, ferroelectric and optical properties. These perovskite type materials find applications in ceramics capacitors, dielectric devices, micro- and opto-electronic devices. 12 The TL response of SrTiO3 doped with Sb and Au has been investigated. 7 CaTiO3 (formally called mineral perovskite) is an inorganic, dielectric and ferroelectric material, relatively abundant on the earth, having low formation energies for deposition and is well-suited for bulk manufacturing techniques. 13
There are various methods for the synthesis of nanostructure CaTiO 3 such as solid state, 14 sol-gel, 15 , 16 co-precipitation, 17 , 18 solvothermal, 19 hydothermal microwave oven, 20 mechanical alloying method, 21 polymeric precursor method, 22 combustion method, 23 powder mixing method 24 and molten salt method, 25 etc. CaTiO3:Ce was synthesized by hydrothermal method for obtaining nanoparticle size of the samples because it is very reliable and efficient for controlling the particle size. 26 Hydrothermal synthesis leads to the single crystal that depends on the solubility of minerals in hot water under high pressure. Hydrothermal method produced nanoparticles with minimum loss of materials at high vapor pressure. 27
In this research work, CaTiO3:Ce nanophosphor was studied for the first time. It was synthesized by hydrothermal method at optimized annealing temperature conditions for the gamma-dosimetric studies using thermolumienescence response in the appropriate gamma dose range. Additionally, some photon sensing parameters, i.e., mass attenuation coefficient (μ/ρ), mass-energy attenuation coefficient (μ en/ρ), effective atomic number for photon interaction (Z eff,PI) and effective atomic number for total photon energy absorption (Z eff,PEA) were also figured out for the synthesized CaTiO3:Ce nanophosphor.
2 Materials and methods
The nanophosphor CaTiO3 and CaTiO3:Ce were prepared via hydrothermal method. Firstly, CaCl2·2H2O (3.532 g), titanium dioxide (1.2 g) and 70 ml deionized water were taken in a 100 ml beaker. This solution was placed on a magnetic stirrer for more than 300 rpm at 55–60 °C for 30 min. Simultaneously, the pH of the solution was adjusted to 13–14 by drop wise addition of KOH as the mineralization reagent. After 30 min of vigorous stirring, Ce (0.1 g) was added into the solution to achieve 0.02 mol% dopant concentration and further stirred for 15 min. This solution is then transferred into teflon-lined stainless steel vessel autoclave at 150–250 °C for 7 h. After cooling at room temperature, the synthesized powder was washed at least 4 to 5 times with ethanol or deionized water. This powder was then further dried at 120 °C for 1.5 h in drying oven (WHL-25 A). The CaTiO3:Ce (0.02 mol%) powder thus synthesized was then thermally annealed at different temperatures out of which 700 °C for 8 h was optimized owing to the best TL response. After annealing the powder was ground at least 15 min. The obtained powder was pressed by cold pressing machine to make the pellets (3.2 mm × 3.2 mm × 0.89 mm) embedded by PVA. Thermoluminescent response of undoped and doped samples was studied by Mikrolab RA94 TLD Reader-Analyzer having heating rate 10 °C/s, for absorbed doses 0–20 mGy from 137Cs γ-source having dose rate 100 mSv/h.
3 Results and discussion
3.1 X-ray diffraction of CaTiO3
Structural properties of synthesized CaTiO3 and CaTiO3:Ce samples, i.e., crystal structure, crystallite size, dislocation density (δ), interplanar spacing (d), micro strain (ε), lattice constants (a, b, c) and unit cell volume (V); have been analyzed by using the XRD technique. 28 A diffractogram between X-ray intensity and diffraction angle was obtained which confirmed the crystallinity of the synthesized material. The crystallite size was calculated by using Debye Scherrer’s formula.
Here, “D” is crystallite size (in nm), 0.9 is the crystallite shape factor, λ is wavelength of X-rays, “β” is full width at half maximum (in degrees) and “θ” is the Bragg angle (in degrees).
Dislocation density of the sample was calculated by using this formula,
Here, “δ” stands for dislocation density. The interplanar spacing of the sample was determined by using Bragg’s law.
Micro strain was calculated as:
The lattice parameter of each sample was determined by using the plane hkl and d-spacing.
The unit cell volume parameter was determined by using the following formula
CaTiO3 and CaTiO3:Ce were characterized by X-ray diffraction [Bruker D8 Advanced, USA, and selecting Cu-K α radiation λ = 0.154 nm] at Hi-Tech Lab of Government -College University Faisalabad. The scanning range (2θ) was from 20 to 60° and the XRD profiles are given in Figure 1.
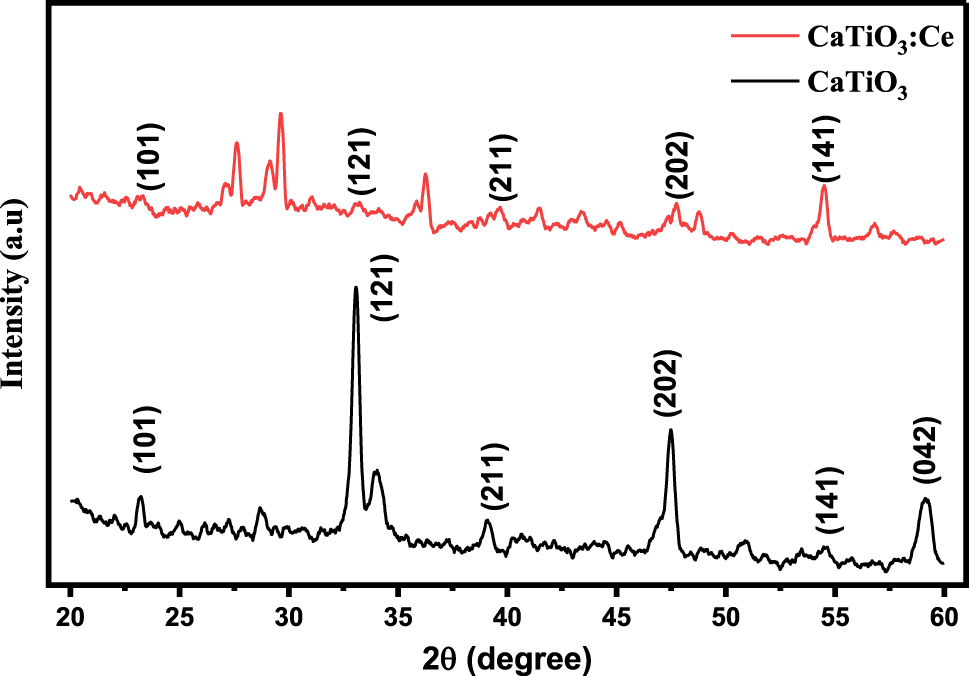
X-ray diffraction patterns of CaTiO3 and CaTiO3:Ce (0.02 mol%).
Figure 1 indicates that CaTiO3 and CaTiO3:Ce (0.02 mol%) were successfully synthesized by the hydrothermal method. The Origin software was used to plot this graph and find out the crystallite size by using XRD data. The peaks were obtained at different 2θ angles such as 23.20, 33.10, 39.14, 47.49, 54.46 and 59.18 with corresponding planes (101), (121), (211), (202), (141) and (042). These planes give the confirmation that calcium titanate has orthorhombic crystal structure (Table 1). The crystallite size of the most intense peak was determined by Equation (1).
Some crystallographic parameters of undoped and doped calcium titanate.
| S. No. | Sample | 2θ (degree) | FWHM β (degree) | d spacing (nm) | Crsytallite size D (nm) | Dislocation density δ
10−3 (nm−2) |
Microstrain ε
10–3 |
Lattice constant (Å) | Volume of unit cell (Å) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CaTiO 3 | 33.07 | 0.405 | 0.2706 | 22.1 | 2.04 | 5.95 | 6.630 | 291.43 |
| 2 | CaTiO 3:Ce | 29.64 | 0.712 | 0.3012 | 48.0 | 0.44 | 2.83 | 7.377 | 401.46 |
The crystallite size of CaTiO3 nanophosphor, calculated by different researchers using different synthesis techniques, is given in Table 2.
Crystallite size of CaTiO3 nanophosphor calculated by different researchers with various methods.
| Author | Sample | Method | Thermal treatment | Crystallite size |
|---|---|---|---|---|
| Zhaung et al., (2014) 29 | CaTiO3 | Hydrothermal method | 180 °C for 12 h | 57.1 nm |
| Oliveira et al., (2015) 22 | CaTiO3 | Polymeric precursor method | 800 °C for 8 h | 59.64 nm |
| Bercmans et al., (2017) 25 | CaTiO3 | Single step molten salt synthesis | 850 °C for 5 h | 50 nm |
| Cherdchom et al., (2019) 30 | CaTiO3 | Combustion synthesis | 1,350 °C for 3 h | 47.6 nm |
| This study | CaTiO3 | Hydrothermal method | 700 °C for 8 h | 22.15 nm |
It is evident from Table 2 that the crystallite size calculated in the present study was smaller as compared to others which is attributed to the high resolution of peaks of the X-ray diffractogram.
3.2 Scanning Electron Microscopy (SEM) of CaTiO3 and CaTiO3:Ce
Scanning Electron Microscopy (SEM) was used to provide the image of the sample by scanning it with the help of high energy beam of electrons. SEM micrograph provides the information about morphology, topography and orientation of material. Calcium titanate doped with cerium (0.1 g) was characterized by SEM (Cube Series, Emcraft, South Korea). The SEM images of calcium titanate doped with cerium, which was synthesized by the hydrothermal method, are shown in Figure 2.
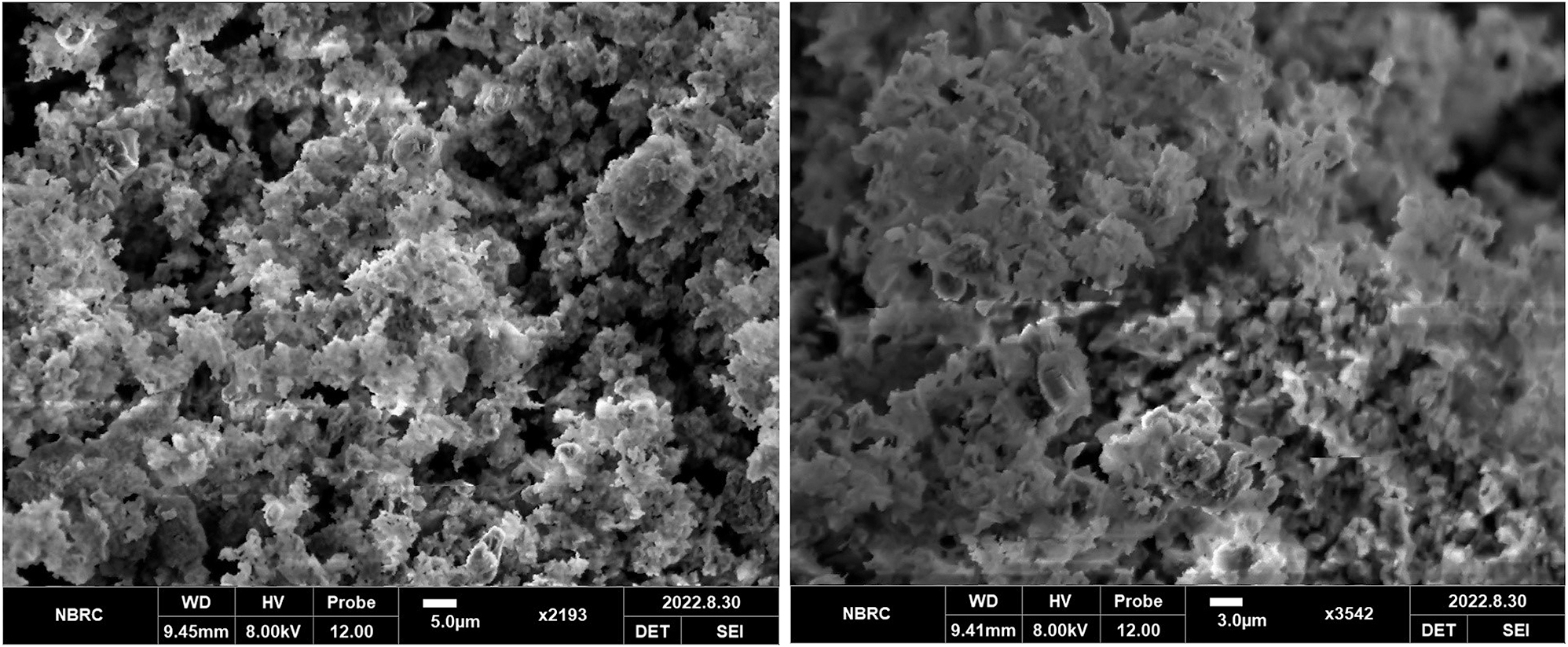
SEM micrographs of calcium titanate doped with cerium.
Figure 2 indicates that calcium titanate doped with cerium (0.1 g) has uniform and homogeneous distribution of particles, yielding a precise particle density. The SEM micrographs indicated a well-defined particle-resembling irregular accumulated morphology. The surface of the sample indicated the pores. The size of phosphor particle was determined by using Image J software as 12 µm.
3.3 Fourier Transform Infrared (FTIR) Spectroscopy of CaTiO3 and CaTiO3:Ce
Fourier Transform Infrared (FTIR) Spectroscopy was used to identify the unknown elements. FTIR spectrometer used to measure the quantity of the absorbance and transmittance of the materials. 31 CaTiO 3 was characterized by FTIR (Tensor 27 IR, Bruker, USA) at National Textile University Faisalabad.
Figure 3 provides FTIR spectra of CaTiO3 and CaTiO3:Ce (0.02 mol%) which were synthesized by the hydrothermal method. A strong IR absorption (or low transmittance) peak appeared at 700 cm−1 which is attributable to the bending mode of vibration, causing a change in the angle between Ti–O–Ti molecules. Another low transmittance peak was observed at 1,489 cm−1 due to stretching or bending mode of CO3
2− bond. In functional group region a peak was observed at 3,644 cm−1 due to stretching mode of –OH bond.
32
The strong IR absorptions appearing at 700 cm−1 and 1,489 cm−1 are attributed to bending in Ti–O–Ti and stretching and bending in
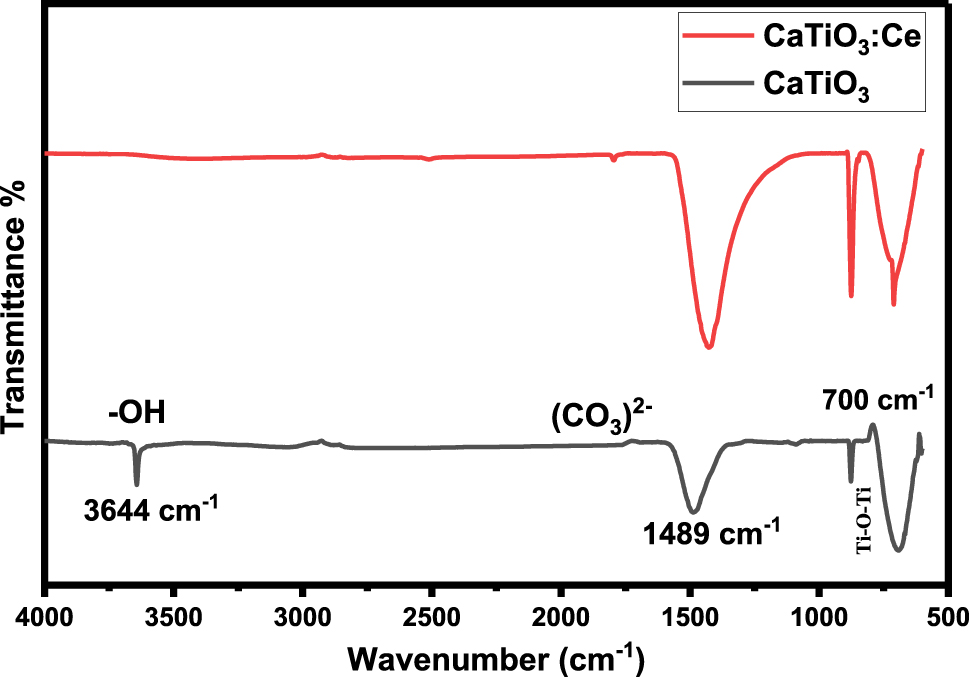
Fourier Transform Infrared (FTIR) Spectrum of CaTiO 3 and CaTiO 3 :Ce (0.02 mol%).
3.4 Raman Spectroscopy of CaTiO3 and CaTiO3:Ce
Calcium titanate undoped and doped with cerium were characterized by Raman Spectroscopy (Model:InVia Raman Microscope, Renishaw, UK). The excitation laser of Raman microscope is 514 nm, laser exposure time is 10 s, grating 1,800 I/mm, objectives ×50L and laser power 100 %. It is located in Department of Physics, COMSAT University Islamabad, Lahore-Campus.
Figure 4 provides Raman spectra of CaTiO3 and CaTiO3:Ce (0.02 mol%), which were synthesized by the hydrothermal method, are recorded in between 100 cm−1 and 1,000 cm−1. The bands were observed at 449 cm−1 and 612 cm−1. The peak observed at 449 cm−1 was due to torsional modes of TiO3. The peak observed at 612 cm−1 was due to stretching modes of vibration of Ti–O. 33
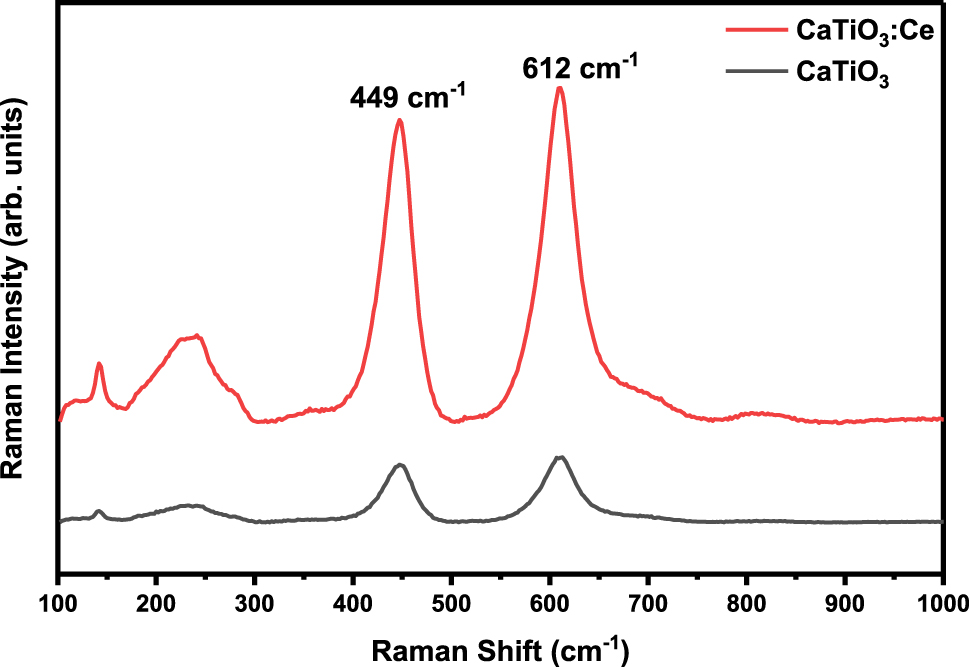
Raman spectra of CaTiO 3 and CaTiO 3 :Ce (0.02 mol%).
3.5 TL dosimetry
Thermoluminescence studies of pure calcium titanate and doped with cerium at optimized annealing temperature of 700 °C for 8 h were performed to test the quality of phosphor and its use in radiation dosimetry. The Mikrolab RA94 TLD Reader-Analyzer was used which can be operated at a heating rate of 0.5–10 °C/s. TL responses of both samples were studied at Pakistan Institute of Engineering and Applied Sciences (PIEAS). The samples were irradiated by using a 137Cs γ-source with dose rate of 100 mSv/h.
Figure 5 provides the dose response in the selected gamma doses. The undoped and doped calcium titanate samples show that the TL intensity increases with the increase in the absorbed gamma dose. The dosimetric behavior of undoped and doped calcium titanate is explained by fitting the mathematical model for the dosimetric quantities i.e., thermoluminescence intensity (I) and the absorbed dose (D) as given in Equations (7) and (8).
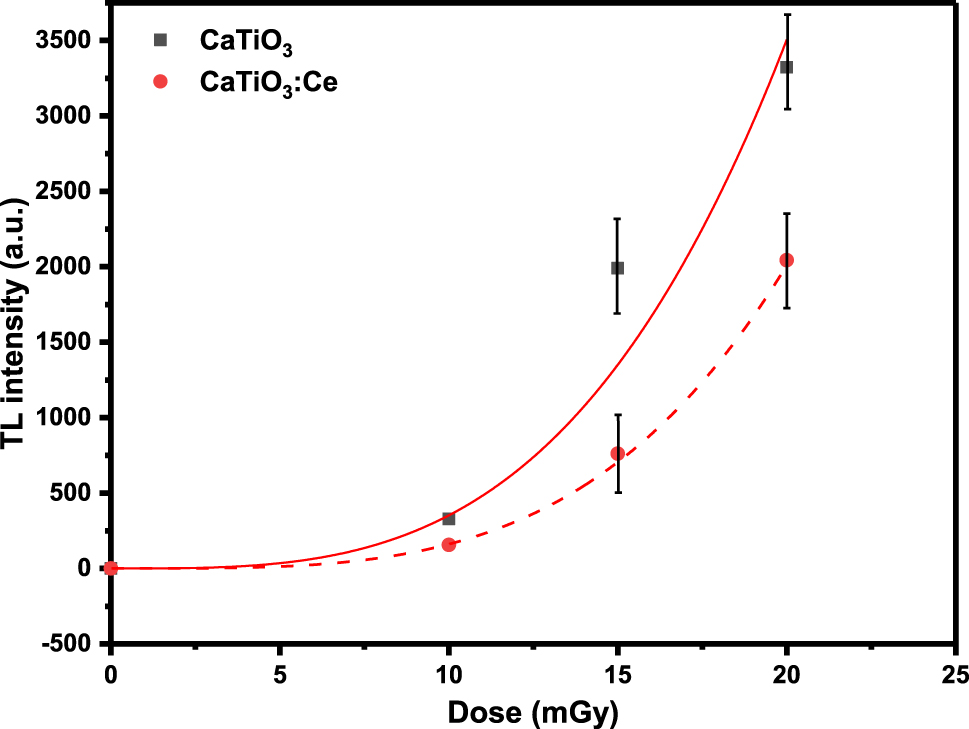
Dose response of pure calcium titanate and doped with cerium.
One may notice that dosimetric thermoluminescent response of undoped calcium titanate is higher than that of the doped calcium titanate. But the undoped sample will saturate much before the doped sample due to higher slope as shown in Equation (7), i.e., since the undoped sample will show too much TL intensity at gamma doses much before the doped sample. The rare earth doped calcium titanate enhances the thermoluminescent response of the selected phosphor calcium titanate. Thus calcium titanate doped with cerium can read much higher gamma doses as compared to undoped calcium titanate. So, our sample of rare earth doped calcium titanate has a potential application in high dose dosimetry.
The change in the thermoluminescent response for undoped and doped samples is well consistent with the fact that doping in host material has significantly created crystal imperfections which in turn has altered the recombination time and frequency of the electrons stimulated thermally.
4 Dosimetric parameters of CaTiO3
4.1 Mass attenuation coefficient (μ/ρ) and mass- energy attenuation coefficients (μ en/ρ) of CaTiO 3
Mass attenuation coefficient (μ/ρ) is a normalization of the linear attenuation coefficient per unit density of a material producing a value that is constant for a given element or compound (i.e., independent of density of the material). 34 Mass attenuation coefficient (μ/ρ) is used to determine the penetration ability of X-ray and photons in matter. 35 The value of μ/ρ was determined by using Bragg’s law.
Here, “
Here “
The value of “
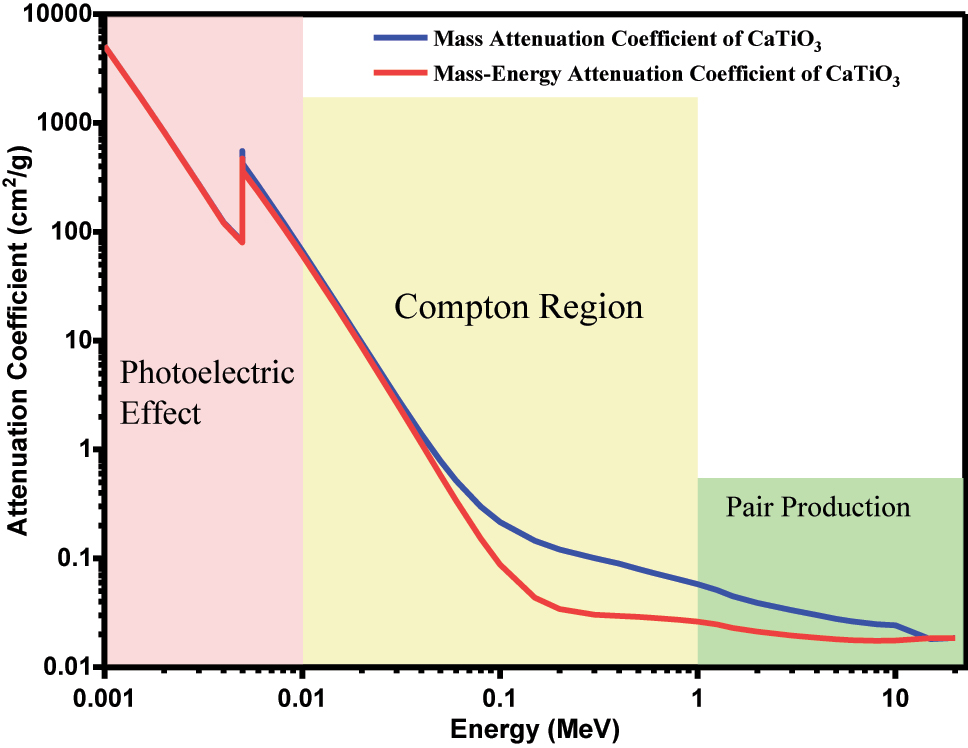
Mass attenuation coefficient ( µ / ρ ) and mass energy attenuation coefficient (μ en /ρ) of CaTiO 3 .
Figure 6 indicates the variation in mass attenuation coefficient (µ/ρ) and mass energy attenuation coefficient (μ en/ρ) of CaTiO 3 and the peak was found to decrease with increase in the photon energy. These variations in µ/ρ and (μ en/ρ) were attributed to radiation-induced interactions of each photon by CaTiO 3. The peaks were obtained at 0.004 MeV, indicating that the incoming photon interacts with the material calcium titanate and the photoelectric interaction takes place in this region. The values of µ/ρ and (μ en/ρ) rapidly decreased with the increasing energy of the photon, especially in medium and high energy regions.
4.2 Effective atomic number for photon interaction Z eff,PI and effective atomic number for photon energy absorption Z eff,PEA of CaTiO 3
Gamma photons interact with matter by different types of interactions such as photoelectric absorption, Compton scattering and pair production. These interactions depend upon the photon energy and the atomic number of the element. The interaction of photon is proportional to Z n the value of n is between 4 and 5 for photoelectric effect, 1 for Compton effect and 2 for pair production. 37
Effective atomic number for photon interaction Z eff,PI was determined by using the formula,
Here, f i stands for molar fraction. Its value was determined by using the formula,
Effective atomic number for total photon energy absorption Z eff,PEA was determined by using the formula,
The effective atomic number for photon interaction Z eff,PI and effective atomic number for total photon energy absorption Z eff,PEA of CaTiO 3 in the energy range of 1–20 MeV is shown in Figure 7.
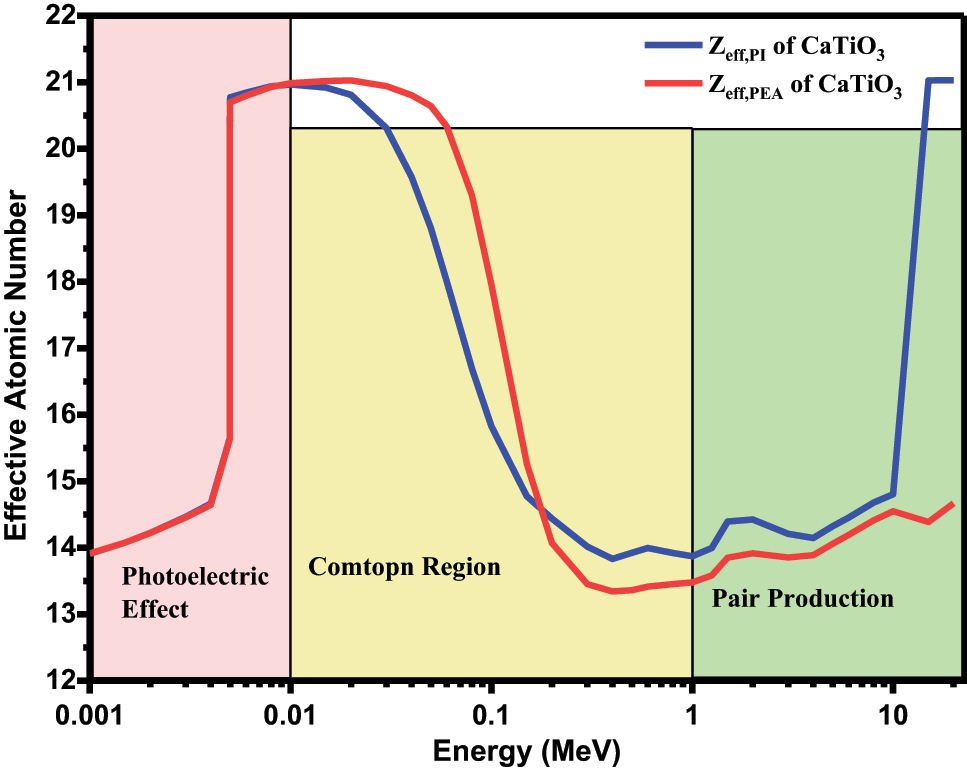
Effective atomic number for photon interaction Z eff,PI and effective atomic number for total photon energy absorption Z eff,PEA of CaTiO 3 .
Figure 7 indicates that the highest peak of Z eff,PI and Z eff,PEA were obtained in low energy region (E < 0.01 MeV), where the photoelectric absorption interaction takes place which gives heavy weight to the elements with the highest atomic number Z 4–5. At 1 keV energy the value of Z eff,PI is 13.9. There is a slight change in the value of Z eff,PI and Z eff,PEA from 1 keV to 4 keV. The change in the peaks gradually decreases from 25 to 200 keV and Z eff,PI values remain constant. There are slight changes in the value of Z eff,PI and Z eff,PEA (almost constant) in medium energy region, where Compton scattering is the main interaction process which is proportional to Z. The lowest values of Z eff,PI and Z eff,PEA were obtained in Compton scattering region. The highest value of Z eff,PI and Z eff,PEA obtained is 21.03 in 10–20 MeV energy region, where the pair production takes place which is proportional to Z 2. The highest peak is obtained in low energy region and high energy region.
5 Conclusions
The undoped CaTiO 3 and doped CaTiO 3:X (X = Zn, Co, Ce) samples were prepared by the hydrothermal method. They were characterized by X-ray diffraction (XRD), Scanning Electron Microscopy (SEM) and Fourier-Transform Infrared (FTIR) Spectroscopy. The XRD results indicate that CaTiO 3 and CaTiO 3:Ce were successfully synthesized, with the crystallite size 22.15 nm and 48.00 nm, respectively. The SEM results of calcium titanate doped with cerium (0.1 g) confirmed that the sample powder has uniform and homogeneous distribution of particles, yielding a precise particle density. The SEM micrographs indicate a well-defined particle-resembling irregular accumulated morphology. The size of phosphor particle is 12 µm. FTIR results indicate that the strongest absorption peaks (low transmittance peaks) were observed at 680–712 cm−1 and 1,436–1,489 cm−1 due to bending mode of vibrations of Ti–O–Ti molecules and CO3 2− bond, respectively. The dosimetric parameters of calcium titanate were determined such as mass attenuation coefficient, mass energy attenuation coefficient, effective atomic number for photon interaction and effective atomic number for photon energy absorption in the energy range 1–20 MeV. Mass attenuation coefficient and mass energy attenuation coefficient decreased with increase in the photon energy. The peaks obtained at 0.004 MeV indicate that the incoming photon interacts with the material calcium titanate and photoelectric interaction takes place in low energy region. The values of both attenuation coefficient decreased in the medium energy region (Compton region) and remained almost constant in the high energy region (pair production). The effective atomic number of calcium titanate is 13.9 when gamma photon (0.662 MeV) of 137Cs interacts with the material calcium titanate. The undoped calcium titanate and doped with cerium were irradiated within a dose range 0–20 mGy from a 137Cs γ-source having dose rate of 100 mSv/h. The undoped and doped calcium titanate samples show that the TL intensity increases with respect to increase in absorbed gamma doses. The rare-earth doped calcium titanate enhanced the thermoluminescent response of the selected phosphor calcium titanate. Since calcium titanate doped with cerium can read much higher gamma doses as compared to undoped calcium titanate, so our sample of rare-earth doped calcium titanate has a potential application in thermoluminescence dosimetric applications.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: This work was supported by Qatar University through a National Capacity Building Program Grant (NCBP), [QUCP-CAM-22/24-463], and was made possible by a UREP award within project [UREP30-105-1-020], granted by the Qatar National Research Fund, a member of The Qatar Foundation. Statements made herein are solely the responsibility of the authors. Open Access funding provided by the Qatar National Library. Also, this work was funded by researchers Supporting Project Number (RSP2024R388) King Saud University, Riyadh, Saudi Arabia.
-
Data availability: Not applicable.
References
1. Murthy, K. V. R.; Virk, H. S. Luminescence Phenomena: An Introduction. Defect Diffusion Forum 2014, 347, 1–34; https://doi.org/10.4028/www.scientific.net/DDF.347.1.Search in Google Scholar
2. Vij, A.; Lochab, S. P.; Singh, S.; Singh, N. Thermoluminescence Study of UV Irradiated Ce Doped SrS Nanostructures. J. Alloys Compd. 2009, 486 (1–2), 554–558. https://doi.org/10.1016/j.jallcom.2009.07.003.Search in Google Scholar
3. McKeever, S. W. S. Thermoluminescence of Solids; Cambridge University Press: London, 1985.10.1017/CBO9780511564994Search in Google Scholar
4. Cameron, J. R.; Suntharalingam, N.; Kenney, G. N. Thermoluminescent Dosimetry; University of Wisconsin Press Ltd: Milwaukee, 1968.Search in Google Scholar
5. Nakajima, T.; Murayama, Y.; Matsuzawa, T.; Koyano, A. Development of a New Highly Sensitive LiF Thermoluminescence Dosimeter and its Applications. Nucl. Instrum. Methods 1978, 157 (1), 155–162. https://doi.org/10.1016/0029-554X(78)90601-8.Search in Google Scholar
6. Wall, B. F.; Driscoll, C. M. H.; Strong, J. C.; Fisher, E. S. The Suitability of Different Preparations of Thermoluminescent Lithium Borate for Medical Dosimetry. Phys. Med. Biol. 1982, 27 (8), 1023–1034; https://doi.org/10.1088/0031-9155/27/8/004.Search in Google Scholar PubMed
7. Yang, B.; Townsend, P. D.; Fan, Y.; Fromknecht, R. Effects of the Ion-Implantation on the Thermoluminescence Spectra of Strontium Titanate. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 226 (4), 549–555. https://doi.org/10.1016/j.nimb.2004.08.002.Search in Google Scholar
8. Spassky, D. A.; Levushkina, V. S.; Mikhailin, V. V.; Zadneprovski, B. I.; Tret’yakova, M. S. Luminescence of Borates with Yttrium and Lutetium Cations. Phys. Solid State 2013, 55, 150–159. https://doi.org/10.1134/S1063783413010319.Search in Google Scholar
9. Nieto, J. A. Thermoluminescence of Metallic Oxides. Development and Applications in Mexico: An Overview. Appl. Radiat. Isot. 2018, 138, 35–39. https://doi.org/10.1016/j.apradiso.2017.07.044.Search in Google Scholar PubMed
10. Zhao, Y.; Wang, Y.; Jin, H.; Yin, L.; Wu, X.; Ma, Y.; Townsend, P. D. Thermoluminescence Spectra of Rare Earth Doped Magnesium Orthosilicate. J. Alloys Compd. 2019, 797, 1338–1347. https://doi.org/10.1016/j.jallcom.2019.05.173.Search in Google Scholar
11. Hussain, T.; Asfora, V. K.; da Nobrega, B. P.; de Barros, V. S. M.; de Azevedo, W. M.; Khoury, H. J. Thermoluminescence Properties of Nanocrystalline BaSO4 Doped with Eu2+ Produced by Solid State Combustion Synthesis. Radiat. Phys. Chem. 2021, 186, 109531. https://doi.org/10.1016/j.radphyschem.2021.109531.Search in Google Scholar
12. Bhargavi, G. N.; Khare, A. Luminescence Studies of Perovskite Structured Titanates: A Review. Opt. Spectrosc. 2015, 118 (6), 902–917; https://doi.org/10.1134/S0030400X15060156.Search in Google Scholar
13. Sakthivadivel, D.; Balaji, K.; Rufuss, D. D. W.; Iniyan, S.; Suganthi, L. Solar Energy Technologies: Principles and Applications; Renewable-Energy-Driven Future, 2021; pp. 3–42.10.1016/B978-0-12-820539-6.00001-7Search in Google Scholar
14. Suriyamurthy, N.; Panigrahi, B. S. Investigations on Luminescence of Rare Earths Doped CaTiO3: Pr3+ Phosphor. J. Rare Earths 2010, 28 (4), 488–492. https://doi.org/10.1016/S1002-0721(09)60138-1.Search in Google Scholar
15. Tahir, M.; Fakhar-e-Alam, M.; Asif, M.; Iqbal, M. J.; Abbas, A.; Hassan, M.; Rehman, J.; Bhatti, Q. A.; Mustafa, G.; Alothman, A. A.; Mohammad, S. Investigation of Gadolinium Doped Manganese Nano Spinel Ferrites via Magnetic Hypothermia Therapy Effect towards MCF-7 Breast Cancer. Heliyon 2024, 10 (3); https://doi.org/10.1016/j.heliyon.2024.e24792.Search in Google Scholar PubMed PubMed Central
16. Mallik, P. K.; Biswal, G.; Patnaik, S. C.; Senapati, S. K. Characterisation of Sol-Gel Synthesis of Phase Pure CaTiO3 Nano Powders after Drying. Mater. Sci. Eng. 2015, 75, 012005; https://doi.org/10.1088/1757-899X/75/1/012005.Search in Google Scholar
17. Asif, M.; Fakhar-e-Alam, M.; Hassan, M.; Sardar, H.; Zulqarnian, M.; Li, L.; Alothman, A. A.; Alangary, A. B.; Mohammad, S. Synergistic Response of PEG Coated Manganese Dioxide Nanoparticles Conjugated with Doxorubicin for Breast Cancer Treatment and MRI Application. Arab. J. Chem. 2024, 17 (10), 105958; https://doi.org/10.1016/j.arabjc.2024.105958.Search in Google Scholar
18. Asif, M.; Iqbal, W.; Fakhar-e-Alam, M.; Hussain, Z.; Saadullah, M.; Hassan, M.; Al-Qahtani, N. H.; Dahlous, K. A. Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity. Pharmaceuticals 2024, 17 (7), 908; https://doi.org/10.3390/ph17070908.Search in Google Scholar PubMed PubMed Central
19. Zhao, H.; Duan, Y.; Sun, X. Synthesis and Characterization of CaTiO3 Particles with Controlled Shape and Size. New J. Chem. 2013, 37 (4), 986–991; https://doi.org/10.1039/C3NJ40974K.Search in Google Scholar
20. Mazzo, T. M.; Moreira, M. L.; Pinatti, I. M.; Picon, F. C.; Leite, E. R.; Rosa, I. L. V.; Varela, J. A.; Perazolli, L. A.; Longo, E. CaTiO3: Eu3+ Obtained by Microwave Assisted Hydrothermal Method: A Photoluminescent Approach. Opt. Mater. 2010, 32 (9), 990–997. https://doi.org/10.1016/j.optmat.2010.01.039.Search in Google Scholar
21. Sahebali, M.; Mojtaba, J. Synthesis of Perovskite CaTiO3 Nanopowders with Different Morphologies by Mechanical Alloying without Heat Treatment. Int. J. Phys. Sci. 2013, 8 (23), 1277–1283; https://doi.org/10.5897/IJPS2013.0014.Search in Google Scholar
22. Oliveira, L. H.; Savioli, J.; Moura, A. P. D.; Nogueira, I. C.; Li, M. S.; Longo, E.; Varela, J. A.; Rosa, I. L. V. Investigation of Structural and Optical Properties of CaTiO3 Powders Doped with Mg and Eu Ions. J. Alloys Compd. 2015, 647 (25), 265–275; https://doi.org/10.1016/j.jallcom.2015.05.226.Search in Google Scholar
23. Shivram, M.; Prashantha, S. C.; Nagabhushana, H.; Sharma, S. C.; Thyagarajan, K.; Harikrishna, R.; Nagabhushana, B. M. CaTiO3:Eu3+ Red Nanophosphor: Low Temperature Synthesis and Photoluminescence Properties. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 120, 395–400. https://doi.org/10.1016/j.saa.2013.09.114.Search in Google Scholar PubMed
24. Mousavi, S. J. The Effect of Sintering Time on the Electrical Properties of CaTiO3 Ceramics. Dig. J. Nanomater. Biostruct. 2014, 9 (3), 1059–1063.Search in Google Scholar
25. Bercmans, L. J.; Sornakumar, T.; Kumar, A. H.; Siva, G.; Venkatesh, G. Synthesis and Characterization of Calcium Titanate and Calcium Zirconate Compound Powders by Molten Salt Method. Nano Hybrids Compos. 2017, 17, 88–95. https://doi.org/10.4028/www.scientific.net/NHC.17.88.Search in Google Scholar
26. Passi, M.; Pal, B. A Review on CaTiO3 Photocatalyst: Activity Enhancement Methods and Photocatalytic Applications. Powder Technol. 2021, 388, 274–304. https://doi.org/10.1016/j.powtec.2021.04.056.Search in Google Scholar
27. Gan, Y. X.; Jayatiissa, A. H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 8917013; https://doi.org/10.1155/2020/8917013.Search in Google Scholar
28. Monshi, A.; Foroughi, M. R.; Monshi, M. R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2 (3), 154–160; https://doi.org/10.4236/wjnse.2012.23020.Search in Google Scholar
29. Zhuang, J.; Tian, Q.; Lin, S.; Yang, W.; Chen, L.; Liu, P. Precursor Morphology-Controlled Formation of Perovskites CaTiO3 and Their Photo-Activity for As(III) Removal. Appl. Catal. B Environ. 2014, 156–157, 108–115; https://doi.org/10.1016/j.apcatb.2014.02.015.Search in Google Scholar
30. Cherdchom, S.; Rattanaphan, T.; Chanadee, T. Calcium Titanate from Food Waste: Combustion Synthesis, Sintering, Characterization, and Properties. Adv. Mater. Sci. Eng. 2019, 2019 (1), 9639016; https://doi.org/10.1155/2019/9639016.Search in Google Scholar
31. Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences a Review. Int. J. Mol. Sci. 2015, 16 (12), 30223–30250. https://doi.org/10.3390/ijms161226227.Search in Google Scholar PubMed PubMed Central
32. Kadam, A. R.; Mishra, G. C.; Dhoble, S. J. Thermoluminescence Study and Evaluation of Trapping Parameters CaTiO3: RE (RE=Eu3+, Dy3+) Phosphor for TLD Applications. J. Mol. Struct. 2020, 1225, 129129. https://doi.org/10.1016/j.molstruc.2020.129129.Search in Google Scholar
33. Moreira, M. L.; Paris, E. C.; Nascimento, G. S. D.; Longo, V. M.; Sambrano, J. R.; Mastelaro, V. R.; Bernardi, M. I. B.; Andres, J.; Varela, J. A.; Longo, E. Structural and Optical Properties of CaTiO3 Perovskite-Based Materials Obtained by Microwave-Assisted Hydrothermal Synthesis: An Experimental and Theoretical Insight. Acta Mater. 2009, 57 (17), 5174–5185; https://doi.org/10.1016/j.actamat.2009.07.019.Search in Google Scholar
34. Gowda, S.; Krishnaveni, S.; Yashoda, T.; Umesh, T. K.; Gowda, R. Photon Mass Attenuation Coefficients, Effective Atomic Numbers and Electron Densities of Some Thermoluminescent Dosimetric Compounds. Pramana 2004, 63 (3), 529–541; https://doi.org/10.1007/bf02704481.Search in Google Scholar
35. Manohara, S. R.; Hanagodimath, S. M.; Gerward, L. Studies on Effective Atomic Number, Electron Density and Kerma for Some Fatty Acids and Carbohydrates. Phys. Med. Biol. 2008, 53 (20), N377. https://doi.org/10.1088/0031-9155/53/20/N01.Search in Google Scholar PubMed
36. Hubbell, J. H.; Seltzer, S. M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest (No. PB-95-220539/XAB; NISTIR-5632), 1995. Available from: http://www.nist.gov/pml/data/xraycoef.10.6028/NIST.IR.5632Search in Google Scholar
37. Hussain, T.; Khalid, N. Stance of Halides towards γ-rays on the Basis of Radiation Interaction Parameters. Radiat. Eff. Defects Solids 2019, 174 (7–8), 647–667. https://doi.org/10.1080/10420150.2019.1632852.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Original Papers
- The collaboratively selective uranyl adsorption of marine fungal modification biosorbent linked by the open-chain polyether terminal with amidoxime
- Assessment of certain theoretical modeling for extraction data of uranium ion by loaded SM-7 with TBP using fixed bed column operation
- Study on separation of ReO4−, a substitute for TcO4−, using functional ionic liquid impregnated extraction chromatography resins
- Synergistic extraction of some divalent cations into nitrobenzene by using dicarbollylcobaltate and substituted calix[5]arenes
- Development of gelatin nanoparticles for positron emission tomography diagnosis in pancreatic cancer
- Radiochemical separation of 161 Tb from neutron irradiated Gd target by liquid-liquid extraction technique
- Influence of gamma irradiation on germination traits, growth and biochemical attributes of dragon fruit (Selenicereus monacanthus)
- Thermoluminescence response of Ce doped CaTiO3 nanophosphor synthesized by hydrothermal method for gamma dosimetry
- Significant influence of La2O3 content on radiation shielding characteristics properties of bismuth sodium borosilicate glasses
- The effect of rare earths (Nd3+, Er3+, Yb3+) additives on the radiation shielding properties of the tungsten oxide modified tellurite glasses
Articles in the same Issue
- Frontmatter
- Original Papers
- The collaboratively selective uranyl adsorption of marine fungal modification biosorbent linked by the open-chain polyether terminal with amidoxime
- Assessment of certain theoretical modeling for extraction data of uranium ion by loaded SM-7 with TBP using fixed bed column operation
- Study on separation of ReO4−, a substitute for TcO4−, using functional ionic liquid impregnated extraction chromatography resins
- Synergistic extraction of some divalent cations into nitrobenzene by using dicarbollylcobaltate and substituted calix[5]arenes
- Development of gelatin nanoparticles for positron emission tomography diagnosis in pancreatic cancer
- Radiochemical separation of 161 Tb from neutron irradiated Gd target by liquid-liquid extraction technique
- Influence of gamma irradiation on germination traits, growth and biochemical attributes of dragon fruit (Selenicereus monacanthus)
- Thermoluminescence response of Ce doped CaTiO3 nanophosphor synthesized by hydrothermal method for gamma dosimetry
- Significant influence of La2O3 content on radiation shielding characteristics properties of bismuth sodium borosilicate glasses
- The effect of rare earths (Nd3+, Er3+, Yb3+) additives on the radiation shielding properties of the tungsten oxide modified tellurite glasses

