Abstract
Background
This case report aims to describe the impact of the bidirectional chemotherapy (BDC) on resecability for initially unresectable malignant peritoneal mesothelioma (MPM).
Methods
We report a case of 55-year-old male with the diagnosis of initially unresecable MPM. The BDC combined intravenous (IV) chemotherapy (Cisplatin–Pemetrexed) and intra peritoneal (IP) chemotherapy (Cisplatin). The response to chemotherapy was assessed by CT – scan and laparoscopy.
Results
Initial evaluation classed the disease as unresecable with PCI at 39. At the reevaluation, CT – scan and laparoscopy showed a macroscopic response, allowing surgery consisting of cytoreductive surgery and hyperthermic intra peritoneal chemotherapy (Doxorubicin and Cisplatin).
Conclusions
BDC (IV and IP) has promising results and allows to undergo surgery for selected patients with borderline or initially unresectable MPM.
We report a case of 55-year-old male with the diagnosis of malignant peritoneal mesothelioma (MPM). Initial evaluation with CT-scan and laparoscopy reveals unresectable peritoneal carcinomatosis with PCI at 39 with thickened omentum (star), small bowel (2 stars) and parietal peritoneum (dash-arrow) deposit, ascitis (plane-arrow) (Figure 1A, B). Bidirectional chemotherapy (BDC) has been performed after three cycles of intravenous (IV) CISPLATIN – PEMETREXED, with intensification combining three cycles of IV PEMETREXED with intraperitoneal (IP) CISPLATIN (Figure 2). At reevaluation, PCI was still at 39 with a macroscopic response (Figure 3A, B). The peritoneal disease was thinner allowing a complete CRS with DOXORUBICIN/CISPLATIN based-HIPEC.
BDC allowed selecting patients with initially unresectable MPM to undergo surgery and increase the overall survival (OS) [1, 2]. New IP delivery with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) reported promising results in palliative treatment of MMP [3] and is under evaluation to increase OS and secondary resectability of huge MMP (Clinical Trials NCT03875144).
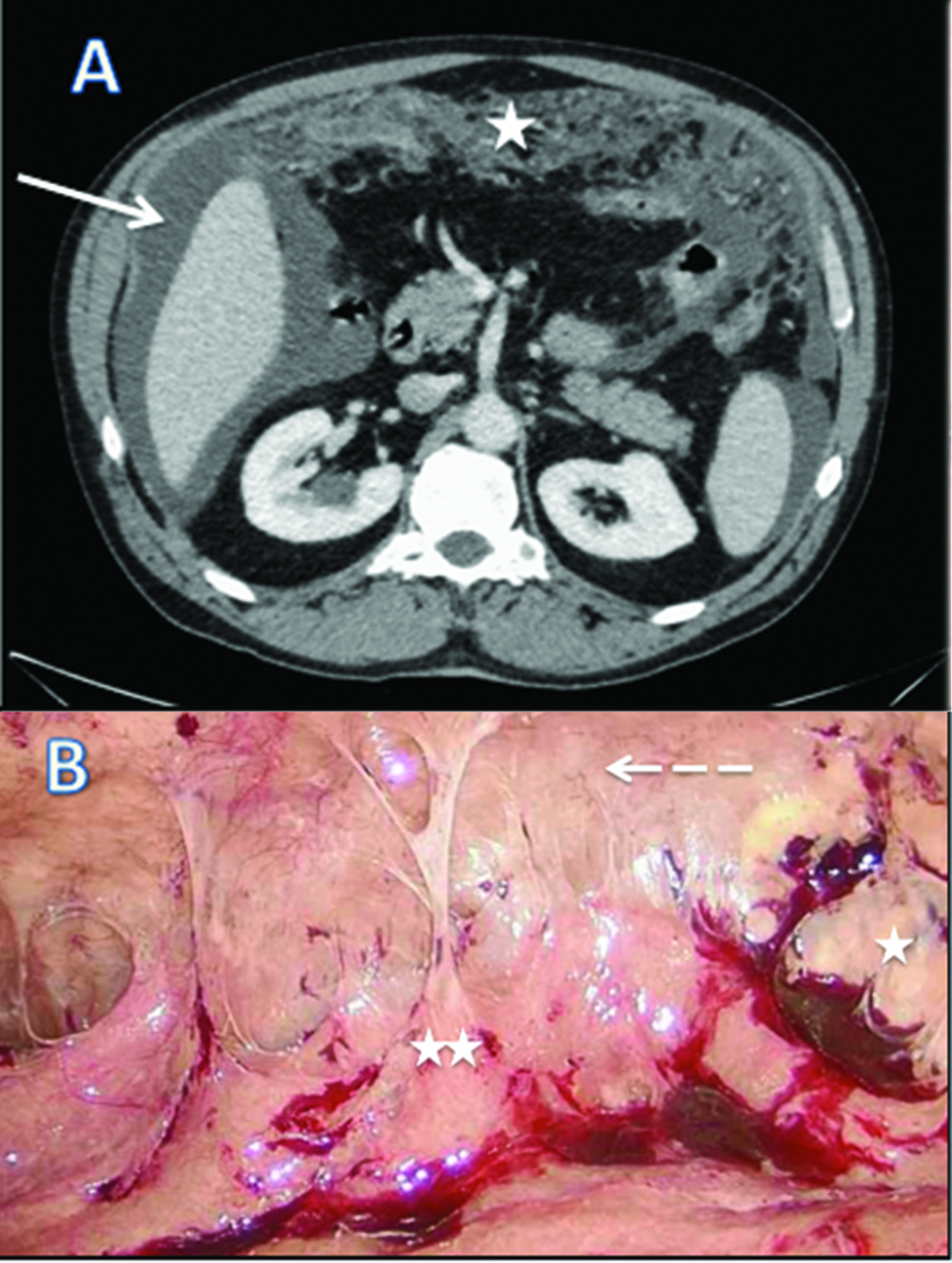
CT scan (A) and laparoscopic evaluation (B) at diagnosis of malignant peritoneal mesothelioma.
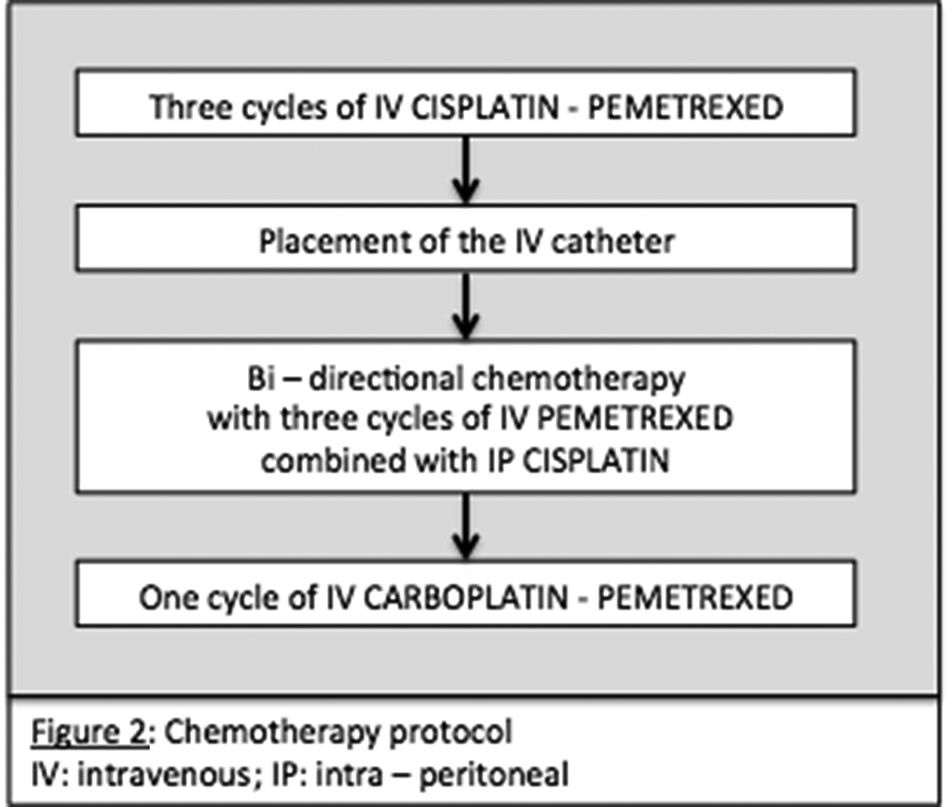
Detailed schedule of chemotherapy.
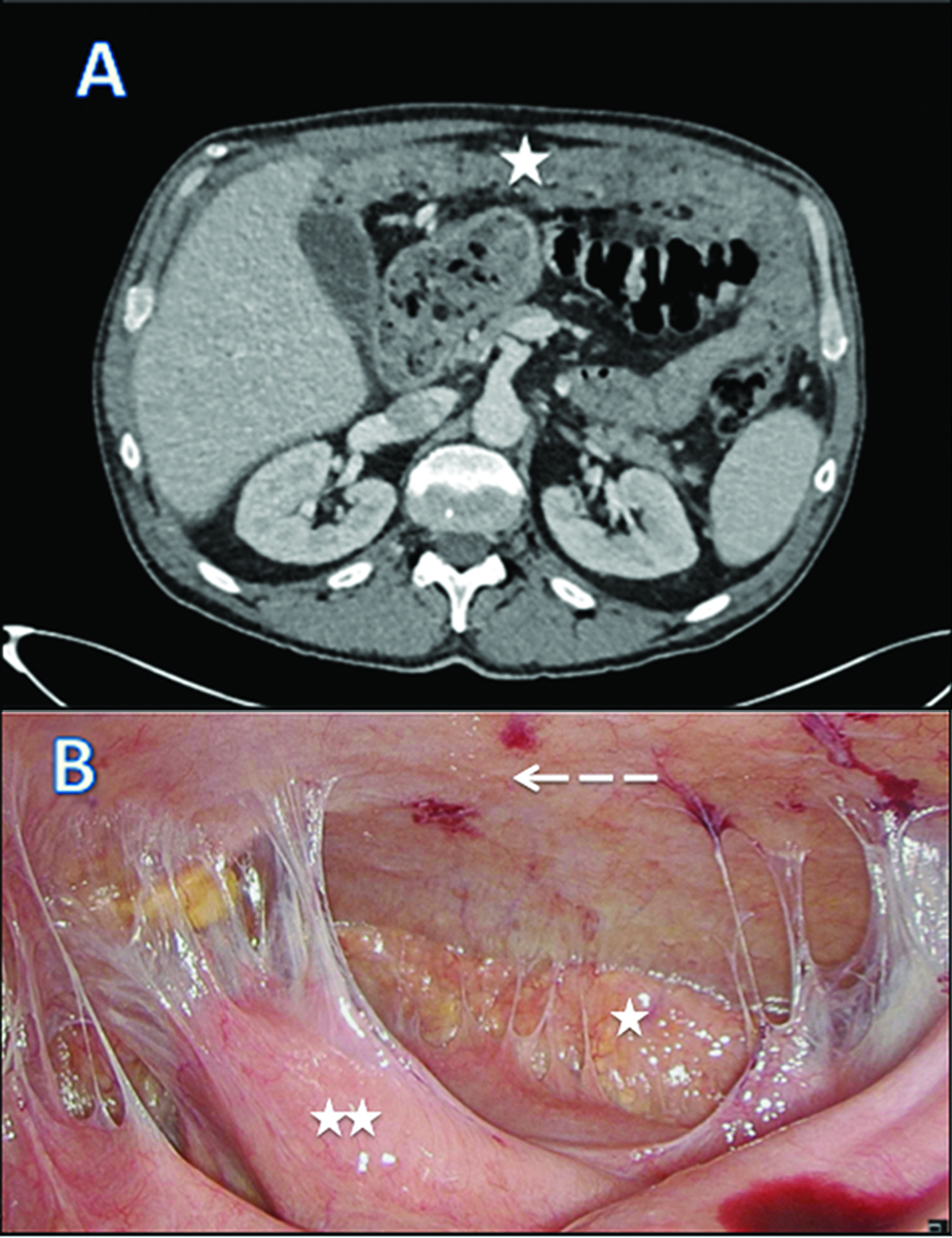
CT scan (A) and laparoscopic evaluation (B) after bi–directional chemotherapy.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Ethical considerations: Informed consent given by the patient (Registration number 24005 in RENAPE network, organization for the treatment of rare tumors of the peritoneum).
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res. 2018;7:599–608.10.21037/tlcr.2018.08.03Suche in Google Scholar PubMed PubMed Central
2. Le Roy F, Gelli M, Hollebecque A, Honoré C, Boige V, Dartigues P, et al. Conversion to complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma after bidirectional chemotherapy. Ann Surg Oncol. 2017;24:3640–6.10.1245/s10434-017-6033-xSuche in Google Scholar PubMed
3. Giger-Pabst U, Demtröder C, Falkenstein TA, Ouaissi M, Götze TO, Rezniczek GA, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer. 2018;18:442.10.1186/s12885-018-4363-0Suche in Google Scholar PubMed PubMed Central
© 2019 Noiret and Eveno, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Editorial
- Pleura – the new section in Pleura and Peritoneum
- Reviews
- Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: A systematic review of the literature
- Morbidity and mortality in women with advanced ovarian cancer who underwent primary cytoreductive surgery compared to cytoreductive surgery for recurrent disease: a meta-analysis
- Research Article
- MESOTIP: Phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma
- Clinical Images
- Bidirectional chemotherapy allowing surgery and HIPEC for malignant peritoneal mesothelioma
Artikel in diesem Heft
- Editorial
- Pleura – the new section in Pleura and Peritoneum
- Reviews
- Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: A systematic review of the literature
- Morbidity and mortality in women with advanced ovarian cancer who underwent primary cytoreductive surgery compared to cytoreductive surgery for recurrent disease: a meta-analysis
- Research Article
- MESOTIP: Phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma
- Clinical Images
- Bidirectional chemotherapy allowing surgery and HIPEC for malignant peritoneal mesothelioma


