Abstract
The analysis of pterins in the cerebrospinal fluid (CSF) is mandatory for the etiologic diagnosis of inborn errors of dopamine and serotonin metabolism. The success of the available therapeutic strategies for preventing the ongoing brain dysfunction is tightly dependent of the early diagnosis of these neurotransmitter disorders. Previous methods of pterins determination in the CSF have in common at least one reversed phase HPLC step coupled to electrochemical or fluorescence detection (FD). They differ in the oxidation procedure of the reduced forms of pterins into their oxidized fluorescent counterparts. Most of the methods using the FD include at least one offline chemical oxidation procedure and cannot allow the direct quantification of tetrahydrobiopterin (BH4). A recent method proposed a single step simultaneous quantification of all forms of pterins including BH4 by HPLC coupled to FD after post-column coulometric oxidation. Nowadays, recent advances in mass spectrometry (MS), notably in term of sensitivity, allow the direct unambiguous determination of all forms of pterins in the CSF by LC-MS/MS.
Introduction
Nowadays, pterins determination in cerebrospinal fluid (CSF) is routinely used for the investigation of inborn errors of neurotransmitters metabolism [1], [2], [3]. Inherited defects leading to neurotransmitter metabolism disorders have been described or predicted at the level of each enzyme involved in the synthesis of the neurotransmitters dopamine, (nor)epinephrine, and serotonin (Figure 1) [2], [3], [4]. These disorders are caused by a defect in the metabolism of tetrahydrobiopterin (BH4), the co-factor for phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH), and tryptophan hydroxylase (TPH), or a defect at the level of TH or aromatic amino-acid decarboxylase (AADC) (Figure 1) [2], [3], [4]. Each of these conditions thus exhibits a characteristic CSF profile of pterins and of the precursors and metabolites of dopamine and serotonin (Table 1).
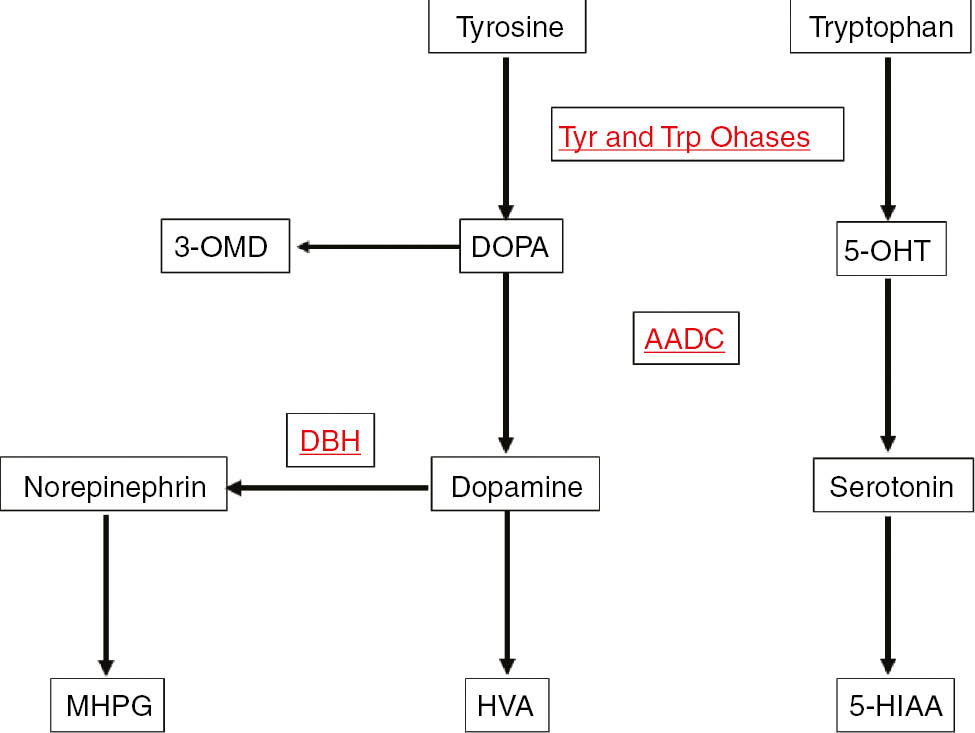
Neurotransmitter metabolism.
Tyr and Trp Ohases, Tyrosine and tryptophan hydroxylases; DBH, dopamine β-hydroxylase; AADC, amino-acid decarboxylase; HVA, homovanillic acid; 5-HIAA, 5-hydroxyindoleacetic acid; 3-OMD, 3-ortho-methyl DOPA; 5-OHT, 5-hydroxytryptophan; MHPG, 3-methoxy-4-hydroxyphenylglycol.
Changes in CSF neurotransmitter metabolites and pterins in disorders of pterin metabolism according to Refs. [1–4].
| Enzymatic defect | Phe | BH4 | BH2 | NH2 | HVA | HIAA | HVA/HIAA | 3-OMD | 5-OHT | MHPG |
|---|---|---|---|---|---|---|---|---|---|---|
| GTPCH AR | ↑ | ↓ | N | ↓ | ↓ | ↓ | N | N | N | ↓ |
| PTPS | ↑ | ↓ | N | ↑ | ↓ | ↓ | N | N | N | ↓ |
| PCD | ↑ | ↓ | N | N | N | N | N | N | N | N |
| DHPR | ↑ | ↓ | ↑ | N | ↓ | ↓ | N | ↓ | ↓ | ↓ |
| GTPCH I AD | N | ↓ | ↓/N | ↓ | ↓ | ↓ | N | N | N | ↓ |
| SR | N | ↓ | ↑ | N | ↓ | ↓ | N | N | N | ↓ |
| TH | N | N | N | N | ↓ | N | ↓ | N | N | ↓ |
| AADC | N | N | N | N | ↓ | ↓ | N | ↑ | ↑ | ↓ |
| DTDS | N | N | N | N | ↑ | N | ↑ | N | N | N |
Phe, Phenylalanine as determined in plasma; N, normal; GTPCH I AD, GTP-cyclohydrolase I deficiency autosomal dominant; GTPCH AR, autosomal recessive; PTPS, 6-pyruvoyl-tetrahydropterin synthase deficiency; SR, sepiapterin reductase deficiency; PCD, pterin-4α-carbinolamine dehydratase deficiency; DHPR, dihydropteridine reductase deficiency; TH, tyrosine hydroxylase deficiency; AADC, amino-acid decarboxylase deficiency; DTDS, dopamine transporter deficiency syndrome. (↓) decreased; (↑) increased.
Several enzymes are involved in BH4 synthesis from guanosine triphosphate (GTP) (Figure 2). Mutations in GTP cyclohydrolase (GTPCH), 6-pyruvoyl-tetrahydropterin synthase (PTPS), sepiapterin reductase (SR), and dihydropteridine reductase (DHPR) may lead to a defect in BH4 synthesis or regeneration (Table 1). Defects of BH4 are usually associated with hyperphenylalaninemia (HPA), but two BH4 deficiencies due to GTPCH-1 or SR defects, normally occur without HPA. The diagnosis of these conditions can be established through the analysis of neopterin (N) and biopterin (B) in CSF [2], [3], [4].
![Figure 2: Tetrahydrobiopterin (BH4) biosynthesis and regeneration (according to [1–4], and [9]).GTP, Guanosine triphosphate; GTPCH, guanosine triphosphate cyclohydrolase; NH2, dihydroneopterin; N, neopterin; PTPS, 6-pyruvoyl-tetrahydropterin synthase; SR, sepiapterin reductase; PCD, pterin-4α-carbinolamine dehydratase; DHPR, dihydropteridin reductase; BH2, dihydrobiopterin; DHFR, dihydrofolate reductase; B, biopterin. Dashed arrows: non-enzymatic.](/document/doi/10.1515/pterid-2017-0001/asset/graphic/j_pterid-2017-0001_fig_002.jpg)
Tetrahydrobiopterin (BH4) biosynthesis and regeneration (according to [1–4], and [9]).
GTP, Guanosine triphosphate; GTPCH, guanosine triphosphate cyclohydrolase; NH2, dihydroneopterin; N, neopterin; PTPS, 6-pyruvoyl-tetrahydropterin synthase; SR, sepiapterin reductase; PCD, pterin-4α-carbinolamine dehydratase; DHPR, dihydropteridin reductase; BH2, dihydrobiopterin; DHFR, dihydrofolate reductase; B, biopterin. Dashed arrows: non-enzymatic.
Neurotransmitter disorders caused by a deficiency in AADC or TH are characterized by a normal CSF pterin (P) pattern (Table 1). In the case of AADC deficiency, there is a strong reduction of CSF concentrations of homovanillic acid (HVA) and 5-hydroxy-indoleacetic acid (5-HIAA) with a concomitant increase of their precursors 3-ortho-methyl DOPA (3-OMD) and 5-hydorxy-tryptophan (5-OHT). As AADC is a pyridoxal 5′-phosphate dependent enzyme, the same CSF profile may be also observed in the case of vitamin B6 deficits or in the case of pyridox(am)ine-5′-phosphate oxidase (PNPO) deficiency. In the case of TH deficiency there is a reduced CSF concentration of HVA and/or a reduced HVA/5-HIAA ratio [2], [3], [4].
Some cases of an isolated decrease of 5-HIAA were also reported but without proof of TPH inherited disorder [2], [3], [4]. More recently, a dopamine transport deficiency syndrome has been described. The involved mechanism affecting the dopamine transport to presynaptic neurons is linked to a defective reuptake leading to dopamine accumulation in the synapse as reflected by the increase of HVA concentration and HVA/5-HIAA ratio in CSF [5].
Early diagnosis of neurotransmitter disorders is of importance notably because some of these diseases can be well treated [6]. The correct diagnosis of these conditions can be achieved through the analysis of HVA, 5-HIAA, 3-OMD, 5-OHT, neopterin, and B in the CSF (Table 1). However, while the analysis of neurotransmitter metabolites can be easily achieved by liquid chromatography coupled to electrochemical detection (ECD) [7] or to mass spectrometry (MS) [8] most of available analytical methods of pterins in CSF cannot allow direct quantification of BH4 [9].
This review will focus on the methods and advances for monitoring pterins in CSF. Current analytical methods are mostly based on earlier BH4 oxidation investigations [10], [11], [12], [13]. They have in common at least one chromatographic separation step. But they differ in the used detection mode and their ability to directly analyze native BH4. Subsequently, LC-MS/MS techniques have been developed that have sufficient sensitivity to quantitate the various pterin species that have been identified in CSF.
After a brief description of the earlier studies on the oxidation of BH4, we will further review the current methods of pterins determination in CSF.
Earlier studies on BH4 oxidation
Since the elucidation of its pivotal role in the hydroxylation reactions of living organisms [10], several studies using liquid chromatography coupled to UV-visible spectroscopy or to fluorescence detection (FD) [11], [12], [13], have been devoted to the elucidation of the mechanisms of oxidation of BH4.
Earlier studies concluded that a large portion of B in tissues and fluids appears to be present in labile reduced forms [11]. As B in its fully oxidized form is stable and has strong fluorescent properties, a selective preliminary oxidation of the biological sample has been considered for pterins analysis. Since, the effects of iodine oxidation on reduced forms of B have been further analyzed by using high performance liquid chromatography (HPLC) [11]. One year later, the electrochemical behavior of BH4 has been extensively studied on pyrolytic graphite and it has been found that oxidation and reduction consisted of two-electron processes [14]. This led to the development of techniques that use ECD for the analysis of reduced pterins in biological materials [15], [16].
Based on the data of these earlier investigations, several chromatographic methods coupled to FD or ECD have been proposed for pterins analysis in CSF. The chromatographic behavior of pterins present in CSF is well known. Their separation can be easily achieved by reversed phase or HILIC modes (Figure 3) [8], [9], [12], [15], [16], [17].
![Figure 3: Pterins chromatographic profile as a function of the separation mode.(A) Atlantis dC18; mobile phase: pH 7.4, 0.05 M sodium citrate/methanol (97/3, v/v). (B) Zic-HILIC; mobile phase: pH 7.4, 0.2 M ammonium formiate/acetonitrile (20/80, v/v). (A, B) Flow rate: 0.6 mL/min at 30°C. Detection 260 nm (Cf. [9] for more details).](/document/doi/10.1515/pterid-2017-0001/asset/graphic/j_pterid-2017-0001_fig_003.jpg)
Pterins chromatographic profile as a function of the separation mode.
(A) Atlantis dC18; mobile phase: pH 7.4, 0.05 M sodium citrate/methanol (97/3, v/v). (B) Zic-HILIC; mobile phase: pH 7.4, 0.2 M ammonium formiate/acetonitrile (20/80, v/v). (A, B) Flow rate: 0.6 mL/min at 30°C. Detection 260 nm (Cf. [9] for more details).
Current HPLC methods can be primarily classified as a function of the detection mode. Alternatively, as these methods differ in the sample preparation procedure and the targeted forms of pterins, they can be also classified as direct or indirect methods.
HPLC-FD methods
The first method of pterins determination in CSF was proposed in 1980 [11]. As oxidized pterins are fluorescent while their reduced counterparts are not, the separation and the quantification of pterins in biological fluids required two HPLC-FD runs including two off-line chemical oxidation procedures for complete analysis [11]. According to the authors, using 0.1% iodine in 0.1 N HCl, 7,8-BH2 and BH4 were shown to be oxidized to B. Hence, the quantification of B after oxidation under acidic conditions will reflect the sum of the concentrations of BH4 and BH2. Under alkaline conditions (0.1 N NaOH), however, the iodine oxidation results in 90% conversion of BH2 to B and 80% conversion of BH4 to pterin (P). After 60 min of incubation the acidic or alkaline mixture is neutralized with NaOH or HCl and excess iodine was reduced by the addition of 1% ascorbic acid solution before injection into the chromatograph [11]. After iodine oxidation in 0.1 N HCl, dihydroneopterin (NH2) and tetrahydroneopterin (NH4) were recovered as N almost quantitatively (90% or more) [11]. After oxidation in 0.1 N NaOH, only a trace of NH4 was recovered as N and 80% of the NH4 was converted to P, whereas about 50% of NH2 was recovered as N.
The authors concluded that iodine oxidation of reduced forms of N shows the same pattern as that observed with reduced B with the exception that NH4 appears to be more labile to alkaline oxidation than BH4 [11].
Pterins in CSF are present in the low nmol/L range. The first HPLC run of the original method [11] thus involves the separation of the oxidation products obtained under acidic conditions whilst the second one is devoted to the separation of the oxidation products obtained under alkaline conditions. Although the oxidation of BH4 and NH2 under alkaline conditions cannot allow their specific quantification, and although very time-consuming, this indirect chemical oxidation method has been widely used since 1980, notably because it has appropriate sensitivity [18], [19], [20], [21].
Methods utilizing post-column chemical or photochemical oxidation procedures [22], [23], [24] were developed later but sensitivity was low likely due to the high post column void volumes.
In 1992, a simplified oxidation procedure using manganese dioxide (MnO2) under acidic conditions was established to diminish the pre-analysis preparation time for CSF analysis [25], [26]. As the oxidation products N and B obtained under acidic conditions are sufficient to establish the correct diagnosis of neurotransmitter disorders (Table 1) some authors proposed a single-step oxidation of NH2, BH4 and BH2 into N and B (BH4+BH2), respectively, by using manganese dioxide under acidic conditions [25], [26]. Samples (200 μL) were mixed with (1 mg of MnO2) and 20 μL of 1 M HCl. After shaking during 10 min at room temperature and centrifuged at 12,000×g before injection of the clear supernatant into the chromatograph [26]. Although faster than the iodine oxidation method, this method obviously does not allow distinguishing BH4 from BH2. Besides, more recently, some authors compared the two proposed chemical oxidation procedures iodine vs. MnO2, and they concluded that the oxidation with iodine results in the most accurate determination of N and B in CSF [25]. However, it is now well established that chemical oxidation methods are to underestimate amounts of reduced forms of N and B [9], [15].
Apart from the diagnosis of neurotransmitter metabolism disorders, direct quantification of BH4, BH2, and NH2 is essential for studying BH4 metabolism (Figure 1) as well as for pharmacokinetic studies or monitoring the efficacy of BH4 supplementation in patients with BH4 deficiency [22], [27]. For instance, two recent papers raised doubts about the ability of BH4 at usual doses (20 or 40 mg/kg/day) to cross the blood-brain barrier in patients with genetic BH4 recycling disorder [28], [29].
Hence, direct methods for the measurement of reduced pterins in CSF are required. For this purpose, as pterins are electroactive compounds, it is preferable to separate the reduced forms and to use an online electro-oxidation method before FD.
HPLC-ECD-FD methods
In 1985, sequential ECD and FD were proposed for the simultaneous quantification of BH4, BH2, and NH2 in CSF [15]. Using a mobile phase with a pH adjusted to 5.2 in a reverse phase mode, BH4 is detected electrochemically in the redox mode, while BH2 and NH2 were detected by FD after post-column coulometric oxidation (PCCO-FD) into B and N, respectively. However, due to the large number of electroactive compounds in CSF, the specific detection of BH4 in the redox mode may be questioned. As ECD is less robust and less sensitive than FD for routine analyses, it may be wondered why the authors did not detect BH4 by FD after coulometric oxidation as they did for BH2 and NH2 [15].
Recently, the effects of pH on the separation and the electro-oxidation of the targeted pterins have been extensively studied [9]. The authors observed that high oxidation yields (≥50%) of BH2 and NH2 can be obtained irrespective of the pH range between pH 3 and pH 8. For BH4, however, a sufficient yield (about 50%) only occurs at pH higher than 7.4. At pH <6 the BH4 oxidation yield is about 20% and then it decreases to become negligible at pH equal to or less than 4. Hence the low oxidation yield of BH4 at pH <6.0 could explain why BH4 was not detected in the original publication using ECD followed by FD. This work allowed the authors to propose an HPLC method able to simultaneously analyze all forms of pterins including BH4, BH2, B and NH2 by FD after post-column coulometric oxidation (Figure 4) [9]. The results obtained in 99 patients who did not suffer from any known inborn error of metabolism showed that P concentrations in CSF vary from 7.6 to 76 nM (median, 32 nM), 3.1 to 17.6 nM (median, 9.5 nM), and 3.9 to 47.3 nM (median, 13.5 nM) for BH4, BH2, and NH2, respectively [9].
![Figure 4: Chromatographic profiles of (A) a normal CSF sample and (B) a CSF sample with elevated NH2 level, according to [9].Obtained under the same conditions as for Figure 2A with fluorescence detection after post-column oxidation at +600 mV. Unknown peaks are unlabeled.](/document/doi/10.1515/pterid-2017-0001/asset/graphic/j_pterid-2017-0001_fig_004.jpg)
Chromatographic profiles of (A) a normal CSF sample and (B) a CSF sample with elevated NH2 level, according to [9].
Obtained under the same conditions as for Figure 2A with fluorescence detection after post-column oxidation at +600 mV. Unknown peaks are unlabeled.
HPLC-MS methods
Since 2009, liquid chromatography coupled to tandem MS was used to detect N and B in plasma [30], as well as for the simultaneous detection of BH4, BH2, and B in urine samples, cell extracts, and rat brains [31], [32], [33], [34]. However, according to the authors, MS/MS detection is less sensitive than FD for pterins quantification [31].
Nowadays, recent developments of MS technology in term of sensitivity allow the determination of compounds present in the low nmol/L range in biological media. As a matter of fact, an LC-MS/MS method has been recently implemented for determination of pterins in CSF [35]. The main compounds that have been identified in CSF include neopterin, NH2, dihydroxanthopterin, BH4, B, 7-8-dihydrobiopterin and sepiapterin. The latter is a yellow fluorescing P and is not normally measured but techniques have been described that allow its measurement to aid in the diagnosis of SR deficiency [35]. The linearity of the method covers a working range of 3–200 nmol/L with a total analytical imprecision <14.4% for all P metabolites [35].
Parameters affecting the CSF concentration of pterins
As pterins are present in labile reduced forms in CSF, it is mandatory to control the technical and biochemical factors that can affect their concentrations, in order to ensure the robustness of the used analytical method and a correct diagnosis.
In order to prevent the auto-oxidation and the oxidation catalyzed by metal ions of BH4 before and during the chromatographic analysis, some authors proposed to add to the sample 1 mg/mL of dithioerythritol (DTE), a thiol reagent, and 1 mg/mL of diethylenetriaminepentaacetic acid (DETAPAC), a chelating agent [15], [35]. According to the authors, these additives protected BH4 for at least 5 h at 4°C and for at least 1 month at −70°C. In order to avoid any BH4 oxidation during the chromatographic step, the authors also propose to add 0.16 mM of DTE to the mobile phase [28].
Recently, the technical and biochemical factors that can affect P concentrations in the CSF have been extensively investigated [26]. According to the authors, N and B concentrations are not affected by the ventriculo-spinal gradient and pretreatment of CSF. Also, the pretreatment of the sample with trichloro-acetic-acid and DTE before oxidation with iodine results in the most accurate determination of N and B [36]. However, by using the methodology of iodine oxidation the authors only looked at total B and N and did not investigate the stability of BH4, BH2 or NH2. Under these conditions it is likely that the reduced pterins auto-oxidized to oxidized forms during sample handling.
More recently, the stability of the CSF samples at 10°C in the dark in the injector was checked after several hours [9]. According to the authors, they did not observe significant differences after 6 h. However, after 8 h, NH2 and BH4 concentrations decreased between 4% and 31% of their initial values. Thus, they concluded that under their conditions, samples should be analyzed not more than 6 h [9]. The authors also checked the stability of pterins in CSF samples frozen at −80°C immediately after collection. As compared with the initial values determined at day 7 after collection they did not observe significant differences after 24 months of storage. Hence, they avoided adding a preservative agent to the samples thus facilitating the collection procedure.
Conclusion
Current methods of pterins determination in CSF can be classified in two categories, those using an offline oxidation procedure of the reduced pterins and those using a post-column electrochemical oxidation. The former are indirect methods because they cannot allow the direct quantification of the reduced forms while the latter are direct methods as they do measure the concentrations of BH4, BH2, and NH2.
Although sufficient to establish the correct diagnosis of neurotransmitter disorders (Table 1), and although many remarkable advances in biomedical research have been made on basis of indirect measurements, the indirect methods of BH4 determination may present some drawbacks. They underestimate the reduced forms of pterins, they are very time consuming, and they are difficult to automate.
In contrast, direct methods are simple, rapid, and easy to automate. Besides, as they can measure BH4 concentrations they can be used for pharmacokinetic studies and for monitoring BH4 supplementations in patients with BH4 deficiency. Direct quantification of BH4 is also essential for studying the metabolism of this cofactor. To all these purposes, the recent LC-MS/MS method, which provides a more comprehensive evaluation of the pterins pathway, offers a significant improvement. At present the LC-MS/MS method should be considered as the only possible reference method.
Conflict of interest statement: All authors have declared no conflicts of interest. All authors contributed to the manuscript and approved its final version.
References
1. Hyland K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin Chem 2008;54:633–41.10.1373/clinchem.2007.099986Suche in Google Scholar
2. Longo N. Disorders of biopterin metabolism. J Inherit Metab Dis 2009;32:333–42.10.1007/s10545-009-1067-2Suche in Google Scholar
3. Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 2011;438:397–414.10.1042/BJ20110293Suche in Google Scholar
4. Kurian MA, Gissen P, Smith M, Heales S, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol 2011;10:721–33.10.1016/S1474-4422(11)70141-7Suche in Google Scholar
5. Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol 2011;10:54–62.10.1016/S1474-4422(10)70269-6Suche in Google Scholar
6. Allen GF, Land JM, Heales SJ. A new perspective on the treatment of aromatic L-amino acid decarboxylase deficiency. Mol Genet Metab 2009;97:6–14.10.1016/j.ymgme.2009.01.010Suche in Google Scholar
7. Hyland K, Surtees RA, Heales SJ, Bowron A, Howells DW, Smith I. Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr Res 1993;34:10–4.10.1203/00006450-199307000-00003Suche in Google Scholar
8. Manini P, Andreoli R, Cavazzini S, Bergamaschi E, Mutti A, Niessen WM. Liquid chromatography-electrospray tandem mass spectrometry of acidic monoamine metabolites. J Chromatogr B: Biomed Sci Appl 2000;744:423–31.10.1016/S0378-4347(00)00285-1Suche in Google Scholar
9. Guibal P, Le N, Doummar D, Giraud N, Roze E, Rodriguez D, et al. Simultaneous determination of all forms of biopterin and neopterin in cerebrospinal fluid. ACS Chem Neurosci 2014;5:533–41.10.1021/cn4001928Suche in Google Scholar PubMed PubMed Central
10. Kaufman S. The structure of the phenylalanine-hydroxylation cofactor. Proc Natl Acad Sci 1963;50:1085–93.10.1073/pnas.50.6.1085Suche in Google Scholar PubMed PubMed Central
11. Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem 1980;102:176–88.10.1016/0003-2697(80)90336-XSuche in Google Scholar
12. Haavik J, Flatmark T. Isolation and characterization of quinonoid dihydropterins by highperformance liquid chromatography. J Chromatogr 1983;251:361–72.10.1016/S0021-9673(01)88192-9Suche in Google Scholar
13. Davis MD, Kaufman S, Milstien S. The auto-oxidation of tetrahydrobiopterin. Eur J Biochem 1988;173:345–51.10.1111/j.1432-1033.1988.tb14004.xSuche in Google Scholar
14. Raghavan R, Dryhurst G. Redox chemistry of the reduced pterin species. J Electroanal Chem 1981;129:189–212.10.1016/S0022-0728(81)80014-9Suche in Google Scholar
15. Hyland K. Estimation of tetrahydro, dihydro and fully oxidised pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J Chromatogr Biomed Appl 1985;343:35–41.10.1016/S0378-4347(00)84565-XSuche in Google Scholar
16. Howells DW, Smith I, Hyland K. Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J Chromatogr Biomed Appl 1986;381:285–94.10.1016/S0378-4347(00)83594-XSuche in Google Scholar
17. Xiong X, Liu Y. Chromatographic behavior of 12 polar pteridines in hydrophilic interaction chromatography using five different HILIC columns coupled with tandem mass spectrometry. Talanta 2016;150:493–502.10.1016/j.talanta.2015.12.066Suche in Google Scholar
18. Suzuki K, Owada M. A simple and sensitive method for the determination of pterins in cerebrospinal fluid. Clinical usefulness for management of tetrahydrobiopterin deficiency. J Inherited Metab Dis 1991;14:825–30.10.1007/BF01799957Suche in Google Scholar
19. Werner ER, Werner-Felmayer G, Wachter H. High-performance liquid chromatographic methods for the quantification of tetrahydrobiopterin biosynthetic enzymes. J Chromatogr B: Biomed Sci Appl 1996;684:51–8.10.1016/0378-4347(95)00507-2Suche in Google Scholar
20. Komori H, Matsuishi T, Yamada S, Ueda N, Yamashita Y, Kato H. Effect of age on cerebrospinal fluid levels of metabolites of biopterin and biogenic amines. Acta Paediatr 1999;88:1344–7.10.1111/j.1651-2227.1999.tb01048.xSuche in Google Scholar
21. Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab 2004;81:45–51.10.1016/j.ymgme.2003.09.014Suche in Google Scholar
22. Tani Y, Ohno T. Analysis of 6R- and 6S-tetrahydrobiopterin and other pterins by reversedphase ion-pair liquid-chromatography with fluorimetric detection by post-column sodium nitrite oxidation. J Chromatogr 1993;617:249–55.10.1016/0378-4347(93)80495-PSuche in Google Scholar
23. Tani Y, Kanai T. Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neurosci Lett 1994;181:169–72.10.1016/0304-3940(94)90586-XSuche in Google Scholar
24. Espinosa-Mansilla A, Munoz de la Pena A, Canada-Canada F, Mancha de Llanos A. LC determination of biopterin reduced forms by UV-photogeneration of biopterin and fluorimetric detection. Talanta 2008;77:844–51.10.1016/j.talanta.2008.07.046Suche in Google Scholar
25. Blau N, Kierat L, Heizmann CW, Endres W, Giudici T, Wang M. Screening for tetrahydrobiopterin deficiency in newborns using dried urine on filter paper. J Inherit Metab Dis 1992;15:402–4.10.1007/BF02435988Suche in Google Scholar PubMed
26. Ormazabal A, García-Cazorla A, Fernández Y, Fernández-Alvarez E, Campistol J, Artuch R. HPLC with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins. J Neurosci Methods 2005;142: 153–8.10.1016/j.jneumeth.2004.08.007Suche in Google Scholar PubMed
27. Cerone R, Schiaffino MC, Fantasia AR, Perfumo M, Birk Moller L, Blau N. Long-term follow-up of a patient with mild tetrahydrobiopterin-responsive phenylketonuria. Mol Genet Metab 2004;81:137–9.10.1016/j.ymgme.2003.11.008Suche in Google Scholar PubMed
28. Leu-Semenescu S, Arnulf I, Decaix C, Moussa F, Clot F, Boniol C, et al. Sleep and rhythm consequences of a genetically induced loss of serotonin. Sleep 2010;33:307–14.10.1093/sleep/33.3.307Suche in Google Scholar PubMed PubMed Central
29. Coughlin CR, Hyland K, Randall R, Ficicioglu C. Dihydropteridine reductase deficiency and treatment with tetrahydrobiopterin: a case report. J Inherited Metab Dis Rep 2012;202:53–6.10.1007/8904_2012_202Suche in Google Scholar PubMed PubMed Central
30. Zhao Y, Cao J, Chen YS, Zhu Y, Patrick C, Chien B, et al. Detection of tetrahydrobiopterin by LC-MS/MS in plasma from multiple species. Bioanalysis 2009;1:895–903.10.4155/bio.09.77Suche in Google Scholar PubMed
31. Giron J, Martın-Tornero E, Hurtado Sanchez MC, Duran Meras I, Espinosa Mansilla A. A simple HPLC-ESI-MS method for the direct determination of ten pteridinic biomarkers inhuman urine A. Talanta 2012;101:465–72.10.1016/j.talanta.2012.09.061Suche in Google Scholar PubMed
32. Kim HR, Kim TH, Hong SH, Kim HG. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem Biophys Res Commun 2012;419:632–7.10.1016/j.bbrc.2012.02.064Suche in Google Scholar PubMed
33. Fismen L, Eide T, Djurhuus R, Svardal AM. Simultaneous quantification of tetrahydrobiopterin, dihydrobiopterin, and biopterin by liquid chromatography coupled electrospray tandem mass spectrometry. Analyt Biochem 2012;430:163–70.10.1016/j.ab.2012.08.019Suche in Google Scholar PubMed
34. Yang BC, Liu FY, Guo JB, Wan L, Wu J, Wang F, et al. Rapid assay of neopterin and biopterin in urine by wooden-tip electrospray ionization mass spectrometry. Anal Methods 2015;7:2913–6.10.1039/C5AY00004ASuche in Google Scholar
35. Arning E, Bottiglieri T. Erratum to: LC-MS/MS analysis of cerebrospinal fluid metabolites in the pterin biosynthetic pathway. JIMD Reports 2014;29:1–9. An erratum to this chapter can be found at http://dx.doi.org/10.1007/8904_2014_372.10.1007/8904_2014_372Suche in Google Scholar PubMed PubMed Central
36. Verbeek MM, Blom AM, Wevers RA, Lagerwerf AJ, van de Geer J, Willemsen MA. Technical and biochemical factors affecting cerebrospinal fluid 5-MTHF, biopterin and neopterin concentrations. Mol Genet Metab 2008;95:127–32.10.1016/j.ymgme.2008.07.004Suche in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Reviews
- Dissecting the enigma of scleroderma: possible involvement of the kynurenine pathway
- Pteridine determination in human serum with special emphasis on HPLC methods with fluorimetric detection
- Pterin determination in cerebrospinal fluid: state of the art
- Original articles
- Serum neopterin concentrations and tryptophan degradation pattern in patients with late stage larynx carcinoma
- Investigation of a potential role for aldose reductase AlrA in tetrahydropteridine synthesis in Dictyostelium discoideum Ax2
Artikel in diesem Heft
- Frontmatter
- Reviews
- Dissecting the enigma of scleroderma: possible involvement of the kynurenine pathway
- Pteridine determination in human serum with special emphasis on HPLC methods with fluorimetric detection
- Pterin determination in cerebrospinal fluid: state of the art
- Original articles
- Serum neopterin concentrations and tryptophan degradation pattern in patients with late stage larynx carcinoma
- Investigation of a potential role for aldose reductase AlrA in tetrahydropteridine synthesis in Dictyostelium discoideum Ax2


