Abstract
In this study, native spores surrounded by fluorescent substances in the spore mass of Dictyostelium discoideum were found to be resistant to relatively strong ultraviolet-C (UV-C) irradiation (2880 J/m2). The remaining emergency activity of the native mass of spores was over 80% even after exposure to strong UV-C irradiation (2880 J/m2). In contrast, the washed spores were very sensitive to weak UV-C irradiation (144 J/m2). The mass of spores in the fruiting body formed by amoebae with a low concentration of fluorescent substances was less resistant to UV-C than that in the fruiting body formed by normally grown amoebae. Based on the remaining emergency activity of washed spores with appropriate lumazine solution, the concentration of fluorescent substances in the native mass of spores was estimated to be equivalent to approximately 5 mmol/L of lumazine.
Introduction
Vegetative amoebae of the cellular slime mold Dictyostelium discoideum grow by binary fission using bacteria as a food source. After deprivation of bacteria, the homogeneous population of slime mold cells aggregates to form a slug-shaped mass of cells (pseudoplasmodium) on a solid substrate. Eventually, the mass of cells forms a fruiting body consisting of spores and a supporting cellular stalk.
Some pteridines have been reported as fluorescent substances of D. discoideum cells. D. discoideum cells were shown to secrete lumazine (2,4-dihydroxypteridine), a deamination product of pterin, which is responsible for extracellular fluorescent products [1–3]. Isoxantholumazine (2,4,7-trihydroxy-pteridine) [1, 3] and dictyolumazine [3] were also reported in the extracellular medium. Furthermore, dictyopterin [6-(D-threo-1,2-dihydroxypropyl)-pterin] is another major intracellular product that has been isolated from vegetative D. discoideum cells [4, 5].
We previously reported the microscopic observation of fluorescence in D. discoideum and D. mucoroides living cells during growth and morphogenesis [6, 7]; fluorescence was observed in the inter-spore space of the spore mass of the fruiting body, but not in the spores themselves.
The spores were found to be more sensitive to ultraviolet-C (UV-C) irradiation than the amoebae in D. discoideum [8]. Although UV-C is harmful to microorganisms, the pteridines absorb UV-C and subsequently emit harmless fluorescence. In the present study, the role of lumazine in the inter-spore space of the spore mass in the response to UV-C exposure was investigated.
Materials and methods
Dictyostelium discoideum NC-4 cells were used in all experiments. The cells were grown with Escherichia coli B/r as a source of food for the myxoamoebae on a solid medium, with each liter containing 10 g of Bacto-Peptone (Difco, Detroit, MI, USA), 10 g of glucose, 0.96 g of Na2HPO4·12H2O, 1.45 g of KH2PO4, and 20 g of Bacto-Agar (Difco) [9]. The cells were grown on a nutrient agar plate at 22°C and were allowed to generate terminal fruiting bodies in order to obtain spores with or without washing by centrifugation.
Cultures in 30 mL of the above mentioned nutrient broth without agar were shaken on a reciprocating shaker (100 strokes per minute) at 22°C. The cells at full growth phase (2–3×107 cells/mL) were harvested from the liquid medium, incubated for 24 h in 40 mmol/L of phosphate buffer (pH 6.4) solution to obtain the interphase amoebae, and were allowed to form the fruiting body on the non-nutrient agar plate. The spores on the fruiting body were used for the UV-C irradiation experiment without washing.
Escherichia coli were harvested from the liquid medium and were suspended in 40 mmol/L of phosphate buffer solution for use in washed E. coli cultures. Cultures in 30 mL of the spores with the washed E. coli were suspended in 40 mmol/L of phosphate buffer (pH 6.4) solution and also shaken on a reciprocating shaker (100 strokes per minute) at 22°C. The harvested amoebae were allowed to form the fruiting body on the non-nutrient agar plate. The spores on the fruiting body were used for the UV-C irradiation experiment without washing.
The spore masses were harvested manually by touching a nickel-chrome wire loop to the spore masses supported by the stalk. The spore masses were washed free from the inter-spore space medium with distilled water by centrifugation with a desktop centrifugal separator (Shimadzu Co., Kyoto, Japan) at 2000×g for 5 min. The washed spores were exposed to UV-C at 1.6 J/m2 for the indicated times, and the spores in the fruiting body on agar plates were also exposed to UV-C irradiation at 1.6 J/m2 for the indicated times. Narrow-band UV-C irradiation (peak at 254 nm) was performed using the GL15 ultraviolet lamp (Toshiba Co., Tokyo, Japan). The ultraviolet intensity was measured using the J225 UV meter (UVP Co., Upland, CA, USA).
All of the UV-C irradiated spores were allowed to germinate homogeneously by heat-shock treatment at 45°C for 30 min in distilled water [10]. They were shaken on a reciprocating shaker (100 strokes per minute) at 22°C for 6 h until each amoeba emerged from the spores. The dormant spores were counted microscopically, and the ratio of amoebae and spores was calculated in the population (approx. 50) for two or three cultures.
The spores irradiated by UV-C (1440 J/m2) in authentic lumazine (Aldrich Chemical Co., Milwaukee, WI, USA) solution were allowed to germinate for 6 h after heat-shock treatment. The lumazine equivalent of the native spore mass was estimated by comparison with germination activity in an appropriate concentration of lumazine solution.
Results and discussion
Almost all of the spores in a fruiting body were observed to germinate by the heat-shock treatment following 6 h of incubation. The germination activity of spores in a fruiting body on solid agar was 82% after UV-C irradiation for 30 min (2880 J/m2) (Figure 1). In contrast, the germination activity of washed spores was reduced after a very short (90 s) UV-C irradiation period (144 J/m2) (Figure 2). The observed sensitivity of washed spores to UV-C coincides well with previous results [11, 12]. Dictyopterin synthesized in the vegetative growth phase of D. discoideum cells was excreted to the outside space of spores during sporulation as its deamination product [2, 6, 7]. The fluorescence excitation spectrum of lumazine and the crude preparation from the spore masses as fluorescent substances show a maximum peak at 322 and 328.5 nm, respectively [13]. Therefore, UV-C might be absorbed by lumazine or lumazine-like substances in the spore mass. As a result, the harmful UV-C is converted to harmless fluorescent light, although the fluorescence excitation spectrum of those under 300 nm is unknown.

Germination activity of the spores in the UV-C-irradiated spore mass on the fruiting body. The spores were harvested from the UV-C-irradiated spore mass on the fruiting body and were then allowed to germinate in the reciprocal incubator after heat-shock treatment. Emerged amoebae and dormant spores were counted microscopically. Data points represent the mean values (with standard deviation) of two or three experiments. The mean at 2880 J/m2 of UV-C irradiation shows a significant difference from that at 0 J/m2 of UV-C irradiation (p<0.05; Sheffe’s F-test).
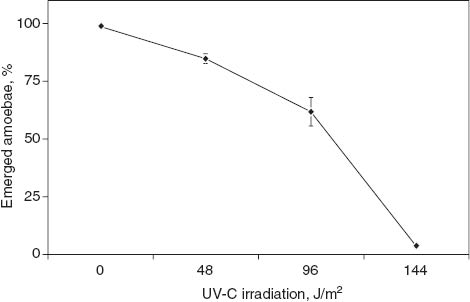
Germination activity of the spores washed before UV-C irradiation. Other procedures are described in the legend for Figure 1. Data points represent the means of two or three experiments with standard deviations.
The resistance level of the spore mass to UV-C was reduced by one-third when the fruiting body was formed by cells that were incubated for 24 h in the phosphate buffer solution after vegetative growth (Table 1). We previously observed by microscope that fluorescent vacuoles were lost from vegetative cells during incubation in a buffer solution [6]. Tatischeff and Klein [14] also showed that the cells secreted fluorescent products in the extracellular medium of the buffer solution. Therefore, these results suggest that the reduction in the resistance level of the spore mass to UV-C was due to the loss of fluorescent substances during incubation of the cells in the phosphate buffer solution.
Germination activity of the UV-C-irradiated (1440 J/m2) spore mass.
| Source of amoebae | Emerged amoebae from spore, % | Fluorescent intensity of auto-fluorescent vacuolesa |
|---|---|---|
| Normally grown amoebaeb | 89.3±7.5c | Strong |
| Amoebae incubated in phosphate bufferd | 34.5±6.1c | Weak |
| Amoebae incubated with washed bacteriae | 49.0±2.5c | Medium |
aFluorescent intensity of auto-fluorescent vacuoles was observed by fluorescent microscope. bThe spore mass was irradiated on the naturally developed fruiting body. The spores were then washed and treated with heat shock and then allowed to incubate for germination. cEach value represents the mean of two or three experiments with standard deviations (mean±SD). dThe spore mass was irradiated on the fruiting body, which was ultimately developed from amoebae incubated for 24 h in phosphate buffer solution after growing in nutrient broth. The other treatment was the same as that mentioned above in table note (b). eThe spore mass was irradiated on the fruiting body, which was ultimately developed from amoebae incubated with washed bacteria in the phosphate buffer solution. The other treatment was the same as that mentioned above in table note (b).
The intensity of fluorescent vacuoles of amoebae grown in the buffer solution with washed bacteria that were fully grown in another culture was weaker than that of amoebae grown with bacteria in two-member cultures (data not shown). The resistance level to UV-C of the spores in the fruiting body formed by amoebae with weak fluorescent vacuoles was almost half of that of the spores in the fruiting body formed by normally grown amoebae (Table 1). As mentioned above, this suggests that the weak fluorescence of the fluorescent vacuoles in the amoebae might be linked to the weak fluorescence of the spore mass.
The germinating activity of spores in the authentic lumazine solution as an alternative of inter-spore substances was determined under UV-C irradiation. The damage by UV-C irradiation (1440 J/m2) was almost completely recovered in 5 mmol/L of lumazine solution, but not in 1 mmol/L of lumazine solution (Figure 3). This suggests that the concentration of fluorescent substances in the inter-spore space of the spore mass is equivalent to approximately 5 mmol/L of lumazine.

Estimation of the fluorescent substances as lumazine equivalent in the spore mass. The fluorescent substance concentration (in lumazine equivalent) in the spore mass was estimated based on the percent of emerged amoebae after spores were UV-C irradiated (1440 J/m2) in indicated concentrations of lumazine solution. Lumazine solution (1, 3, 5, and 7 mmol/L) was used for the experiment. Data points represent the mean values of two or three experiments with standard deviations. The mean at 3 mmol/L of lumazine shows a significant difference from those at 5 and 7 mmol/L of lumazine (p≤0.05; Sheffe’s F-test).
Conclusion
This study demonstrated that the spores were, at least partially, protected from UV-C by the fluorescent substances in the spore mass. Furthermore, these results suggested that the fluorescent substances in the spore mass might absorb the harmful UV-C and subsequently emit harmless fluorescence. Based on the remaining emergency activity of washed spores with appropriate lumazine solution, the concentration of fluorescent substances in the native mass of spores was estimated to be equivalent to approximately 5 mmol/L of lumazine.
References
1. Gerisch G, Blank G, Schweiger M, Fuchs D, Hausen A, Reibneger G, et al. Pteridines released from Dictyostelium discoideum cells into the extracellular medium. In: Wachter H, Curtius HC, Pfleiderer W, editors. Biochemical and clinical aspects of pteridines, vol. 1. Berlin: Walter de Gruyter, 1982:253–6.Search in Google Scholar
2. Tatischeff I, Klein R, Tham G. Extracellular lumazine from aggregating Dictyostelium discoideum cells. Influence of pH on its fluorescence. Hoppe-Seyler’s Z Physiol Chem 1984;365:1255–62.10.1515/bchm2.1984.365.2.1255Search in Google Scholar
3. Klein R, Tatischeff I, Tham G, Mano N. Chiral lumazines: preparation, properties, enantiomeric separation. Chirality 1994;6:564–71.10.1002/chir.530060709Search in Google Scholar
4. Klein R, Thiery R, Tatischeff I. Dictyopterin, 6-(D-threo-1,2-dihydroxypropyl)-pterin, a new natural isomer of L-biopterin. Isolation from vegetative cells of Dictyostelium discoideum and identification. Eur J Biochem 1990;187:665–9.10.1111/j.1432-1033.1990.tb15351.xSearch in Google Scholar
5. Klein R. Determination of the stereoconfiguration of natural pterin by chiral high-performance liquid chromatography. Anal Biochem 1992;203:134–40.10.1016/0003-2697(92)90053-ASearch in Google Scholar
6. Uchiyama S, Nagai S, Maruyama K. Localization of fluorescent substances in the cellular slime mold Dictyostelium discoideum cells during growth and development. J Plant Res 1993;106:345–9.10.1007/BF02345979Search in Google Scholar
7. Uchiyama S, Nagai S, Maruyama K. Fluorescence in the migrating pseudoplasmodium of the cellular slime mold Dictyostelium mucoroides. Cell Struct Func 1994;19:159–63.10.1247/csf.19.159Search in Google Scholar PubMed
8. Hashimoto Y, Wada M. Comparative study of the sensitivity of spores and amoebae of Dictyostelium discoideum to ultraviolet light. Radiat Res 1980;83:688–95.10.2307/3575348Search in Google Scholar
9. Bonner JT. Growth on solid media. In: The cellular slime mold, 2nd ed. New Jersey: Princeton University Press, 1967:81–6.Search in Google Scholar
10. Ashworth JM, Dee J. General biology of the cellular slime moulds. In: The biology of slime moulds. London: Edward Arnold, 1975:32–45.Search in Google Scholar
11. Ford WT Jr, Deering RA. Survival, spore formation and excision repair of UV-irradiated developing cells of Dictyostelium discoideum NC-4. Photochem Photobiol 1979;30:653–9.10.1111/j.1751-1097.1979.tb07195.xSearch in Google Scholar PubMed
12. Okaichi K. RNA synthesis during germination of UV-irradiated Dictyostelium discoideum spores. J Radiat Res 1987;28:172–85.10.1269/jrr.28.172Search in Google Scholar PubMed
13. Uchiyama S, Nagai S, Maruyama K. Lumazine-like fluorescence in a mass of spores of the cellular slime mold, Dictyostelium discoideum. J Plant Res 1997;110:383–6.10.1007/BF02524938Search in Google Scholar
14. Tatischeff I, Klein R. Fluorescent products secreted by Dictyostelium discoideum cells which are able to aggregate. FEBS Lett 1982;138:265–9.10.1016/0014-5793(82)80457-2Search in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Urinary neopterin concentrations during radiotherapy for gynecological cancer
- Protection of spores from ultraviolet-C irradiation by auto-fluorescent substances in the spore mass of the cellular slime mold Dictyostelium discoideum
- Abstracts
- Abstracts 33rd International Winter Workshop Clinical, Chemical and Biochemical Aspects of Pteridines and Related Topics
Articles in the same Issue
- Frontmatter
- Original articles
- Urinary neopterin concentrations during radiotherapy for gynecological cancer
- Protection of spores from ultraviolet-C irradiation by auto-fluorescent substances in the spore mass of the cellular slime mold Dictyostelium discoideum
- Abstracts
- Abstracts 33rd International Winter Workshop Clinical, Chemical and Biochemical Aspects of Pteridines and Related Topics

