Abstract
This chapter analyzes the advantages of the use of bioartificial polymers as carriers and the main strategies used for their design. Despite the enormous progresses in this field, more studies are required for the fully evaluation of these nanovectors in complex organisms and for the characterization of the pharmacodynamic and pharmacokinetic of the loaded drugs. Moreover, progresses in polymer chemistry are introducing a wide range of functionalities in the bioartificial polymeric material (BPM) nanostructures leading to a second generation of bioartificial polymer therapeutics based on novel and heterogeneous architectures with higher molecular weight and predictable structures, in order to achieve greater multivalency and increased loading capacity. Therefore, research on bioartificial polymeric nanovectors is an “on-going” field capable of attracting medical interest.
1 Introduction
1.1 Bioartificial polymeric materials
“Bioartificial polymeric materials” (BPMs) are a class of polymeric composites based on the blend between synthetic and natural polymers, designed to produce new materials combining the biocompatibility of the biological components with the physical and mechanical features of the synthetics [1, 2, 3].These materials are engineered for different applications such as biodegradable delivery systems, leak proof membranes, systems of proteins purification, dialysis membranes, wound dressing, artificial skin, cardiovascular devices, nerve guide channels, implantable devices, bone graft substitutes [4, 56] and have an enormous repercussion in the human life quality [7]. Table 1summarizes the main applications of these materials (Table 1).
Uses of bioartificial polymeric materials.
| Area | Examples |
|---|---|
| Replacement of damaged parts | Artificial joint |
| Healing devices | Sutures, bone plates |
| Assistance of functionality | Cardiac pacemaker, ocular devices |
| Diagnosis | Probes |
| Aesthetics | Breast implants |
| Drug delivery | Nanoparticles, Nanofibers |
1.1.1 Natural polymers for BPMs
Natural polymers are macromolecules produced by living organisms (e.g. plants, mammals, crustaceans) with structural or functional purposes. The main classes of natural polymers are polynucleotides, polypeptides and polysaccharides [8]. Polynucleotides act as carriers of the genetic information, polypeptides function as structural materials or catalysts, polysaccharides are components of membranes and enhance intracellular communication [9]. Natural polymers are broadly used as advanced materials for the production of fibers, adhesives, coatings, gels, thermoplastics, resins, etc. and most of them have medical applications [9, 10]. (Table 2). These polymers possess several inherent advantages such as bioactivity, the ability to present receptor-binding ligands to cells, susceptibility to cell-triggered proteolytic degradation and natural remodeling. However, their immunogenicity, variability in purity across groups, complex structure, strength inadequacies and difficulty in controlling material degradability limit their utilization [11].
Natural polymers used for bioartificial polymeric materials.
| Polymer | Structure | Natural derivation |
|---|---|---|
| Albumin | NH2-E-A-H-K-S-E- (N terminal sequence of the protein) | Blood plasma |
| Chitosan | (C6H11NO4)n | Shrimp and other crustacean shells |
| Collagen | -(-G-Hyp-P-A-Hyp-P-)- (repeated sequence of the glycoprotein) | Animal connective tissues |
| Fibrin | NH2-Q-G-V-N-D-N- (N terminal sequence of the protein) | Blood plasma |
| Fibroin | -(-G-S-G-A-G-A-)- (repeated sequence of the protein) | Silk |
| Heparin | C12H19NO20S3 | Blood plasma |
| Hyaluronic acid | (C14H21NO11)n | Animal synovial fluids Microbial production |
1.1.2 Synthetic polymers for BPMs
Synthetic polymers are petroleum-based products produced by chemical reactions. These materials are important components of BPMs thanks to their inert nature, high resistance of chemical linkages to hydrolytic and oxidative degradation and ability to tailor mechanical properties. Synthetic polymers contribute to the efficient functioning of devices providing mechanical support to implants such as articulating surfaces and scaffolds (e.g. knee and hip implants), protective coatings to improve blood compatibility, electrically stimulating devices (e.g. pacemakers, heart valves), catheters and dialysis tubing, vascular grafts and implantable drug delivery systems (e.g. drug eluting coatings on vascular stents). The main classes of synthetic polymers used in BPMs include poly(olefins), poly(urethanes), poly(carbonates), poly(siloxanes), poly(amides), poly(ethers), poly(sulfones) and poly(esters) [12, 13, 14, 15]. Table 3summarizes their chemical structures and general properties (Table 3).
Synthetic polymers used for bioartificial polymeric materials.
| Polymer | Structure | Key properties |
|---|---|---|
| Poly(ethylene) | (C2H4)n | Excellent chemical resistance Low- and high-density grades Thermoplastics features |
| Poly(propylene) | (C3H6)n | Flexible with good fatigue resistance Thermoplastics features |
| Poly(methyl methacrylate) | (C5O2H8)n | Good impact strength Lightweight, transparent Poor chemical resistance Thermoplastics features |
| Poly(dimethyl siloxane) |  | Excellent viscoelastic properties Transparent, elastic, inert, nontoxic |
| Poly(ether ether ketone) |  | Excellent chemical resistance Excellent mechanic properties Thermoplastics features |
| Polyurethane |  | Biodegradable Biostable Thermoplastics features |
1.2 Nanotechnology and medicine
Nanotechnology (NT) is the science of manipulating matter at the atomic or molecular scale and holds the promise of providing significant improvements in the technologies intended to enhance human well-being and protect the environment [15]. NT is often regarded as a product of the latter part of the twentieth century but it influenced human evolution from the earliest civilization. Indeed, the ancient Greeks used permanent hair-dying recipes composed of 5 nm lead sulfide crystals and European medieval artists colored stained glass using metal nanoparticles. Modern NT, started in 1959 when physicist Richard Feynman recognized the possibility to build machines able to manufacture objects with atomic precision and explained that, at the nanoscale, surface phenomena dominate the behavior of the objects [16]. The term NT, however, was introduced in 1974 by Norio Taniguchi referring to the “production technology to get the extra-high accuracy and ultra-fine dimensions” [17]. Practical application of NT started with the description of the molecular manufacturing [18] and the invention of the scanning tunneling microscope (STM) that allowed the first direct manipulation of individual atoms [17]. Nowadays, NT is a dynamic field where over 50,000 articles published annually and more than 2,500 patents filed [19].
Nanomedicine, an offshoot of NT, uses nano-sized tools for the diagnosis, prevention and treatment of diseases (Figure 1). Applicative examples are biosensors, implantable devices, prostheses components and drug delivery platforms. This chapter focuses on the delivery of therapeutic substances through bioartificial polymeric nanovectors, a novel and interesting aspect of nanomedicine.

Nanovectors direction to the FDA approval.
2 Bioartificial polymeric nanovectors
Drug delivery is the method of administering pharmaceutical compounds to achieve therapeutic effects in humans or animals [20]. The delivery vehicles are films, plasters, gels and polymeric-based nanovectors. Among these, nano-sized delivery systems have a significant role in the alteration of bioavailability, pharmacokinetic and pharmacodynamic properties of drug molecules thanks to their favorable chemical-physical characteristics due to the reduced dimensions, their ability of delivering therapeutic agents directly into the intended site of action and their capability to overcome tight junction membrane barriers (e.g. blood brain barrier and blood-ocular-barrier) [21, 22, 23, 24]. Nanovectors are particularly useful to transport drugs that have poor solubility or a short half-life and have numerous biological applications such as cancer therapy, stabilization and protection of molecules, proteins, peptides and DNA, analysis of environmental hazards, protein and gene delivery, action as self-regulated devices bio-recognizable systems and stimuli-controlled vectors [25, 26, 27].
BPMs are widely used as nano-sized drug delivery systems due to the synthetically controllable size, surface charge and morphology, solubility, mechanical properties and pharmacokinetic [24, 28,29, 30]. Bioartificial polymeric nanovectors are targeted to the biological substrate using three different mechanisms: active targeting, passive targeting and endocytosis. Active targeting is an internalization method that uses receptors, surface ligands, antigen-antibody combinations or aptamers to enter targeted tissues or cells. Passive targeting takes advantage of nanosystems’ physicochemical properties (e.g. small size, surface functionalization, morphology) to accumulate in target tissues. In particular, the nanovehicles are able to enter into the cells through van der Waals forces, electrostatic charges, steric interactions or interfacial tension based on the pathophysiological characteristics of the tissues (e.g. extravasation of nanovectors through the “leaky” endothelium of tumor tissue). Finally, endocytosis, the major route for nanomedicines, allows transport of nanodelivery systems across cell membrane and is generally classified into phagocytosis and pinocytosis [31, 32, 33, 34]. The synthesis of BPM nanovectors depends on the polymeric units of the material and follows top down or bottom up approaches. “Top down” approach refers to the reduction of a bulk material to get nano-sized particle, while “bottom up” allows the build of nanoparticles starting from the monomers [35]. The technique used greatly impacts the physical, chemical and biological properties of the produced vehicles and influences their size, shape and surface chemistry [36, 37, 38]. However, clinical utilization of BPM-based nanovectors is still at the early stages and the commercialized vehicles are mainly composed of synthetic polymers. Table 4summarizes the marketed polymeric nanovectors for drug delivery applications (Table 4).
Commercialized polymeric nanovectors for drug delivery applications.
| PRODUCT | NANO-SYSTEM | PAYLOAD | THERAPEUTIC INDICATION |
|---|---|---|---|
| Copaxone® | Polymeric drug | Poly(alanine, lysine, glutamic acid, tyrosine) | multiple sclerosis |
| Renagel® | Polymeric drug | Poly(allylamine) | end stage renal failure |
| Emmelle® gel | Polymeric drug | dextrin-2-sulphate | HIV/AIDS - vaginalvirucide |
| Adagen® | Polymer-protein conjugate | PEG-adenosine deaminase | severe combined immunodeficiency syndrome |
| Zinostatin Stimalmer® | Polymer-protein conjugate | SMANCS | cancer - hepatocellularcarcinoma |
| Oncaspar® | Polymer-protein conjugate | PEG-L-asparaginase | acute lymphoblastic leukamia |
| PEG-intron™ | Polymer-protein conjugate | PEG-a-interferon 2b | hepatitis C |
| PEG-Asys® | Polymer-protein conjugate | PEG-a-interferon 2a | hepatitis C |
| Pegvisomant® | Polymer-protein conjugate | PEG-human growth hormone | acromegaly |
| Neulasta™ | Polymer-protein conjugate | PEG-GCSF | prevention of neutropeniaassociated with cancerchemotherapy |
2.1 Nanospheres and Nanocapsules
Nanospheres (NSs) and nanocapsules (NCs) are nano-sized vectors composed of amphiphilic copolymers structured with hydrophobic chains forming the inner part of the particles and hydrophilic portions on the surface. NSs have homogeneous solid matrices [39] while NCs exhibit a core-shell structure in which the drug is confined to a reservoir or within a cavity surrounded by a polymer membrane [40]. Both NSs and NCs allow the fine tuning of their properties through surface functionalization, the use of different shell materials and with the regulation of their size [41, 42, 43]. Poly-lactic acid (PLA), poly-glycolic acid (PGA), poly-lactic-co-glycolic acid (PLGA), poly ε-caprolactone (PCL), chitosan (CS) and polyethylene glycol (PEG) are the main materials used for the synthesis of these systems due to their wide biocompatibility and biodegradability [44, 45, 46, 47, 48]. NS drugs are dissolved, entrapped, encapsulated, chemically bound or adsorbed to the constituent polymer matrix [49, 50] while NCs carry the active substance in the core, or on their surfaces or absorbed in the polymeric membrane [51, 52, 53]. The use of NSs and NCs is an attractive strategy for the vectorization of a variety of active substances such as antineoplastics, antiinflammatories, immunosuppressants, antigens, hormones, antivirals, antibacterials, antifungals, diuretics, antipneumocystics and vitamins. Moreover, these systems are useful to mask unpleasant tastes, to provide controlled release properties, to protect vulnerable molecules from degradation and to increase the therapeutic efficacy of active molecules [54, 55]. Finally, NSs and NCs have high intracellular uptake and require a low amount of polymer for each particle, resulting in high drug loading [46, 49]. NSs and NCs have common preparation techniques that are classified into two general categories depending on the starting material. The use of monomers requires emulsion polymerization, interfacial polymerization or ionic gelation methods. Differently, for preformed polymers, nanoparticle preparation is achieved through emulsification/solvent evaporation, emulsification/solvent diffusion, salting out, dialysis and nanoprecipitation. The emulsion polymerization method is carried out using organic or aqueous solvents as continuous phase [46]. Surfactants or protective soluble polymers are used to prevent aggregation in the early stages of the polymerization. The polymerization process starts using an initiator molecule (ion or free radical), or activating the monomer with high-energy radiations. Incorporation of active principles is obtained dissolving the substance in the same phase of the monomers. The interfacial polymerization is a process with a similar mechanism that involves the dissolution of two reactive agents into two phases (i.e., continuous- and dispersed-phase), with the reaction that takes place at the interface of the two liquids [56]. Interfacial polymerization permits to modulate the formation of NSs or NCs using different eluents. In fact, to promote NC formation, aprotic solvents are used, while protic liquids induce the formation of NSs [57]. Incorporation of active principles is obtained dissolving the drug into the dispersed phase. Ionic gelation permits the preparation of polymeric nanoparticles using biodegradable hydrophilic polymers such as CS, gelatin and sodium alginate. This method requires the mixture of two aqueous phases, one containing the hydrophilic polymer and the other a crosslinker (e.g. poly-anion sodium tripolyphosphate). The positive groups of the polymer interact with negative charged crosslinkers to form nano-sized vectors [58]. Drug is added in the same phase of the hydrophilic polymer [58]. Emulsification/solvent evaporation is a method that requires the preparation of the polymer solution in lipophilic volatile solvent with a subsequent formation of an emulsion, adding water and stabilizers. The lipophilic solvent diffuses through the emulsion and its evaporation lead to the formation of a nanoparticle suspension. High-speed homogenization or sonication is utilized to improve the diffusion, while the solvent evaporation is favored by continuous magnetic stirring at room temperature or under reduced pressure. The solidified nanoparticles are collected by centrifugation and washed with distilled water to remove additives [59]. Drug loading is carried dispersing the substance in the volatile solvent. Similarly, in the emulsification/solvent diffusion method, the encapsulating polymer is dissolved in a partially water soluble eluent and saturated with water to ensure the diffusion. Subsequently, the polymer-water saturated solvent phase is emulsified in an aqueous solution containing stabilizers, leading to the solvent diffusion to the external phase and to the formation of nanovectors. Drug loading is achieved dissolving the substance in the polymer phase [46]. Salting out is a modification of the emulsification/solvent diffusion in which polymer and drug are initially solubilized in the volatile solvent which is emulsified into an aqueous gel containing salting-out agents (e.g. electrolytes, such as magnesium chloride, calcium chloride, and magnesium acetate, or non-electrolytes such as sucrose) and colloidal stabilizers (e.g. polyvinylpyrrolidone or hydroxyethylcellulose). This oil/water emulsion is diluted with aqueous solutions to enhance the diffusion. The salting out agents improve the encapsulation efficiency of the drug [46]. In the dialysis methodology, the polymer is dissolved in an organic solvent and placed inside a dialysis tube. Dialysis is performed against a non-solvent miscible with the lipophilic eluent. The progressive aggregation of polymer and the formation of nanoparticles is the consequence of the displacement of the organic solvent inside the membrane [59]. Drug incorporation is obtained adding the active principle in the same eluent of the polymer. Finally, in the nanoprecipitation method, the polymer is solubilized in a water-miscible solvent and is injected into a stirred aqueous solution containing a surfactant. The stirring causes a fast diffusion of the solvent and the polymer deposition on the interface between the water and the organic eluent, leading to the instantaneous formation of a colloidal suspension [60]. The aqueous solution must be a non-solvent of the polymer [61]. Drug encapsulation is achieved solubilizing the drug into the organic solvent [61]. A schematic representation of the described preparation techniques is available in Figure 2. Bioartificial polymers are widely studied as components of NSs and NCs. Such systems are described by Bellotti et al. as composed of butyl methacrylate, poly(ethylene glycol) methyl ether methacrylate, 2-(dimethylamino) ethyl methacrylate crosslinked with trimethylolpropane trimethacrylate and functionalized with folic acid on their surface in order to specific target enclosed anticancer drug to cancer cells [62]. The antitumor activity is the subject of research of several other authors. For example, Cui et al. formed ionically assembled nanoparticles from poly(ionic liquid-co-N-isopropylacrylamide) with deoxycholic acid through electrostatic interactions. These nanoparticles exhibit dual-responsive properties based on pH and thermal environment conditions with practical applications as drug delivery carriers, as shown by the encapsulation of doxorubicin. In particular, low pH and high temperature provoke structural collapse of the ionically assembled nanoparticle and the release of doxorubicin. In fact, 80 % of drug molecules are released within 48 h at pH 5.2, 43 °C, but only 30 % of doxorubicin is released within 48 h at 37 °C and pH 7.4 [63]. Bahadur et al. designed nanoparticles formed by poly(2-(pyridin-2-yldisulfanyl)ethyl acrylate) conjugated with PEG and cyclo(Arg-Gly-Asp-d-Phe-Cys) peptide. These nanovectors are loaded with doxorubicin. The size of the vehicle is 50.13 ± 0.5 nm in PBS. Such vectors are stable in physiological condition and release doxorubicin with the trigger of acidic pH and redox potential. Moreover, these acrylate-based nanoparticles show a two-phase release kinetics, providing both loading and maintenance doses for cancer therapy. The conjugation with the peptide enhances the cellular uptake and nuclear localization. In fact, these vectors exhibit significantly higher anticancer efficacy compared to that of free doxorubicin at concentrations higher than 5 μM [64]. Barick et al. synthesized glycine functionalized magnetite (Fe3O4) nanoparticles by Michael addition/amidation reaction. These nanocarriers have average size of about 10 nm and are resistant to protein adsorption in physiological medium. Moreover, the terminal amino acids on the shell of the magnetic nanocarriers allow outer functionalization and potential conjugation with drug molecules. The encapsulation of doxorubicin as model drug revealed high loading affinity, sustained release profile, magnetic-field-induced heating and substantial cellular internalization. Moreover, the enhanced toxicity to tumor cells using a local magnetic field suggests their potential for combination therapy involving hyperthermia and chemotherapy [65]. Similarly, Zhao et al. produced arginine–glycine–aspartic acid-modified Fe3O4 nanoparticles to control the delivery and release of doxorubicin. The conjugation of these targeted magnetite nanoparticles with the drug is via acid-labile imine bond. Such linkage gives magnetic control, specific targeting and pH-responsivity to the nanocarriers. The cell toxicity assays indicate higher anticancer activity of these pH-sensitive magnetic nanocarriers compared to free doxorubicin and increased cytotoxicity consequent to the conjugation with arginine–glycine–aspartic acid peptides [66]. Cheng et al. developed nanoparticles of carboxy-terminated poly(d,L-lactide-co-glycolide)-block-poly(ethylene glycol) conjugated with A10 RNA aptamers, able to bind the prostate specific membrane antigens. Such nanoparticles deliver docetaxel and paclitaxel to tumor cells. These nanovectors are evaluated in a xenograft mouse model of prostate cancer. The surface functionalization with A10 aptamers significantly enhances the delivery to tumors [67]. Patil et al. synthesized copolymer PLA–PEG nanoparticles functionalized with biotin or folic acid and incorporating paclitaxel, by solvent polymerization technique. The addiction of the ligands significantly enhances nanoparticles accumulation in tumor cells in vitro and results in improved efficacy of in a mouse xenograft tumor model [68]. Farokhzad et al. synthesized a bioconjugate composed of PLA-block-PEG copolymer and aptamers for targeted delivery to prostate cancer cells. These nanovectors encapsulate the model drug rhodamine labeled with dextran. Such nanoparticles present carboxylic acid groups on the particle surface, useful for functionalization and for covalent conjugation with amine-modified aptamers. Moreover, the coating of PEG enhances circulating half-life and decreases the uptake into non-targeted cells. The bioconjugation with RNA aptamers permits the targeting on prostate LNCaP epithelial cells [69]. Schiffelers et al. produced self-assembling nanoparticles with siRNA and polyethyleneimine PEGylated with an Arg-Gly-Asp (RGD) peptide ligand attached at the distal end of the PEG. These nanovectors deliver siRNA inhibiting vascular endothelial growth factor receptor-2 expression into tumor neovasculature expressing integrins. Intravenous administration of this system into tumor-bearing mice results in selective tumor uptake, siRNA sequence-specific inhibition of protein expression within the tumor and reduction of both tumor angiogenesis and growth rate [70]. Cho et al. synthesized retinoic acid loaded poly(L-lactic acid) nanoparticles coated with galactose-carrying polymer for hepatocyte-specific targeting using galactose ligands as recognition signals to asialoglycoprotein receptors. The authors study the effects of released retinoic acid on morphology and DNA synthesis of hepatocytes. Such drugs modify in vitro shapes of hepatocytes. Moreover, fluorescence and confocal laser microscopic studies confirm the positive influence of galactose-carrying polymers coating on nanoparticles internalization [71] Soppimath et al. synthesized core-shell nanoparticles, self-assembled from the amphiphilic tercopolymer poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide-co-10-undecenoic acid) in which 10-undecenoic acid is employed as hydrophobic and pH-sensitive segment. The temperature responsiveness of the core-shell nanoparticles is triggered by a change in the environmental pH. The shell of these nanoparticles is composed of amine groups able to conjugate biological signals for specific affinities to certain cell types. Such nanoparticles, loaded with doxorubicin, are stable in PBS at 37 °C but precipitate in acidic environment, triggering the release of the enclosed drug molecules [72]. Shu et al. produced crosslinked hollow polyelectrolyte NCs composed of cysteamine conjugated CS and dextran sulfate by adsorption on β-cyclodextrin functionalized silica spheres. These NCs have enhanced physical stability against acidic pH conditions and decrease the loss of protein caused by the gastric cavity and the release of drugs in the intracellular environment after glutathione reduction. Bovine serum albumin (BSA) used as model drug exhibits spherical morphology, dimension of 120 nm, with a good polydispersion index and sustained release without the initial burst [73]. The cited vehicles are summarized in Table 5.

Schematic representation and manufacturing methods of nanospheres –left- and nanocapsules –right.
Bioartificial polymeric nanospheres and nanocapsules.
| Nanovectors | Composition | Encapsulated drug | Reference |
|---|---|---|---|
| Nanospheres | Butyl methacrylate, poly(ethylene glycol) methyl ether methacrylate, 2-(dimethylamino) ethyl methacrylate crosslinked with trimethylolpropane trimethacrylate and functionalized with folic acid | Anti-cancer drugs | [62] |
| Ionically assembled nanoparticles from poly(ionic liquid-co-N-isopropylacrylamide) with deoxycholic acid | Doxorubicin | [63] | |
| Poly(2-(pyridin-2-yldisulfanyl)ethyl acrylate) conjugated with polyethylene glycol and cyclo(Arg-Gly-Asp-d-Phe-Cys) peptide | Doxorubicin | [64] | |
| Glycine functionalized Fe3O4 magnetic nanoparticles | Doxorubicin | [65] | |
| Arginine–glycine–aspartic acid (RGD)-modified Fe3O4 nanoparticles | Doxorubicin | [66] | |
| Poly(d,L-lactide-co-glycolide)-block-poly(ethylene glycol) conjugated with A10 RNA aptamers | Docetaxel and paclitaxel | [67] | |
| Polylactide–polyethylene glycol nanoparticles functionalized with biotin or folic acid | Paclitaxel | [68] | |
| Bioconjugate composed of poly(lactic acid)-block-polyethylene glycol copolymer and aptamers | Rhodamine-labeled dextran | [69] | |
| Polyethyleneimine PEGylated with an Arg-Gly-Asp peptide ligand attached at the distal end of the polyethylene glycol | siRNA | [70] | |
| Poly(L-lactic acid) nanoparticles coated with galactose-carrying polymer | Retinoic acid | [71] | |
| Nanocapsules | Butyl methacrylate, poly(ethylene glycol) methyl ether methacrylate, 2-(dimethylamino) ethyl methacrylate crosslinked with trimethylolpropane trimethacrylate and functionalized with folic acid | Anti-cancer drugs | [62] |
| Poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide- co-10-undecenoic acid) | Doxorubicin | [72] | |
| Cysteamine conjugated chitosan and dextran sulfate | Bovine serum albumin | [73] |
2.2 Nanohydrogels and nanoaggregates
Nanohydrogels (NHYs) are nano-sized networks of polymer chains able to incorporate H2O in their structure. Usually, NHYs are macromolecular hydrocolloids with numerous hydrophilic functional groups [74]. Their composition ranges from linear water-soluble polymers to water insoluble molecules that act as swellable networks stabilized by crosslinking agents. In general, these substances have high molecular weight (5000–10000 Da) and cannot cross-biological membranes. Further, they include cellulosic components like sodium carboxymethyl cellulose or polyanion bioadhesives like polyacrylic acid. These nanovectors are classified on the basis of the presence or absence of electrical charge located on the crosslinked chains. In fact, they are grouped as nonionic, anionic, cationic, amphoteric electrolytes or zwitterions. The surface charge regulates the adhesivity. For example NHYs, due to their capability of forming strong non-covalent bonds with the mucin, have prolonged ocular residence time and reduced dosing frequency [75]. Production of hydrogels requires the simple preparation of polymer solutions in low or intermediate concentrations and the formation of crosslinks for the prevention of the dissolution. Many crosslinking methods are currently available for hydrogel synthesis. Generally, physically crosslinked gels are those whereas physical interactions exist between polymer chains (e.g. hydrogen bonds, amphiphilic graft) while chemically crosslinked hydrogels are synthesized with covalent bonds (e.g. crosslink with aldehydes, free radical polymerization). The nanodimensions are usually obtained with sonication [76]. The medical application of nano-sized hydrogels is limited by the difficult administration of an accurate dose of active principle due to the variable release of gelified systems [75]. Nanoaggregates (NAGs) are colloidal carriers formed from amphiphilic block copolymers. In some cases, further molecules act as crosslinker agents. NAGs possess inherent properties such as high loading efficiency and in vivo stability. These vehicles are able to provide site-specific drug delivery via either a passive or active targeting mechanisms. NAGs are suitable for encapsulation of poorly water-soluble drugs by covalent conjugation as well as physical encapsulation. Active transport is achieved by conjugating a drug with vectors or ligands that bind specific receptors [77]. The synthesis of NHYs and NAGs is summarized in Figure 3. The use of bioartificial materials for the preparation of NHYs and NAGs is a novel research interest. Despite of this, a number of papers are available in literature. For example, CS-poly (acrylamide-co-methacrylic acid) hydrogels were synthesized by Ullah et al. They use different coupling agents (3-dimethylaminopropyl)-3-ethylcarbodimide hydrochloride and 3-aminopropyltriethoxysilane) and the functionalization with phenylboronic acid, a glucose sensing moiety, to design multifunctional NHYs with enhanced glucose sensitivity, stability, drug loading and release profile. Moreover, the authors study the glucose-induced volume phase transition and release profile at physiological conditions of the model drug Alizarin Red (a compound with 1,2-diol structure, similar to insulin) in order to find potential application in self-regulated insulin delivery with enhanced sensitivity toward glucose [78]. Jaiswal et al. synthesized poly N-isopropylacrylamide – CS-based NHYs encapsulating iron oxide (Fe3O4) magnetic nanoparticles through free radical polymerization of the acrylate in presence of CS. These NHSs are spherical shaped with size ranging from 50 nm to 200 nm on the base of the feed ratios of CS. The encapsulation of Fe3O4 nanoparticles into poly N-isopropylacrylamide–CS based NHYs is confirmed by transmission electron microscopy. This system shows optimal magnetization, good specific absorption rate and excellent cytocompatibility, finding potential applications in hyperthermia treatment of cancer and targeted drug delivery [79]. Yoon et al. produced self-assembled NAGs co-encapsulating doxorubicin and oligonucleotides through the conjugation of four-arm poly(ethylene glycol) with doxorubicin and anti-bcl-2 oligonucleotides. These conjugates are hydrophobically self-assembled into NAGs in aqueous solutions. Elemental scanning of the products reveals a core-shell structure with the drug located at the core of the vectors and the genetic materials at the shell. Analysis by dynamic light scattering and electron microscopy proves the complete disappearance of the particles under reducing conditions and the liberation of oligonucleotides at low pH. In vitro studies confirm the uptake of drug and oligonucleotides in cells treated with NAGs [80]. Table 6recaps the described nanovectors (Table 6).

Schematic representation and manufacturing methods of nanoaggregates –top- and nanohydrogels –bottom.
Bioartificial polymeric nanohydrogels and nanoaggregates.
| Nanovectors | Composition | Encapsulated drug | Reference |
|---|---|---|---|
| Nanohydrogels | Chitosan-poly (acrylamide-co-methacrylic acid) | Alizarin red Insulin | [78] |
| Poly N-isopropylacrylamide-chitosan-based nanohydrogels | Anticancer drugs | [79] | |
| Nanoaggregates | Four-arm poly(ethylene glycol),doxorubicin, anti-bcl-2 oligonucleotides | Doxorubicin Anti-bcl-2 oligonucleotides | [80] |
2.3 Micelles (MCs) and solid lipid nanoparticles (SLNs)
Micelles (MCs) are colloidal dispersions belonging to a large family of systems consisting of particulate matter (the “dispersed phase”), distributed within a continuous phase (the “dispersion medium”), usually constituted by water. MCs form spontaneously at certain concentration (critical micelle concentration) and temperature (critical micelle temperature) values from amphiphilic or surface active agents. Usually, these vehicles have particle size ranging between 5 and 100 nm [81, 82]. Regarding the structure, the hydrophobic fragments of amphiphilic molecules form the core of MCs, while hydrophilic fragments the shells. The formation of MCs is driven by the decrease of free energy in the system because of the removal of hydrophobic fragments from the continuous phase, and the re-establishing of hydrogen bond network in water. Moreover, additional energy results from the formation of van der Waals bonds between hydrophobic blocks in the core of the formed MCs [83]. MCs possess high stability both in vitro and in vivo, good biocompatibility and are able to solubilize a broad variety of poorly soluble pharmaceuticals through the interaction of the lipophilic substances with their hydrophobic core [84]. Many of these drug-loaded vehicles are currently at different stages of preclinical and clinical trials [85]. Micellar nano-drug delivery systems have increased water solubility, improved bioavailability, reduction of toxicity, enhanced permeability across the physiological barriers, substantial changes in drug biodistribution, extended blood half-life and protection from degradation [86]. Moreover, MCs have spontaneous interstitial penetration into the body compartments with leaky vasculature (tumors and infarcts) [87]. Active targeting of MCs is obtained through surface chemical attachment of driving molecules [88]. MCs are prepared simply dissolving the amphiphiles in water. These vectors are thermodynamically stabilized against disassembly if the amphiphilic concentration remains above the CMC. While, upon dilution below the CMC, MCs disassemble with a rate depending on the structure of the amphiphiles and on the interactions between the chains [89]. The encapsulation of molecules is obtained dissolving the substance into the micellar solution [89]. A further method of MCs preparation, useful to encapsulate non-water soluble molecules, is the thin-film hydration method. The amphiphilic copolymer and the lipophilic drug are dissolved in organic solvent. Then, the apolar eluent is removed to get the drug-containing lipid membrane. This film is resuspended in a polar solvent for nano-MCs self-assembly. The driving force of this process is the hydrophobic effect between the non-polar segments of the polymers. The hydrophobic effect also plays an important role in the drug encapsulation, stabilizing the intermolecular interaction between the substance and the hydrophobic segment [90]. SLNs are colloidal carrier systems, generally spherical in shape, composed of a high melting point lipids, as a solid core, coated by aqueous surfactants. The core lipids are fatty acids, acylglycerols and waxes, whereas phospholipids, sphingomyelins, bile salts and sterols are utilized as stabilizers [91]. SLNs are useful for the delivery of poor water soluble drugs [92]. The particle diameters are in the range of 10–1000 nm. SLNs are characterized by high biocompatibility, high bioavailability, physical stability, protection of incorporated labile drugs from degradation, excellent tolerability, prevention of problems related with multiple routs of administration, avoidance of the use of organic solvents during the preparation, formation of films over the skin showing occlusive properties and absence of problems concerning large-scale production and sterilization [93, 94]. However, common disadvantages of SLNs are particle growth, unpredictable gelation tendency, uncertain diffusion of the drug within the lipid matrix of the vector, unexpected dynamics of polymorphic transitions and inherent low incorporation rate due to the crystalline structure of the solid lipid [95, 96]. SLNs are prepared through homogenization, solvent-evaporation, microemulsion and film-ultrasound dispersion techniques. In the homogenization method, the homogenizers push a liquid with high pressure (100–2000 bar) through a narrow gap. The high shear stress and cavitation forces disrupt the particles down to the submicron range. This technique is carried in hot or cold conditions. Hot homogenization requires temperatures above the melting point of the lipid and necessitates the preparation of a pre-emulsion of the drug loaded lipid melt with the aqueous emulsifier. Higher temperatures result in lower particle sizes. In cold conditions, the drug containing lipid melt is cooled and dispersed into a cold surfactant solution. This pre-suspension is homogenized at or below room temperature, breaking the lipids in solid nanoparticles. In the solvent-evaporation method, the lipids are dissolved in a water-immiscible organic solvent that is emulsified in an aqueous solvent. The nanoparticles dispersion is obtained upon evaporation of the eluent that leads to lipid precipitation. The microemulsion technique is operated stirring a mixture of low melting fatty acids, emulsifiers and water, at 65–70 °C. This hot liquid is dispersed in cold water (2–3 °C) under stirring. The high-temperature gradient facilitates rapid lipid crystallization and prevent aggregation. Finally, in the film-ultrasound dispersion method, the lipid and the drug are put into suitable organic solution that is evaporated to form a lipid film. The following addition of an aqueous solvent results in an emulsion that is sonicated giving SLNs with uniform particle size [97]. A recap of the production methods for both MCs and SLNs is available in Figure 4. Bioartificial polymers are widely used in the preparation of both these vesicular systems. For example, Zhang et al. used the core crosslinking method to generate MCs able to increase curcumin delivery to HeLa cells (immortalized cancer cells) in vitro and improve tumor accumulation in vivo. These MCs are designed with folic acid-PEG as the hydrophilic unit, pyridyldisulfide as the crosslinkable and hydrophobic unit, and disulfide bond as the crosslinker. Such nanovectors show spherical shape with a diameter of 91.2 nm and high encapsulation efficiency. Cytotoxicity effectiveness is demonstrated by the high cellular uptake and the positive in vitro antitumor studies. The linkage with folate targets the curcumin against cancer cells and enhances the in vivo efficacy of these MCs [98]. Similarly, Lee et al. produced pH-sensitive polymeric MCs composed of poly(l-histidine), PEG and poly(L-lactic acid) block copolymers with folate conjugation, delivering adriamycin. These MCs are investigated for pH-dependent drug release, folate receptor-mediated internalization and cytotoxicity using MCF-7 cells (human breast adenocarcinoma cell line) in vitro. These nanovectors show accelerated drug release only at acidic pH. Moreover, the conjugation with folic acid enhances tumor cell kill due to folate receptor-mediated tumor uptake [99]. Li et al. reported linear PEG and dendritic cholic acids block copolymers MCs stabilized with boronate esters at the core–shell interface for efficient anticancer drug delivery. Such system is loaded with paclitaxel to assess its capacity to retain the encapsulated drug under physiological conditions and release the payload when triggered by the lower pH value of the tumor environment or by the presence of competitive diols (e.g. mannitol). This nanovector shows minimal premature drug release at physiological glucose level and physiological pH values in blood circulation and simple activation at the acidic tumor microenvironment or by the additional intravenous administration of mannitol as an on-demand triggering agent [100]. Paclitaxel-loaded mixed polymeric MCs consisting of poly(ethylene glycol) distearoyl phosphoethanolamine conjugates, solid triglycerides and cationic lipofectin lipids were prepared by Wang et al. Optimized MCs have average size of about 100 nm, and zeta-potential of about −6 mV. Such vehicles are stable when stored at 4°C or at room temperature. Release of paclitaxel starts at 37 °C and, approximately, 16 % of the drug is dispensed in 72 h. In vitro anticancer effects of the nanovectors are evaluated using human mammary adenocarcinoma (BT-20) and human ovarian carcinoma (A2780) cell lines. The results show enhanced anti-cancer activity due to the ability of the MCs to escape from endosomes and enter the cytoplasm of BT-20 and A2780 cancer cells [101]. Lee et al. developed paclitaxel-loaded sterically stabilized SLNs for parenteral administration. These nanovectors, prepared using the hot homogenization method, are composed of trimyristin as a solid lipid core and egg phosphatidylcholine and pegylated phospholipid as stabilizers. The particles are spherical in shape, with sizes and zeta potentials of around 200 nm and −38 mV. Paclitaxel is loaded to the solid cores at a w/w ratio of 6 % with high encapsulation efficiency. In vitro drug release studies show a slow sustained release and high cytotoxicity on OVCAR-3 human ovarian cancer cell line and MCF-7 breast cancer cell line [102]. Gao et al. prepared poly(ethylene glycol)/phosphatidyl ethanolamine (PEG−PE) conjugates for the solubilization and delivery of various poorly soluble anticancer drugs such as m-porphyrin, tamoxifen and taxol. These MCs are stable and have the size of 10 to 40 nm [103]. Wong et al. investigated the in vivo efficacy, unwanted toxicity and loco-regional distribution of doxorubicin-loaded polymer-lipid hybrid nanoparticles formulation in a murine solid tumor model after intratumoral injection. These SLNs are prepared by dispersing the drug in stearic acid and tristearin, with subsequent addition of the hydrolyzed polymers of epoxidized soybean oil to enhance doxorubicin incorporation into the lipids. This formulation is injected intratumorally in murine solid tumors of approximately 0.3 g. In vivo, SLNs-treated tumors develop substantially larger central necrotic regions compared to the untreated tumors, with minimal systemic toxicity [104]. Kukowska-Latallo et al. produced polyamidoamine dendritic polymers conjugated to folic acid as targeting agent for methotrexate. These conjugates are injected intravenous into immunodeficient mice bearing human KB tumors overexpressing the folic acid receptors, resulting in high internalization into the tumor cells [105]. Table 7 acts as a recap of the described nanosystems.

Schematic representation and manufacturing methods of micelles –left- and solid lipid nanoparticles –right.
Bioartificial polymeric micelles and solid lipid nanoparticles.
| Nanovectors | Composition | Encapsulated drug | Reference |
|---|---|---|---|
| Micelles | Folic acid – polyethylene glycol and pyridyldisulfide | Curcumin | [91] |
| Poly(l-histidine), polyethylene glycol and poly(L-lactic acid) block copolymers with folate conjugation | Adriamycin | [92] | |
| Linear polyethylene glycol and dendritic cholic acids block copolymers stabilized with boronate esters | Paclitaxel | [93] | |
| Poly(ethylene glycol)-distearoyl phosphoethanolamine conjugates, solid triglycerides and cationic lipofectin lipids | Paclitaxel | [94] | |
| Poly(ethylene glycol)/phosphatidyl ethanolamine | m-porphyrin, tamoxifen and taxol | [95] | |
| Solid lipid nanoparticles | Trimyristin, egg phosphatidylcholine and pegylated phospholipid | Paclitaxel | [96] |
| Stearic acid, tristearin, hydrolyzed polymer of epoxidized soybean oil | Doxorubicin | [97] | |
| Polyamidoamine dendritic polymers conjugated with folic acid | Methotrexate | [98] |
2.4 Nanofibers
Nanofibers (NFs) are fibers with diameters less than 100 nanometers that exhibit special properties due to the extremely high surface to weight ratio, low density, high pore volume and tight pore size [106]. These properties make NFs suitable for applications ranging from medical (e.g. drug delivery systems) to industrial and high-tech fields (e.g. aerospace, capacitors, transistors, battery separators, energy storage, fuel cells) [106]. In nanomedicine, NFs are widely used due to their similarity to the extracellular matrix (ECM), the possibility to use several materials for their synthesis (in fact, natural and synthetic polymers along with several solvent systems are effectively used to create NFs) and the possibility to change their architecture in regard to porosity, diameter, mechanical properties, structure arrangement and structure functionalization [107]. The synthetic techniques for NFs are self-assembly, phase separation and electrospinning. All of these techniques require the preparation of a homogeneous drug–polymer solution that is loaded in the electrospinning machine (electrospinning technique), is simply mixed (self-assembly method) or is treated to obtain gelation and freeze-drying (phase separation) (Figure 5) [106, 107]. Research about NFs as drug delivery systems is at the early stage of exploration and most of the works focus on the sustained release profiles of model drugs (e.g. small molecules, herbs, proteins, DNA, genes and vaccines) using biodegradable hydrophilic, hydrophobic or amphiphilic polymers and, recently, BPMs [108]. Zhang et al. reported degradable heparin-poly (ε-caprolactone) fiber mats fabricated by electrospinning. The highly sulfated heparin heteropolymer remains homogenous in the spinning solution and is distributed throughout the fabricated polymers. The NFs release heparin for 14 days with a diffusionally controlled kinetics over this period. The drug retains its biological properties and functionality [109]. Chew et al. investigated the encapsulation of human β-nerve growth factor (NGF), stabilized in BSA carrier proteins, in a copolymer of ε-caprolactone and ethyl ethylene phosphate. The proteins are randomly dispersed throughout the electrospun fibrous mesh in an aggregated form. The sustained release of NGF by diffusion is detectable for at least 3 months and the bioactivity of the drug is retained throughout this period [110]. Feng et al. produced CS polyethylene oxide NFs with uniform diameter of 112 nm and the potential modulation of CS viscosity and surface tension through the use of different CS molecular weight and polyethylene oxide quantities. These vehicles exhibit excellent biocompatibility with hepatocytes [111]. Similarly, Bhattarai et al. reported that CS\polyethylene oxide nanofibrous scaffolds promote the attachment of human osteoblast and chondrocytes, maintaining their characteristic morphology and viability. This matrix is of particular interest for tissue engineering, drug delivery and tissue remodeling [112]. Moreover, Subramanyan et al. prepared CS\polyethylene oxide NFs for cartilage tissue engineering. These scaffolds are used for cell attach and deliver of growth factors [113]. Park et al. produced chitin/poly glycolic acid NFs with BSA coating to improve human epidermal fibroblasts attach and spread [114]. Shalumon et al. developed bioactive and biocompatible NFs composed of carboxymethyl cellulose\polyvinyl alcohol blend. Such nanomaterials are tested for cytotoxicity and cell attachment, resulting in a safe application for tissue engineering and drug delivery [115]. Nanofibrous scaffold of CS\polyvinyl alcohol and carboxyethylchitosan\polyvinyl alcohol are also prepared by Zhou et al. These materials, tested on L929 fibroblast culture, have good cell attachment and growth [116]. CS hydroxyapatite nanofibrous scaffolds are reported by Yang et al. This nanomaterial significantly stimulates the bone forming ability due to the excellent osteoconductivity of hydroxyapatite [117]. Finally, Junkasem et al. described the fabrication of α-chitin whisker-reinforced poly(vinyl alcohol) NFs by electrospinning. The α-chitin whiskers are prepared from α-chitin flakes by acid hydrolysis. Such vectors exhibit average length and width of about 549 and 31 nm, respectively. The incorporation of the chitin whiskers within the poly(vinyl alcohol) is verified by infrared spectroscopy and thermogravimetry, resulting in an increased Young’s modulus of the bioartificial polymer of about 4–8 times compared to the unmodified poly(vinyl alcohol) [118]. Table 8summarizes the described bioartificial polymeric NFs (Table 8).
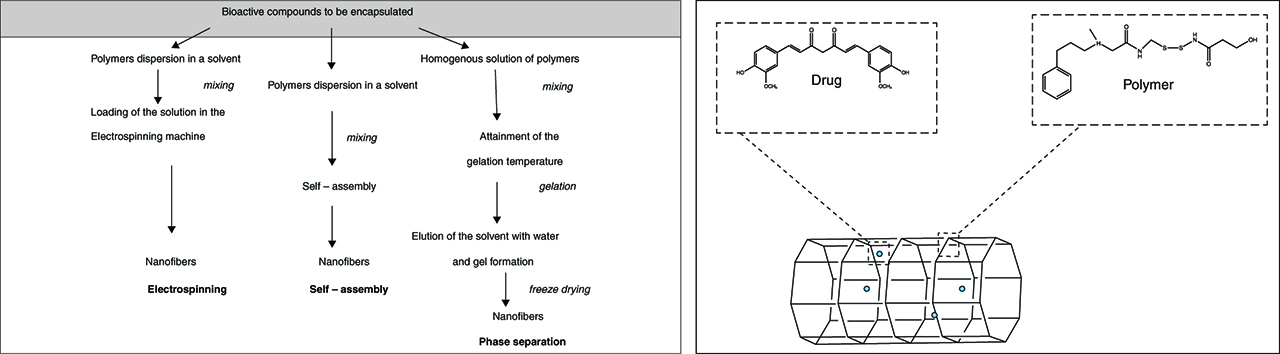
Schematic representation and manufacturing methods of nanofibers.
Bioartificial polymeric nanofibers.
| Nanovectors | Composition | Encapsulated drug | Reference |
|---|---|---|---|
| Nanofibers | Heparin-poly (ε-caprolactone) fiber mats | Heparin | [102] |
| Copolymer of bovine serum albumin (BSA) ε-caprolactone and ethyl ethylene phosphate | β-nerve growth factor | [103] | |
| Chitosan polyethylene oxide | Generic drug | [104] |
3 Conclusion
This chapter analyzes the advantages of the use of bioartificial polymers as carriers and the main strategies used for their design. Despite the enormous progresses in this field, more studies are required for the fully evaluation of these nanovectors in complex organisms and for the characterization of the pharmacodynamic and pharmacokinetic of the loaded drugs. Moreover, progresses in polymer chemistry are introducing a wide range of functionalities in the BPM nanostructures leading to a second generation of bioartificial polymer therapeutics based on novel and heterogeneous architectures with higher molecular weight and predictable structures, in order to achieve greater multivalency and increased loading capacity. Therefore, research on bioartificial polymeric nanovectors is an “on-going” field capable of attracting medical interest.
Acknowledgments
This work was supported by Progetto PON—“Ricerca e Competitività 2007–2013”—PON01_01802: “Sviluppo di molecole capaci di modulare vie metaboliche intracellulari redox-sensibili per la prevenzione e la cura di patologie infettive, tumorali, neurodegenerative e loro delivery mediante piattaforme nano tecnologiche”, PON01_02512: “Ricerca e sviluppo di bioregolatori attivi sui meccanismi epigenetici dei processi infiammatori nelle malattie croniche e degenerative”, PON03_00106: “Materiali Avanzati per la Ricerca ed il comparto Agroalimentare, Laboratorio Pubblico-Privato, MAReA” and PRIN 2012 (prot. 201288JKYY): “Nanotecnologie per variare i programmi di sviluppo osseo nella parete vasale per la prevenzione e trattamento delle patologie associate alla calcificazione ectopica arteriosa”.
This article is also available in: Tylkowski, Polymer Engineering. De Gruyter (2017), isbn 978–3–11–046828–1.
References
1. Lazzeri L. Trends in polymer science. Cambridge, UK: Elsevier Trends Journals, 1996.Suche in Google Scholar
2. Barbani N, Lazzeri L, Lelli L, et al. Physical and biological stability of dehydro-thermally crosslinked collagen—Poly(vinyl alcohol) blends. J Mater Science Mater Med. 1994;5:882–886.10.1007/BF01172030Suche in Google Scholar
3. Cascone MG. Dynamic–mechanical properties of bioartificial polymeric materials. Polym Int. 1997;43:55–69.10.1002/(SICI)1097-0126(199705)43:1<55::AID-PI762>3.0.CO;2-#Suche in Google Scholar
4. Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909.10.1016/j.biomaterials.2009.07.027Suche in Google Scholar
5. Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75.10.1146/annurev.bioeng.6.040803.140027Suche in Google Scholar
6. Scotchford CA, Cascone MG, Downes S, Giusti P. Osteoblast responses to collagen-PVA bioartificial polymers in vitro: the effects of cross-linking method and collagen content. Biomaterials. 1998;19:1–11.10.1016/S0142-9612(97)00236-6Suche in Google Scholar
7. Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492.10.1038/nature02388Suche in Google Scholar
8. Joyce Y, Wong JD. Biomaterials. Boca Raton, FL: CRC Press, 2007.Suche in Google Scholar
9. Long Y. Biodegradable polymer blends and composites from renewable resources. New Jersey, USA: John Wiley & Sons, 2009.Suche in Google Scholar
10. Sionkowska A. Natural polymers as components of blends for biomedical applications. In: Severian Dumitriu VP, editors. Polymeric biomaterials: structure and function Vol. 1. Boca Raton, FL: CRC Press, 2013.Suche in Google Scholar
11. Parthasarathy M, Sethuraman S. Hierarchical characterization of biomedical polymers. In: Laurencin CT, Deng M, editors. Natural and synthetic biomedical polymers. Oxford, UK: Elsevier, 2014:33–42.10.1016/B978-0-12-396983-5.00002-8Suche in Google Scholar
12. Martina M, Hutmacher DW. Biodegradable polymers applied in tissue engineering research: a review. Polym Int. 2007;56:145–157.10.1002/pi.2108Suche in Google Scholar
13. Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762–798.10.1016/j.progpolymsci.2007.05.017Suche in Google Scholar
14. Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polymer Sci B Polymer Phys. 2011;49:832–864.10.1002/polb.22259Suche in Google Scholar
15. Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–1151.10.1039/b811392kSuche in Google Scholar
16. Bostrom A, Löfstedt RE. Nanotechnology risk communication past and prologue. Risk Anal Off Publ Soc Risk Anal. 2010;30:1645–1662.10.1111/j.1539-6924.2010.01521.xSuche in Google Scholar
17. Koodali RT, Klabunde KJ. Nanotechnology: fundamental principles and applications. In: Kent AJ, editors. Handbook of industrial chemistry and biotechnology. Boston, MA: Springer US, 2012:249–263.10.1007/978-1-4614-4259-2_8Suche in Google Scholar
18. Drexler KE. Engines of creation. London, UK: Fourth Estate, 1996.Suche in Google Scholar
19. Huang C, Notten A, Rasters N. Nanoscience and technology publications and patents: a review of social science studies and search strategies. J Technol Transfer. 2010;36:145–172.10.1007/s10961-009-9149-8Suche in Google Scholar
20. Jain KK. Drug delivery systems – an overview. In: Jain KK, editor. Drug delivery systems. Totowa, NJ: Humana Press, 2008:1–50.10.1007/978-1-59745-210-6Suche in Google Scholar
21. Davies AG, Thompson JM. Advances in nanoengineering: electronics, materials and assembly. London, UK: Imperial College Press, 2007.10.1142/p484Suche in Google Scholar
22. Mansur HS. Quantum dots and nanocomposites. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:113–129.10.1002/wnan.78Suche in Google Scholar
23. Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc Natl Acad Sci. 2003;100:10603–10606.10.1073/pnas.1534701100Suche in Google Scholar
24. Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Controlled Release Off J Controlled Release Soc. 2001;70:1–20.10.1016/S0168-3659(00)00339-4Suche in Google Scholar
25. Takeuchi H, Yamamoto H, Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv Drug Deliv Rev. 2001;47:39–54.10.1016/S0169-409X(00)00120-4Suche in Google Scholar
26. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347.10.1016/S0169-409X(02)00228-4Suche in Google Scholar
27. Cao X, Lai S, Lee LJ. Design of a self-regulated drug delivery device. Biomed Microdevices. 2001;3:109–117.10.1023/A:1011494008729Suche in Google Scholar
28. Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14:239–247.10.1038/nrd4503Suche in Google Scholar PubMed PubMed Central
29. Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17:2950–2962.10.1016/j.bmc.2009.02.043Suche in Google Scholar PubMed
30. Doshi N, Mitragotri S. Designer biomaterials for nanomedicine. Adv Funct Mater. 2009;19:3843–3854.10.1002/adfm.200901538Suche in Google Scholar
31. Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010.10.1039/c2cs15344kSuche in Google Scholar PubMed PubMed Central
32. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf, B. 2010;75:1–18.10.1016/j.colsurfb.2009.09.001Suche in Google Scholar PubMed
33. Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934.10.1073/pnas.0600997103Suche in Google Scholar PubMed PubMed Central
34. Sahoo B, Devi KS, Banerjee R, Maiti TK, Pramanik P, Dhara D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl Mater Interfaces. 2013;5:3884–3893.10.1021/am400572bSuche in Google Scholar PubMed
35. Nagavarma BV, Yadav HK, Ayaz A, Vasudha LS, Shivakumar HG. Different techniques for preparation of polymeric nanoparticles- A review. Asian J Pharm Clin Res. 2012;5:16–23.Suche in Google Scholar
36. Dutta RK, Sharma PK, Kobayashi H, Pandey AC. Functionalized biocompatible nanoparticles for site-specific imaging and therapeutics. In: Kunugi S, Yamaoka T, editors. Polymers in nanomedicine. Berlin, Germany: Springer Berlin Heidelberg, 2012:233–275.Suche in Google Scholar
37. Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16.10.1146/annurev-bioeng-071811-150124Suche in Google Scholar PubMed
38. Li YP, Pei YY, Zhang XY, Gu Z, Zhou Z, Yuan W, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Controlled Release. 2001;71:203–211.10.1016/S0168-3659(01)00218-8Suche in Google Scholar
39. Amit Singh GG, Sharma PK. Nanospheres: a novel approach for targeted drug delivery system. Int J Pharm Sci Rev Res. 2010;5:84–88.Suche in Google Scholar
40. Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J Controlled Release Off J Controlled Release Soc. 2008;128:185–199.10.1016/j.jconrel.2008.02.007Suche in Google Scholar
41. Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48.10.1016/0169-409X(95)00039-ASuche in Google Scholar
42. Scherrmann JM, Temsamani J. The use of pep: tran’s vectors for the delivery of drugs into the central nervous system. Int Congress Ser. 2005;1277:199–211.10.1016/j.ics.2005.02.023Suche in Google Scholar
43. Sarti S, Bordi F. Polymeric hollow micro and nanospheres for biotechnological applications: a focused review. Mater Lett. 2013;109:134–139.10.1016/j.matlet.2013.07.003Suche in Google Scholar
44. Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24.10.1016/S0169-409X(97)00048-3Suche in Google Scholar
45. Yow HN, Routh AF. Formation of liquid core–polymer shell microcapsules. Soft Matter. 2006;2:940–949.10.1039/B606965GSuche in Google Scholar
46. Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed Nanotechnol Biol Med. 2006;2:8–21.10.1016/j.nano.2005.12.003Suche in Google Scholar PubMed
47. Galindo-Rodriguez S, Allemann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21:1428–1439.10.1023/B:PHAM.0000036917.75634.beSuche in Google Scholar
48. Prabhakar VT, Yadav A, Ratan R. Magic magic bullets - nanocapsules in future medicine. Int J Pharma Sci. 2013;3:303–308.Suche in Google Scholar
49. Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147–157.10.1177/117739280700200002Suche in Google Scholar
50. Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318.Suche in Google Scholar
51. Khoee S, Yaghoobian M. An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. Eur J Med Chem. 2009;44:2392–2399.10.1016/j.ejmech.2008.09.045Suche in Google Scholar
52. Radtchenko I, Sukhorukov G, Mohwald H. A novel method for encapsulation of poorly water-soluble drugs: precipitation in polyelectrolyte multilayer shells. Int J Pharm. 2002;242:219–223.10.1016/S0378-5173(02)00161-8Suche in Google Scholar
53. Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–142.10.1016/j.ijpharm.2009.10.018Suche in Google Scholar
54. Whelan J. Nanocapsules for controlled drug delivery. Drug Discovery Today. 2001;6:1183–1184.10.1016/S1359-6446(01)02055-4Suche in Google Scholar
55. Barratt GM. Therapeutic applications of colloidal drug carriers. Pharm Sci Technolo Today. 2000;3:163–171.10.1016/S1461-5347(00)00255-8Suche in Google Scholar
56. Karode SK, Kulkarni SS, Suresh AK, Mashelkar RA. New insights into kinetics and thermodynamics of interfacial polymerization. Chem Eng Sci. 1998;53:2649–2663.10.1016/S0009-2509(98)00083-9Suche in Google Scholar
57. Puglisi G, Fresta M, Giammona G, Ventura CA. Influence of the preparation conditions on poly(ethylcyanoacrylate) nanocapsule formation. Int J Pharm. 1995;125:283–287.10.1016/0378-5173(95)00142-6Suche in Google Scholar
58. Dustgani A, Farahani EV, Imani M. Preparation of chitosan nanoparticles loaded by dexamethasone sodium phosphate. Iran J Pharm Sci. 2008;4:111–114.Suche in Google Scholar
59. Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36:887–913.10.1016/j.progpolymsci.2011.01.001Suche in Google Scholar
60. Quintanar-Guerrero D, Allémann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm. 1998;24:1113–1128.10.3109/03639049809108571Suche in Google Scholar
61. Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliv Rev. 2003;55:519–548.10.1016/S0169-409X(03)00041-3Suche in Google Scholar
62. Bellotti E, Barbani N, Cascone MG, Cristallini C. Development and characterization of new intelligent nanoparticles for drug targeting. In: R. Alessandro, editor Biocompatible materials for medicine. Mantova, Italy: Universitas Studiorum, 2014:187–190.Suche in Google Scholar
63. Cui W, Lu X, Cui K, Niu L, Wei Y, Lu Q. Dual-responsive controlled drug delivery based on ionically assembled nanoparticles. Langmuir ACS J Surf Colloids. 2012;28:9413–9420.10.1021/la3016436Suche in Google Scholar
64. Remant Bahadur KC, Thapa B, Xu P. pH and redox dual responsive nanoparticle for nuclear targeted drug delivery. Mol Pharm. 2012;9:2719–2729.10.1021/mp300274gSuche in Google Scholar
65. Barick KC, Singh S, Jadhav NV, Bahadur D, Pandey BN, Hassan PA. pH-responsive peptide mimic shell cross-linked magnetic nanocarriers for combination therapy. Adv Funct Mater. 2012;22:4975–4984.10.1002/adfm.201201140Suche in Google Scholar
66. Zhao Z, Huang D, Yin Z, Chi X, Wang X, Gao J. Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: magnetic control and pH-responsive release. J Mater Chem. 2012;22:15717–15725.10.1039/c2jm31692gSuche in Google Scholar
67. Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, et al. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–876.10.1016/j.biomaterials.2006.09.047Suche in Google Scholar
68. Patil YB, Toti US, Khdair A, Ma L, Panyam J. Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials. 2009;30:859–866.10.1016/j.biomaterials.2008.09.056Suche in Google Scholar
69. Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64:7668–7672.10.1158/0008-5472.CAN-04-2550Suche in Google Scholar
70. Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149.10.1093/nar/gnh140Suche in Google Scholar
71. Cho CS, Cho KY, Park IK, Kim SH, Sasagawa T, Uchiyama M, et al. Receptor-mediated delivery of all trans-retinoic acid to hepatocyte using poly(L-lactic acid) nanoparticles coated with galactose-carrying polystyrene. J Controlled Release Off J Controlled Release Soc. 2001;77:7–15.10.1016/S0168-3659(01)00390-XSuche in Google Scholar
72. Soppimath KS, Tan DC, Yang YY. pH-triggered thermally responsive polymer core–shell nanoparticles for drug delivery. Adv Mater. 2005;17:318–323.10.1002/adma.200401057Suche in Google Scholar
73. Shu S, Zhang X, Wu Z, Wang Z, Li C. Gradient cross-linked biodegradable polyelectrolyte nanocapsules for intracellular protein drug delivery. Biomaterials. 2010;31:6039–6049.10.1016/j.biomaterials.2010.04.016Suche in Google Scholar
74. Robinson JR, Mlynek GM. Bioadhesive and phase-change polymers for ocular drug delivery. Adv Drug Deliv Rev. 1995;16:45–50.10.1016/0169-409X(95)00013-WSuche in Google Scholar
75. Gurtler F, Gurny R. Patent literature review of ophthalmic inserts. Drug Dev Ind Pharm. 1995;21:1–18.10.3109/03639049509048094Suche in Google Scholar
76. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–121.10.1016/j.jare.2013.07.006Suche in Google Scholar
77. Sharma VK, Jain A, Soni V. Nano-aggregates: emerging delivery tools for tumor therapy. Crit Rev Ther Drug Carrier Syst. 2013;30:535–563.10.1615/CritRevTherDrugCarrierSyst.2013007706Suche in Google Scholar
78. Ullah F, Othman MB, Javed F, Ahmad Z, Akil HM, Rasib SZ. Functional properties of chitosan built nanohydrogel with enhanced glucose-sensitivity. Int J Biol Macromol. 2016;83:376–384.10.1016/j.ijbiomac.2015.11.040Suche in Google Scholar
79. Jaiswal MK, Banerjee R, Pradhan P, Bahadur D. Thermal behavior of magnetically modalized poly(N-isopropylacrylamide)-chitosan based nanohydrogel. Colloids Surf, B. 2010;81:185–194.10.1016/j.colsurfb.2010.07.009Suche in Google Scholar
80. Yoon S, Kim WJ, Yoo HS. Dual-responsive breakdown of nanostructures with high doxorubicin payload for apoptotic anticancer therapy. Small (Weinheim an Der Bergstrasse, Germany). 2013;9:284–293.10.1002/smll.201200997Suche in Google Scholar
81. Tanford C. The hydrophobic effect: formation of micelles and biological membranes, 2nd ed Somerset, NJ: John Wiley & Sons, 1980.Suche in Google Scholar
82. Ruckenstein E, Nagarajan R. Critical micelle concentration. Transition point for micellar size distribution. J Phys Chem. 1975;79:2622–2626.10.1021/j100591a010Suche in Google Scholar
83. Jones M, Leroux J. Polymeric micelles – a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48:101–111.10.1016/S0939-6411(99)00039-9Suche in Google Scholar
84. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16.10.1007/s11095-006-9132-0Suche in Google Scholar
85. Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Controlled Release Off J Controlled Release Soc. 2014;190:465–476.10.1016/j.jconrel.2014.06.042Suche in Google Scholar
86. Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Controlled Release Off J Controlled Release Soc. 2001;73:137–172.10.1016/S0168-3659(01)00299-1Suche in Google Scholar
87. Gabizon AA. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Adv Drug Deliv Rev. 1995;16:285–294.10.1016/0169-409X(95)00030-BSuche in Google Scholar
88. Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci CMLS. 2004;61:2549–2559.10.1007/s00018-004-4153-5Suche in Google Scholar
89. Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf, B. 1999;16:3–27.10.1016/S0927-7765(99)00058-2Suche in Google Scholar
90. Ai X, Zhong L, Niu H, He Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J Pharm Sci. 2014;9:244–250.10.1016/j.ajps.2014.06.006Suche in Google Scholar
91. Wissing SA, Muller RH. A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles. Int J Cosmet Sci. 2001;23:233–243.10.1046/j.1467-2494.2001.00087.xSuche in Google Scholar
92. Schwarz C, Mehnert W, Lucks JS, Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Controlled Release. 1994;30:83–96.10.1016/0168-3659(94)90047-7Suche in Google Scholar
93. Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharmaceutics Biopharm. 2000;50:161–177.10.1016/S0939-6411(00)00087-4Suche in Google Scholar
94. Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54 Suppl 1 S131–S155.10.1016/S0169-409X(02)00118-7Suche in Google Scholar
95. Attama AA, Muller-Goymann CC. Effect of beeswax modification on the lipid matrix and solid lipid nanoparticle crystallinity. Colloids and Surf, A. 2008;315:189–195.10.1016/j.colsurfa.2007.07.035Suche in Google Scholar
96. Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discov Technol. 2012;9:2–16.10.2174/157016312799304552Suche in Google Scholar
97. Ekambaram P, Hasan Sathali AA, Priyanka K. Solid lipid nanoparticles: a review. Scientific Rev Chem Communications. 2012;2:80–102.Suche in Google Scholar
98. Zhang Y, Zhou J, Yang C, Weiwei W, Liping C, Fan H, et al. Folic acid-targeted disulfide-based cross-linking micelle for enhanced drug encapsulation stability and site-specific drug delivery against tumors. Int J Nanomed. 2016;11:1119–1130.10.2147/IJN.S347786Suche in Google Scholar
99. Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Controlled Release. 2003;91:103–113.10.1016/S0168-3659(03)00239-6Suche in Google Scholar
100. Li Y, Xiao W, Xiao K, Berti L, Juntao L, Tseng HP, et al. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic ph values and cis-diols. Angew Chem Int Ed. 2012;51:2864–2869.10.1002/anie.201107144Suche in Google Scholar PubMed PubMed Central
101. Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol) -lipid conjugate and positively charged lipids. J Drug Target. 2005;13:73–80.10.1080/10611860400011935Suche in Google Scholar PubMed PubMed Central
102. Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials. 2007;28:2137–2146.10.1016/j.biomaterials.2007.01.014Suche in Google Scholar PubMed
103. Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982.10.1021/nl025604aSuche in Google Scholar
104. Wong HL, Rauth AM, Bendayan R, Wu XY. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik Ev. 2007;65:300–308.10.1016/j.ejpb.2006.10.022Suche in Google Scholar PubMed
105. Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–5324.10.1158/0008-5472.CAN-04-3921Suche in Google Scholar
106. Pisignano D. Structural and surface properties of polymer nanofibers and their applications. In: Dario Pisignano, editor. Polymer nanofibers: building blocks for nanotechnology. Cambridge, UK: The Royal Society of Chemistry, 2013:189–235.Suche in Google Scholar
107. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211.10.1089/ten.2006.12.1197Suche in Google Scholar
108. Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–2253.10.1016/S0266-3538(03)00178-7Suche in Google Scholar
109. Yu DG, Zhu LM, White K, Branford-White C. Electrospun nanofiber-based drug delivery systems. Health. 2009;01:67–75.10.4236/health.2009.12012Suche in Google Scholar
110. Chew SY, Wen J, Yim EK, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–2024.10.1021/bm0501149Suche in Google Scholar PubMed
111. Feng ZQ, Leach MK, Chu XH, Wang YC, Tian T, Shi XL, et al. Electrospun chitosan nanofibers for hepatocyte culture. J Biomed Nanotechnol. 2010;6:658–666.10.1166/jbn.2010.1159Suche in Google Scholar PubMed
112. Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26:6176–6184.10.1016/j.biomaterials.2005.03.027Suche in Google Scholar PubMed
113. Subramanian A, Vu D, Larsen GF, Lin HY. Preparation and evaluation of the electrospun chitosan/PEO fibers for potential applications in cartilage tissue engineering. J Biomater Sci Polym Ed. 2005;16:861–873.10.1163/1568562054255682Suche in Google Scholar PubMed
114. Park KE, Kang HK, Lee SJ, Min BM, Park WH. Biomimetic nanofibrous scaffolds: preparation and characterization of PGA/chitin blend nanofibers. Biomacromolecules. 2006;7:635–643.10.1021/bm0509265Suche in Google Scholar PubMed
115. Shalumon KT, Binulal NS, Selvamurugan N, Naira SV, Menona D, Furuikeb T, et al. Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr Polym. 2009;77:863–869.10.1016/j.carbpol.2009.03.009Suche in Google Scholar
116. Zhou Y, Yang D, Chen X, Xu Q, Lu F, Nie J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9:349–354.10.1021/bm7009015Suche in Google Scholar PubMed
117. Yang D, Jin Y, Zhou Y, Ma G, Chen X, Lu F, et al. In situ mineralization of hydroxyapatite on electrospun chitosan-based nanofibrous scaffolds. Macromol Biosci. 2008;8:239–246.10.1002/mabi.200700221Suche in Google Scholar PubMed
118. Junkasem J, Rujiravanit R, Supaphol P. Fabrication of α-chitin whisker-reinforced poly(vinyl alcohol) nanocomposite nanofibres by electrospinning. Nanotechnology. 2006;17:4519–4528.10.1088/0957-4484/17/17/039Suche in Google Scholar
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Chromatographic Techniques for Rare Earth Elements Analysis
- Developments in the use of rare earth metal complexes as efficient catalysts for ring-opening polymerization of cyclic esters used in biomedical applications
- Controlled Chemical Synthesis in CVD Graphene
- Combining catalytical and biological processes to transform cellulose into high value-added products
- Recent advances in “bioartificial polymeric materials” based nanovectors
Artikel in diesem Heft
- Chromatographic Techniques for Rare Earth Elements Analysis
- Developments in the use of rare earth metal complexes as efficient catalysts for ring-opening polymerization of cyclic esters used in biomedical applications
- Controlled Chemical Synthesis in CVD Graphene
- Combining catalytical and biological processes to transform cellulose into high value-added products
- Recent advances in “bioartificial polymeric materials” based nanovectors

