Molecular architecture of pterin deaminase from Saccharomyces cerevisiae NCIM 3458
-
Thandeeswaran Murugesan
Abstract
As early as 1974, reports have confirmed the anticancer activity of pterin deaminase isolated from fungi. The enzyme has also been reported in bacteria, fungi and slime mold genera, but the enzyme characterization was effetely done. The present study attempted to purify and characterize pterin deaminase enzyme from Saccharomyces cerevisiae NCIM 3458. The protein was extracted from the extracellular extract by using the ethanol precipitation method. Partial purification of pterin deaminase enzyme was achieved by ion exchange chromatography (Hi-Trap QFF) by fast protein liquid chromatography (AKTA purifier). The molecular weight of the protein was apparently determined by SDS-PAGE, and the presence of pterin deaminase was confirmed by activity staining. The purified enzyme was further biochemically characterized. Molecular docking studies showed higher binding affinity towards folic acid interaction. The structural characterization of this protein may open the windows for new drug targets for cancer therapy.
Introduction
Pteridine research has been renowned as eminent for scores of natal process [1]. It also acts as essential cofactors in the process of cell metabolism and has turned into a focal point of cancer screening research because of reported varying pteridine levels in cancers [2]. In the pathway of pteridines, one of the most momentous uncharted enzymes was pterin deaminase. Most of the studies have focused only on the pteridines and its derivatives, whereas the studies on pterin deaminase are countable. Pterin deaminase is an enzyme belonging to the family of hydrolases, acting on carbon-nitrogen bonds other than peptide bonds. The reaction took place as follows [3].

The pterin deaminases were disseminated as intracellular, membrane-bound or extracellular enzyme in a spacious variety of prokaryotic and eukaryotic sources [4]. The first report on the intracellular pterin deaminase was isolated from the bacterium Alcaligenes metalcaligenes by Levenberg and Hayaishi in 1959 [5]. However, extracellular pterin deaminase was reported in eukaryotic organisms such as Aspergillus sp., Mucor sp., Rhizopus sp. and Penicillium sp. [6], [7], [8]. Rembold and Simmersbach [9] revealed that the pterin deaminase present in rat liver showed the highest substrate specificity. Besides this report in mammalian system, there are no other research findings in mammalian pterin deaminase. The 3D structure of the pterin deaminase enzyme is not available in the database plow at present. This uncharacteristic pterin deaminase is lighting an interest in developmental and neuronal biologist. The structure and function of the pterin deaminase enzyme might prove to pave the way for resolving unsolved quests in the biological systems. Saccharomyces cerevisiae, being utilized as a model system by pioneers of powerful functional genomics, systems and synthetic biology for recombination and fermentation technology [10], was selected as the test organism in the present study. This study was divulged to produce and purify enzyme pterin deaminase from S. cerevisiae 3458. In addition, the study involved the structure prediction of pterin deaminase through bioinformatic tools.
Materials and methods
Collection of strain
Lyophilized culture of S. cerevisiae (NCIM no. 3458) was procured from the National Collection of Industrial Microorganism (NCIM), Pune. The cells were activated by inoculating in yeast extract peptone dextrose (YPD) medium and incubated in an aerobic environment with agitation of 120 rpm at 30 °C for 48 h. This served as the candidate for pterin deaminase enzyme production.
Preparation of extracellular enzyme
After incubation, the cells were harvested by centrifugation at 10,000 rpm at 4 °C for 15 min. The supernatant was collected from the broth culture and served as a crude enzyme.
Enzyme assay
Enzyme activity (IU mL−1) against the substrate 0.01 M folic acid was estimated in triplicates in extracellular culture filtrates by Nesslerization method [11]. The assay conditions used for the estimation of pterin deaminase were as follows: 340 μL of 0.01 M folic acid was incubated with 50 μL of enzyme for 10 min at 30 °C in the presence of 40 μL of 0.01 M phosphate-buffered saline buffer (pH 7), with a simultaneous run of blank with same reaction mixture without enzyme. The reaction was terminated using 1.5 M trichloroacetic acid. The resulting precipitate was centrifuged to collect the ammonia suspended in supernatant. For estimation, 100 μL of the supernatant was mixed with distilled water (800 μL) and Nessler’s reagent (100 μL) making the final reaction volume to be 1 mL for determination of ammonia. The reaction mixture was incubated for 10 min, and the absorbance was read at 480 nm using spectrophotometer. Ammonium sulfate (0.2–1 μM) was used as standard for ammonia estimation. Pterin deaminase activity was expressed as micromoles of ammonia released per minute per milliliter against folic acid as substrate.
Protein precipitation
Concentration of protein was tried by overnight incubation with chilled ethanol ranging from 3 to 9 volumes. After incubation, the tubes were centrifuged at 8000 rpm for 10 min. The protein pellet formed was suspended with 0.01 M potassium phosphate buffer (pH 7) for further protein and enzyme analysis. The ethanol concentration, which yielded the highest protein content [12] and enzyme activity, was chosen for further purification with column chromatography.
Ion exchange chromatography (FPLC)
Ion exchange chromatography was performed at 4 °C with pre-packed Hi Trap Q FF (1 mL) column, an anion exchanger that was connected to the AKTA purifier FPLC system (GE Healthcare, Sweden). The column was equilibrated with 10 column volumes of binding buffer (buffer A: 0.01 M potassium phosphate buffer, pH 7). Ethanol precipitated sample was injected to the column through a sample loop. Then the column was washed with buffer A and eluted with gradient buffer (mixture of buffer A and buffer B). Buffer B (1 M NaCl) was mixed gradually with buffer A to give a gradient mode of 0%–100%. Flow through and elute were collected with a fraction collector included in the system (Frac-920) at a fixed flow rate (1 mL/min). All the fractions under the observed protein peaks were subjected to protein and enzyme assays. The active fractions with enzyme activity was used for further protein analysis. SDS-PAGE was done with the active fractions according to the method proposed by Laemmli [13]. Silver staining was done for the chosen fraction by the method of Switzer et al. [14].
Native PAGE
Native PAGE staining was performed by adopting the enzyme assay method of Levenberg and Hayaishi [5]. The enzyme samples (crude, ethanol precipitated and active fractions of ion exchange chromatography) were run on 12% Native-PAGE at 4 °C under non-denaturing conditions according to Davis [15]. The staining was done by incubating the gel in 0.01 M folic acid and 0.05 M Tris-HCl buffer at 4 °C in dark for 20 min, washed in distilled water for 30 s and immediately observed for the fluorescence band under UV light.
Enzyme characterization
Optimum pH was determined by incubating the reaction mixture in various pH ranging from 4 to 10, and optimum temperature for enzyme activity was ascertained by incubating the reaction mixture in various temperatures ranging from 30 °C to 90 °C. Ammonia liberation in all the reactions was calculated by Nessler’s Reagent for calculating enzyme activity.
The kinetic constants Km and Vmax were estimated following the method of Lineweaver and Burk [16] using various concentrations (5 mM–15 mM) of folic acid as substrate.
Inhibition assay
The effect of inhibitors on pterin deaminase was determined by using different inhibitors such as ethylene diamine tetra acetic acid (EDTA), dithiothreitol (DTT), phenyl methyl sulfonyl fluoride (PMSF), SDS, Triton X100 and 1,10-phenanthroline. The enzyme was incubated for 30 min with various concentrations of inhibitors like 10 mM, 1 mM and 0.1 M. After incubation, the enzyme activity was estimated by assaying the ammonia released by enzyme by deamination the substrate folic acid by a modified method of Wurster and Butz [17].
In silico analysis of pterin deaminase
A complete genome of S. cerevisiae (NC_001143.9) and a sequence of pterin deaminase (WP_012652141.1) were retrieved from NCBI through Z28140.1 identifier (NCBI-Gene sequence). Pterin deaminase perfectly matched with a small part of the sequence present in the S. cerevisiae. Further, folic acid structure was retrieved from PubChem database with ID of CID_6037. Using Prime modules of Schrodinger suit 2016, a model protein structure of pterin deaminase was predicted and validated. The modeled structure of pterin deaminase and folic acid from PubChem database was used for interaction studies by glide modeling followed by extra precision method and rigid docking parameter.
Results
Extraction of extracellular enzyme
Saccharomyces cerevisiae (NCIM 3458) used for this study was procured from NCIM, Pune, and maintained in YPD medium. The extracellular enzyme was extracted from the yeast cells through centrifugation, and the crude enzyme retained in the supernatant was used as crude enzyme extract. The protein content of the extracellular enzyme was found to be 1.1 mg/mL, and the enzyme activity using folic acid as substrate was found to be 38.97 U/mL.
Maximum extracellular enzyme was precipitated (44.60 U/mL) with nine volumes of ethanol, whereas the other concentrations (varying from three to eight volumes) clearly showed less protein yield. The highest concentration of protein being precipitated at a 1:9 ratio (crude extract-ethanol) validated nine volumes as the preferred concentration of ethanol.
Purification of pterin deaminase
The ethanol precipitated enzyme, after suspension with potassium phosphate buffer, was estimated to have 44.60 U/mL of pterin deaminase activity, and protein content was measured to be 0.96 mg/mL. The specific activity was increased from 35.42 to 46.45 U/mg, and the purification fold was increased to 1.311 (Table 1).
Purification step of pterin deaminase.
| Purification steps | Volume, mL | Enzyme activity, U/mL | Total protein, mg/mL | Specific activity, U/mg | Purification (fold) |
|---|---|---|---|---|---|
| Crude | 40 | 38.97 | 1.1 | 35.42 | 1 |
| Ethanol precipitation | 10 | 44.60 | 0.96 | 46.45 | 1.311 |
| Trap Q FF (ion-exchange column) | 1 | 82.8 | 0.26 | 310.11 | 8.76 |
The ion exchange column purification process increased the purity of the enzyme, which was revealed by the increase in enzyme activity to 82.8 U/mL with the specific activity of 310.11 U/mg. The purification fold was increased to 8.76. Although the chromatogram revealed five active peaks, the fractions from 12 to 15 under the peak alone exhibited enzyme activity corresponding to 20% NaCl gradient solution (Table 1; Figure 1).

Ion exchange chromatogram of purified pterin deaminase.
The protein profile of the crude enzyme showed more than four bands and appeared very thin due to very low concentration of loaded protein. The precipitated protein sample showed similar band pattern as crude but denser conforming the increased concentration of protein. Single band was visualized upon PAGE analysis of the active fractions (pooled) of ion exchange chromatography, and apparent molecular weight of the enzyme was approximately 60 kDa (Figure 2).

SDS-PAGE (silver staining): lane 1, marker; lane 2, crude (20 μL); lane 3, ethanol precipitation (30 μL); lane 4, purified fraction (35 μL); lane 5, purified fraction (40 μL); lane 6, purified fraction (45 μL); lane 7, purified fraction (50 μL).
Confirmation of pterin deaminase
For further confirmation, the enzyme sample was run on 12% Native-PAGE at 50 V for 3 h. Upon incubation with the substrate (folic acid), the gel revealed fluorescence under UV light and confirmed the presence of pterin deaminase in the purified sample. The fluorescence observed was attributed to the presence of product lumazine, which has blue fluorescence. The band was clearly visible in precipitated protein where the protein concentration was higher (Figure 3).

Activity staining of purified pterin deaminase: lane 1, FPLC purified fraction; lane 2, FPLC purified fraction; lane 3, ethanol precipitate.
Optimization of purified pterin deaminase
The optimum temperature was found to be 30 °C, which corresponded to the maximum enzyme activity of 51 U/mL (Figure 4), whereas alkaline pH 8 was optimized condition for pterin deaminase activity (Figure 5). Double reciprocal plot showed the Km as 10 mM of folic acid and Vmax of 317.30 U/mg (Figure 6). The overall characteristic of pterin deaminase was summarized in Table 2.

Effect of temperature on enzyme activity.

Effect of pH on enzyme activity.

Double reciprocal plot.
Characteristics of purified pterin deaminase from Saccharomyces cervisiae.
| Sample no. | Parameters | Units |
|---|---|---|
| 1 | Optimum pH | 8 |
| 2 | Optimum temperature °C | 30 °C |
| 3 | Molecular weight | Approximately 60 kDa |
| 4 | Km, μM | 10 mM |
| 5 | Vmax, IU/mg | 317.30 |
Effect of inhibitors
To predict the nature of the enzyme, inhibitor studies were carried out with purified enzyme. The enzyme was inhibited by EDTA and 1,10-phenanthroline at 10 mM concentration (Figure 7), and inhibitors such as DTT and PMSF also partially inhibited the enzyme activity at 10 mM concentration. EDTA disodium salt inhibited the enzyme activity, which indicated that the enzyme to be a metallo enzyme.

Effect of inhibitors on pterin deaminase activity.
Homology modeling of protein sequence
Pterin deaminase sequence from Agrobacterium radiobacter K84 was retrieved from NCBI GenBank (GI: WP_012652141.1). Domain region of retrieved sequence was aligned with Saccharomyces genome sequence and found 47% similarity matches with pterin deaminase (Figure 8). Aligned sequence was then subjected to translate for six frame translations, which have high consensus similarity. 1AF2, 1ALN, 1CTT, 1CTU, 1JTK, 1MQ0, 1A4L, 1A4M, 1ADD, 1FKW, 1FKX and 1KRM as PDB ID were chosen as a template to predict structure of pterin deaminase (Table 3). All the template selection was kept on the basis of functionally similar as deaminase activity with metals such as Mg, zinc and copper. Knowledge-based homology modeling runs as high complexity and low sequence identity alignment between query and subject from irrespective of organism specification and enzyme classification. Further templates were utilized to predict the structure using prime module from Schrodinger suite. The modeled structure was then hypothesized to undergo energy minimization to optimize geometrical conformations. Modeled protein structure was plotted against Ramachandran Rotomer library conformations and screened, allowed and disallowed region of amino acids stability (Figure 9).

Alignment matrix of target sequence (WP_012652141) and query sequence (TRS85_851686).
List of DNA and protein sequences and template structures retrieved from databases.
| Sample no. | Sample | ID | Organisms | Database | Details |
|---|---|---|---|---|---|
| 1 | DNA sequence | WP_012652141.1 | Agrobacterium radiobacter K84 | NCBI | Gene imparted with pterin deaminase activity as per earlier report (Fan et al. [18]) |
| 2 | DNA sequence | TRS85 Trs85p | Saccharomyces cerevisiae | NCBI gene | Resultant gene sequence when gene of Agrobacterium radiobacter K84 was blasted against Saccharomyces cerevisiae |
| 3 | DNA | NC_001143.9 | Saccharomyces cerevisiae | NCBI genome | Whole genome |
| 4 | Template protein structure | 1A4L_A (adenosine deaminase) | Mus musculus | PDB | Ada structure complexed with deoxycoformycin at pH 7 |
| 1A4M_A (adenosine deaminase) | Mus musculus | PDB | Ada structure complexed with purine riboside at pH 7 | ||
| 1ADD_A (adenosine deaminase) | Mus musculus | PDB | A pre-transition state mimic of an enzyme: X-ray structure of adenosine deaminase with bound 1-deaza-adenosine and zinc-activated water | ||
| 1AF2_A (cytidine deaminase) | Escherichia coli | PDB | Crystal structure of cytidine deaminase complexed with uridine | ||
| 1ALN_A (cytidine deaminase) | Escherichia coli | PDB | Crystal structure of cytidine deaminase complexed with 3-deazacytidine | ||
| 1CTT_A (cytidine deaminase) | Escherichia coli | PDB | Transition-state selectivity for a single oh group during catalysis by cytidine deaminase | ||
| 1CTU_A (cytidine deaminase) | Escherichia coli | PDB | Transition-state selectivity for a single oh group during catalysis by cytidine deaminase | ||
| 1FKW_A (adenosine deaminase) | Mus musculus | PDB | Murine adenosine deaminase (d295e) | ||
| 1FKX_A (adenosine deaminase) | Mus musculus | PDB | Murine adenosine deaminase (d296a) | ||
| 1JTK_A (cytidine deaminase) | Bacillus subtilis | PDB | Crystal structure of cytidine deaminase from Bacillus subtilis in complex with the inhibitor tetrahydrodeoxyuridine | ||
| 1KRM_A (cytidine deaminase) | Bos taurus | PDB | Crystal structure of bovine adenosine deaminase complexed with 6-hydroxyl-1,6-dihydropurine riboside | ||
| 1MQ0_A (cytidine deaminase) | Homo sapiens | PDB | Crystal structure of human cytidine deaminase | ||
| 5 | Ligand | CID 6037 | - | PubChem | Folic acid |

Plotting modeled amino acids conformations for stereo chemical quality and protein moiety check with Z-score analysis.
Interaction profile of modeled protein
Interaction profile of modeled pterin deaminase with folic acid showed that the ligand interacted at three sites of the protein having residual atom types ARG 370 (H), ARG 370 (H), ASN 337 (O) receptor via O (oxygen) and H (hydrogen) atom types forming oxygen and hydrogen bonds with the bond distance 2.214, 1.547 and 2.417 A, respectively (Figure 13). ARG and ASP have Schiff base and mediate meta portion amino group in the folic acid structure. Higher interaction of model protein versus folic acid obtained the glide score of −12.94 as higher interaction. Docking studies revealed that the protein sequence had folic acid binding capacity. Hence, using this as the base sequence of the gene responsible for pterin deaminase in S. cerevisiae 3458 further recombination and expression studies could be performed to confirm the function of the gene sequence. Analog conformations of folic acids interacted with metal binding proteins. Adenosine (Figure 10), guanine (Figure 11) and cysteine deaminase (Figure 12) proteins were selected to undergo docking procedure as to confirm functionality of deaminase. All obtained docking scores are lower than modeled pterin deaminase protein, i.e. −11.9, −4.1 and −8.3, respectively. Arginine is common amino acids that have interaction with all folic binding deaminase proteins.

Interaction profile of folic acids and adenosine deaminase in the functional site as ribbon representation.
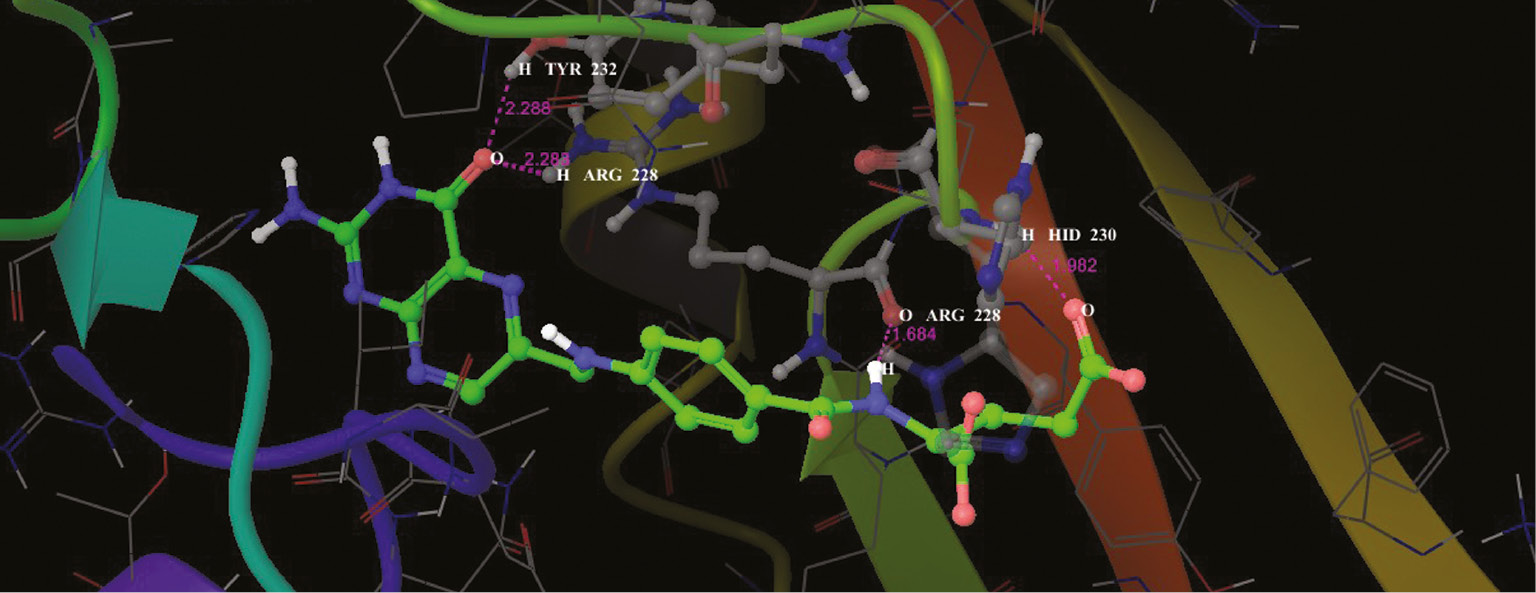
Interaction profile of folic acids and cytosine deaminase in the functional site as ribbon representation.
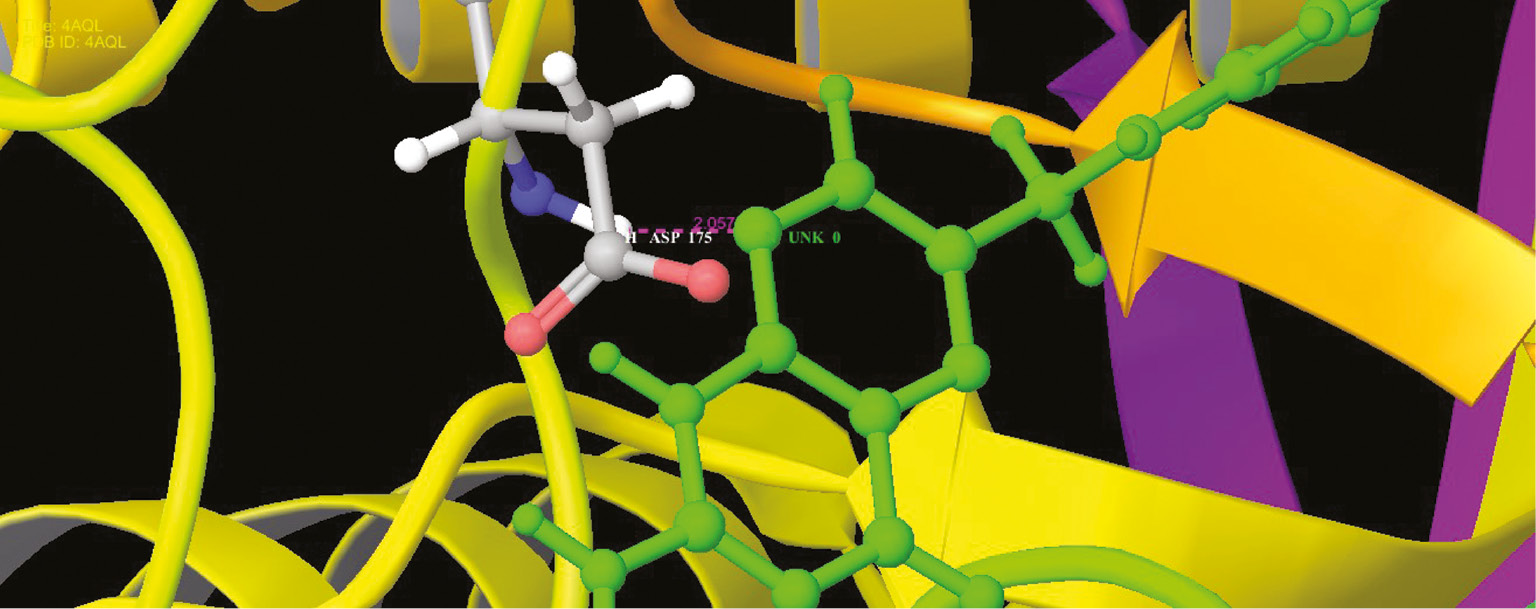
Interaction profile of folic acids and guanine deaminase in the functional site as ribbon representation.
Discussion
Saccharomyces cerevisiae is the most meticulously investigated eukaryotic microorganism and has been used in the production of food and alcoholic beverages, and these days this organism is also used in a number of different processes within the pharmaceutical industry [19]. Thus, it is now possible to introduce the production of anticancer enzyme pterin deaminase from this persuasive organism. Saccharomyces cerevisiae (NCIM 3458) has been so far explored for the production of aminohydrolase enzymes such as L-asparaginase and allantoinase, whereas this is the first attempt to pronounce the presence of pterin deaminase in the organism.
Folic acid, which is otherwise named as pteroyl glutamic acid, has pterin ring attached to glutamic acid by para amino benzoic acid. Hence, folic acid was used as substrate for the enzyme, which acts on amino group at the second position of the pterin ring. The report of Levenberg and Hayaishi [5] used pteroyl glutamic acid as the substrate in A. metalcaligenes, whereas Rembold and Simmersbach [9] reported isoxanthopterin, tetrahydropterin and pterin as substrates of pterin deaminase.
During the extraction process in this study, the higher protein precipitation was achieved at 1:9 ratios of protein-ethanol, and the purification fold was increased from 35.42 to 46.45 U/mg. Although the protein precipitation was reported to be complete and instantaneous with maximum recovery at all temperatures [20], only at lower temperature recovery of activated protein is a maximum that varies with pH [21]. Hence, the precipitation of the enzyme with 90% cold ethanol was substantiated.
Literally, there were no reports based on the purification of pterin deaminase enzymes in yeast, whereas Kusakabe et al. [7] reported the enzyme was purified from fungi Aspergillus Y8-5 (ATCC 20413) by DEAE cellulose column chromatography and obtained 84,975 units of pterin deaminase with the enzyme activity 825 U/mg of powder (1428 U/mg of protein). In this view, in the present study, DEAE cellulose column has been used from which the enzyme was eluted earlier at around 20% NaCl concentration with 10 mM phosphate buffer of pH 7. This indicated that the protein is negatively charged with less net charge, as binding with the matrix is not strong requiring only low salt concentration for its elution. In this study, the binding buffer of pH 7.0 has been used for the purification of this enzyme of alkaline nature as it was proved that 7.6 binding buffer resulted in higher resolution peaks for alkaline enzyme than those with higher pH binding buffer [22].
The molecular weight of the pterin deaminase was found to be 110 kDa in Bacillus megaterium [23]. In our study, the presence of pterin deaminase in S. cerevisiae was apparently the molecular weight was 60 kDa, which is similar to the previous report of Fan et al. [18] 47 kDa in Agrobacterium sp.
Confirmation of the presence of the enzyme was achieved by activity staining. The fluorescent property of the substrate and product was exploited for locating the enzyme in the native gel [5]. Upon incubation with the substrate and subsequent washing, the unbound substrate was removed leaving only the enzyme bound compounds. As both the substrate and product possess fluorescent capacity, the appearance of fluorescent band in native gel was attributed to the presence of enzyme illuminated by bound substrate or product.
The pterin deaminase characterized so far has been found to be active at alkaline pH [23], [24], [25], [26] owing to the release of ammonia during the reaction, and this has been confirmed in the present study also. Notably, this enzyme has been observed to be stable at higher temperature of 100 °C with around 10% of residual enzyme activity. The thermostability of the enzyme is imperative as the deamination process is an exothermic reaction, which requires the catalyzing enzyme to be thermostable [7]. Thus, the enzyme pterin deaminase was proven to be alkaline and thermostable enzyme. In earlier reports, pH 8 was measured as optimized condition for pterin deaminase activity, and it was capable of tolerating a pH range of 6.5–8.5 and also temperatures up to 90 °C [8], [22], [24], [25], [26], [27], [28].
Folic acid, an efficient substrate, was prepared for substrate kinetic determination. Elevation activity of folic acid and pterin deaminases was higher in S. cerevisiae (Km 10 mM and Vmax 317.30 IU/mg) than other prokaryotic organisms. Similarly, pterin deaminase catalyzing the hydrolytic deamination of pteridines was found in the bacterium, and B. megaterium showed the Km value for 6-carboxypterin at 1.3 mM [23]. The Km value of the enzyme from slime mold Dictyostelium discoideum strain Ax-2 was established as 0.002 mM folic acid for isoform I and for isoform II as 0.023 mM and 0.03 mM pterin. The pterin deaminase extracted from rat liver exhibited a Km value of 30 μM for pterin [9]. The wide variation in Km value may be attributed to the diversified sources (slime mold, bacteria and rat), which might be having varied concentration of substrate (pterin derivatives) metabolism.
Scientific reports suggest that the research groups were unable to identify efficient inhibitors for pterin deaminase. In spite of these, some of the inhibitors have been used and identified for the characterization of pterin deaminase in S. cerevisiae. The report of this study showed that the enzyme was deprived by EDTA and 1,10-phenanthroline at 10 mM concentration. Similarly, Levenberg and Hayaishi [5] found p-Cl-Hg benzoate and sodium or potassium fluoride caused severe inhibition for pterin deaminase activity when compared with other halide ions. In 1981, Wurster et al. [29] analyzed that the kinetic data for pterin deamination in the presence of 0.05 μM folic acid yielded typical competitive inhibition. Kim et al. [30] revealed KCN inhibited dihydropterin deaminase activity strongly (98.6%). Inhibition studies of the enzymes revealed some structural characters of the enzyme. The inhibition of enzyme activity by 1,10-phenanthroline demonstrates that the enzyme has metal component at 1,10-phenanthroline, forming strong complexes with zinc ions [31]. Further, the inhibition of the enzyme by EDTA corroborates the involvement of metal ion in the enzyme activity as EDTA chelates with heavy metals [32]. Interference of DTT in enzyme activity may be attributed to the presence of cysteine disulfide bonds in the enzyme as DTT reduces the disulfide bonds. Less inhibition of DTT might be interpreted as less disulfide bonds exposed to reducing reaction as DTT cannot reduce the disulfide bonds buried in the folds of the proteins [33], [34]. The reduced impact of PMSF on the enzyme activity portends the presence of less serine residues in the active site of enzyme activity as PMSF reacts with only hyperactive serine residues at the active site [35]. Thus, an inhibitory study prophesies the pterin deaminase to be a metallo enzyme with disulfide bridges of cystein and a few hyperactive serine residues.
The role of pterin deaminase in the body metabolism of S. cerevisiae is unknown, which may be revealed by silence gene mutation for which the gene sequence has to be determined. In this view, the present study was attempted to predict the gene sequence of the enzyme. Besides, this study added a further significant unique result of the protein structure of pterin deaminase in eukaryotic organisms S. cerevisiae. The reported gene sequence of pterin deaminase of A. radiobacter K84 was taken as the reference sequence that was used to pick out the pterin deaminase gene in S. cerevisiae genome. The protein structure of pterin deaminase was predicted and validated using Prime modules of Schrodinger suit 2016. The modeled structure of pterin deaminase and other folic binding proteins was interacted using glide modeling followed by extra precision method and rigid docking parameter. Folic acid is used as a substrate interacted with modeled pterin deaminase structure with Extra-Precision energy composition from Glide procedure. The higher interaction of model protein obtained the glide score −12.94. Docking score showed that it had good interaction with the enzyme, thus endorsing the functional property of pterin deaminase activity [18]. The marginal amount of score to consider is −12.94, and it hosts functional properties with folic complexity. Hence, the modeled pterin deaminase was proved for the ID: TRS85_851686 (Figure 13) of S. cerevisiae from proposed interaction profile, and the pterin deaminase structure can be taken into account for further biological activity. More recently, Fan et al. [18] determined a multidisciplinary approach that integrated homology modeling, molecular docking screens of a metabolite library and physical library screening by kinetic assays in Arad3529 from A. radiobacter K84 regarding pterin deaminase.
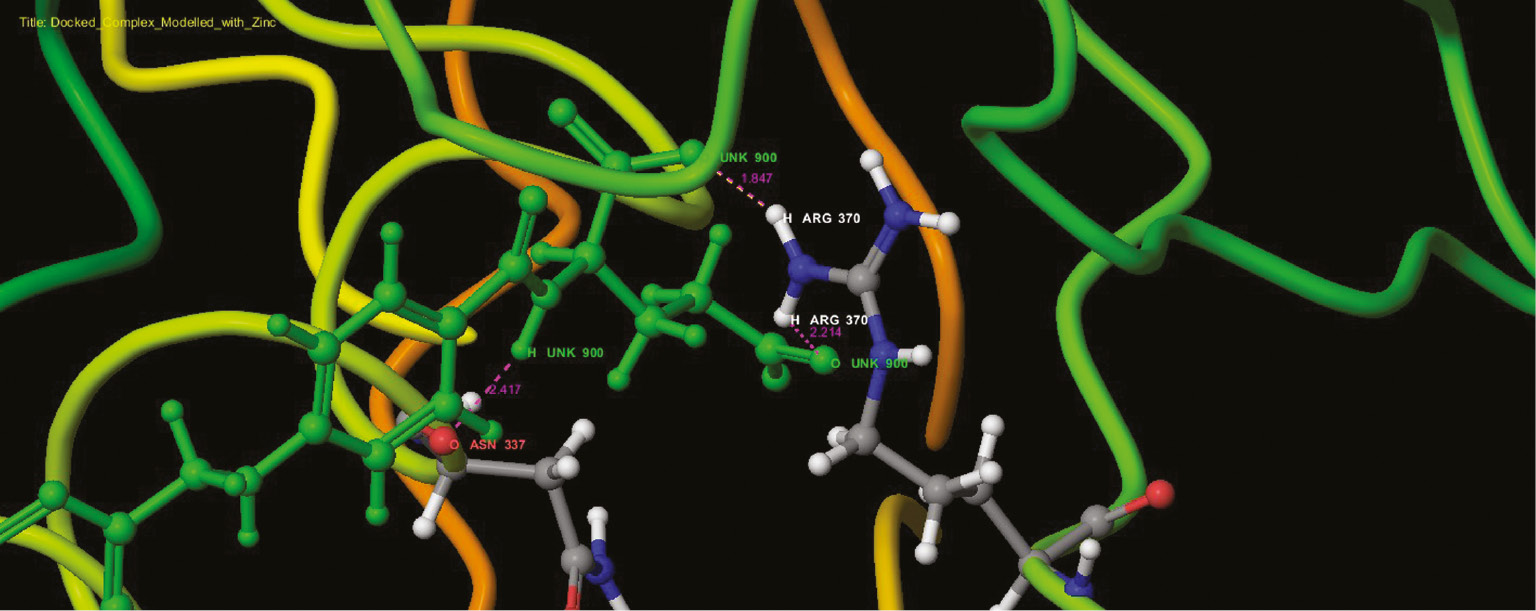
Interaction profile of folic acids and modeled pterin deaminase in the functional site as ribbon representation.
Conclusion
Therapeutically, enzymology offers a means of treatment in tumors, although certainly insufficiently investigated to date. This study revealed novel data about the structural characterizes of pterin deaminase from S. cerevisiae. This study highlighted the possibility of extracting pterin deaminase enzyme from S. cerevisiae 3458 and also shed light on the biochemical properties of the enzyme activity. The confirmation of the predicted structure of pterin deaminase protein might lead to better understanding of the biological functions of this enzyme. Moreover, this study summarized herein appears to the first report of pterin deaminase from S. cerevisiae, and this may just open the windows for new drugs for cancer therapy.
Conflict of interest statement: All authors have declared no conflicts of interest.
References
1. Milstien S, Kapatos G, Levine RA, Shane B. 12th Symposium chemistry and biology of pteridines and folates, Bethesda, MD, 17 – 22.6.2001, Abstracts. Pteridines 2002;12:29–92.10.1515/pteridines.2001.12.2.29Search in Google Scholar
2. Gamagedara S, Gibbons S, Ma Y. Investigation of urinary pteridine levels as potential biomarkers for noninvasive diagnosis of cancer. Clinica Chimica Acta 2011;412:120–8.10.1016/j.cca.2010.09.015Search in Google Scholar
3. Jayaraman A, Thandeeswaran M, Priyadarsini U, Sabarathinam S, Ayub KA, Palaniswamy M. Characterization of unexplored amidohydrolase enzyme-pterin deaminase. Appl Microbiol Biotechnol 2016;100:4779–89.10.1007/s00253-016-7513-9Search in Google Scholar PubMed
4. Ziegler I. The pteridine pathway in zebrafish: regulation and specification during the determination of neural crest cell-fate. Pigment Cell Res 2003;16:172–82.10.1034/j.1600-0749.2003.00044.xSearch in Google Scholar PubMed
5. Levenberg B, Hayaishi O. Bacterial pterindeaminase. J Biol Chem 1959;234:955–61.10.1016/S0021-9258(18)70211-1Search in Google Scholar PubMed
6. Nawa S, Taira T, Sakaguchi B. Pterin dehydrogenase found in drosophila melanogaster. Proc Japan Acad 1958;34:115–9.10.2183/pjab1945.34.115Search in Google Scholar
7. Kusakabe H, Kodama K, Midorika Y, Machida H, Kuninaka A, Yoshino H. Process for producing pterin deaminases having antitumour activity. US patent 1976;32:3930955.Search in Google Scholar
8. Kusakabe H, Kodama K, Midorika Y, Machida H, Kuninaka A, Yoshine H. Inhibition of growth of L5178y leukemia-cells by a fungal folate-deaminating enzyme. Agric Biol chem 1974;38:1753–4.10.1080/00021369.1974.10861412Search in Google Scholar
9. Rembold H, Simmersbach F. Catabolism of pteridine cofactors II. A specific pterin deaminase in rat liver. Biochim Biophys Acta 1969;184:589–96.10.1016/0304-4165(69)90273-6Search in Google Scholar
10. Sasano Yu, Nagasawa K, Kaboli S, Sugiyama M, Harashima S. CRISPR-PCS: a powerful new approach to inducing multiple chromosome splitting in Saccharomyces cerevisiae. Sci Rep 2016;6:30278.10.1038/srep30278Search in Google Scholar PubMed PubMed Central
11. Imada A, Igarasi S, Nakahama K, Isono M. Asparaginase and glutaminase activities of microorganisms. J General Microbiol 1973;76:85–99.10.1099/00221287-76-1-85Search in Google Scholar PubMed
12. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem 1951;193:265–75.10.1016/S0021-9258(19)52451-6Search in Google Scholar PubMed
13. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5.10.1038/227680a0Search in Google Scholar PubMed
14. Switzer RC, Merril CR, Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem 1979;98:231–7.10.1016/0003-2697(79)90732-2Search in Google Scholar PubMed
15. Davis BJ. Disc electrophoresis-II: method and application to human serum proteins. Ann NY Acad Sci 1964;121:404–27.10.1111/j.1749-6632.1964.tb14213.xSearch in Google Scholar PubMed
16. Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.10.1021/ja01318a036Search in Google Scholar
17. Wurster B, Butz U. Reversible binding of the chemoattractant folic acid to cells of D. discoideum. Eur J Biochem 1980;109: 613–8.10.1111/j.1432-1033.1980.tb04834.xSearch in Google Scholar PubMed
18. Fan H, Hitchcock DS, Seidel RD, Hillerich B, Lin H, Almo SC, et al. Assignment of pterin deaminase activity to an enzyme of unknown function guided by homology modeling and docking. J Am Chem Soc 2013;135:795–803.10.1021/ja309680bSearch in Google Scholar PubMed PubMed Central
19. Ostergaard S, Olsson L, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev 2000;64:34–50.10.1128/MMBR.64.1.34-50.2000Search in Google Scholar PubMed PubMed Central
20. Marino MA, Freitas S, Miranda EA. Ethano precipitation of glycosyl hydrolases produced by Trichoderma harzianum P49P11. Braz J Chem Eng 2015;32:325–33.10.1590/0104-6632.20150322s00003268Search in Google Scholar
21. Schubert FP, Finn KR. Alcohol precipitation of proteins: the relationship of denaturation and precipitation for catalase. Biotechnol Bioeng 1981;23:2569–90.10.1002/bit.260231114Search in Google Scholar
22. Nooralabetu PK. Efficiency of ion exchange chromatography to resolve alkaline phosphatase from hepatopancreas of tiny shrimp, Parapenaeopsis stylifera. Int J Chromatogr Sci 2013;3:35–44.Search in Google Scholar
23. Takikawa S, Kitayamayokokawa C, Tsusue M. Pterin deaminase from Bacillus megaterium- purification and properties. J Biochem 1979;85:785–90.Search in Google Scholar PubMed
24. Bacher A, Rappold H. Bacterial degradation of folic acid. Meth Enzymol 1980;66:652–56.10.1016/0076-6879(80)66521-5Search in Google Scholar PubMed
25. Tsusue M, Takikawa S, Yokokawa CK. The bacterial catabolism of pteridines. Dev Biochem 1978;4:153–8.Search in Google Scholar
26. Priyadharshini U, Angayarkanni J, Sabarathinam S, Palaniswamy M. Purification and characterization of unexplored enzyme pterin deaminase from native isolate Micrococcus luteus BI252. Pteridines 2009;20:31.Search in Google Scholar
27. Almatarneh HM, Flinn GC, Poirier AR. Mechanism for the deamination reaction of cysteine with H2O/OH− and 2H2O/OH− : a computational study. J Chem Inf Model 2008;48:831–43.10.1021/ci7003219Search in Google Scholar
28. Kusakabe H, Kodama K, Machida H, Kuninaka A. Antitumor activity of pterin deaminase. Agric Biol chem 1979;43:1983–4.10.1271/bbb1961.43.1983Search in Google Scholar
29. Wurster B, Bek F, Butz U. Folic acid and pterin deaminases in D. discoideum kinetic properties and regulation by folic acid, pterin and adenosine 31, 51-phosphate. J Bacteriol 1981;148:183–92.10.1128/jb.148.1.183-192.1981Search in Google Scholar
30. Kim J, Park SI, Ahn C, Kim H, Yim J. Guanine deaminase functions as dihydropterin deaminase in the biosynthesis of aurodrosopterin, a minor red eye pigment of Drosophila. J Biol Chem 2009;284:23426–35.10.1074/jbc.M109.016493Search in Google Scholar PubMed
31. Branden C, Jurnvall H, Eklund H, Furugren B. 3 Alcohol dehydrogenases. Enzymes 1975;11:103–90.10.1016/S1874-6047(08)60211-5Search in Google Scholar
32. Oviedo C, Rodriguez J. EDTA: the chelating agent under environmental scrutiny. Quim Nova 2003;26:901–5.10.1590/S0100-40422003000600020Search in Google Scholar
33. Holst B, Tachibana C, Winther RJ. Active site mutations in yeast protein disulfide isomerase cause dithiothreitol sensitivity and a reduced rate of protein folding in the endoplasmic reticulum. J Cel Biol 1997;138:1229–38.10.1083/jcb.138.6.1229Search in Google Scholar PubMed PubMed Central
34. Trivedi VM, Laurence SJ, Siahaan JT. The role of thiols and disulfides in protein chemical and physical stability. Curr Protein Pet Sci 2009;10:614–25.10.2174/138920309789630534Search in Google Scholar PubMed PubMed Central
35. Sharma A, Kishan RK. Serine protease inhibitor mediated peptide bond re-synthesis in diverse protein molecules. FEBS Lett 2011;585:3465–70.10.1016/j.febslet.2011.10.004Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Photosensitization of peptides and proteins by pterin derivatives
- Polyamines, folic acid supplementation and cancerogenesis
- Mini review
- Medical significance of simultaneous application of red blood cell distribution width (RDW) and neopterin as diagnostic/prognostic biomarkers in clinical practice
- Original articles
- Molecular architecture of pterin deaminase from Saccharomyces cerevisiae NCIM 3458
- Quantitative analysis by flow cytometry of green fluorescent protein-tagged human phenylalanine hydroxylase expressed in Dictyostelium
- Age-dependance of pteridines in the malaria vector, Anopheles stephensi
- Seasonality of blood neopterin levels in the Old Order Amish
- Correlation of salivary neopterin and plasma fibrinogen levels in patients with chronic periodontitis and/or type 2 diabetes mellitus
- Positive association between Toxoplasma gondii IgG serointensity and current dysphoria/hopelessness scores in the Old Order Amish: a preliminary study
- Sleep onset insomnia, daytime sleepiness and sleep duration in relationship to Toxoplasma gondii IgG seropositivity and serointensity
- Concentrations of neopterin, kynurenine and tryptophan in wound secretions of patients with breast cancer and malignant melanoma: a pilot study
- Comparison of performance of composite biomarkers of inflammatory response in determining the prognosis of breast cancer patients
- Association of peripheral blood cell count-derived ratios, biomarkers of inflammatory response and tumor growth with outcome in previously treated metastatic colorectal carcinoma patients receiving cetuximab
- Neoadjuvant combination therapy with trastuzumab in a breast cancer patient with synchronous rectal carcinoma: a case report and biomarker study
Articles in the same Issue
- Frontmatter
- Reviews
- Photosensitization of peptides and proteins by pterin derivatives
- Polyamines, folic acid supplementation and cancerogenesis
- Mini review
- Medical significance of simultaneous application of red blood cell distribution width (RDW) and neopterin as diagnostic/prognostic biomarkers in clinical practice
- Original articles
- Molecular architecture of pterin deaminase from Saccharomyces cerevisiae NCIM 3458
- Quantitative analysis by flow cytometry of green fluorescent protein-tagged human phenylalanine hydroxylase expressed in Dictyostelium
- Age-dependance of pteridines in the malaria vector, Anopheles stephensi
- Seasonality of blood neopterin levels in the Old Order Amish
- Correlation of salivary neopterin and plasma fibrinogen levels in patients with chronic periodontitis and/or type 2 diabetes mellitus
- Positive association between Toxoplasma gondii IgG serointensity and current dysphoria/hopelessness scores in the Old Order Amish: a preliminary study
- Sleep onset insomnia, daytime sleepiness and sleep duration in relationship to Toxoplasma gondii IgG seropositivity and serointensity
- Concentrations of neopterin, kynurenine and tryptophan in wound secretions of patients with breast cancer and malignant melanoma: a pilot study
- Comparison of performance of composite biomarkers of inflammatory response in determining the prognosis of breast cancer patients
- Association of peripheral blood cell count-derived ratios, biomarkers of inflammatory response and tumor growth with outcome in previously treated metastatic colorectal carcinoma patients receiving cetuximab
- Neoadjuvant combination therapy with trastuzumab in a breast cancer patient with synchronous rectal carcinoma: a case report and biomarker study

