Urinary neopterin concentrations during radiotherapy for gynecological cancer
-
Sachin Vipin Trivedi
Abstract
Neopterin is a biomarker of host response to neoplasia. In the present study, urinary neopterin was determined during the course of (chemo)radiation in ten patients with gynecological tumors (nine patients with cervical carcinoma and one patient with carcinoma of the vulva). Baseline urinary neopterin concentrations were, generally, above the normal range. Neopterin concentrations were relatively stable during the first 5 weeks of combined (chemo)radiation. Marked peaks of neopterin concentrations reflected the emergence of complications. Neopterin could represent a useful biomarker for the assessment of the condition of the patients during this aggressive therapy.
Introduction
Immune response and inflammatory reaction play a fundamental role in the control of tumor growth or progression [1]. There is also mounting evidence indicating that immune and inflammatory responses are activated during anticancer therapy. It is currently widely recognized that surgical interventions elicit systemic inflammatory response. An induction of systemic inflammatory response has also been described for cytotoxic chemotherapy.
Neopterin, a heterocyclic compound, is produced from guanosine triphosphate (GTP) by activated macrophages. The activity of GTP cyclohydrolase, the enzyme responsible for the production of neopterin, is induced by interferon-γ, a cytokine produced by T-lymphocytes and natural killer cells [2]. Because of the interactions within the cytokine network [3], neopterin concentrations reflect both systemic immune and inflammatory responses. Neopterin may be determined in the serum or in urine, and both serum and urinary neopterin concentrations have been validated as biomarkers of systemic immune and inflammatory response in a wide range of disorders ranging from acute myocardial infarction, autoimmune diseases, infections to virtually all malignant tumors [4–9].
In previous investigations, increased neopterin concentrations have been reported during the administration of biological agents or systemic chemotherapy [10, 11]. More recently, we have described an increase in urinary neopterin concentrations in patients with head and neck carcinoma treated with external beam radiation [12, 13]. Pelvic radiotherapy is associated with irradiation of the intestines or bone marrow that could be expected to be accompanied by an even more extensive activation of the immune system than in the case of head and neck tumors. In the present pilot study, we investigated urinary neopterin in patients treated with pelvic radiotherapy.
Patients and methods
Nine patients with carcinoma of the uterine cervix and one patient with carcinoma of the vulva treated with pelvic radiotherapy were included in the present exploratory analysis (Table 1). Patients were staged according to the Fédération Internationale de Gynécologie et d′Obstétrique (FIGO) classification [14]. All patients with cervical carcinoma were treated with concomitant weekly cisplatin (40 mg/m2), while the patient treated for carcinoma of the vulva received radiotherapy alone.
The patient cohort.
| Patient | Age, years | Histology | FIGO stage | Baseline urinary neopterin, μmol/mol creatinine |
|---|---|---|---|---|
| 1 | 49 | SCC | III.B | 301 |
| 2 | 36 | SCC | III.B | 205 |
| 3 | 60 | Adenocarcinoma | III.B | 62 |
| 4 | 42 | SCC | III.B | 110 |
| 5 | 70 | Adenocarcinoma | III.B | 307 |
| 6 | 45 | SCC | II.B | 198 |
| 7 | 50 | Adenocarcinoma | III.B | 394 |
| 8 | 49 | Adenocarcinoma | II.B | 359 |
| 9 | 51 | Adenocarcinoma | II.B | 267 |
| 10 | 76 | SCC (vulva) | Recurrent | 353 |
FIGO, Fédération Internationale de Gynécologie et d′Obstétrique; SCC, squamous cell carcinoma.
Patients with carcinoma of the uterine cervix were treated with whole-pelvis three-dimensional conformal radiotherapy using a linear accelerator with 18-MV photons. Dose was prescribed at the ICRU (International Commission on Radiation Units and Measurement) point and was 50 Gy in 25 fractions (2 Gy per fraction). In patients with cervical cancer, treated with intracavitary high-dose-rate brachytherapy, the dose was prescribed to a selected reference “point A” (defined as a point 2 cm lateral to the cervical canal and 2 cm superior to the ovoids). Dose for organs at risk was reported using individual points for the bladder and rectum. Patients underwent six fractions of brachytherapy, 4 Gy per fraction, three fractions per week. The dose in the patient with recurrent carcinoma of the vulva was 50 Gy in 25 fractions to the vulva and bilateral inguinofemoral lymph nodes, with a boost of 16 Gy in 8 fractions to the left groin (to the tumor mass).
Early morning urine specimens were collected before and during the course of radiotherapy. The sample collection was usually not done on days when patients were not in the hospital (usually during weekends or pauses caused by bank holidays) or during interruptions of treatment. Thus, at the visit of the same number, the timing of sample collection from treatment start differed in individual patients. The samples were stored at -20°C until analysis. Urinary neopterin was determined with a modification of the method described previously [15]. Briefly, after centrifugation (45 s, 12,000×g) and dilution of 100 μL of urine specimens with 1.0 mL of mobile phase containing disodium-EDTA (2 g/L), the samples were filtered using a microtiter filter plate (AcroPrep 96, 0.2 μm/350 μL; Pall Life Science, Ann Arbor, MI, USA) and a vacuum manifold (Pall Life Science), and then injected into a column. Neopterin was determined using a high-performance liquid chromatography system (Prominence LC20, Shimadzu, Kyoto, Japan) consisting of a special autosampler with Rack changer/C for the microtitration plates, a degasser (DGU-20A5), two liquid chromatograph pumps (LC-20 AB), an auto sampler (SIL-20AC), a column oven (CTO-20AC thermostat), a fluorescence detector (RF-10AXL), a diode array detector (SPD-M20A) and a communications bus module (CBM-20A). Phosphate buffer (15 mmol/L, pH 6.4), with a flow rate of 0.8 mL/min, was used as the mobile phase. Separation was performed using a hybrid analytical column (Gemini Twin 5 μ, C18, 150×3 mm; Phenomenex, Torrance, CA, USA) at 25°C; the injection volume was 1 μL. Neopterin was identified by its native fluorescence (353 nm excitation, 438 nm emission wavelength). Creatinine was monitored simultaneously in the same urine specimen with a diode array detector at 235 nm. Time of analysis for urinary neopterin and creatinine was 6 min, and the analytes were quantified by external standard calibration. Neopterin concentrations were expressed as neopterin/creatinine ratio (μmol/mol creatinine).
Urinary neopterin concentrations before and during radiotherapy were compared using the Wilcoxon signed rank test and the Friedman test. The decision on statistical significance was based on a p=0.05 level. The analyses were performed with NCSS software (Number Cruncher Statistical Systems, Kaysville, UT, USA).
Results
Baseline urinary neopterin concentrations were, generally, above the normal range (Table 1). Urinary neopterin concentrations were relatively stable during the first 5 weeks of combined (chemo)radiation (Figures 1–3). Marked peaks of neopterin concentrations reflected the emergence of complications. No statistically significant changes were observed when neopterin concentrations at each visit were compared to baseline using the Wilcoxon signed rank test. In nine patients with cervical cancer treated with chemoradiation, no significant difference was observed between urinary neopterin concentrations before and at the end of treatment after (mean±standard deviation) 47±12 days (245±111 vs. 285±121 μmol/mol creatinine, p=0.477). In addition, the Friedman test performed on the data of cervical cancer patients treated with chemoradiation also revealed no significant trend (p=0.861).
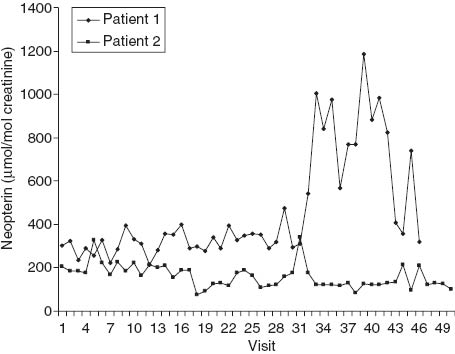
Urinary neopterin concentrations during the course of chemoradiation in patients 1 and 2. Patient 1, who had stage III.B cervical carcinoma and increased baseline urinary neopterin concentrations (301 μmol/mol creatinine), started external beam radiotherapy on the day of visit 1 and the first administration of cisplatin on the day of visit 6. Uterovaginal brachytherapy was initiated on the day of visit 30 and continued for six fractions, with the last fraction administered on the day of visit 39. On the day of visit 37, the patient reported a burning sensation in the genital area. Systemic administration of ciprofloxacin and metronidazol was started and continued for 10 days. This episode coincided with a marked increase in urinary neopterin concentrations. A peak urinary neopterin level of 1187 μmol/mol creatinine was observed on the day of visit 39 (on this day the patient reported chills). Patient 2 also had stage III.B cervical carcinoma and baseline neopterin concentrations on the upper limit of the normal range (205 μmol/mol creatinine). External beam radiotherapy was started on the day of visit 1, and administration of cisplatin was initiated on the day of visit 4. Uterovaginal brachytherapy was started the day before visit 35. Clinically, the course of treatment of this patient was uneventful. The therapy was complicated only by mild (grade 2) leukopenia. Only mild fluctuations of urinary neopterin concentrations were observed that were not accompanied by clinical symptoms.
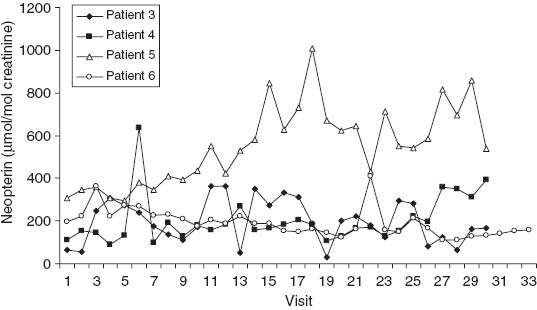
Urinary neopterin concentrations during the course of chemoradiation in patients 3–6, who had cervical carcinoma. The marked increase in urinary neopterin concentrations in patient 5 starting with visit 11 was associated with dyspeptic complaints accompanied by diarrhea and fatigue. Subsequently, the patient had skin rash. The peak urinary neopterin concentrations in patients 3 and 4 were accompanied only by minor complaints.
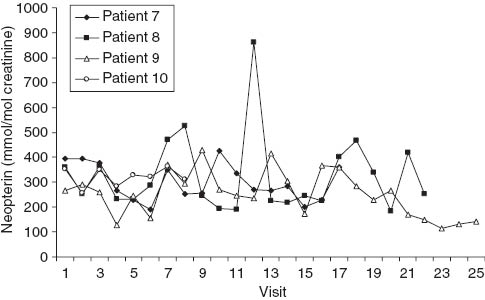
Urinary neopterin concentrations during the course of chemoradiation in patients 7–10. In patient 8, the peak urinary concentration on the day of visit 12 coincided with the manifestation of skin rash on the lower extremities. Other peak neopterin values were accompanied only by minor complaints. Patients 7, 8 and 9 had cervical carcinoma. Patient 10 had recurrent carcinoma of the vulva and was followed only shortly because local reaction made the collection of urine specimens difficult.
Discussion
In the present pilot study, we could not detect any significant increase in urinary neopterin concentrations during external beam radiation in patients with gynecological cancer, mostly cervical carcinoma. Present data indicate that, in the absence of complications, urinary neopterin concentrations show only mild fluctuation throughout the course of therapy, without a significant trend. These negative findings contrast with observation recently reported in a cohort of patients with head and neck carcinoma of similar size [12, 13]. In fact, it might be expected that the chemoradiation regimen used in cervical cancer would result in a marked activation of systemic immune response reflected in increased neopterin concentrations. All major cell populations responsible for the host response to neoplasia are present in the peritoneal cavity, including monocytes/macrophages [16], and these cells may be activated by therapeutic manipulations [17]. Moreover, both chemotherapy and radiation cause a significant damage to the intestinal barrier [18] that results in the activation of the systemic immune response. This could not be observed in the present cohort, and only in individual patients was a marked increase in urinary neopterin concentration noted that reflected the emergence of the complications of therapy rather than a direct effect of the treatment itself. Similarly to the present study, no significant increase in urinary neopterin concentrations was reported earlier in patients with rectal cancer treated with chemoradiation [18].
There are several possible explanations for the negative findings in the present pilot study. First, the size of the cohort was small and may have been insufficient to detect a more limited change in urinary neopterin concentrations. Moreover, the pretreatment urinary neopterin concentrations were relatively high and above normal range in most patients. High neopterin concentrations may decrease as a consequence of tumor control. The samples were usually not collected during treatment interruptions, making comparison of values obtained at the same visit in different patients difficult. Consequently, statistical analyses performed here have to be regarded as exploratory.
Although biomarkers play an increasingly important role in the management of cancer patients, the use of biomarkers associated with the host response to neoplasia is still limited [19]. Neopterin is a well-established biomarker of immune system activation [4, 20]. The prognostic significance of increased systemic neopterin concentrations has been demonstrated across a spectrum of malignant disorders [8]. Increased urinary neopterin concentration is also an independent parameter associated with poor prognosis in cervical cancer [21]. However, in the present study, the number of patients examined was too small to analyze an association between neopterin concentrations before or during chemoradiation with the outcome.
High neopterin concentrations in cancer patients may be associated with a down-regulation of immune response [22, 23]. The immune system plays an important role in the progression of abdominal and pelvic neoplasms [16]. The fact that neopterin concentrations may also increase as a result of non-neoplastic disorders [4] represents an advantage, and assessment of neopterin levels may help to detect a wide range of different complications.
Pelvic chemoradiation is an effective therapeutic modality in adjuvant treatment as well as in patients with inoperable cervical carcinoma [24–26]. In contrast, chemoradiation is an aggressive therapy that results in a significant percentage of serious, in extreme cases even lethal, complications. Timely management of complications of therapy is of great importance. Present data indicate that urinary neopterin concentrations are relatively stable during the course of pelvic (chemo)radiation. A rise in urinary neopterin concentration may indicate the presence of complications. While some side effects of anticancer therapy, e.g., skin or eye toxicity [27], may be assessed directly by visual inspection, most adverse events of the treatment are not so easy to detect or evaluate. Measurement of neopterin in the urine offers a non-invasive approach for the assessment of the condition of the patient, and it could be of special value in the outpatient setting. Future investigations on a larger cohort of patients should investigate the potential of neopterin as a biomarker for early detection of complications during pelvic radiotherapy as well as the association of the changes in urinary neopterin concentrations during the treatment with the outcome.
In conclusion, the present investigation failed to detect an increase in urinary neopterin concentration in patients treated with pelvic (chemo)radiation. Urinary neopterin concentrations may reflect the complications of therapy and could be used to follow the condition of the patient during the treatment.
Acknowledgments
This study was supported by the research project Biomedreg CZ.1.05/2.1.00/01.0030 and by a grant from the Internal Grant Agency of the Czech Republic (NT/13566-4).
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74.10.1016/j.cell.2011.02.013Search in Google Scholar
2. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 1984;160:310–6.10.1084/jem.160.1.310Search in Google Scholar
3. Henderson DC, Sheldon J, Riches P, Hobbs JR. Cytokine induction of neopterin production. Clin Exp Immunol 1991;83:479–82.10.1111/j.1365-2249.1991.tb05664.xSearch in Google Scholar
4. Wachter H, Fuchs D, Hausen A, Reibnegger G, Werner ER. Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem 1989;27:81–141.10.1016/S0065-2423(08)60182-1Search in Google Scholar
5. Melichar B, Gregor J, Solichova D, Lukes J, Tichy M, Pidrman V. Increased urinary neopterin in acute myocardial infarction. Clin Chem 1994;40:338–9.10.1093/clinchem/40.2.338Search in Google Scholar
6. Solichova D, Melichar B, Blaha V, Klejna M, Vavrova J, Palicka V, et al. Biochemical profile and survival in nonagenarians. Clin Biochem 2001;34:563–9.10.1016/S0009-9120(01)00261-2Search in Google Scholar
7. Plata-Nazar K, Jankowska A. Clinical usefulness of determining the concentration of neopterin. Pteridines 2011;22:77–89.10.1515/pteridines.2011.22.1.77Search in Google Scholar
8. Reibnegger G, Fuchs D, Fuith LC, Hausen A, Werner ER, Werner-Felmayer G, et al. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect Prev 1991;15:483–90.Search in Google Scholar
9. Fuchs D, Hausen A, Reibnegger G, Werner ER, Dierich MP, Wachter H. Neopterin as a marker for activated cell mediated immunity: application in HIV infection. Immunol Today 1988;9:150–5.10.1016/0167-5699(88)91203-0Search in Google Scholar
10. Brown RR, Lee CM, Kohler PC, Hank JA, Storer BE, Sondel PM. Altered tryptophan and neopterin metabolism in cancer patients treated with recombinant interleukin 2. Cancer Res 1989;49:4941–4.Search in Google Scholar
11. Melichar B, Urbanek L, Krcmova L, Kalábová H, Melicharová K, Malírová E, et al. Urinary neopterin, hemoglobin and peripheral blood cell counts in breast carcinoma patients treated with dose-dense chemotherapy. Anticancer Res 2008;28:2389–96.Search in Google Scholar
12. Holečková P, Krčmová L, Létal J, Svobodník A, Kalábová H, Kašparová M, et al. Urinary neopterin concentration and toxicity of radiotherapy in patients with head and neck carcinoma during external beam radiation. Anticancer Res 2013;33:4097–101.Search in Google Scholar
13. Holecková P, Krcmová L, Kalábová H, Kašparová M, Plíšek J, Pála M, et al. Prognostic significance of serum retinol, serum alpha-tocopherol, and urinary neopterin in patients with head and neck carcinoma treated with external beam radiation. Int J Vitamin Nutr Res 2012;82:77–84.10.1024/0300-9831/a000096Search in Google Scholar
14. Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstet 2006;95:S43–103.10.1016/S0020-7292(06)60030-1Search in Google Scholar
15. Melichar B, Solichova D, Melicharova K, Malirova E, Cermanova M, Zadak Z. Urinary neopterin in patients with advanced colorectal carcinoma. Int J Biol Markers 2006;21:190–8.10.1177/172460080602100309Search in Google Scholar PubMed
16. Melichar B, Freedman RS. Immunology of the peritoneal cavity: Relevance for host-tumor relation. Int J Gynecol Cancer 2002;12:3–17.10.1046/j.1525-1438.2002.01093.xSearch in Google Scholar PubMed
17. Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, et al. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and, immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin Cancer Res 2003;9:5228–37.Search in Google Scholar
18. Dvorak J, Melichar B, Hyspler R, Krcmová L, Urbánek L, Kalábová H, et al. Intestinal permeability, vitamin A absorption, alpha-tocopherol, and neopterin in patients with rectal carcinoma treated with chemoradiation. Med Oncol 2010;27:690–6.10.1007/s12032-009-9270-4Search in Google Scholar PubMed
19. Melichar B. Laboratory medicine and medical oncology: the tale of two Cinderellas. Clin Chem Lab Med 2013;51:99–112.10.1515/cclm-2012-0496Search in Google Scholar PubMed
20. Melichar B, Solichová D, Freedman RS. Neopterin as an indicator of immune activation and prognosis in patients with gynecological malignancies. Int J Gynecol Cancer 2006;16:240–52.10.1111/j.1525-1438.2006.00294.xSearch in Google Scholar PubMed
21. Reibnegger G, Bichler AH, Dapunt O, Fuchs DN, Fuith LC, Hausen A, et al. Neopterin as a prognostic indicator in patients with carcinoma of the uterine cervix. Cancer Res 1986;46:950–5.Search in Google Scholar
22. Melichar B, Touskova M, Solichova D, Kralickova P, Kopecky O. CD4+ T-lymphocytopenia and systemic immune activation in patients with primary and secondary liver tumours. Scand J Clin Lab Inv 2001;61:363–70.10.1080/003655101316911404Search in Google Scholar PubMed
23. Melichar B, Jandik P, Krejsek J, Solichova D, Drahosova M, Skopec F, et al. Mitogen-induced lymphocyte proliferation and systemic immune activation in cancer patients. Tumori 1996;82:218–20.Search in Google Scholar
24. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–43.10.1056/NEJM199904153401501Search in Google Scholar PubMed
25. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–53.10.1056/NEJM199904153401502Search in Google Scholar PubMed
26. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–61.10.1056/NEJM199904153401503Search in Google Scholar PubMed
27. Melichar B, Nemcová I. Eye complications of cetuximab therapy. Eur J Cancer Care 2007;16:439–43.10.1111/j.1365-2354.2006.00763.xSearch in Google Scholar PubMed
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Urinary neopterin concentrations during radiotherapy for gynecological cancer
- Protection of spores from ultraviolet-C irradiation by auto-fluorescent substances in the spore mass of the cellular slime mold Dictyostelium discoideum
- Abstracts
- Abstracts 33rd International Winter Workshop Clinical, Chemical and Biochemical Aspects of Pteridines and Related Topics
Articles in the same Issue
- Frontmatter
- Original articles
- Urinary neopterin concentrations during radiotherapy for gynecological cancer
- Protection of spores from ultraviolet-C irradiation by auto-fluorescent substances in the spore mass of the cellular slime mold Dictyostelium discoideum
- Abstracts
- Abstracts 33rd International Winter Workshop Clinical, Chemical and Biochemical Aspects of Pteridines and Related Topics

