Investigation of incompatibilities of the parenteral drugs etacrynic acid and theophylline with other commonly used intravenous drugs in a paediatric cardiological intensive care unit
Abstract
Objectives
Critically ill paediatric patients often receive a large number of parenteral drugs via only a few available intravenous accesses. This poses the risk of incompatibilities. The aim of our study was to investigate the compatibility of etacrynic acid and theophylline with other parenteral drugs used in a paediatric cardiological intensive care unit. These include ampicillin and sulbactam, cefazolin, furosemide, glucose 50 % solution, meropenem, pantoprazole, paracetamol, potassium chloride 7.46 % solution and sodium bicarbonate 8.4 % solution.
Methods
Drugs were mixed in various concentrations representing infant and adult dosage. The physicochemical compatibility was investigated over a period of up to 96 h using several methods. This was done by visual inspection, pH determination, turbidimetric measurements at 350 nm, 410 and 530 nm and content determination via high performance liquid chromatography.
Results
Fifty drug combinations were tested. No incompatibilities were found in 37 drug combinations, indicating these as physicochemically compatible. Depending on the concentration, 13 drug combinations namely etacrynic acid + theophylline, etacrynic acid + ampicillin + sulbactam, etacrynic acid + cefazolin, theophylline + cefazolin and theophylline + meropenem were evaluated as incompatible.
Conclusions
These results will enable healthcare professionals and pharmacists to optimise infusion therapy for paediatric patients and substantially reduce the risk of incompatibilities regarding etacrynic acid and theophylline.
Introduction
Infusion management of critically ill patients is a challenge for healthcare professionals. Several drugs and, if necessary, parenteral nutrition have to be administered to patients via only a few available central or peripheral venous accesses. Due to this fact, incompatibilities between the drugs can occur with serious consequences. Incompatibilities of intravenous drugs are defined as the reaction of two or more drugs or a drug and the container material resulting in solutions that are no longer optimal for the patient [1]. The frequency of incompatibilities in ICUs depends on many factors. These include for example the number of administered drugs, the number of venous accesses, the type of ICU, the patients or the experience of the nurses. Furthermore, the design of studies which investigate the incidence of incompatibilities is very heterogeneous, so that the incidence is reported between 2.16 and 68 % [1], [2], [3], [4], [5]. Incompatibilities can result in an ineffective therapy for the patient and increase the risk of thrombosis, embolisms, infection due to the more frequent manipulation of the infusion line or a dangerous systematic inflammatory response syndrome [6]. In general, two types of intravenous applications can be distinguished in intensive care units: intermittent and continuous infusions. Intermittent infusions are administered for only a few minutes up to 1 h, whereas continuous infusions are administered for several hours and often over a period of 24 h. In contrast to intermittent infusions, it is not possible to rinse the catheter with a neutral solution, for example 0.9 % saline, after administration of a drug to avoid incompatibilities [7], [8], [9]. In addition, continuous infusions often have a lower flow rate and thus longer contact time between the individual drugs, especially in paediatric patients. Therefore, continuous infusions have an increased risk for the occurrence of incompatibilities [3]. One strategy to avoid incompatibilities is the use of multilumen catheters [1]. In adults, 5-lumen catheters are often used. As the venous diameter is smaller, 3-lumen catheters can be used in children, and single- or sometimes 2-lumen catheters can be used in children under 2 years of age. At the same time, venous catheter placement is particularly difficult in infants [10], and each catheter must be kept open by flushing with heparin or normal saline [7], 8], 11], 12], which may be counterproductive to the intended volume constriction in infants following surgery for congenital heart disease [13]. In addition, each extra venous access increases the risk of infections [14]. It is therefore often unavoidable in paediatric intensive care to infuse multiple drugs through the same lumen of a catheter. Unfortunately, data on drug compatibility is limited for the lower doses used in paediatric units [15], 16]. In this study, we aimed to investigate the physicochemical compatibility of intravenous drugs used in the paediatric cardiological intensive care unit of the Clinic for Congenital Heart Defects and Paediatric Cardiology of the UKSH Campus Kiel in order to increase the safety management of drug therapy. Two of the frequently used drugs in this ICU are etacrynic acid and theophylline. The loop diuretic etacrynic acid is currently not a first-choice diuretic. The other loop diuretics torasemide and furosemide have much better side effect profiles [17], so that etacrynic acid is used at most as a reserve diuretic. However, in paediatric cardiological patients, studies have found a benefit in the use of etacrynic acid alone or in combination with furosemide [18], 19]. A similar situation applies for theophylline. Formerly used for the treatment of asthma or COPD, it is hardly used any more for these indications [20], 21]. In addition to the bronchodilating and anti-inflammatory effects, a diuretic effect of theophylline has been described. Especially in paediatric cardiological patients after surgery, urine output was increased and fluid overload was reduced with theophylline [22], 23].
Thereby, we focused on the investigation of the compatibility of etacrynic acid (Eca) and theophylline (Tph) in combination with ampicillin (Amp) + sulbactam (Sul), cefazolin (Cef), furosemide (Fur), 50 % glucose solution (G50), meropenem (Mero), pantoprazole (Panto), paracetamol (acetaminophen, PCM), potassium chloride solution (KCl) or sodium bicarbonate solution (SBC) (the formulae of the drugs are shown in Figure 1).
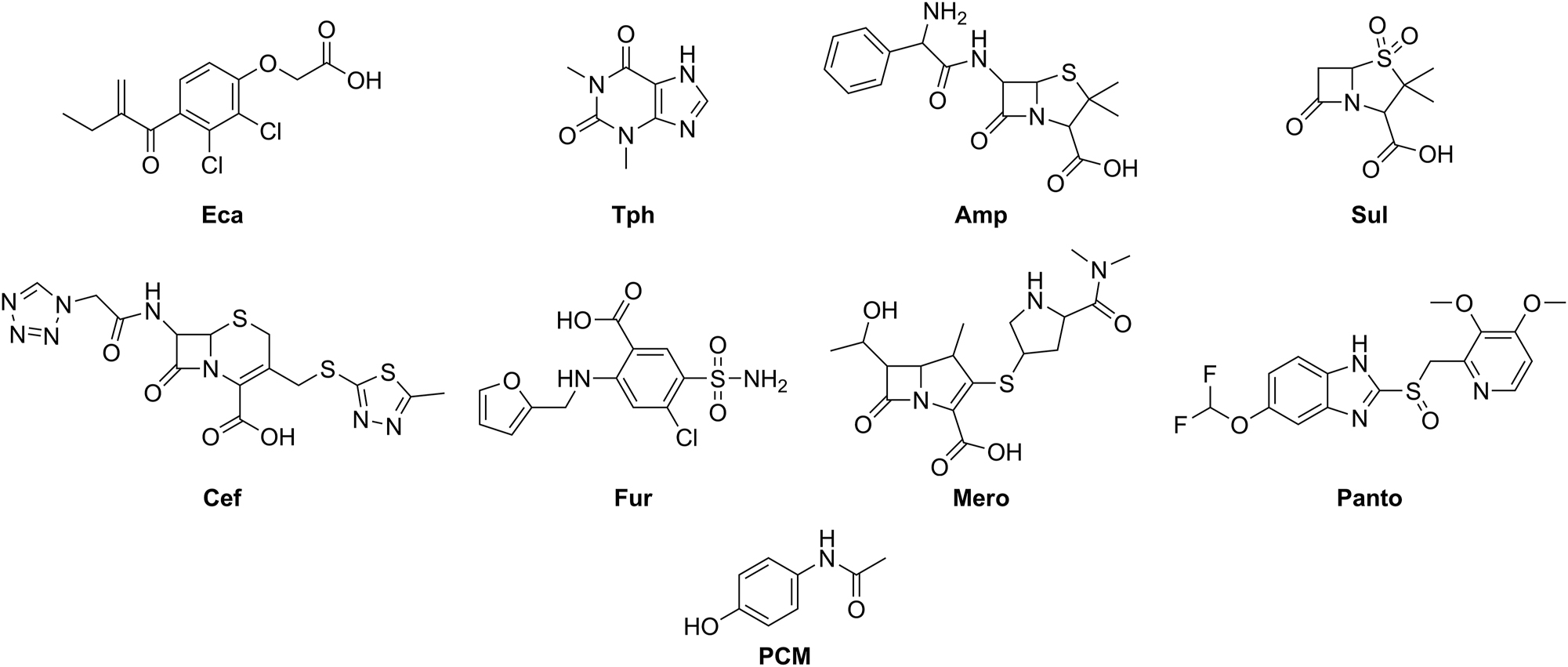
Formulae of the investigated drugs (without glucose, potassium chloride and sodium bicarbonate). Eca: etacrynic acid, Tph: theophylline, Amp: ampicillin, Sul: sulbactam, Cef: cefazolin, Fur: furosemide, Mero: meropenem, Panto: pantoprazole, PCM: paracetamol (acetaminophen).
Materials and methods
Chemicals and reagents
The drugs as well as 50 mL polypropylene syringes (Original Perfusor® B. Braun Melsungen AG or BD™ Medical™ Plastipak™) and sterile 0.9 % saline solution (B. Braun Melsungen AG or Fresenius Kabi) were kindly provided by the hospital pharmacy of the UKSH Campus Kiel. Table 1 gives the drug formulations, pH of the solution (after reconstitution), batches and concentrations used. At the time of testing all solutions were within the stated expiry time. LC-MS grade water was obtained from VWR (83,645.320), acetonitrile gradient grade 99.9 % from honeywell (34,851), acetic acid from Sigma-Aldrich (1.00066) and sodium dihydrogen phosphate from Carl Roth (K300.2).
Drugs tested in this compatibility study.
| Drug | Salt | Galenic form | Corporate name (company) | pH | Excipients | Batch | min.–max. or fix conc., mg/ml |
|---|---|---|---|---|---|---|---|
| Ampicillin + Sulbactam | Ampicillin sodium + Sulbactam sodium | PSII | Unacid® 1.5 g (Pfizer) | 8.0–10.0 | n/a | DA787402; DL253401 | 20 + 10 |
| Cefazolin | Cefazolin sodium | PSII | Cefazolin-saar® 2000 mg i.v. (Chephasaar) | 4.0–6.0 | n/a | 3075900; 3076000; 3117100 | 10–100 |
| Etacrynic acid | Etacrynate sodium | PSII | Reomax 50 mg/20 mL (Bioindustria L.I.M.) | 6.3–7.7 | Mannitol, pHBA | 063162; 079178 | 0.1–0.8 |
| Furosemide | Furosemide sodium | IS | Lasix® 20 mg/2 mL (Sanofi-Aventis) | 8.7 | NaOH | G0767; J0712; J0273; J1033; K0933 | 0.2–2 |
| 50 % glucose | n/a | IS | Glucosteril® 50 % (Fresenius Kabi) | 3.0–4.5 | n/a | 14NE48 | Undiluted |
| Meropenem | Meropenem trihydrate | PSII | Meropenem Hikma 1 g (Hikma Pharma) | 7.3–8.3 | Na2CO3 | MDEA1230; MDEA1091 | 2–20 |
| Pantoprazole | Pantoprazole sodium sesquihydrate | PSII | Pantoprazol EVER Pharma 40 mg (EVER Pharma) | 8.0–11.0 | NaEDTA | R6681; R7FE1; R75V1 | 1 |
| Paracetamol (acetaminophen) | n/a | IS | Paracetamol B. Braun 10 mg/mL (B. Braun) | 4.5–5.5 | Mannitol, NaCitr, AcOH | 20185406; 20183403; 21187413 | 10 |
| 7.46 % potassium chloride | n/a | IS | Kaliumchlorid-Lösung 7.46 % (SERAG-WIESSNER) | 4.5–7.5 | n/a | 2000317 | Undiluted |
| 8.4 % sodium bicarbonate | n/a | IS | Natrium-hydrogencarbonat 1 M 8.4 % (SERAG-WIESSNER) | 7.0–8.5 | n/a | 227182 | Undiluted |
| Theophylline | n/a | IS | afpred® forte-THEO 200 mg (Leyh-Pharma) | 8.5–9.5 | NaOH | 70601; 80641; 90371 | 0.8–4 |
-
AcOH, acetic acid; IS, injection solution; NaCitr, sodium citrate; NaEDTA, sodium ethylenediaminetetraacetate; Na2CO3, sodium carbonate; NaOH, sodium hydroxide; pHBA, p-hydroxybenzoic acid; PSII, powder for solution for injection or infusion. Injection solutions contain water for injection.
Study design
In cooperation with the paediatric cardiological intensive care unit (pcICU) of the Clinic for Congenital Heart Defects and Paediatric Cardiology and the hospital pharmacy of the UKSH Campus Kiel, frequently used drugs were identified. Since both newborns and young adults are treated in the pcICU, the drugs are used in a wide range of doses or concentrations. It is quite possible that concentrations vary by a factor of 10 depending on the age of the patient. In addition, it is conceivable that the infusion rates of two drugs are different. When two or more drugs are infused through the same lumen of an infusion catheter, a different infusion rate will result in different concentrations of the drugs. To simulate different infusion rates in compatibility studies, 10:1 and 1:10 mixtures are often investigated in addition to the 1:1 mixture [24], 25]. However, this was not considered appropriate for our study due to the wide range of drug concentrations on the pcICU. Instead, we decided to use a mixing scheme as shown in Figure 2. For each drug, a maximum and a minimum concentration was defined. To represent a worst-case scenario, the drugs were then mixed together in different concentrations as follows: maximum concentration drug A- maximum concentration drug B (a); maximum concentration drug A- minimum concentration drug B (b); minimum concentration drug A- maximum concentration drug B (c); minimum concentration drug A- minimum concentration drug B (d). Drugs, which are used in the pcICU only in one concentration (so-called fix concentration), were mixed only with the maximum (e) or minimum (f) concentration of the other drug.
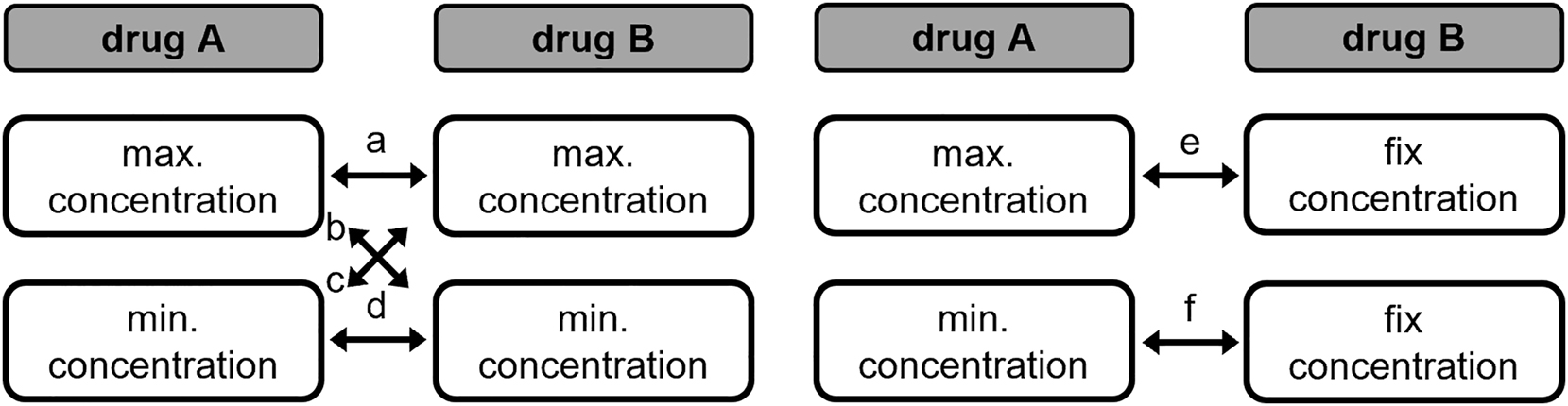
Mixing scheme. Resulting combinations are a, b, c, d, e, f.
Each combination was prepared in duplicates. A contact time between the two drugs of 4 h was defined as the clinically relevant time period, as this is considered to be the maximum likely contact time of drugs when administered simultaneously through the same lumen of a catheter [24], 26], 27]. If no relevant changes occurred within these 4 h, the combination was considered to be compatible. However, the samples were measured over a total period of 96 h in order to provide more evidence for the data obtained.
Literature and database research
Firstly, published compatibility data were searched. Therefore, the online databases King® Guide (last updated 03/2020), ASHP® Injectable drug information™ (last updated 06/2020) and stabilis (last updated 02/2023) as well as the prescribing information were used. In addition, the literature database PubMed® was searched for compatibility data using the keywords compatibility or incompatibility or Y-site or stability or coinfusion and name of the drug.
Sample preparation
The drugs were reconstituted, if necessary, with 0.9 % saline (Reomax with 5 % glucose solution provided) according to the manufacturer’s instructions and then further diluted with 0.9 % saline. These drug solutions were mixed in a 1:1 ratio in 50 mL syringes as described in the mixing scheme (Figure 2). The filled syringes were sealed with a combi-stopper (B. Braun Melsungen) and stored at room temperature and under laboratory light. Immediately after mixing, as well as after 2 h, 4 h, 8 h, 24 h, 48 h and 96 h, 2 mL of solution were taken for pH determination and turbidimetric measurement. At each measurement point, the samples were also checked for visual conspicuities (e.g. particles, discolouration, production of gas and turbidity) in front of a white and black background using an adapted pharmacopoeia method (Ph. Eur. 10.0: 2.9.20 Particulate Contamination: Visible Particles) [28]. In addition, 0.8 mL of sample solution was taken directly after mixing and after 2 h, 4 h, 24 h and 96 h, aliquoted to 0.2 mL and frozen in liquid nitrogen. The 0.2 mL aliquots were stored at −80 °C until high performance liquid chromatography (HPLC) measurements. Prior to HPLC measurement, the samples were freshly thawed, diluted with 0.9 % saline solution if necessary and measured in triplicate.
pH measurement
1 mL sample solution was placed in a test tube and the pH was measured after a latency time of 10 min with a Hanna pH metre (HI 2211 + HI 1053), which was calibrated daily with ready-to-use buffer solutions (Carl Roth, A517.1, P713.1, 8086.4).
Turbidimetry
To examine the sample solutions for non-visible changes 0.8 mL of the sample solution was placed in a semi-micro cuvette (Brand®, 759115) and measured in triplicates at 350 nm, 410 and 530 nm in a photometer (Varian Cary® 50 UV-Vis Spectrophotometer) based on the recommendations from Bardin et al. [29]. Changes in absorption at these wavelengths can indicate chemical changes, discolouration or turbidity of the solution.
HPLC and validation
For the combinations evaluated as physically compatible, the drug content was determined, with the exception of potassium chloride, glucose and sodium bicarbonate. For this purpose, an HPLC method was developed that allowed the quantification of all selected drugs. The HPLC method follows a method already described in Jirschitzka et al. [30].
The HPLC system consisted of a degasser (Biotech Degasi® Classic), Waters™ 1,525 binary HPLC pump, Waters™ 717plus autosampler, column oven (Techlab GmbH) and a Waters™ 2,487 Dual λ absorbance detector (254 nm, 270 nm). An Agilent® Poroshell 120EC-C18, 2.7 μm, 3.0 mm × 50 mm column with Phenomenex® SecurityGuard™ Ultra cartridge, UHPLC C18 3.0 mm inclusive guard holder was used. The flow rate was set to 0.8 mL/min. A gradient was used. Mobile phase A consisted of a 10 mM sodiumphosphate buffer pH 3.2 and mobile phase B consisted of acetonitrile/sodiumphosphate buffer pH 3.2 60:40. The gradient started at 95 % A for 3 min, then changed to 80 % A within 2 min to continue to 40 % A until 16 min. Within another 2 min the mobile phase A decreased further to 30 % to remained there for 1 min. Then, the gradient changed within 1 min–95 % A and re-equilibration started for 5 min. The entire gradient, including re-equilibration, ran for 25 min. The column oven was set to 20 °C. Waters™ BreezeTM-software (version 3.3) was used for data acquisition and processing.
The HPLC method was validated according to ICH guideline [31]. Therefore the linearity of the method was determined by a five-point calibration and the precision and reproducibility were checked measuring the maximum, middle and minimum concentrations of the range in triplicates. To validate the freezing process, the maximum and minimum concentrations were measured both before and after freezing and thawing in triplicates.
Limit values for assessing drug combinations as incompatible
In the literature, different limit values for the methods usually used in compatibility studies are discussed to decide whether a combination can be considered compatible or incompatible. In particular, there are different views on the maximum acceptable deviation of the pH value. For example, in some compatibility studies, a pH deviation of more than 0.2 compared to the beginning is already considered as a sign of incompatibility [32], 33]. In other studies, however, deviations of the pH value of maximum two are also accepted [34]. As a limit value for turbidimetric measurements, absorbance changes of 0.01 at 530 nm or 0.04 at lower wavelengths are often defined [25], 35]. One study even accepted changes in absorbance of 0.1 [26]. However, since a visually perceptible discolouration is already observable from an absorbance change at 410 nm of approximately 0.015, a maximum absorbance change of 0.04 as a limit value for 410 nm seemed unsuitable to us. Therefore, we have decided to use a lower limit value for the absorbance at 410 nm. To describe the chemical compatibility of intravenous drugs, a residual content of at least 90 % is generally considered acceptable [29]. In summary, in this study, drug combinations were considered as physicochemical compatible if no changes occurred seen with the unaided eye within 4 h, the pH did not change by more than 1, the absorbance did not change by more than 0.04 at 350 nm and 0.01 at 410 and 530 nm, and drug content did not decrease by more than 10 %.
LC-MS
To identify degradation products, LC-MS and LC-MS/MS experiments were carried out for conspicuous combinations according to a method described in Jirschitzka et al. [30]. LC-MS measurements were performed with an Agilent 1260 HPLC system (G1379B 1260 μ-degasser, G1312B bin pump, G1367E 1260 HiP ALS + G1330B, G1316A 1260 TCC, G4212B 1260 diode array detector) with gradient elution (A: 0.2 % acetic acid in water–B: 0.2 % acetic acid in acetonitrile) coupled to a Bruker Amazon SL mass spectrometer (Bruker Daltonik, Bremen, Germany) with an ion trap and MSn facility. The gradient started with a flow rate of 1 mL/min at 95 % A for 2 min, then continued to 65 % A in 0.5 min and further to 20 % A in 3.5 min. The gradient changed to 3 % A in 0.5 min and remained there for 1.5 min. Finally, the gradient changed to 95 % A in 0.5 min and re-equilibration started at an increased flow rate of 1.3 mL/min for 1 min. The MS was switched on from min two to min 9.2 in positive mode. The column oven was set to 25 °C. A Phenomenex® Luna® 3 μm C18(2) 100 Å, 100 × 4.6 mm column inclusive Phenomenex® SecurityGuard™ Ultra cartridge, UHPLC C18 3.0 mm with guard holder was used. Hystar 3.2 SR two software was used to control the HPLC, Bruker Trap Control 7.0 software was used to control the MS and the MS data were analysed with Bruker Data Analysis 4.0.
Results
Literature research
Only two compatibility studies were found in PubMed® for the drug combinations selected here. Firstly, Allen et al., who confirmed the physical compatibility between Eca and KCl [36], and secondly, Kershaw et al., who investigated the compatibility between Tph, Amp, Cef, KCl and Sul and rated them as visually compatible [37]. The three databases (King® guide, ASHP® Injectable drug information™ and stabilis) refer to these two studies when searching for compatibility data for Eca and Tph in combination with the other drugs. Compatibility data beyond this were not given in the databases. Unfortunately, precise information on compatibility with other drugs is also missing in the prescribing information. In the prescribing information for Reomax, at least, it is stated that Eca is incompatible with solutions with a pH below 5. By contrast, the prescribing information of afpred® forte-THEO only states that no substances other than the diluting solution should be added in order to avoid incompatibilities. The prescribing information of the other drugs either mentions an acidic pH, which should be avoided (Lasix®), gives no further information except that the respective drugs should be administered separately (Meropenem Hikma, Pantoprazol EVER Pharma, Paracetamol B. Braun) or states specific incompatibilities, but not for the drugs used in this study (Unacid®, Cefazolin-saar®). As a result of the literature research, the cross-table shown in Figure 3 became the basis for our compatibility study.
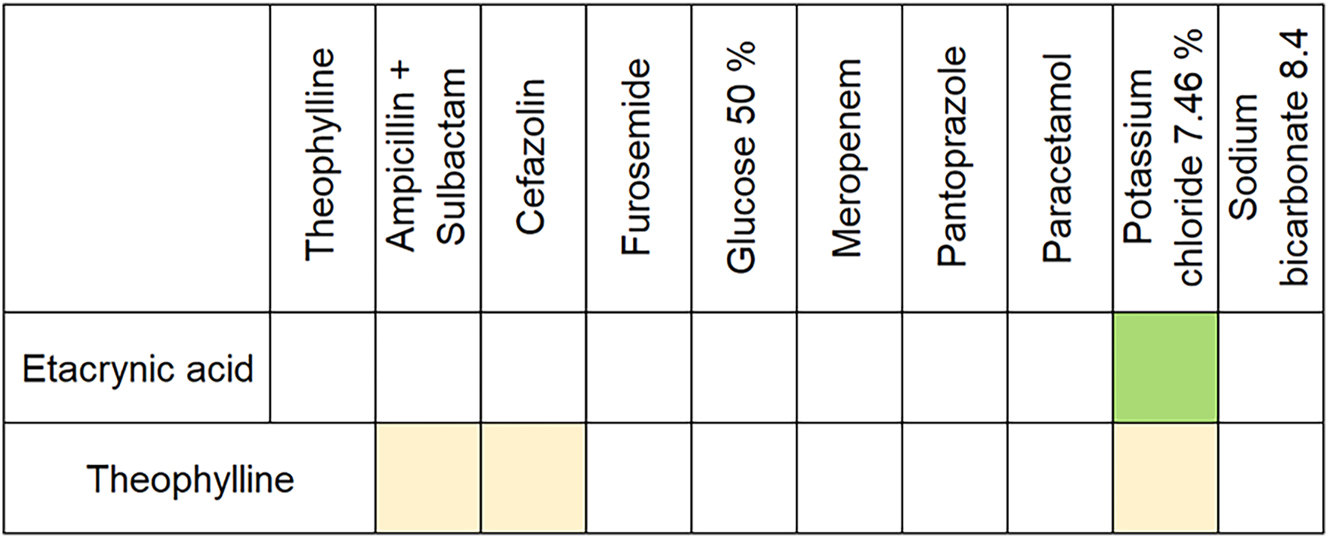
Compatibility chart displayed as a cross-table based on the conducted literature research. Colour indicates the type of compatibility. beige: visually compatible, green: physically compatible, white: no information available.
Validation HPLC
An HPLC method was developed for the identification and quantification of the drugs. Figure 4 shows an overlayed representative chromatogram of all drugs. With the exception of Amp and Mero, all peaks are baseline separated. However, since Amp and Mero did not need to be identified at the same time, this was not problematic. Table 2 shows the results of the validation and the retention times. All drugs showed a linear relation between peak area and concentration over the whole concentration range. The coefficient of variation was always less than 2 %. The maximum and minimum recoveries for both direct measurement and frozen and thawed samples were between 97.86 and 101.99 %. This was considered acceptable for the quantification of the drugs. Therefore, the developed HPLC method was found to be appropriate.
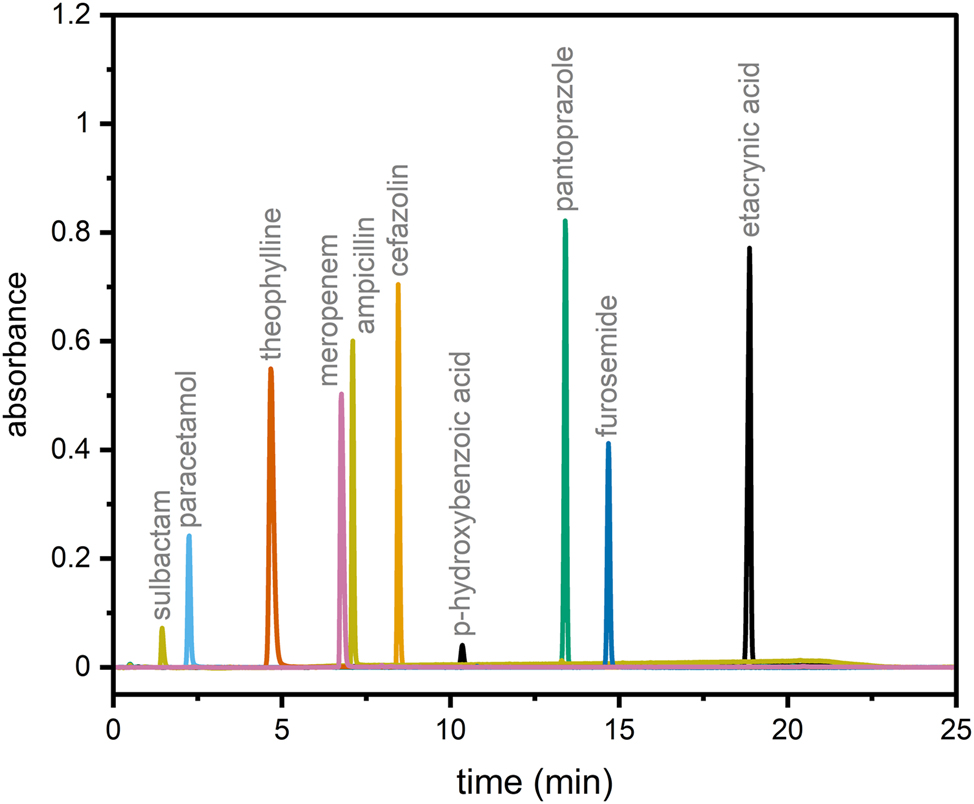
Overlayed HPLC-UV chromatogram of tested drugs.
Results of the validation of the HPLC method.
| Drug | λ, nm | Linearity, mg/ml | tR, min | R2 | Equation regression line | max. CV, % | Recoverya
min. – max., % |
|---|---|---|---|---|---|---|---|
| Amp | 220 | 0.02–0.6 | 7.2 | 1.000 | y=69,370,85x + 19,697 | 0.9 Ø 0.5 ± 0.3 |
98.1–101.9 Ø 100.5 ± 1.1 |
| Cef | 254 270 |
0.02–0.6 | 8.5 | 1.000 1.000 |
y=72,312,11x + 2,461 y=89,256,16x + 4,391 |
1.9 Ø 0.8 ± 0.5 |
98.7–101.2 Ø 99.8 ± 0.8 |
| Eca [30] | 254 270 |
0.01–0.6 | 18.8 | 1.000 1.000 |
y=38,516,26x + 649 y=41,368,90x + 448 |
1.9 Ø 0.9 ± 0.6 |
97.9–101.3 Ø 99.1 ± 0.8 |
| Fur | 333 | 0.02–0.6 | 14.6 | 1.000 | y=47,095,31x + 71 | 1.2 Ø 0.5 ± 0.5 |
98.8–101.9 Ø 99.8 ± 1.3 |
| Mero | 254 270 |
0.2–1.2 | 6.8 | 1.000 1.000 |
y=24,217,53x – 2,164 y=36,867,63x + 4,119 |
0.9 Ø 0.4 ± 0.3 |
98.1–101.5 Ø 99.6 ± 1.2 |
| Panto | 254 270 |
0.02–0.6 | 13.9 | 1.000 1.000 |
y=66,414,97x – 4,860 y=9,136,36x – 1,845 |
1.9 Ø 1.1 ± 0.8 |
98.6–102.0 Ø 100.5 ± 1 |
| PCM | 254 270 |
0.04–0.12 | 2.5 | 0.999 0.999 |
y=175,219,65x – 14,972 y=59,179,13x – 5,008 |
2.0 Ø 1 ± 0.7 |
98.4–101.9 Ø 100.7 ± 0.7 |
| Sul | 220 | 0.01–0.3 | 1.6 | 1.000 | y=19,977,74x – 2,446 | 1.3 Ø 0.6 ± 0.5 |
98.2–100.9 Ø 99.7 ± 1.1 |
| Tph [30] | 254 270 |
0.01–0.6 | 4.6 | 1.000 1.000 |
y=86,143,03x + 281 y=181,218,56x + 303 |
1.6 Ø 0.7 ± 0.4 |
98.6–100.9 Ø 99.5 ± 0.5 |
-
λ, wavelength; tR, mean retention time; R2, correlation coefficient; CV, coefficient of variation; Amp, ampicillin; Cef, cefazolin; Eca, etacrynic acid; Fur, furosemide; Mero, meropenem; Panto, pantoprazole; PCM, paracetamol; Sul, sulbactam; Tph, theophylline; Ø, mean±SD. aIncludes both the intraday and interday recovery after freezing and thawing.
Physicochemical compatibility
When examining the drug mixtures with the unaided eye in front of a black and white background according to Ph. Eur. 10.0: 2.9.20, no changes were noticed in the clinically relevant time period of 4 h. In Figure 5, the results of the compatibility study are presented in form of a cross-table. A total of 50 combinations of drugs were investigated, each in duplicates. Of these, 37 combinations were assessed as physicochemically compatible and 13 combinations were assessed as incompatible. Drug combinations assessed as incompatible include etacrynic acid + theophylline, etacrynic acid + ampicillin + sulbactam, etacrynic acid + cefazolin 100 mg/mL, theophylline + cefazolin and theophylline 4 mg/mL + meropenem 2 mg/mL.
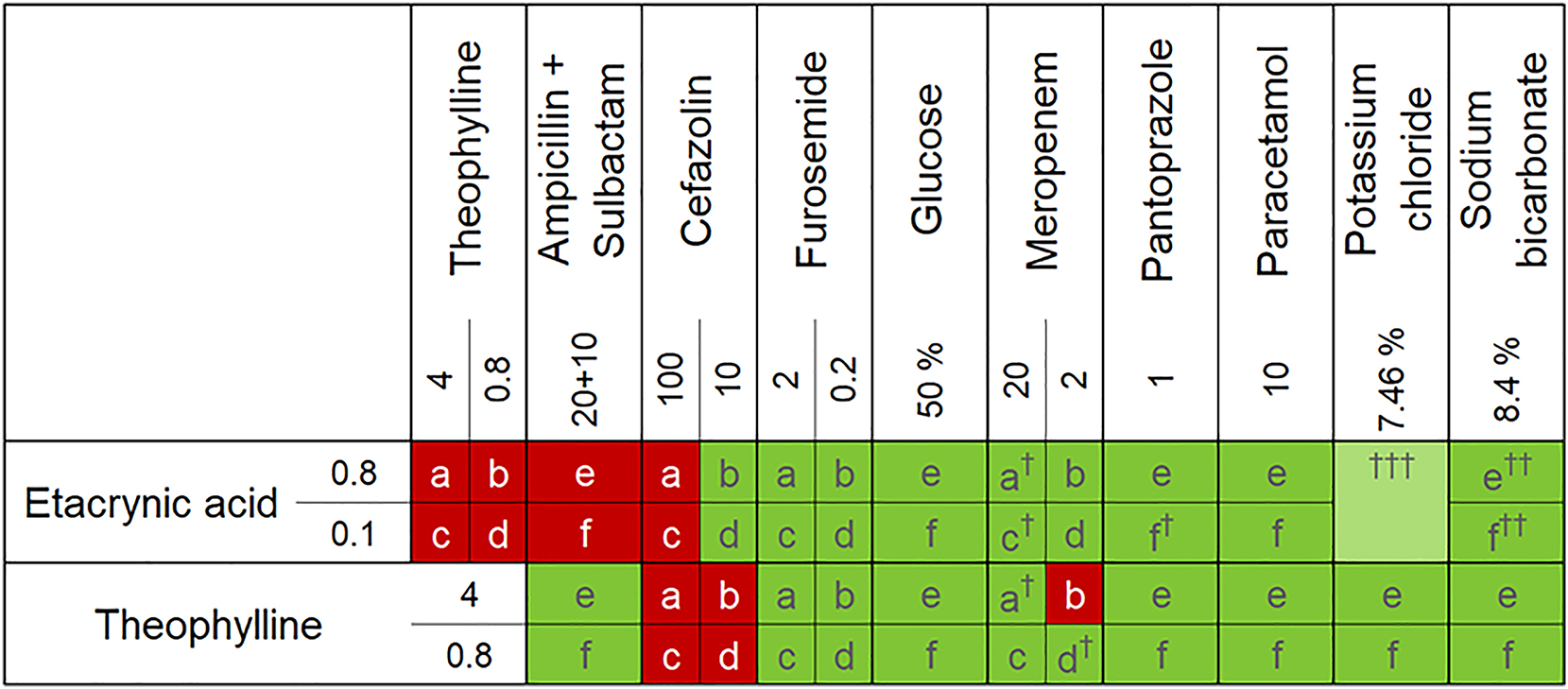
Compatibility table displayed as a cross-table. a,b,c,d,e,f represents the drug combinations according to Figure 2. The respective concentrations in mg/ml, unless otherwise stated, are entered in the columns or rows. Colour indicates the type of compatibility. light green: physically compatible, dark green: physicochemically compatible, red: incompatible, †relative concentration decreases by more than 3 % within 4 h, ††appearance of an increasing degradation product, †††published data, concentration not included.
When determining the contents of the combinations of etacrynic acid with sodium bicarbonate, an additional increasing peak was observed in the chromatograms (see Figure 6). LC-MS and LC-MS/MS experiments were carried out to identify the unknown substance. A molecular ion (M+H+) with m/z 321 was found and allocated to the unknown substance. Together with the fragmentation pattern obtained from the LC-MS/MS experiment of m/z 321 ± 2 (see Figure 7), the substance was identified as 2-(2,3-dichloro-4-(2-(hydroxymethyl)butanoyl)phenoxy)acetic acid (OH-Eca).
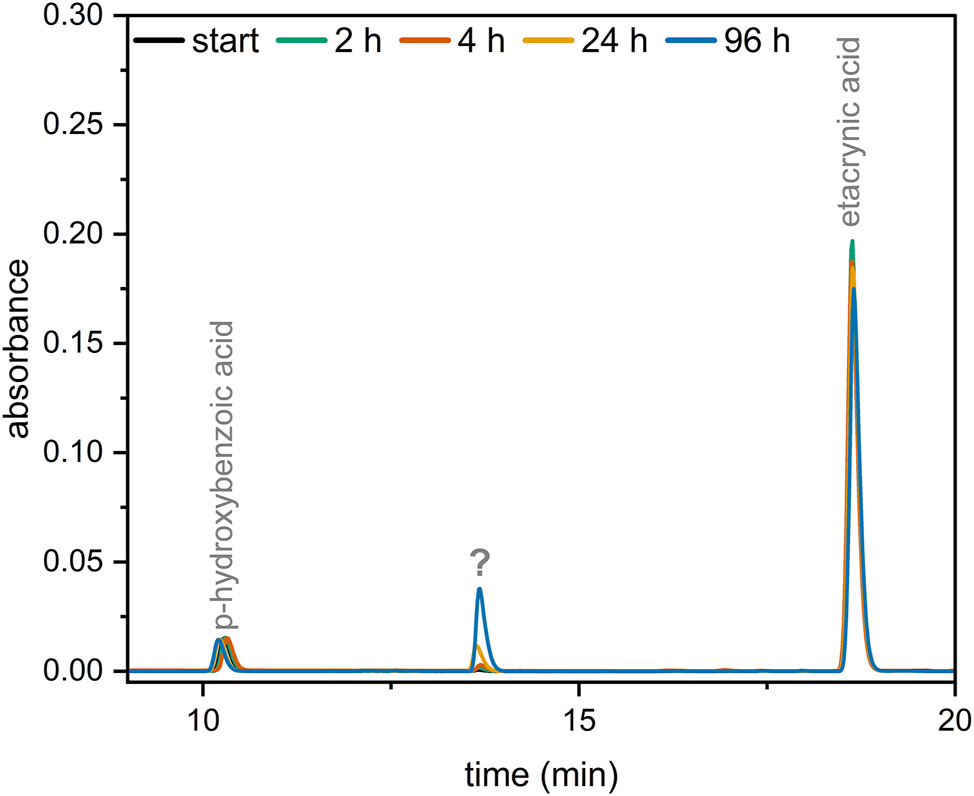
Overlayed chromatogram of etacrynic acid 0.8 mg/mL + sodium hydrogencarbonate 8.4 %. ?: unknown substance.
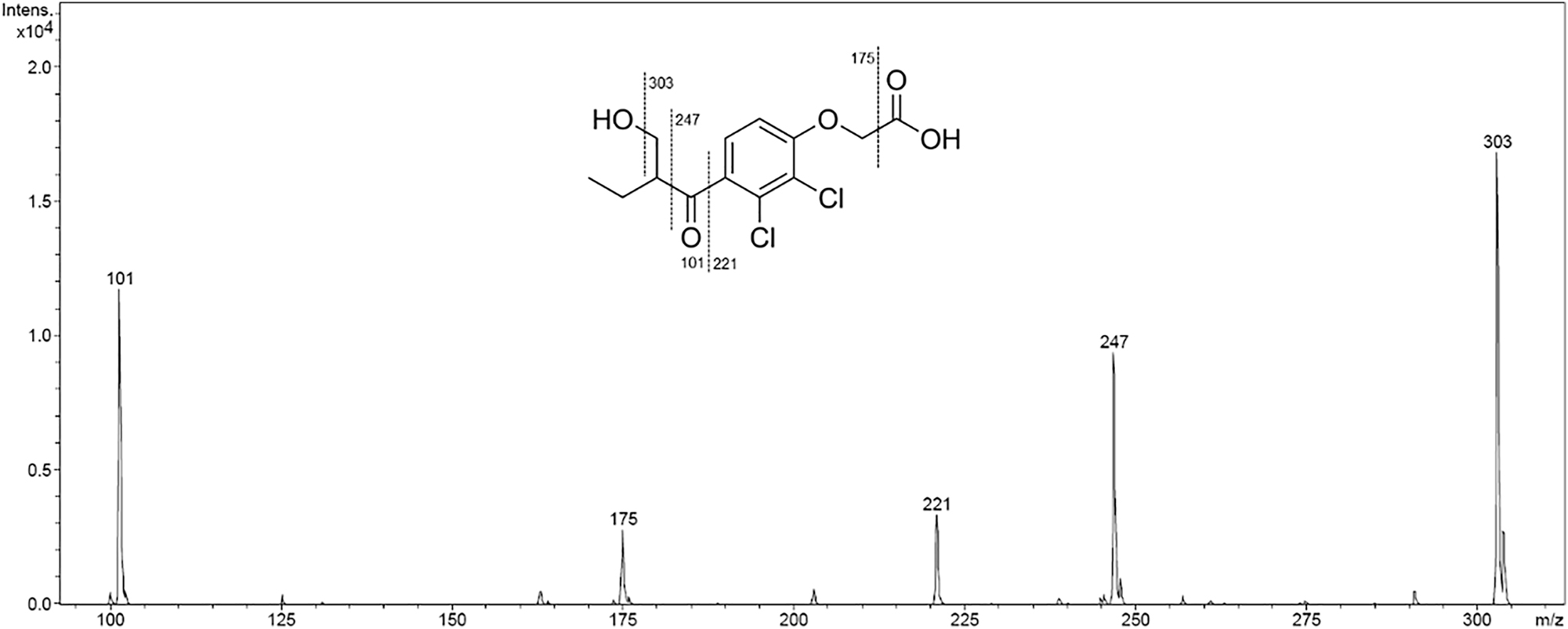
MS/MS experiment (Bruker Amazon SL massspectrometer with ion trap and MSn facility) of m/z 321 ± 2 and presumed fragmentation of 2-(2,3-dichloro-4-(2-(hydroxymethyl)butanoyl)phenoxy)acetic acid.
A graphical representation of the results of the individual drug combinations over 96 h can be found in the supplementary materials.
Discussion
Etacrynic acid and Theophylline represent two drugs in frequent use in the pcICU of the Clinic for Congenital Heart Defects and Paediatric Cardiology of UKSH Kiel.
In addition to Eca and Tph, patients often receive many other drugs, including Amp + Sul, Cef, Fur, G50, Mero, Panto, PCM, KCl or SBC either as continuous infusion or short infusion. Concerning the possible resulting drug combinations, only two studies were found in the literature which investigated their compatibility [36], 37]. In the study from Kershaw et al., only a visual assessment of Tph in combination Amp, Cef, KCl and Sul was carried out, so the evidence regarding the compatibility of these drugs is limited. To fill this gap, we performed a compatibility study of our own and investigated the physicochemical compatibility between etacrynic acid and theophylline with other drugs commonly used in the pcICU.
Out of 50 combinations tested, 13 incompatible combinations were identified (see Figure 5). Interestingly, some of these incompatible combinations initially showed no relevant changes visually, turbidimetrically or in terms of their pH, and thus these combinations could be assessed as physically compatible. However, when the content of these combinations was determined by HPLC, it was found that the concentration of one or more drugs decreased by more than 10 % within 4 h and therefore these drug combinations had to be assessed as incompatible. These physically-not-detectable-incompatible combinations include etacrynic acid 0.8 mg/mL + theophylline 0.8 mg/mL, etacrynic acid 0.1 mg/mL + theophylline 4 mg/mL or 0.8 mg/mL and etacrynic acid 0.8 mg/mL or 0.1 mg/mL + ampicillin 20 mg/mL + sulbactam 10 mg/mL. In the case of etacrynic acid in combination with theophylline, an addition product of the two drugs is formed, which is described elsewhere in detail [30]. In view of the fact that the contact time of the drug solutions is shorter with Y-site administration compared to a mixture of drugs in the same container and that chemical reactions usually take longer to occur, physical tests are already considered sufficient in the literature for assessing the compatibility of drugs administered via Y-site infusion [3], 24]. The occurrence of the physically-not-detectable-incompatible drugs shows that even with physically inconspicuous drug combinations, the content can drop drastically within a few hours. Therefore, whenever possible, a determination of the drug content should be part of compatibility studies and the evaluation of a drug combination as physically compatible should be carefully interpreted.
Additionally, we found some combinations that were conspicuous because the relative concentration of the drugs dropped by more than 3 % in 4 h (see supplementary materials). These drug combinations include meropenem and pantoprazole together with etacrynic acid or theophylline. With prolonged storage, these combinations would then also be assessed as incompatible, as the concentration falls below 90 %. In other studies, meropenem and pantoprazole were frequently part of drug combinations that were assessed as incompatible [7], 24], 26], 27]. However, since the content of these drugs is in our study within the defined limit and the other methods did not show any further conspicuous features, these drug combinations were nevertheless assessed as compatible over a period of 4 h, despite the fact that it is evident that chemical changes have already been started. Thus, if possible, meropenem and pantoprazole should be infused separately or at least with special caution.
Another interesting drug combination is etacrynic acid with sodium bicarbonate. When combining etacrynic acid with sodium bicarbonate, an additional peak that increases over time was observed in the chromatograms (Figure 6). LC-MS experiments were performed to identify this unknown substance. The molecule ion with m/z 321 found via LC-MS strongly suggests that the unknown substance is the vinyl-C hydroxylated product of etacrynic acid (OH-Eca). OH-Eca is a known degradation product of etacrynic acid [38]. ESI-MS/MS experiments of m/z 321 ± 2 allowed us to generate further evidence for the identification of the unknown substance as OH-Eca. In particular, the fragment with m/z 101 confirms this assumption (see Figure 7). Since the concentration of etacrynic acid in combination with sodium bicarbonate does not decrease by more than 10 % even with prolonged storage, these combinations were also assessed as compatible. However, caution should be exercised in this case and, if possible, sodium bicarbonate should be administered alone.
A detailed presentation of the experimental results of the drug combinations can be found in the supplementary materials. With this, an individual assessment of the compatibility of the investigated drug combinations is possible, that may go beyond 4 h. It should be noted that the results of this study are not necessarily transferable to drugs of other manufacturers than those used here due to the possibly different formulation, with regards to the salts, buffers or excipients used. In addition, only 2-drug combinations were investigated in this study. Therefore, the results can be applied to mixtures of three or more drugs only to a limited extent.
Conclusions
In this study, we investigated the compatibility of etacrynic acid and theophylline with other drugs commonly used in a paediatric cardiological intensive care unit. 50 drug combinations have been tested in concentrations that reflect the wide dosing differences between infants and young adults. No incompatibility was detected in 37 drug combinations. 13 drug combinations were found to be incompatible and should not be administered through the same lumen of a catheter. It has been shown that the concentration of the individual drugs plays a decisive role in the occurrence of incompatibilities. Therefore, we recommend that compatibility studies always examine several concentrations of a drug. Our findings help healthcare professionals and pharmacists to optimise the infusion regime and thus contribute to improving the safety management of drug therapy in paediatrics.
Acknowledgments
We would like to thank the staff of the hospital pharmacy of the UKSH Kiel for the good cooperation and the compatibility team of the paediatric cardiological intensive care unit of the Clinic for Congenital Heart Defects and Paediatric Cardiology UKSH Kiel for their support in planning this study. Furthermore, we would like to thank Sven Wichmann for performing the LC-MS/MS measurements.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Machotka, O, Manak, J, Kubena, A, Vlcek, J. Incidence of intravenous drug incompatibilities in intensive care units. Biomed Pap Med Fac Palacky Univ Olomouc Czech Repub 2015;159:652–6. https://doi.org/10.5507/bp.2014.057.Suche in Google Scholar PubMed
2. Taxis, K, Barber, N. Incidence and severity of intravenous drug errors in a German hospital. Eur J Clin Pharmacol 2004;59:815–7. https://doi.org/10.1007/s00228-003-0689-9.Suche in Google Scholar PubMed
3. Marsilio, NR, Da Silva, D, Bueno, D. Incompatibilidades medicamentosas em centro de tratamento intensivo adulto de um hospital universitário. Rev Bras Ter Intensiva 2016;28:147–53. https://doi.org/10.5935/0103-507X.20160029.Suche in Google Scholar PubMed PubMed Central
4. Gikic, M, Di Paolo, ER, Pannatier, A, Cotting, J. Evaluation of physicochemical incompatibilities during parenteral drug administration in a paediatric intensive care unit. Pharm World Sci 2000;22:88–91. https://doi.org/10.1023/a:1008780126781.10.1023/A:1008780126781Suche in Google Scholar PubMed
5. Tissot, E, Cornette, C, Demoly, P, Jacquet, M, Barale, F, Capellier, G. Medication errors at the administration stage in an intensive care unit. Intensive Care Med 1999; 25 (1999):353–9, https://doi.org/10.1007/s001340050857.Suche in Google Scholar PubMed
6. Flamein, F, Storme, L, Maiguy-Foinard, A, Perez, M, Décaudin, B, Masse, M, et al.. Avoid drug incompatibilities: clinical context in neonatal intensive care unit (NICU). Pharm Technol Hosp Pharm 2017;2. https://doi.org/10.1515/pthp-2017-0009.Suche in Google Scholar
7. Sriram, S, Aishwarya, S, Moithu, A, Sebastian, A, Kumar, A. Intravenous drug incompatibilities in the intensive care unit of a tertiary care hospital in India: are they preventable? J Res Pharm Pract 2020;9:106–11. https://doi.org/10.4103/jrpp.jrpp_20_11.Suche in Google Scholar PubMed PubMed Central
8. Benlabed, M, Martin Mena, A, Gaudy, R, Perez, M, Genay, S, Hecq, J-D, et al.. Analysis of particulate exposure during continuous drug infusion in critically ill adult patients: a preliminary proof-of-concept in vitro study. Intensive Care Med Exp 2018;6:38. https://doi.org/10.1186/s40635-018-0205-2.Suche in Google Scholar PubMed PubMed Central
9. Oduyale, MS, Patel, N, Borthwick, M, Claus, S. Co-administration of multiple intravenous medicines: intensive care nurses’ views and perspectives. Nurs Crit Care 2020;25:156–64. https://doi.org/10.1111/nicc.12497.Suche in Google Scholar PubMed
10. Schindler, E, Schears, GJ, Hall, SR, Yamamoto, T. Ultrasound for vascular access in pediatric patients. Paediatr Anaesth 2012;22:1002–7. https://doi.org/10.1111/pan.12005.Suche in Google Scholar PubMed
11. Bradford, NK, Edwards, RM, Chan, RJ. Normal saline (0.9% sodium chloride) versus heparin intermittent flushing for the prevention of occlusion in long-term central venous catheters in infants and children. Cochrane Database Syst Rev 2020;4:CD010996. https://doi.org/10.1002/14651858.CD010996.pub3.Suche in Google Scholar PubMed PubMed Central
12. Goossens, GA. Flushing and locking of venous catheters: available evidence and evidence deficit. Nurs Res Pract 2015;2015:985686. https://doi.org/10.1155/2015/985686.Suche in Google Scholar PubMed PubMed Central
13. Hanot, J, Dingankar, AR, Sivarajan, VB, Sheppard, C, Cave, D, Garcia Guerra, G. Fluid management practices after surgery for congenital heart disease: a worldwide survey. Pediatr Crit Care Med 2019;20:357–64. https://doi.org/10.1097/pcc.0000000000001818.Suche in Google Scholar
14. Lorente, L, Henry, C, Martín, MM, Jiménez, A, Mora, ML. Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care 2005;9:R631–5. https://doi.org/10.1186/cc3824.Suche in Google Scholar PubMed PubMed Central
15. Hanifah, S, Ball, P, Kennedy, R. Medication incompatibility in intravenous lines in Paediatric Intensive Care Units PICUs of Indonesian hospital. Crit Care Shock 2018;21:118–27.Suche in Google Scholar
16. Neininger, MP, Buchholz, P, Kiess, W, Siekmeyer, M, Bertsche, A, Bertsche, T. Incompatibilities in paediatric intensive care - pitfalls in drug information. Pharmazie 2018;73:605–8. https://doi.org/10.1691/ph.2018.8585.Suche in Google Scholar PubMed
17. Alisky, JM, Tuttle, TF. Ethacrynic acid can be effective for refractory congestive heart failure and ascites. South Med J 2003;96:1148–50. https://doi.org/10.1097/01.smj.0000082004.40613.d7.Suche in Google Scholar
18. Kim, KE, Onesti, G, Moyer, JH, Swartz, C. Ethacrynic acid and furosemide. Am J Cardiol 1971;27:407–15. https://doi.org/10.1016/0002-9149(71)90438-3.Suche in Google Scholar PubMed
19. Ricci, Z, Haiberger, R, Pezzella, C, Garisto, C, Favia, I, Cogo, P. Furosemide versus ethacrynic acid in pediatric patients undergoing cardiac surgery: a randomized controlled trial. Crit Care 2015;19:2. https://doi.org/10.1186/s13054-014-0724-5.Suche in Google Scholar PubMed PubMed Central
20. Akademie für Ethik in der Medizin e. V., Arzneimittelkommission der Deutschen Apotheker, Arzneimittelkommission der deutschen Ärzteschaft, Bundesarbeitsgemeinschaft SELBSTHILFE e. V., Deutsche Atemwegsliga e. V., Deutsche Forschungsgruppe Pneumologie in der Primärversorgung e. Vet al. NVL COPD – teilpublikation der Langfassung, 2. Auflage. Berlin: Bundesärztekammer (BÄK); Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF); 2021.Suche in Google Scholar
21. Arzneimittelkommission der Deutschen Apotheker, Arzneimittelkommission der deutschen Ärzteschaft, Bundesarbeitsgemeinschaft SELBSTHILFE, Deutsche Atemwegsliga, Deutsche Forschungsgruppe Pneumologie in der Primärversorgung, Deutsche Gesellschaft für Allergologie und klinische Immunologieet al. NVL asthma – Langfassung, 4. Auflage. Berlin: Bundesärztekammer (BÄK); Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF); 2018.Suche in Google Scholar
22. Yavuz, Y, Isildak, FU. Effect of intraoperative theophylline use on acute kidney injury in paediatric cardiac surgery. Cardiol Young 2022:1–9. https://doi.org/10.1017/s1047951122000245.Suche in Google Scholar PubMed
23. Bell, M, Jackson, E, Mi, Z, McCombs, J, Carcillo, J. Low-dose theophylline increases urine output in diuretic-dependent critically ill children. Intensive Care Med 1998;24:1099–105. https://doi.org/10.1007/s001340050723.Suche in Google Scholar PubMed
24. D’Huart, E, Vigneron, J, Demoré, B. Physical compatibility of intravenous drugs commonly used in intensive care units: an observational study and physical compatibility laboratory tests on anti-infective drugs. Pharm Technol Hosp Pharm 2019;4:29–40. https://doi.org/10.1515/pthp-2019-0005.Suche in Google Scholar
25. Gersonde, F, Eisend, S, Haake, N, Kunze, T. Physicochemical compatibility and emulsion stability of propofol with commonly used analgesics and sedatives in an intensive care unit. Eur J Hosp Pharm 2017;24:293–303. https://doi.org/10.1136/ejhpharm-2016-001038.Suche in Google Scholar PubMed PubMed Central
26. Ayari, G, D’Huart, E, Vigneron, J, Demoré, B. Y-site compatibility of intravenous medications commonly used in intensive care units: laboratory tests on 75 mixtures involving nine main drugs. Pharm Technol Hosp Pharm 2022;7. https://doi.org/10.1515/pthp-2022-0002.Suche in Google Scholar
27. Lessard, J-J, Caron, E, Schérer, H, Forest, J-M, Leclair, G. Compatibility of Y-site injection of meropenem trihydrate with 101 other injectable drugs. Hosp Pharm 2020;55:332–7. https://doi.org/10.1177/0018578719844168.Suche in Google Scholar PubMed PubMed Central
28. European Pharmacopoeia, 10th edition 2019. English: subscription to main volume + supplement 1 + supplement 2, 1. Auflage. Stuttgart: Deutscher Apotheker Verlag; 2019.Suche in Google Scholar
29. Bardin, C, Astier, A, Vulto, A, Sewell, G, Vigneron, J, Trittler, R, et al.. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference: table 1., vol 19; 2012. p. 278–85. https://doi.org/10.1136/ejhpharm-2012-000112.Eur J Hosp Pharm3Suche in Google Scholar
30. Jirschitzka, S, Girreser, U, Kunze, T. An aza-Michael addition product causes incompatibility between etacrynic acid and theophylline in a paediatric cardiological ICU. Pharmazie 2023;78:27–30. https://doi.org/10.1691/ph.2023.2564.Suche in Google Scholar PubMed
31. EMA. ICH Q2(R2) Validation of analytical procedures: Note for guidance on validation of analytical procedures: text and methodology(CPMP/ICH/381/95); 1995. Available from: URL: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures.Suche in Google Scholar
32. Hines, S, Pleasance, S. Compatibility of an injectable high strength oxycodone formulation with typical diluents, syringes, tubings, infusion bags and drugs for potential co-administration. Eur J Hosp Pharm Pract 2009;15:32–8.Suche in Google Scholar
33. Tomczak, S, Gostyńska, A, Nadolna, M, Reisner, K, Orlando, M, Jelińska, A, et al.. Stability and compatibility aspects of drugs: the case of selected cephalosporins. Antibiotics (Basel) 2021;10. https://doi.org/10.3390/antibiotics10050549.Suche in Google Scholar PubMed PubMed Central
34. Kanji, S, Lam, J, Johanson, C, Singh, A, Goddard, R, Fairbairn, J, et al.. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit Care Med 2010;38:1890–8. https://doi.org/10.1097/ccm.0b013e3181e8adcc.Suche in Google Scholar
35. Knudsen, L, Eisend, S, Haake, N, Kunze, T. Physicochemical compatibility of commonly used analgesics and sedatives in the intensive care medicine. Eur J Hosp Pharm 2014;21:161–6. https://doi.org/10.1136/ejhpharm-2014-000444.Suche in Google Scholar
36. Allen, LV, Levinson, RS, Phisutsinthop, D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm 1977;34:939–43. https://doi.org/10.1093/ajhp/34.9.939.Suche in Google Scholar
37. Kershaw, BP, Monnier, HL, Mason, JH. Visual compatibility of premixed theophylline or heparin with selected drugs for i.v. administration. Am J Hosp Pharm 1993;50:1360, 1362–3. https://doi.org/10.1093/ajhp/50.7.1360.Suche in Google Scholar
38. Yarwood, RJ, Moore, WD, Collett, JH. The influence of the ammonium ion on the stability of ethacrynic acid in aqueous solution. J Pharm Biomed Anal 1987;5:369–78. https://doi.org/10.1016/0731-7085(87)80043-2.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/pthp-2023-0009).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Paediatric orodispersible lozenges produced by moulding process: quality and stability control
- Research Articles
- Investigation of incompatibilities of the parenteral drugs etacrynic acid and theophylline with other commonly used intravenous drugs in a paediatric cardiological intensive care unit
- Focused physicochemical stability study of reconstituted trastuzumab biosimilar SB3 (Ontruzant®) stored in the original vials over 28 days
- Guideline
- Recommendations on training objectives and staff qualification for the manual preparation of capsules in pharmacy
Artikel in diesem Heft
- Paediatric orodispersible lozenges produced by moulding process: quality and stability control
- Research Articles
- Investigation of incompatibilities of the parenteral drugs etacrynic acid and theophylline with other commonly used intravenous drugs in a paediatric cardiological intensive care unit
- Focused physicochemical stability study of reconstituted trastuzumab biosimilar SB3 (Ontruzant®) stored in the original vials over 28 days
- Guideline
- Recommendations on training objectives and staff qualification for the manual preparation of capsules in pharmacy

