1 Introduction
Transition metal-catalyzed cross-couplings are among the most powerful methods in organic synthesis to form carbon–carbon bonds and these reactions have received considerable attention from chemists over the past eighty years. Among these coupling reactions, Grignard reagents were used extensively and were coupled with Grignard reagents, halides or pseudo-halides. To induce these couplings, different transition metal catalysts can be used such as palladium, nickel, iron, cobalt, manganese, copper, as well as silver and more scarcely, titanium, chromium, zirconium, rhodium or ruthenium. Among these metal catalysts, the most commonly used metals are probably palladium and nickel, however in the context of “sustainable chemistry”, chemists have to develop methods that are eco-friendly and other metals, than palladium and nickel catalysts have to be utilized. As iron and cobalt catalysts are more eco-friendly than palladium and nickel catalysts, they have been extensively used. Even if silver catalysts have not been extensively studied, these catalysts should not be neglected as some reactions can be efficiently be performed with silver salts and not with other metal catalysts. Silver-catalyzed couplings have been reported to form carbon–carbon bonds in between two sp2 carbons, in between one sp2 and one sp3 carbons and mainly in between two sp3 carbons.
2 Coupling reactions of aryl Grignard reagents
2.1 With aryl Grignard reagents
2.1.1 Homocoupling
Since the early work of Gardner and Borgstrom, in 1929 [1], it has been shown that silver(I) salts and, particularly silver bromide (AgBr), are working as stoichiometric oxidants to realize the synthesis of symmetrical biaryls from aryl magnesium halides. The coupling was realized by the addition of an aryl magnesium halide to a well-stirred suspension of AgBr in ether, followed by the addition of benzene. After heating, biphenyl (66 %), 4, 4’-dimethoxy biphenyl (48 %) and bi-p-tolyl (72 %) were obtained in satisfactory purity and yields when the coupling reaction was performed on small scale (0.05 mol). However, when the reaction was scaled up, the yield in the bi-aryl derivatives was reduced. For example, the yield in bi-p-tolyl was reduced from 72 % to 25 %, when the reaction was performed on 0.35 mol of Grignard reagent instead of 0.05 mol [1] (Fig. 1).
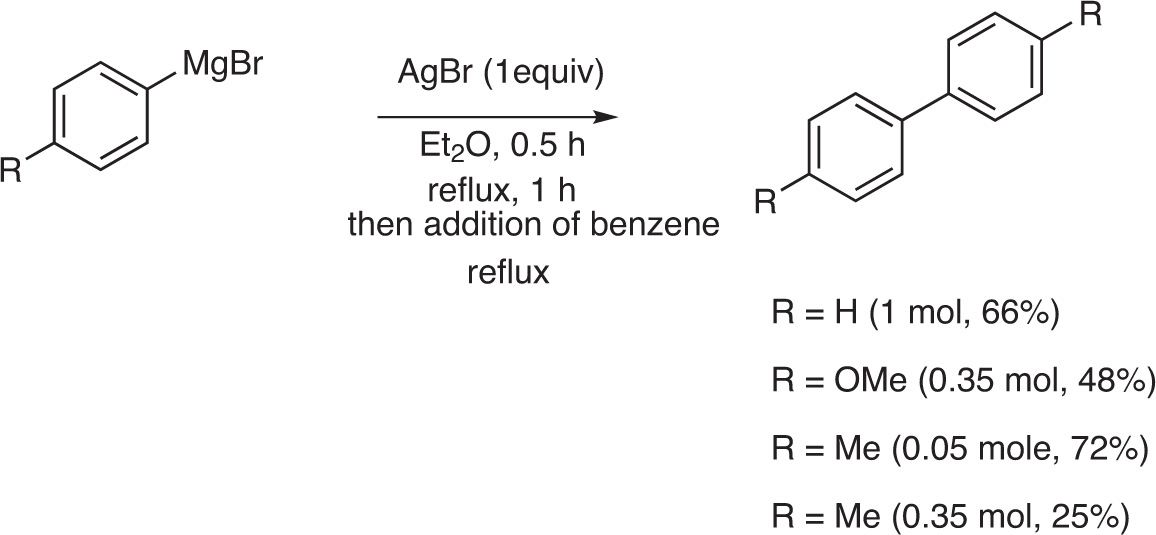
Cross-coupling of aryl Grignard reagents.
Gardner and Borgstrom [1] reported that radical intermediates are responsible for the formation of the coupling products and that these radicals are formed by the interaction of the Grignard reagents with silver bromide, assuming that the reaction takes place according to eqs. 1 and 2 represented in Fig. 2.
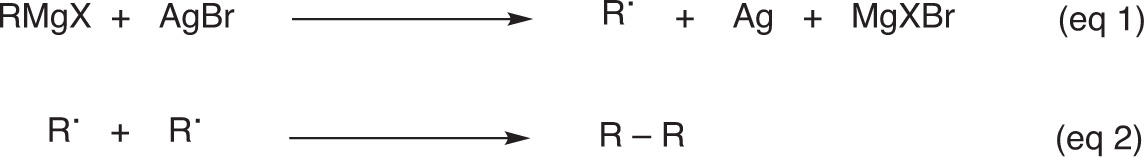
Radicals involved in the coupling reactions.
2.1.2 Heterocoupling
In 1937, Gardner, Joseph and Gollub realized a coupling in between two different Grignard reagents by treating a mixture of phenylmagnesium bromide and p-anisole magnesium bromide with AgBr [2]. To separate and identify the resulting formed compounds, the mixture was boiled with hydroiodic acid and acetic acid and, after a base/acidic treatment, the products were isolated by fractional crystallization to afford the coupling products. 4-Hydroxybiphenyl was isolated in 8.2 % yield, accompanied by the homocoupling products, biphenyl and 4, 4’-dihydroxybiphenyl which were isolated in 46 % yield and 19.4 %, respectively. Even if the yield in the heterocoupling product is low, it was demonstrated for the first time that a heterocoupling was possible when two different aryl Grignard reagents were treated with a silver catalyst (Fig. 3).
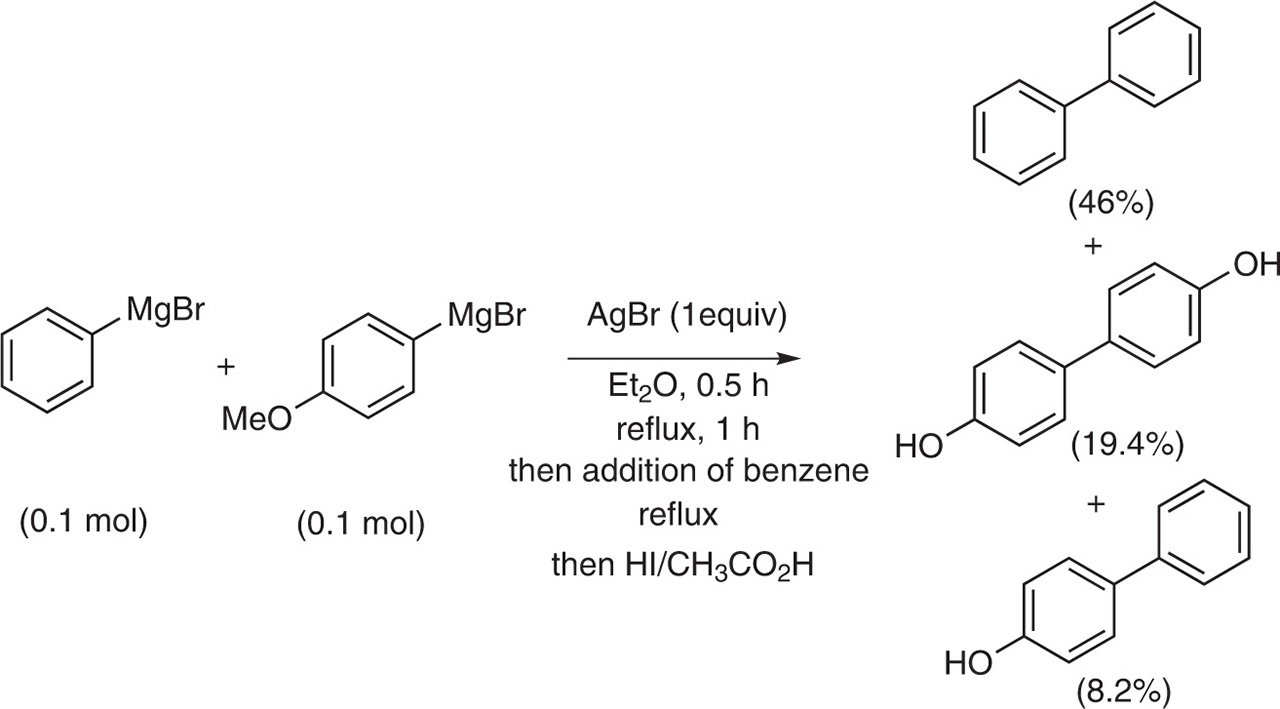
Heterocoupling between two different Grignard reagents.
2.2 With alkyl halides
The alkylation of an aryl Grignard reagent by an alkyl halide can be realized in the presence of silver catalysts. Oshima et al. showed that the cross-coupling of an aryl magnesium bromide with an alkyl bromide was possible in the presence of AgBr (10 mol %) and triphenylphosphite [P(OPh)3 (10 mol %)] as the additive (Fig. 4). It is worth noting that acyclic primary, secondary and tertiary alkyl bromides as well as cyclic bromides can be involved in a coupling reaction and that the coupling products were isolated in good yields (> 60 %). However for tertiary bromides, the reaction took 10 h instead of 5 h for primary and secondary halides (Fig. 4). It seems that the coupling reaction is not sensitive to steric hindrance as the reaction of o-tolyl magnesium bromide with bromocyclohexane furnished the coupling product in 90 % yield [3] (Fig. 4).
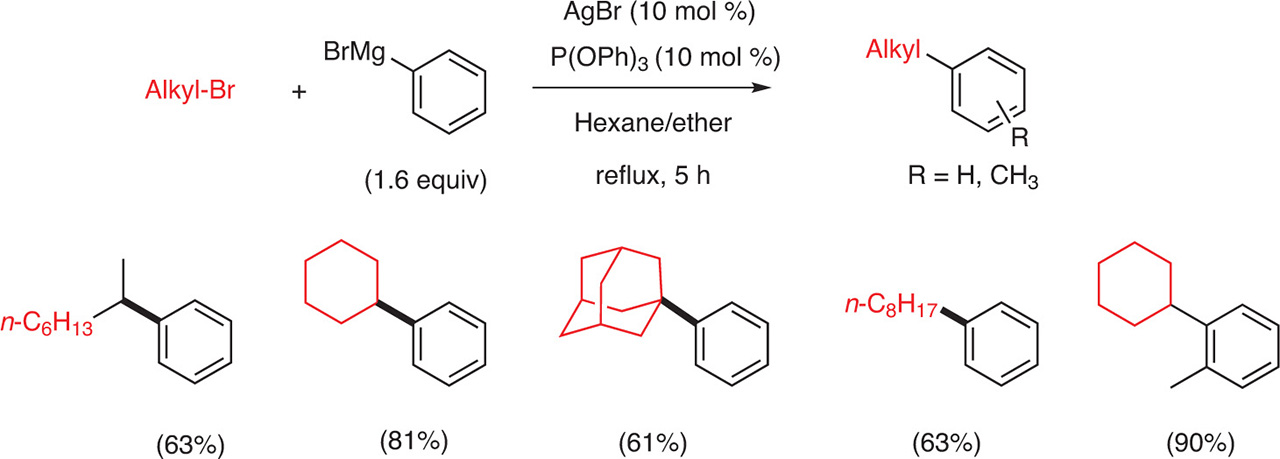
Coupling between an aryl Grignard and an alkyl halide.
3 Coupling of vinyl Grignard reagents with alkyl halide
Only one example was reported in the literature for the coupling of a vinyl Grignard reagent with a halide, catalyzed by silver salts. When cis-propenyl magnesium bromide was treated with methyl bromide, in the presence of a silver(I) salt, cis-but-2-ene was produced with retention of configuration of the vinyl Grignard showing that under these conditions, the coupling is highly stereoselective [4] (Fig. 5). To our knowledge, no other coupling reactions, catalyzed by silver salts, were reported in between a vinyl Grignard reagent and a halide.

Coupling between a vinyl Grignard and an alkyl halide.
4 Coupling of alkyl Grignard reagents
The coupling of an alkyl Grignard reagent with another alkyl Grignard reagent or with an alkyl halide, catalyzed by silver salts, is the most commonly reported coupling in the literature.
4.1 With alkyl Grignard reagents
4.1.1 Intermolecular homocoupling
In their early work, in 1929, Gardner and Borgstrom have reported that a homocoupling can take place when an alkyl Grignard is treated with a catalytic amount of AgBr. n-Butyl, benzyl-, cyclohexyl-Grignard reagents respectively led to n-octane, bi-benzyl, bi-cyclohexyl in moderate to good yields (40–72 %) [1]. With iso-butyl-, and sec-butyl Grignard reagents, these authors reported, at first, that they do not observe any coupling products [1]. However, 10 years later, in 1939, Gardner and Joseph reported that iso-butyl, and sec-butyl Grignard were reacting in the presence of AgBr to produce 2,5-dimethylhexane (37.5 %) and 3,4-dimethylhexane (13 %), respectively [5] (Fig. 6).
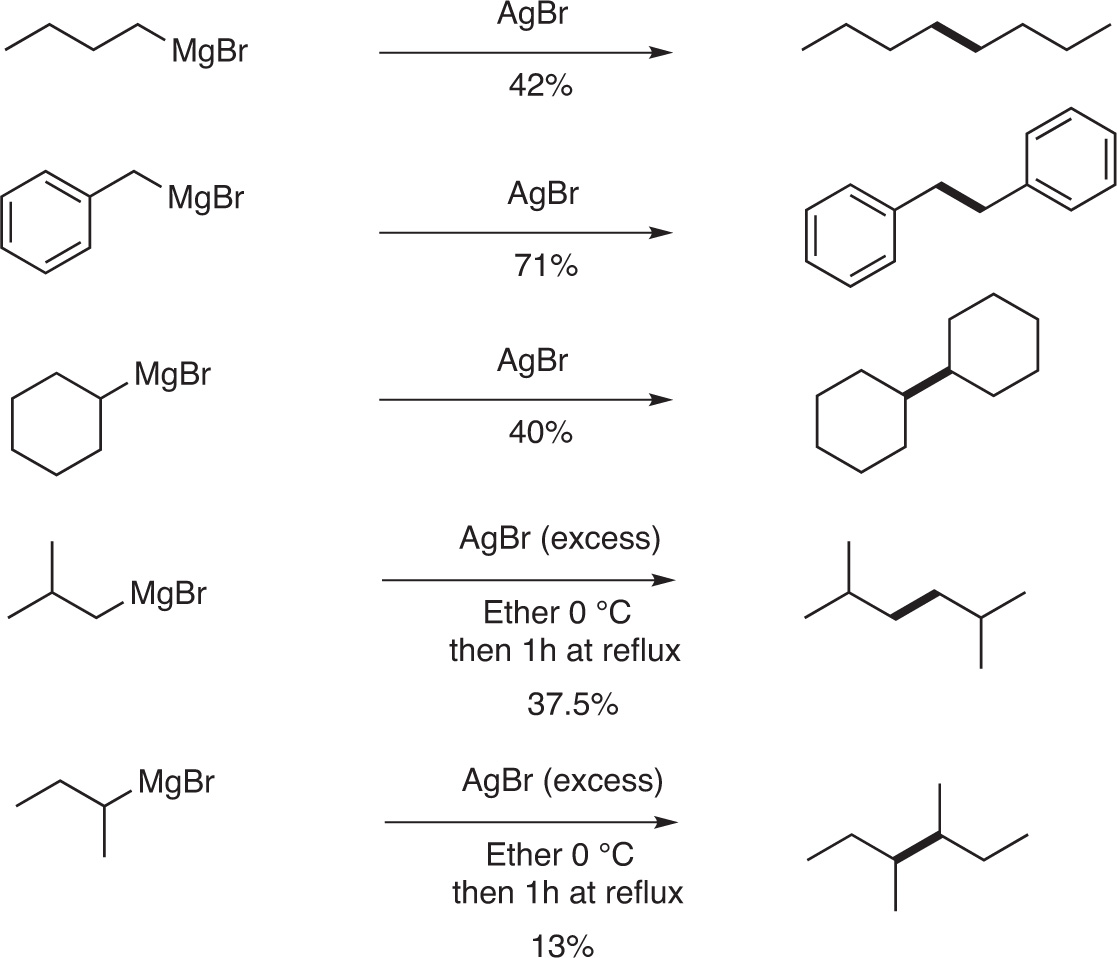
Homocoupling of alkyl Grignard reagents catalyzed by AgBr.
When the coupling was achieved with AgBr in excess, the yields in the homocoupling products were moderate to low. On the contrary, when the reaction was realized with a catalytic amount of AgOTs (1 mol %) in the presence of a reoxidant such as dibromoethane (1.2 equiv), the yields in the homocoupling products were excellent even with Grignard reagents prepared from secondary halides [6] (Fig. 7).
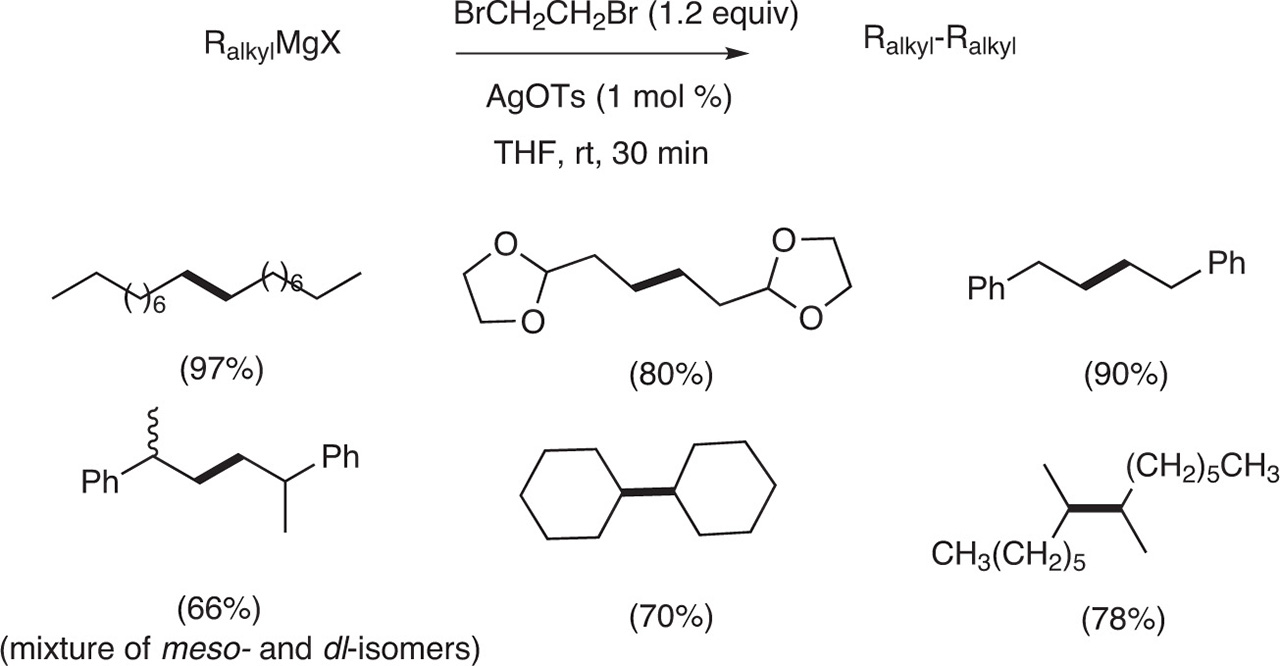
Homocoupling of akyl Grignard catalyzed by AgOTs/dibromoethane.
4.1.2 Intramolecular homocoupling
The coupling of two alkyl Grignard reagents was realized in an intramolecular version. When α,ω-di-Grignard derivatives were prepared in THF, by addition of magnesium onto α, ω-di-halides and, after treatment of the resulting α,ω-di-Grignard with a catalytic amount of tetrakis iodo(tri-n-butylphosphine)silver(I) [AgIPBu3]4], cyclobutane, cyclopentane and cyclohexane were formed in good yields (83–93 %). On the contrary, cyclooctane was obtained in only 23 % yield. It is worth nothing that the homo-coupling is not effecient to form cyclooctane and cyclodecane as these latter were only detected (yields inferior to 1 %) [7] (Fig. 8).
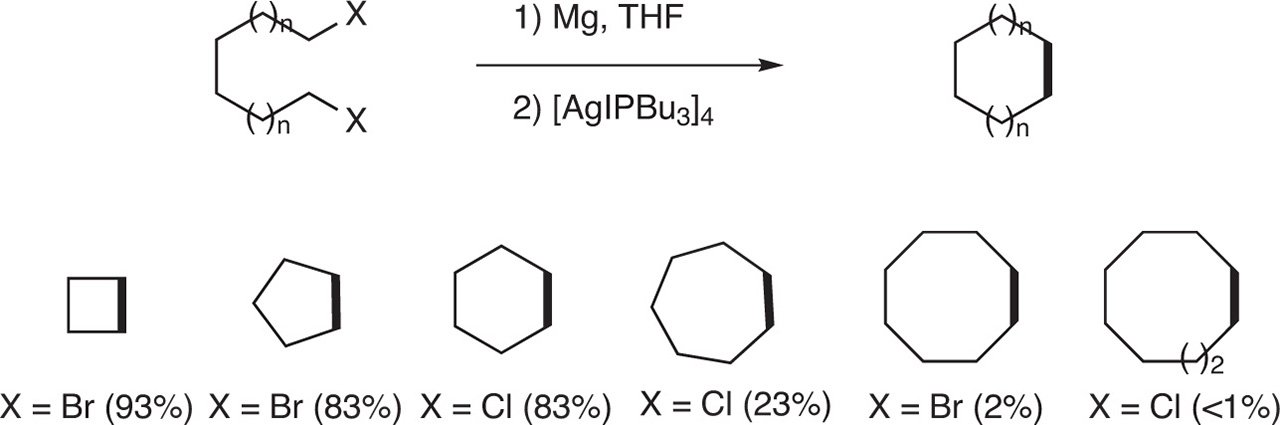
Formation of cyclic compounds by Intramolecular homocoupling of α,ω-di-Grignard reagents.
Other silver salts such as silver triflate (AgOTf), silver bromide (AgBr) as well as silver nitrate (AgNO3) can be used for an intramolecular coupling. Bicyclic compounds were formed in good yields when α,ω-dichlorides were treated with magnesium and then with Ag(OTf) [7] (Fig. 9).
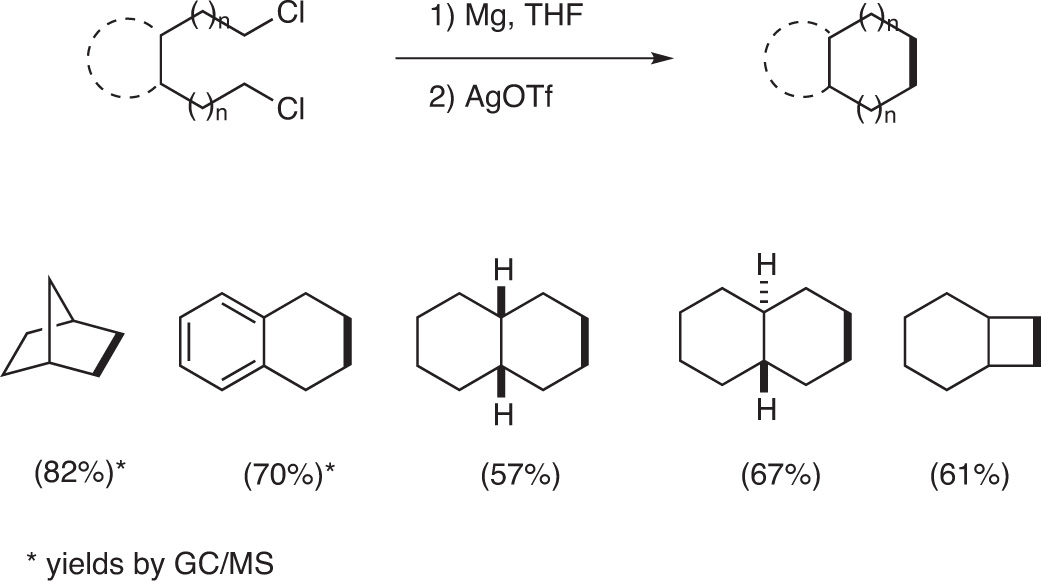
Formation of bicyclic compounds by intramolecular coupling of α,ω-di-Grignard reagents.
4.2 With alkyl halides
Silver salts are also effective catalysts for the coupling of Grignard reagents with alkyl halides to produce the coupling products in good yields if the alkyl group of the Grignard is identical to the alkyl group of the halide (Fig. 10, eq 1). On the contrary, when the alkyl group of the Grignard is different from the alkyl group of the halide, a mixture of the coupling products is formed (Fig. 10). For example, when a Grignard reagent is formed from a primary halide and is reacting with a primary halide different from the one that was used to prepare the Grignard, a mixture of three coupled products was obtained in an equimolar ratio [8] (Fig. 10, eq 2).
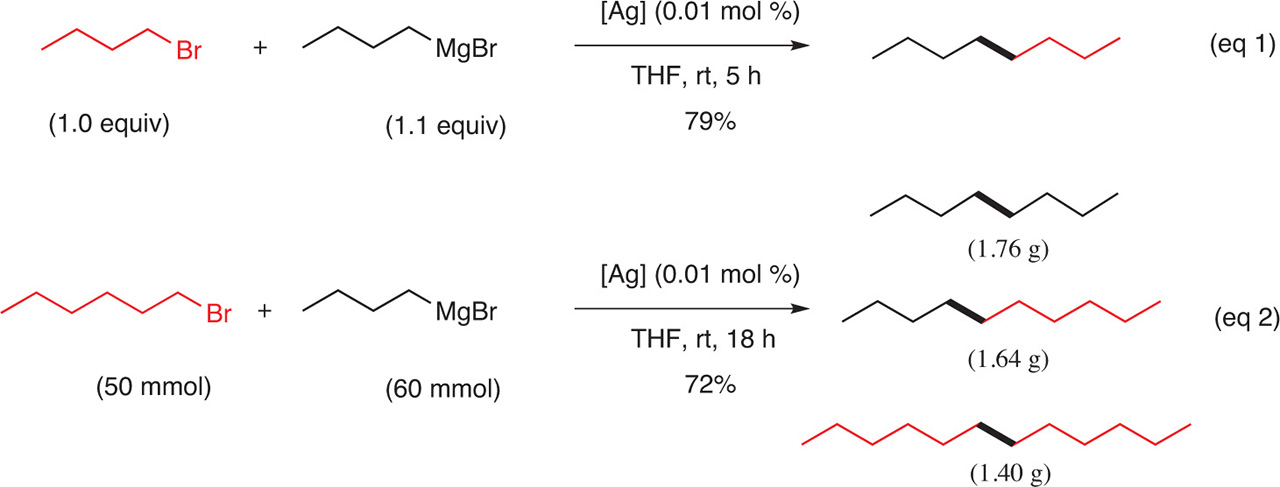
Coupling of an alkyl Grignard with an alkyl halide.
In 1971, it was demonstrated by Tamura and Kochi that the rate of the catalytic coupling of EtMgBr with an alkyl halide is much faster for tertiary halides (tert-BuBr) than for secondary halides (iPrBr) than for primary halides (n-PrBr) in a rate of 20:3:1. It is worth noting that structural changes in the Grignard reagents do not induce an apparent systematic variation in the reaction rate [9]. This observation was used several years later by Oshima et al. to realize a coupling between benzylmagnesium bromide and secondary as well as tertiary bromides in the presence of AgNO3 in ether, to produce the corresponding cross-coupling products in high yields [10] (Fig. 11).
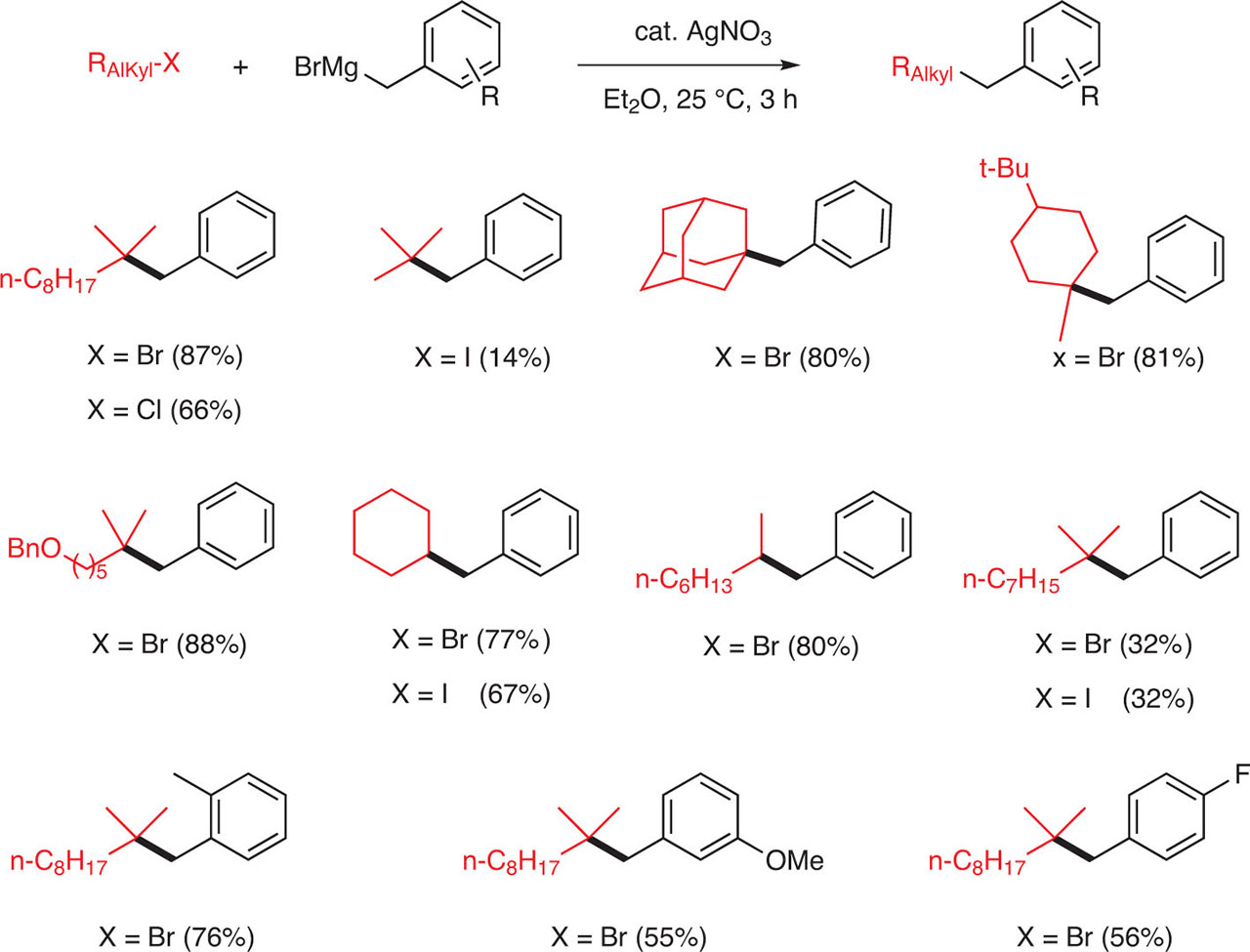
Coupling between benzyl magnesium bromide and alkyl halides.
As secondary halides react faster than primary halides, the coupling of halides with Grignard reagents is very chemioselective [3]. The reaction can be performed by using AgCl, AgBr, AgBr/KF or AgBr/P(OPh)3, in a mixture of ether/CH2Cl2 [3]. For example, when 1 was treated with Grignard 2 and AgBr/P(OPh)3as the catalystic system, compound 3 was formed in 55 % yield and 4 was not detected (Fig. 12). When the Grignard reagent is phenylmagnesium bromide, under the silver-catalyzed arylation conditions (AgBr/KF), the monophenylated product 6 and the diphenylated one, 7, were isolated in 43 % and 16 % yields, respectively. With phenylmagnesium bromide, the reaction is less chemioselective than with alkyl Grignard reagents. (Fig. 12).
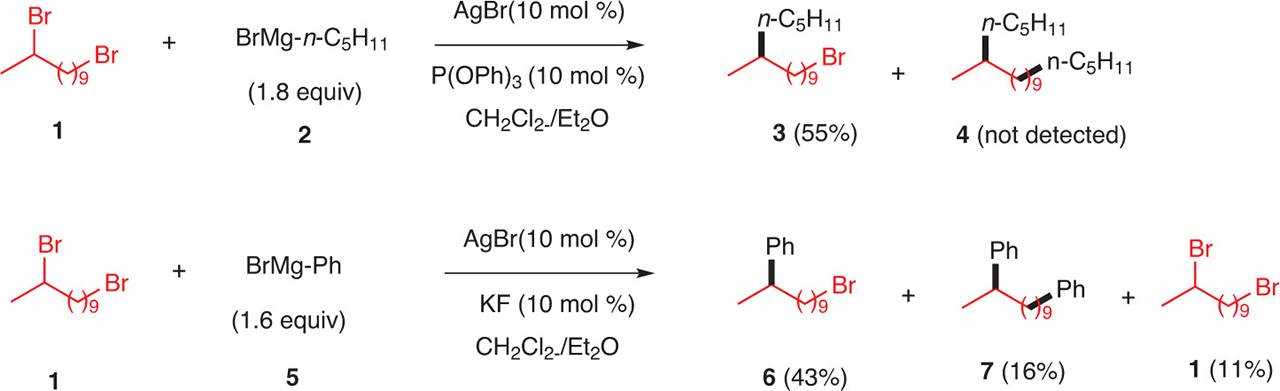
Chemoselective coupling of phenylmagnesium bromide with dibromo alkyl derivatives.
The coupling reaction between an alkyl Grignard and an alkyl halide, catalyzed by Ag(0), was used to prepare glycerol triethers in good yields. It is worth noting that the Grignard reagent was prepared from an iodide and that the alkyl halide was an alkyl chloride [11] (Fig. 13).
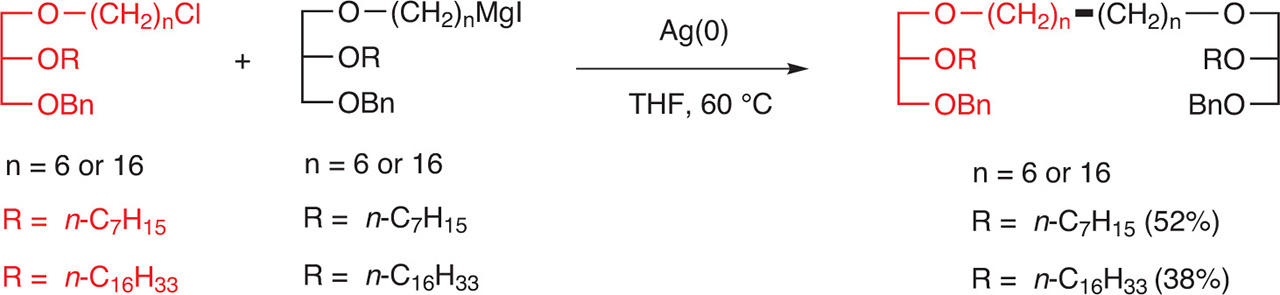
Synthesis of glycerol triethers by coupling of alkyl Grignard reagents with alkyl halides.
The silver-catalyzed coupling can proceed also with allylic Grignard reagents (Fig. 14). Allylations and methallylations of tertiary and secondary allyl bromides were realized under smooth conditions. Unfortunately, silver-catalyzed prenylation and crotylation of alkyl halides led to a poor regioselectivity [10] Fig. 14.
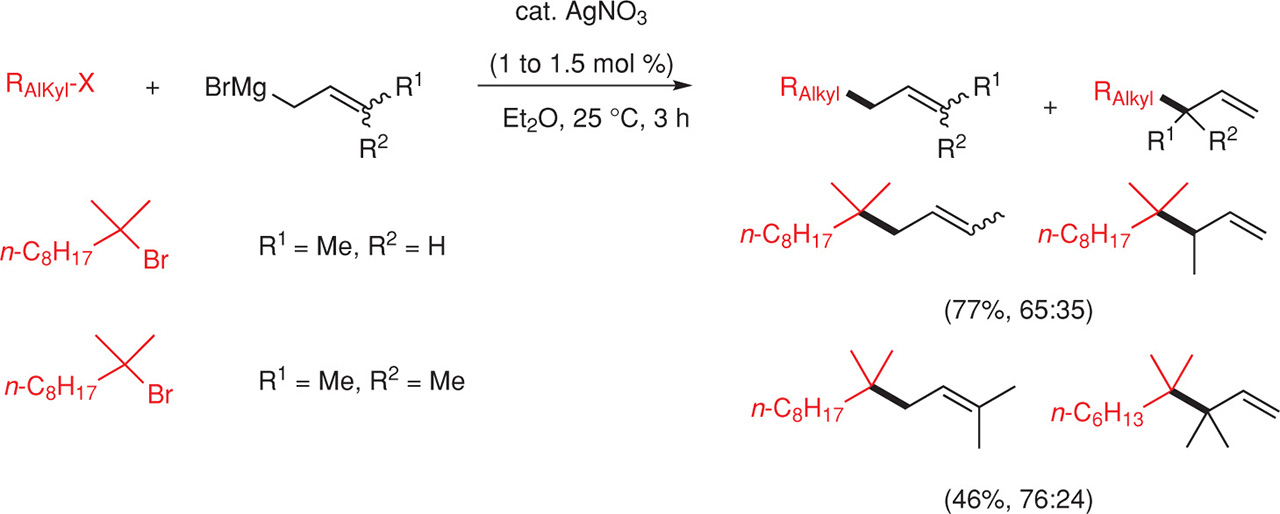
Coupling of allyl and methallyl Grignard reagents with alkyl halides.
4.5 With vinyl halide
If Z-vinyl Grignard reagents were coupled with alkyl halides with retention of configuration, on the contrary the reverse combination, for example the coupling of an alkyl Grignard reagent with a cis-vinyl halide, did not lead to the substituted olefin with the retention of configuration of the vinyl halide. For example, the coupling of cis-propenyl bromide with CH3MgBr, in the presence of Ag(I), led to a mixture of cis- and trans-but-2-ene [11] (Fig. 15).
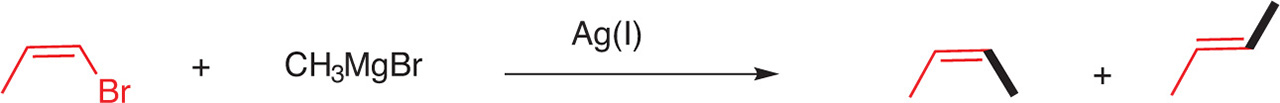
Coupling of alkyl Grignard reagents with vinyl halides.
5 Mechanism
According to the early work of Gardner and Borgstrom [1] and then Kochi and Tamura [9], it has been postulated that radical intermediates were formed when a Grignard reagent and a halide were treated with silver salts to form the coupling products. In 2008, radical intermediates have been highlighted by Oshima et al. [10] by using a radical clock. Thus, when 8 was treated with PhCH2MgBr in the presence of AgNO3 in ether at 25 °C (3 h), the functionalized cyclized product 9 was isolated and then transformed to 10, proving that radical intermediates were formed (Fig. 16).
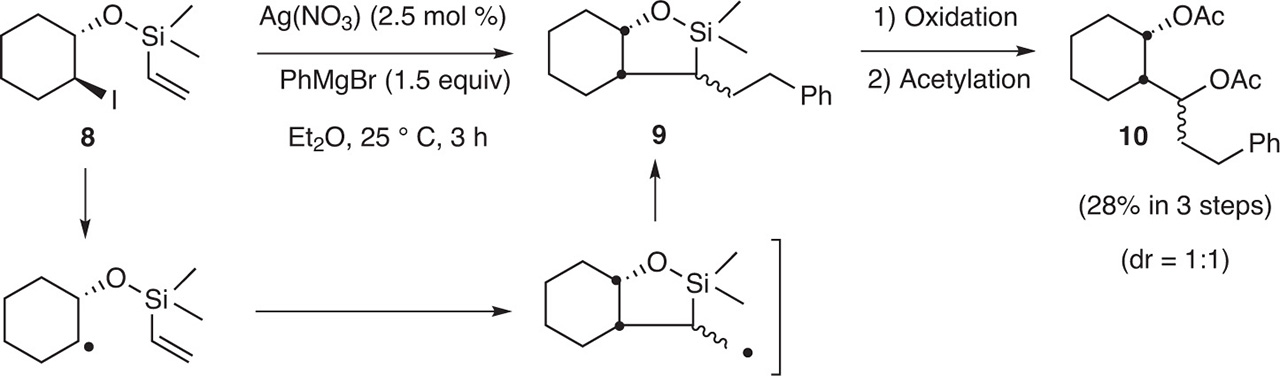
Trapping of the radical intermediates.
Based on the studies on silver-catalyzed couplings [9, 10, 12], a mechanism has been proposed by Oshima et al. [10]. A Grignard reagent such as, for example, benzyl magnesium bromide would first react with the silver(I) salt to produce silver(0) (which can be multi-atomic or clustered and might not be single atom), and diphenylethane (steps A and B). The zero valent silver would affect a Single Electron Transfer (SET) to the alkyl halide to form the corresponding alkyl halide radical and silver halide (step C). The alkyl radical would couple with another zero valent silver to yield the alkyl silver intermediate (step D). The silver halide formed by the SET would then react with benzylmagnesium bromide to generate a benzyl silver intermediate (step E). Reductive coupling of the benzyl silver with the alkyl silver intermediate will afford the coupling product and will regenerate the zero valent silver (step F). We have to point out that the reasons for the predominant cross-coupling over a possible homocoupling are not clear at this stage. In addition, the formation of a carbocation cannot be excluded (Fig. 17).
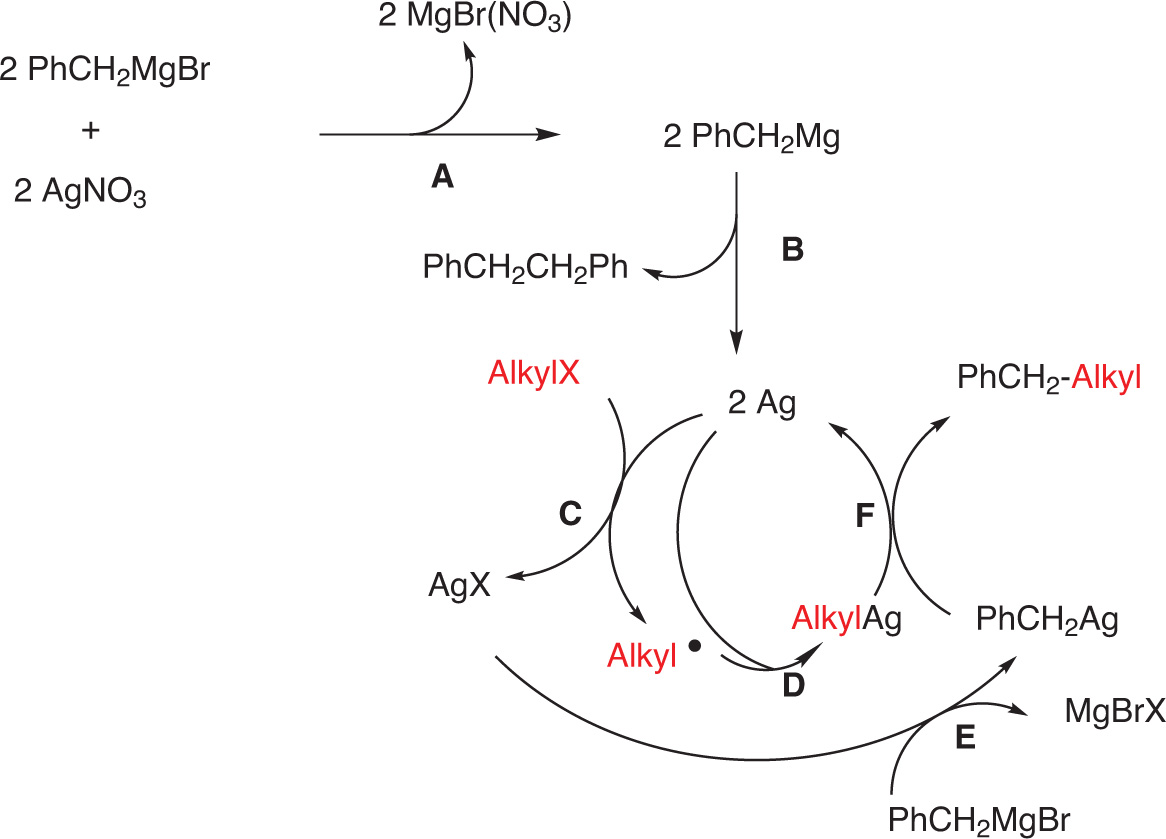
Proposed mechanism.
6 Conclusion
Since the preliminary results of Gardner and then Kochi, only a few examples of coupling reactions catalyzed by silver salts were reported in the literature. Silver salts can catalyze the coupling of alkyl, allyl, vinyl and aryl Grignard with alkyl, vinyl and aryl Grignard reagents or halides. These couplings are very stereoselective when a vinyl Grignard is used, as well as chemoselective as tertiary halides are more reactive than secondary halides and more reactive than primary halides. As all the coupling possibilities using silver-catalyzed cross-couplings, in between a Grignard reagent and another Grignard reagent or a halide, have not been examined extensively, useful synthetic methods could be developed in the future. In addition, the development of enantioselective coupling reactions is highly desirable.
Acknowledgements
This article is also available in: Cossy, Grignard Reagents and Transition Metal Catalysts. De Gruyter (2016), isbn 978–3–11–035266–5.
References
[1]Gardner JH, Borgstrom PA. J Am Chem Soc 1929; 51:3375–3377.10.1021/ja01386a027Search in Google Scholar
[2]Gardner JH, Joseph L, Gollub F. J Am Chem Soc 1937, 59:2583–2584.10.1021/ja01291a032Search in Google Scholar
[3]Someya H, Yorimitsu H, Oshima K. Tetrahedron Lett 2009, 50:3270–3272.10.1016/j.tetlet.2009.02.040Search in Google Scholar
[4]Kochi JK. J Organomet Chem 2002, 653:11–19.10.1016/S0022-328X(02)01265-2Search in Google Scholar
[5]Gardner JH, Joseph, L. J. Am. Chem. Soc. 1939, 61:2551–2552.10.1021/ja01878a502Search in Google Scholar
[6]Nagano T, Hayashi T. Chem. Letters 2005, 34:1152–1153.10.1246/cl.2005.1152Search in Google Scholar
[7]Whitesides GM, Gutowski FD. J Org Chem 1976, 41:2882–2885.10.1021/jo00879a019Search in Google Scholar
[8]Tamura T, Kochi J. Synthesis 1971, 303–305.10.1055/s-1971-35043Search in Google Scholar
[9]Tamura M, Kochi, J. J Amer Chem 1971, 93:1483–1485.10.1021/ja00735a028Search in Google Scholar
[10]Someya H, Ohmiya O, Yorimitsu H, Oshima K. Org Lett 2008, 10:969–971.10.1021/ol800038aSearch in Google Scholar PubMed
[11]Berkowitz WF, Pan D, Bittman R.Tetrahedron Lett 1993, 34:4297–4300.10.1016/S0040-4039(00)79333-6Search in Google Scholar
[12]Kochi JK. J Organomet Chem 2002, 653:11–19.10.1016/S0022-328X(02)01265-2Search in Google Scholar
© 2016 by Walter de Gruyter Berlin/Boston
Articles in the same Issue
- Grignard reagents and Copper
- Novel nanoparticle materials for drug/food delivery-polysaccharides
- Neutron Activation Analysis of the Rare Earth Elements (REE) – With Emphasis on Geological Materials
- Grignard Reagents and Silver
- Biological routes to itaconic and succinic acids
Articles in the same Issue
- Grignard reagents and Copper
- Novel nanoparticle materials for drug/food delivery-polysaccharides
- Neutron Activation Analysis of the Rare Earth Elements (REE) – With Emphasis on Geological Materials
- Grignard Reagents and Silver
- Biological routes to itaconic and succinic acids

