Abstract
Objectives
Pleural effusion, defined as an abnormal accumulation of fluid in pleural space, can be of two types: transudative and exudative. The primary aim of the study was to assess the predictive accuracy of procalcitonin (PCT) and pentraxin-3 (PTX-3) in comparison to other biochemical markers such as C-reactive protein (CRP), and adenosine deaminase (ADA) in the differential diagnosis of pleural effusions.
Methods
A cross-sectional analytical study was conducted on patients with pleural effusion. Multiple comparisons and receiver-operating characteristics (ROC) analyses were made to evaluate the diagnostic significance of biochemical markers.
Results
Sixty-six patients with exudative pleural effusion classified as malignant, tuberculous, and parapneumonic effusions (malignant pleural effusion [MPE], tuberculous [TPE], and parapneumonic [PPE]) were included. Significant differences in pleural fluid levels in both PCT (p-value: 0.001) and PTX-3(p-value: 0.001), as well as serum levels of PCT (p-value: 0.001), were observed between the three groups. ROC analysis showed both PTX-3 and PCT having favorable discrimination ability with high sensitivity (≥90%) and specificity to predict PPE from TPE and MPE.
Conclusions
Evaluation of serum and pleural fluid PCT and levels of PTX-3 in the pleural fluid may be used as an early biomarker to differentiate the etiology of pleural effusion.
Introduction
Pleural effusion is defined as an abnormal accumulation of fluid in pleural space [1]. It can be of two types: transudative and exudative. Diseases like cirrhosis of the liver, nephrotic syndrome, and congestive heart failure lead to transudative effusion, while infection and malignancy are the most common causes for exudative effusion [2]. Various modalities are available to diagnose parapneumonic (PPE), tuberculous (TPE), and malignant pleural effusion (MPE). These modalities include biochemical [3], microbiological [4], cytopathological analyses [5], and radiological techniques [6]. In addition to these modalities, there are at least two biomarkers showing promising results in the early diagnosis of pleural effusions: adenosine deaminase (ADA) and acute-phase proteins like C-reactive protein (CRP). These established biochemical markers (CRP and ADA) aid in the differential diagnosis of various etiologies’ pleural effusions. ADA is useful in diagnosing TPE [7], while acute-phase proteins like CRP aid in diagnosing PPE [8].
Recently, two further biomarkers have been evaluated in the early diagnosis of pleural effusions: pentraxin-3 (PTX-3) and procalcitonin (PCT). PTX-3 pleural fluid levels distinguish PPE and other exudative effusions [9], [10], [11], [12]. PTX-3, also known as tumor necrosis factor (stimulated gene 14), is a Pentraxin superfamily member. Although Pentraxin proteins are synthesized in the liver, they can also be produced locally in response to pro-inflammatory stimuli. PTX-3 is secreted by various cells like neutrophils, alveolar cells fibroblasts, adipocytes, and smooth muscle cells. PCT, a marker used in sepsis diagnosis, is also significant in discriminating PE of different etiologies [13], [14]. The lung and liver produce PCT after infections, especially bacterial infections [15], [16].
Thus, much research has been carried out in the last few years to validate biomarkers able to differentiate pleural effusions’ etiology. However, an adequate diagnostic accuracy of these biomarkers has not yet been demonstrated [17]. We planned a comparative analysis to assess the predictive accuracy and diagnostic significance of PCT and PTX-3 compared to established biochemical markers (CRP and ADA) in the early differential diagnosis of pleural effusions.
Materials and methods
Study population
Patients who underwent diagnostic or therapeutic thoracocentesis during the past year in the Department of Pulmonary Medicine, Himalaya Institute of Medical Science, Swami Rama Himalaya University, Dehradun, Uttrakhand, India, were enrolled in the study. Only patients with exudative pleural effusion were included in the study. Patients with pyothorax, hemothorax, or transudative effusion were excluded. Pleural fluid and serum samples from such patients were collected after obtaining informed written consent. Approval of the Institutional Review Board (IRB) was received before the study, and the IRB approval number was HIMS/RC/2017/40 dated 31/01/2017.
Study design
This is an analytical cross-sectional study. All patients with exudative pleural effusion were divided into three groups after routine pleural fluid analysis based on widely accepted Light’s criteria [18].
Group 1: Patients with TPE: 33 patients
Tuberculous effusions were confirmed by the presence of acid-fast bacilli (AFB) and TB-PCR testing.
Group-2: Patients with MPE: 23 patients
Malignant effusions were confirmed by cytology/histology examination. All 23 MPE cases were cytologically confirmed and were further divided into primary and secondary.
Group-3: Patients with PPE: 10 patients
PPE was confirmed by purulent sputum and response to antibiotics.
Samples were collected with stringent aseptic precaution from the Department of Pulmonary Medicine and send to the central diagnostic laboratory for evaluation.
Laboratory analysis
Biochemical parameters, including protein, lactate dehydrogenase (LDH), CRP, and pentraxin, were performed immediately on automated analyzers. Serum/pleural fluid PCT sample was preserved at −20 °C before analysis as recommended by the kit insert. After routine cytological and microbiological examination, samples of serum and pleural fluid were analyzed for PTX3 (detection limit, 0.025 ng/mL), CRP (detection limit, 10 μg/mL), and PCT (detection limit, 27 pg/mL) using commercial kits on MR-96A ELISA reader and COBAS-6000 modular analyzer. ADA was estimated by the enzymatic method on Unicel DXC800 (Beckman Coulter India [Pvt.] Ltd). Assay kits were calibrated, and quality controls were analyzed daily at the National Accreditation Board for testing and calibration laboratories (NABL) accredited lab in Himalaya Institute of medical science. The diagnostic labs also participate in EQAS (monthly external quality assurance services) assurance programs to maintain the accuracy and precision of laboratory results.
Statistical aspects
Data were analyzed by using statistical software SPSS 20. Categorical data were expressed as frequency and percentage. Quantitative data were expressed as mean ± SD and median (min–max) for normal and skewed distribution. Spearman correlation coefficient was used to find the correlation between biomarkers in serum and pleural fluid. Kruskal Wallis H test followed by Dunn’s posthoc multiple comparison tests to compare median values among the three groups. Receiver operating characteristics (ROC) curve was used to find cut-off values for pneumonia considering TB and lung cancer reference one by one. A ROC analysis was performed, and the area under the curve (AUC) was calculated.
Results
Patient’s baseline characteristics
Out of the total 80 patients enrolled, 14 were excluded after diagnosis of pyothorax, hemothorax, or transudative effusion. The remaining study group included 66 patients (52 males, 14 females) with a mean age of 53.9 years. Out of these patients, 33 (50%) had TPE, 23 (34%) had MPE, and 10 (15%) had PPE. Out of 23 MPE, 19 were due to primary lung cancer (14 adenocarcinoma, three small cell carcinoma, one squamous cell carcinoma, one large cell carcinoma). The remaining four effusions were secondary to other malignancies. The pleural fluid characteristics of the 66 patients are shown in Table 1.
Baseline characteristics of patients with pleural effusion.
| Variable | Tuberculous pleural effusion (TPE) n=33 | Malignant pleural effusion (MPE) n=23 | Parapneumonic pleural effusion (PPE) n=10 | |
|---|---|---|---|---|
| Age, years | 47.6 ± 20.2 | 63.7 ± 13.6 | 52.4 ± 13.3 | |
| Sex | Male | 25 (75.7%) | 21 (91.3%) | 6 (60.0%) |
| Female | 8 (24.2%) | 2 (8.7%) | 4 (40.0%) | |
| Protein, g/dL | Pleural | 3.85 ± 0.30 | 4.6 ± 0.20 | 3.72 ± 0.43 |
| Serum | 6.29 ± 0.58 | 6.70 ± 0.55 | 6.06 ± 0.65 | |
| PF/serum ratio | 0.61 | 0.68 | 0.61 | |
| LDH, IU/L | Pleural | 422.1 ± 117.4 | 507.2 ± 149.3 | 510.6 ± 171.8 |
| Serum | 209.9 ± 22.09 | 272 ± 41.57 | 258.8 ± 41.59 | |
| PF/serum ratio | 1.91 | 1.83 | 1.98 | |
TPE, tuberculous pleural effusion; MPE, malignant pleural effusion; PPE, parapneumonic pleural effusion.
Biomarkers levels in the serum
Analysis of serum biomarkers revealed variations across the three groups (TPE, MPE, and PPE). However, only PCT showed a substantial difference between all three groups (TPE vs. MPE, TPE vs. PPE, and MPE vs. PPE) by multiple comparisons. ADA showed a significant difference only between TPE and PPE. Serum CRP and PTX-3 levels showed no statistical differences between the groups (Table 2).
Biomarkers levels in serum according to etiology of pleural effusion.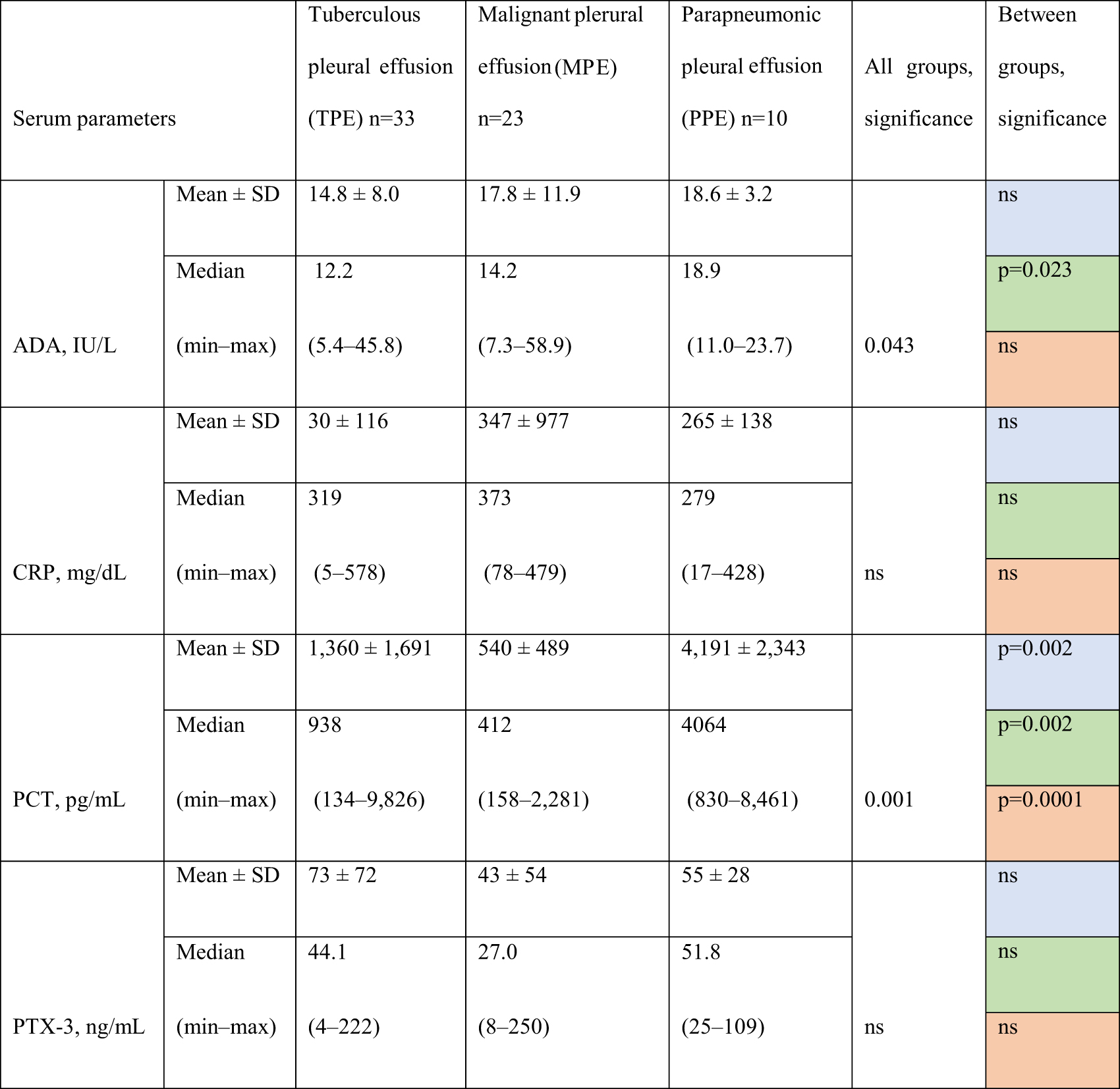
Comparisons. Blue fields: TPE vs. MPE. Green fields: TPE vs. PPE. Red fields: MPE vs. PPE. ADA, adenosine deaminase; PCT, procalcitonin; PTX-3, pentraxin-3; CRP, C-reactive protein; ns, not significant.
Biomarkers levels in the pleural fluid
ADA, PCT, and PTX-3 levels in pleural fluid revealed notable differences across the groups. Only PCT showed a significant difference between all three groups (TPE vs. MPE, TPE vs. PPE, MPE vs. PPE) by multiple comparisons. ADA and PTX-3 levels were significantly different between two groups (TPE vs. MPE and MPE vs. PPE) and (TPE vs. PPE, MPE vs. PPE), respectively. CRP levels showed no statistically significant difference between the groups (Table 3).
Biomarkers levels in the pleural fluid according to the cause of the effusion.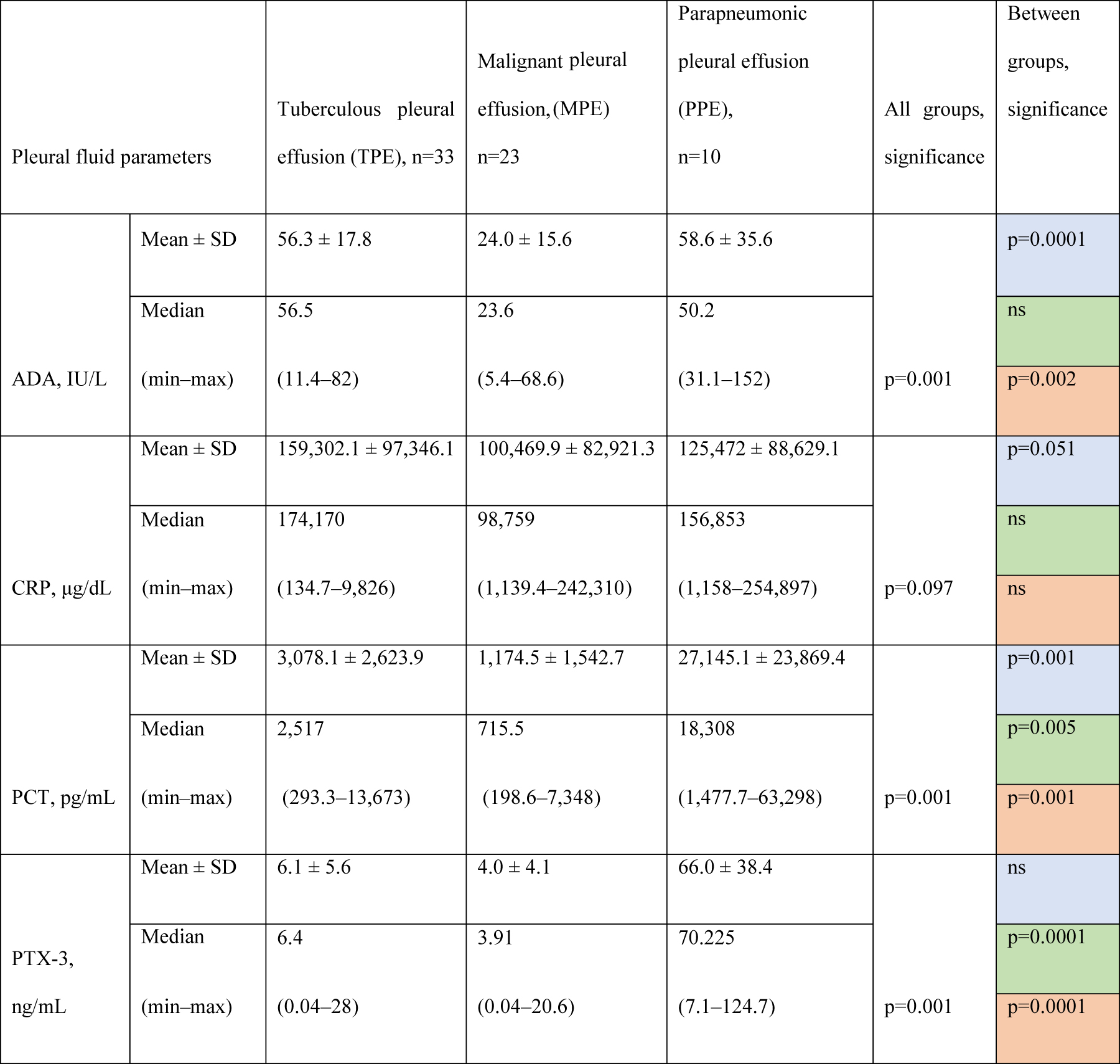
Comparisons. Blue fields: TPE vs. MPE. Green fields: TPE vs. PPE. Red fields: MPE vs. PPE; SD, standard deviation; ADA, adenosine deaminase; PCT, procalcitonin; PTX-3, pentraxin-3; CRP, C-reactive protein; ns, not significant.
ROC analysis of the diagnostic performance of PCT and PTX3
To differentiate PPE from TPE, pleural fluid PCT levels at the cut-off value of 6,173 had a sensitivity and specificity of 90%, and PTX-3 levels at the cut-off 9.2 had a sensitivity and specificity of 90 and 88%, respectively. To differentiate the PPE from MPE, pleural fluid PCT levels at the cut-off value of 2,189.7 had a sensitivity and specificity of 90 and 87%, respectively; PTX-3 levels at the cut off 7.19 had a sensitivity and specificity of 100 and 95.6%, respectively (Table 4, Figure 1). Correlation analysis showed a significant positive correlation for CRP, PCT, and PTX-3 in serum and pleural fluid (Figure 2).
Predictive values of CT and PTX-3 for distinguishing a parapneumonic effusion (PPE) vs. malignant pleural effusion (MPE), respectively, a tuberculous pleural effusion (TPE).
| Biomarker | Cut-off value | AUC (95% CI) | Sensitivity | Specificity | LR+ | LR− | |
|---|---|---|---|---|---|---|---|
| To predict PPE from TPE | PCT, pg/mL | ≤6,173 | 0.90 (0.77–1.00) | 90 | 90 | 9.9 | 0.11 |
| PTX-3, ng/mL | ≥9.2 | 0.94 (0.86–1.00) | 90 | 88 | 7.4 | 0.11 | |
| To predict PPE from MPE | PCT, pg/mL | ≤2,189.7 | 0.97 (0.94–1.00) | 90 | 87 | 6.9 | 0.11 |
| PTX-3, ng/mL | ≥7.19 | 0.99 (0.97–1.00) | 100 | 95.6 | 23.01 | 0.000 |
AUC, area under the curve; LR+, likelihood ratio positive; LR−, likelihood ratio negative; PCT, procalcitonin, PTX-3, pentraxin-3; PPE, parapneumonic effusion; MPE, malignant pleural effusion; TPE, tuberculous pleural effusion.
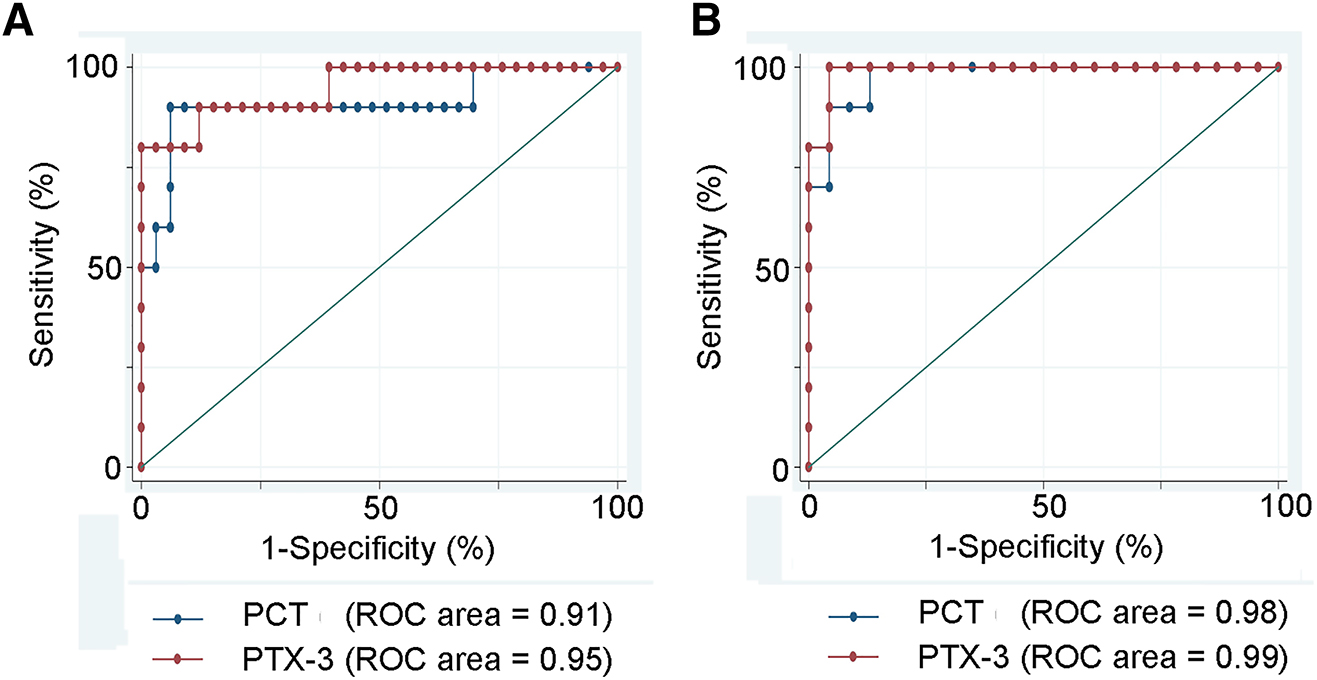
Prediction of a parapneumonic nature of pleural effusion.
(A) Prediction of a parapneumonic nature of a pleural effusion (PPE) vs. a tuberculous nature (TPE). (B) Prediction of a parapneumonic effusion (PPE) vs. a malignant pleural effusion (MPE). The prediction, represented by the ROC area, was in both situations excellent (over 90%). The best prediction was reached using PTX-3 for PPE (ROC area = 99%). Areas under the curve (AUCs) produced by receiver operating characteristics.
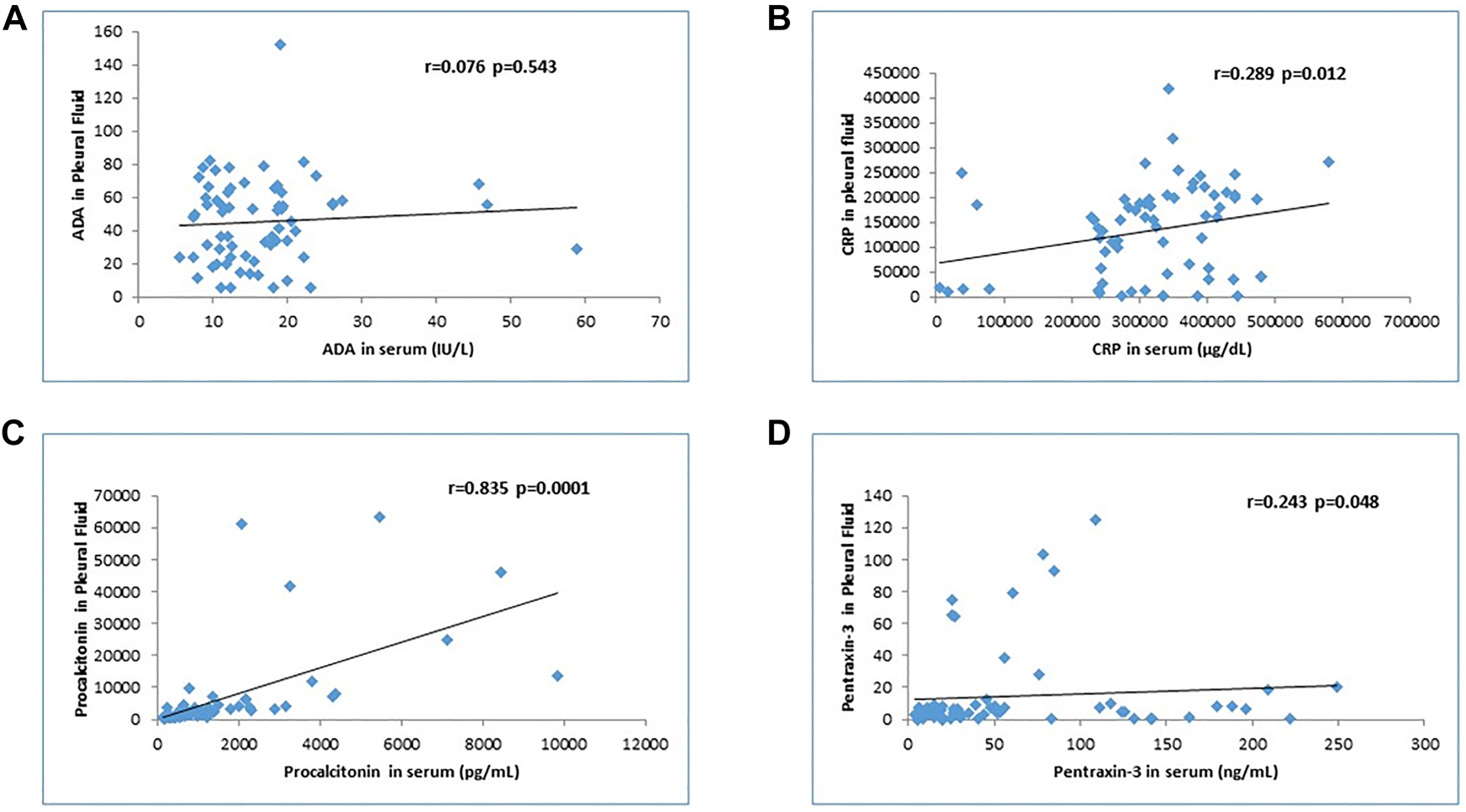
Analysis of the correlation between serum and pleural fluid levels of four biomarkers (ADA, CRP, PCT, and PTX-3).
(A) Adenosine deaminase (ADA). (B) C-reactive protein (CRP). (C) Procalcitonin (PCT). (D) Pentraxin-3 (PTX-3). There is a significant correlation for CRP, PCT, and PTX-3 but not for ADA.
Discussion
The purpose of our study was to find unsurpassed abilities of biochemical markers in diagnosing and differentiating pleural effusion. We evaluated two established (ADA, CRP) and two more recent biomarkers (PTX-3 and PCT) in serum and pleural fluid separately to find their accuracy and efficiency to discriminate pleural effusion. Our study’s novelty was the analysis of different biomarkers simultaneously in the serum and pleural fluid, revealing that PTX-3 measurement in the pleural fluid is more accurate than in the serum. In contrast, both serum and pleural fluid levels of PCT provided a higher accuracy rate in discrimination of pleural effusions of different etiologies.
Analysis of serum biomarkers for discriminating the origin of pleural effusions
The prior literature shows that PCT in serum and pleural fluid can differentiate between PPE and MPE [19]. Elevated serum PCT levels were also found in community-acquired pneumonia (CAP) vs. tuberculous patients [20]. The present comparative analysis of four biomarkers found that significantly elevated PCT in serum and pleural fluid differentiates between TPE, PPE, and MP in agreement with the previous studies.
Analysis of pleural fluid biomarkers for discriminating the origin of pleural effusions
In the present study, biomarker analyses in the pleural fluid revealed that ADA, PCT, and PTX-3 in pleural fluid significantly differentiate PPE from TPE and MPE. ADA levels were found to be significantly higher in tuberculous exudative vs. nontuberculous exudative pleural effusions [21], [22], [23]. Similarly, PTX-3 in the pleural fluid could distinguish between PE of various etiologies and differentiate PPE from TPE and MPE. This result confirms previous data showing that pleural fluid levels of PTX-3 could significantly differentiate between PE of different etiologies [24], [25]. Estimating pleural fluid PCT levels in our study showed that PCT significantly distinguishes between PPE, TPE, and MPE. Again, this confirms previous studies showing that PCT levels can diagnose between PPE and non-PPE [25], [26]. Various studies suggested pleural fluid CRP is a better indicator to differentiate between PE of different etiologies [27], [28]. However, we found that pleural fluid CRP did not significantly distinguish between PPE, TPE, and MPE.
After determining the area under the curve of PCT and PTX-3 levels in the pleural fluid at a particular cut-off value, we found both biomarkers suitable to differentiate PPE from TPE. We set the cut-off value based on literature data. Wang et al. [26] reported that PCT levels could differentiate PPE from non-PPE at a cut-off point of 0.18 ng/mL with an AUC 0.776 (sensitivity, 69.7%; specificity, 72.1%). With the same cut-off point for PCT (0.18 ng/mL), Lin et al. [29] reported an AUC of 0.752 (sensitivity, 66.7%; specificity, 77.4%). Yeo et al. [30] found that PTX3 yielded the most constructive discriminating ability to predict PPE from MPE or TPE by providing the following: AUC, 0.74 (95% CI, 0.63–0.84), sensitivity, 62%; and specificity, 81% at a cut-off point of 25.00 ng/mL. Contrastingly, Porcel et al. [18] determined that PCT levels in the pleural fluid were of little value. Gabhale et al. [31] reported pleural fluid CRP to have good sensitivity (97.05%) and specificity (71.76%) in distinguishing TPE from non-TPE.
Our study also has certain limitations. Firstly, the sample size was relatively small, and there was a variation in cases accretion. Thus, our data has exploratory value, and the obtained cut-offs from ROC must be re-assessed in a large population to validate the findings. Secondly, sampling bias may have been present during the pleural fluid tapping procedure, which may have influenced the validity of laboratory measurements. We did not analyze the demographic and baseline patient characteristic (urban/rural, smoking, alcohol). Further laboratory data such as serum/pleural levels of glucose/HbA1c, albumin and serum/fluid cytology were not considered. A possible influence of the treatment applied (e.g., administration of chemotherapy, steroids, or antibiotics) on the laboratory results cannot be excluded.
There is no gold standard diagnostic technique for differential diagnosis of pleural effusion. The available traditional methods like cytology and microbiology are time-consuming. Depending on the laboratory consultant’s expertise, there is a chance of subjective bias affecting the patients’ management. Radiological investigations (USG/CT/MRI) are rapid but cannot distinguish the cause of the pleural effusion. Radiological studies are costlier, require specific technical infrastructure as well as diagnostic confirmation. The present study shows that newer biomarkers might be sensitive and specific enough compared to traditional methods. Although we did not evaluate these aspects, biomarker testing might be more cost-effective, rapid, reliable, and easy to perform than available comparators, improving the capacity to differentiate pleural effusion. Serum and pleural fluid PCT and PTX-3 levels might be useful early biomarkers to diagnose pleural effusions’ etiology. However, more extensive, adequately powered prospective studies are required to validate our findings.
Funding source: Himalaya Institute of Medical Science, Swami Rama Himalaya University
Award Identifier / Grant number: SRHU/HIMS/RC/2017/23
Research funding: This study was supported by a grant from Himalaya Institute of Medical Science, Swami Rama Himalaya University (SRHU/HIMS/RC/2017/23).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: Approval of the Institutional Review Board was taken before the study, and the IRB approval number was HIMS/RC/2017/40 dated 31/01/2017.
References
1. Fishman, AP, Elias, JA, Fishman, JA, Michael, GA, Robert, SM, Pack, AI. Non-malignant pleural effusion. In: Fishman Alfred, P, editor. Fishmans pulmonary diseases and disorders, 4th ed. New Delhi: McGraw-Hill; 2008:1487–9.Search in Google Scholar
2. Heffner, JE. Discriminating between transudates and exudates. Clin Chest Med 2006;27:241–52. https://doi.org/10.1016/j.ccm.2005.12.008.Search in Google Scholar
3. Samanta, S, Sharma, A, Das, B, Mallick, AK, Kumar, A. Significance of total protein, albumin, globulin, serum effusion albumin gradient and LDH in the differential diagnosis of pleural effusion secondary to tuberculosis and cancer. J Clin Diagn Res 2016;10:14–8.10.7860/JCDR/2016/20652.8379Search in Google Scholar PubMed PubMed Central
4. Maskell, NA, Batt, S, Hedley, EL, Davies, CW, Gillespie, SH, Davies, RJ, et al.. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817–23. https://doi.org/10.1164/rccm.200601-074oc.Search in Google Scholar
5. Daniil, ZD, Zintzaras, E, Kiropoulos, T, Papaioannou, AI, Koutsokera, A, Kastanis, A, et al.. Discrimination of exudative pleural effusions based on multiple biological parameters. Eur Respir J 2007;30:957–64. https://doi.org/10.1183/09031936.00126306.Search in Google Scholar
6. Davies, HE, Davies, RJO, Davies, CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii41–ii53. https://doi.org/10.1136/thx.2010.137000.Search in Google Scholar
7. Chaudhary, S, Patel, AK. Role of pleural fluid adenosine deaminase (ADA) for the diagnosis of tuberculous pleural effusion. Calicut Med J 2010;8:e4.Search in Google Scholar
8. Izhakian, S, Wasser, WG, Fox, BD, Vainshelboim, B, Kramer, MR. The diagnostic value of the pleural fluid C - reactive protein in parapneumonic effusions. Dis Markers 2016;2016:7539780. https://doi.org/10.1155/2016/7539780.Search in Google Scholar
9. Liu, S, Qu, X, Liu, F, Wang, C. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediat Inflamm 2014;2014:421429. https://doi.org/10.1155/2014/421429.Search in Google Scholar
10. Bottazzi, B, Bastone, A, Doni, A, Garlanda, C, Valentino, S, Deban, L, et al.. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol 2006;79:909–12. https://doi.org/10.1189/jlb.1005557.Search in Google Scholar
11. Wisniewski, HG, Vilcek, J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev 2004;15:129–46. https://doi.org/10.1016/j.cytogfr.2004.01.005.Search in Google Scholar
12. Camozzi, M, Zacchigna, S, Rusnati, M, Coltrini, D, Ramirez-Correa, G, Bottazzi, B, et al.. Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler Thromb Vasc Biol 2005;25:1837–42. https://doi.org/10.1161/01.atv.0000177807.54959.7d.Search in Google Scholar
13. Lee, SH, Lee, EJ, Min, KH, Hur, GY, Lee, SY, Kim, GH, et al.. Procalcitonin as a diagnostic marker in differentiating parapneumonic effusion from tuberculous pleurisy or malignant effusion. Clin Biochem 2013;46:1484–8. https://doi.org/10.1016/j.clinbiochem.2013.03.018.Search in Google Scholar
14. Al-Aarag, AH, Mohammed, SA, Ibrahim, HY, Mohammad, OI. Diagnostic value of procalcitonin in pleural effusion. Egypt J Chest Dis Tuberc 2020;69:392–8.10.4103/ejcdt.ejcdt_162_19Search in Google Scholar
15. Mehanic, S, Baljic, R. The importance of serum procalcitonin in diagnosis and treatment of serious bacterial infections and sepsis. Mater Sociomed 2013;25:277–81. https://doi.org/10.5455/msm.2013.25.277-281.Search in Google Scholar
16. Uzzan, B, Cohen, R, Nicolas, P, Cucherat, M, Perret, GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 2006;34:1996–2003. https://doi.org/10.1097/01.ccm.0000226413.54364.36.Search in Google Scholar
17. Porcel, JM, Vives, M, Cao, G, Bielsa, S, Ruiz-González, A, Martínez-Iribarren, A, et al.. Biomarkers of infection for the differential diagnosis of pleural effusions. Eur Respir J 2009;34:1383–9. https://doi.org/10.1183/09031936.00197208.Search in Google Scholar
18. Light, RW. Clinical practice: pleural effusion. N Engl J Med 2002;346:1971–7. https://doi.org/10.1056/nejmcp010731.Search in Google Scholar
19. Wafaa, S, Shimy, EL. Diagnostic value of procalcitonin and C-reactive protein in differentiation between some benign and malignant pleural effusions. Egypt J Chest Dis Tuberc 2014;63:923–30.10.1016/j.ejcdt.2014.06.006Search in Google Scholar
20. Ujagin, M. Usefulness of serum procalcitonin levels in pulmonary tuberculosis. Eur Respir J 2011;37:371–5.10.1183/09031936.00011910Search in Google Scholar PubMed
21. Gupta, BK, Bharat, V, Bandyopadhyay, D. Role of adenosine deaminase estimation in differentiation of tuberculous and non-tuberculous exudative pleural effusions. J Clin Med Res 2010;2:79–84. https://doi.org/10.4021/jocmr2010.03.280w.Search in Google Scholar
22. Samanta, S, Sharma, A, Das, B, Mallick, AK, Kumar, AP. A co-relative study of ADA and CYFRA 21-1 in serum and pleural effusion secondary to tuberculosis and cancer. Scholars J Appl Med Sci 2016;4:3020–7.Search in Google Scholar
23. Yadav, D Taparia, P, Mishra, S, Agnihotri, SP. Usefulness of pleural fluid ada level in differential diagnosis of exudative pleural effusion – a pilot study. Int J Curr Med Pharmaceut Res 2016;2:513–9.Search in Google Scholar
24. Ozsu, S, Abul, Y, Mentese, A, Bektas, H, Uzun, A, Ozlu, T, et al.. Pentraxin-3: a novel biomarker for discriminating para-pneumonic from other exudative effusions. Respirology 2013;18:657–62. https://doi.org/10.1111/resp.12038.Search in Google Scholar
25. Ciftci, F, Bilgin, G, Ozcan, AN, Dogan, O, Yuksel, A, Erol, S, et al.. Eur Respir J 2017;50:PA365.Search in Google Scholar
26. Wang, CY, Hsiao, YC, Jerng, JS, Ho, CC, Lai, CC, Yu, CJ, et al.. Diagnostic value of procalcitonin in pleural effusions. Eur J Clin Microbiol Infect Dis 2011;30:313–8. https://doi.org/10.1007/s10096-010-1082-0.Search in Google Scholar
27. Turay, UY, Yildirim, Z, Turkoz, Y, Biber, C, Erdogan, Y, Keyf, AI, et al.. Use of pleural fluid C-reactive protein in diagnosis of pleural effusions. Respir Med 2000;94:432–5. https://doi.org/10.1053/rmed.1999.0759.Search in Google Scholar
28. Chierakul, N, Kanitsap, A, Chaiprasert, A, Viriyataveekul, R. A simple C-reactive protein measurement for the differentiation between tuberculous and malignant pleural effusion. Respirology 2004;9:66–9. https://doi.org/10.1111/j.1440-1843.2003.00521.x.Search in Google Scholar
29. Lin, MC, Chen, YC, Wu, JT, Ko, YC, Wang, CC. Diagnostic and prognostic values of pleural fluid procalcitonin in parapneumonic pleural effusions. Chest 2009;136:205–11. https://doi.org/10.1378/chest.08-1134.Search in Google Scholar
30. Yeo, CD, Kim, JW, Cho, MR, Kang, JY, Kim, SJ, Kim, YK, et al.. Pleural fluid pentraxin-3 for the differential diagnosis of pleural effusions. Tuberc Respir Dis 2013;75:244–9. https://doi.org/10.4046/trd.2013.75.6.244.Search in Google Scholar
31. Gabhale, SD, Taparia, P, Yadav, D, Agnihotri, SP. Usefulness of pleural fluid crp level in differential diagnosis of exudative pleural effusion – a pilot study. Int J Clin Biochem Res 2015;2:97–109.Search in Google Scholar
© 2021 Anita Sharma et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Trace amounts of irinotecan found in the blood of a surgeon after performing HIPEC: what does it imply?
- Research Articles
- Is the blood of a surgeon performing HIPEC contaminated by irinotecan, its major metabolites and platinum compounds?
- Nasogastric- vs. percutaneous gastrostomy tube for prophylactic gastric decompression after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
- Study of oxaliplatin penetration into ovaries of patients treated with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases of colorectal and appendiceal origin using mass spectrometry imaging
- Prevalence, clinical characteristics, and outcome of pleural effusions in ovarian cancer
- Efficacy of procalcitonin and pentraxin-3 as early biomarkers for differential diagnosis of pleural effusions
Articles in the same Issue
- Frontmatter
- Editorial
- Trace amounts of irinotecan found in the blood of a surgeon after performing HIPEC: what does it imply?
- Research Articles
- Is the blood of a surgeon performing HIPEC contaminated by irinotecan, its major metabolites and platinum compounds?
- Nasogastric- vs. percutaneous gastrostomy tube for prophylactic gastric decompression after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
- Study of oxaliplatin penetration into ovaries of patients treated with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases of colorectal and appendiceal origin using mass spectrometry imaging
- Prevalence, clinical characteristics, and outcome of pleural effusions in ovarian cancer
- Efficacy of procalcitonin and pentraxin-3 as early biomarkers for differential diagnosis of pleural effusions


