Abstract
Background
Development of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) requires adequate preclinical models.
Methods
The model should be easy to use, reproducible and cost-effective. It should have a volume similar to the human abdominal cavity, and an oval shape. The inner surface should be lined with serosa. The model should allow pharmacological and biological analysis, including histology. No living animals should be used.
Results
The fresh urinary bladder is explanted from an adult bovine in the slaughterhouse. A 4-cm incision is performed into the bladder neck. The bladder can be inverted through the incision, which allows exposition of the serosa on its inner side. A balloon trocar is inserted through the incision and a ligature placed, ensuring full tightness. The therapeutic capnoperitoneum is installed. The bovine bladder has a volume somewhat smaller (2–3 L) than the human abdominal cavity (3–5 L). Costs are minimal. There is no significant bacteriological contamination. Manipulation is simple.
Conclusions
The (inverted) bovine urinary bladder is an innovative and versatile ex vivo model for optimizing drug delivery with therapeutic aerosols both onto the mucosa or the serosa. This model can be used for pharmaceutical Quality-by-Design approaches and will replace a large number of experiments in the animal.
Introduction
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) has received much attention in recent years due to its superior pharmacological properties, which offers potential clinical advantages [1]. In particular, locoregional administration of a therapeutic substance as an aerosol under pressure allows higher tissue penetration, optimal exposition of the surface of the target organ to the drug, dose reduction and low systemic exposure. Therapeutic pressurized aerosols have many potential applications in cancer, in particular for treating diseases of the peritoneal [2] and pleural cavities, but also within hollow organs such as the esophagus [3] or the urinary bladder.
A particular problem is to optimize the physico-chemical characteristics of therapeutic aerosol in order to optimize drug delivery into the target tissues. For example, Khosrawipour et al. has demonstrated recently that the homogeneity of the therapeutic aerosol applied during PIPAC is not perfect [4]. However, the experimental model used by these authors (tissue fragments placed into a plastic container box) was suboptimal because the plastic surfaces are not absorbing any substances so that the aerosols droplets are falling down rapidly to the floor of the container, due to gravity.
The present paper presents an innovative model for optimizing homogeneity of drug delivery using therapeutic pressurized aerosols. This model consists of the fresh bovine urinary bladder ex vivo. This model can be used for evaluating the target effect of therapeutic aerosol both onto the mucosa or, by inverting the organ, onto the serosa. The (inverted) bovine urinary bladder is a novel ex-vivo model in which physico-chemical characteristics of a therapeutic aerosol can be optimized easily.
Materials and methods
Ethical approval
The conducted research is not related to either human or animals use. This is an ex vivo study on fresh bovine organ. No authorization of an animal protection committee was needed.
Specifications list
A list of specifications was defined a priori for the model intended. The model should be easy to use, reproducible and cost-effective. It should have a volume similar to the human abdominal cavity, and an oval shape. The inner surface should be lined by serosa. The model should allow pharmacological and biological analysis, including histological analysis. A further condition was not to sacrifice living animals for the experiment.
Material
Fresh organs were obtained from the slaughterhouse and transported to the experimental laboratory at a temperature of 4–8 °C. The organs were toughly-rinsed with physiological solution. The current investigation involved sampling and analyzing various organs of bovine origin (stomach, urinary bladder). Two gastric organs were analyzed, followed by eight urinary bladders.
The bovine stomachs were opened, inverted, an adequate portion of the multilobulated organ dissected. Then, the organ was closed (mucosa outside, serosa inside) with a running suture of Mersilene® 1. Then, a 12 mm balloon trocar (Kii®, Applied Medical, Düsseldorf, Germany) was inserted through the parietal wall and a capnoperitoneum of 12 mmHg installed.
The bovine urinary bladder was prepared as following: a 4-cm incision was performed at the bladder neck and the organ inverted through the incision. Then a pursuing suture (Mersilene 1) was placed along the edges of the incision, a 12 mm balloon trocar (Kii®, Applied Medical, Düsseldorf, Germany) was inserted through the open bladder neck, the suture closed and a capnoperitoneum of 12 mmHg installed.
Application of therapeutic aerosol
Application of the therapeutic aerosol was performed as described previously [1]. Shortly, 30 mL of a saline solution stained with methylene blue 0.0003 % were aerosolized with a nebulizer (Capnopen®, Capnomed, Villingendorf, Germany) at a pressure of 15 mmHg and room temperature (24.5 °C) and maintained for 15 min.
Then the organs were opened and the serosa examined. Homogeneity of the distribution was first studied qualitatively by visual examination. Results were photo-documented.
Occupational health safety
Additional experiments were performed within a class-2 safety hood certified for application of cytostatic drugs (Maxisafe 2000, ThermoFisher Scientific, Dreieich, Germany). 150 mL NaCl containing 7.5 mg Cisplatin (Teva, Blaubeuren, Germany) were aerosolized into the safety hood. Occupational healthy safety conditions were examined by an independent certified company (DEKRA industrials, Stuttgart, Germany). The probe sampling system used was a Gravikon VC25 device combined with a dust detector (Ströhlein, Kaarst, Germany). Air was collected on a cellulose nitrate filter with a diameter of 50 mm, with a flow of 22.5 m3/h. Toxicological research analysis of cisplatin levels was performed according to a standard protocol (NIOSH 7300). The detection limit was 0.3 ng/sample. Sampling and analysis were performed by engineers of the Division for Hazardous Substances at the Laboratory for Environmental and Product Analysis of DEKRA Industrial GmbH.
Results
The bovine inverted urinary bladder was well suited for optimizing therapeutic aerosols ex vivo. A 4 cm incision was performed into the bladder neck of the explanted organs. A pursuing suture was placed along the edges of the incision. Then a balloon trocar (Kii®, Applied Medical Systems) was inserted through the incision and the ligature knotted, ensuring full tightness (Figure 1A). CO2 was then insufflated through the trocar, and the therapeutic capnoperitoneum was installed using the nebulizer, applying a macromolecule as staining substance (toluidine blue). Tightness of the organ was easy to achieve. The inverted urinary bladders had a volume (2–3 L) somewhat smaller than the human abdominal cavity (3–5 L).
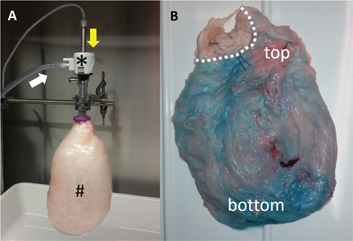
The (inverted) bovine urinary bladder model insufflated with CO2 at a pressure of 15 mmHg at room temperature. Panel (A) shows the experimental setup with (#) the inverted urinary bladder (mucosa outside, serosa inside), (*) the balloon trocar (Kii®, Applied Medical), (white arrow) the CO2-insufflation line and (yellow arrow) the nebulizer (Capnopen®, Capnomed). Panel (B) shows a homogeneous repartition of the staining substance (toluidine blue) onto the serosa after aerosolization into the inverted organ. Dotted line: bladder neck.
As an embodiment, the organ was inverted through the bladder neck incision, which then allowed exposition of the serosa on the inner surface of the model. This is possible because the anatomy of the bovine urinary bladder differs from the human one: the bovine urinary bladder is protruding into the abdominal cavity and is almost completely covered by peritoneum. In this case, a simple surgical dissection of the paravesical fatty tissue was needed prior inversion of the organ. First qualitative analysis of the homogeneity of repartition of the therapeutic aerosol was performed, showing a relatively homogeneous blue staining (Figure 1B). Specifically, it was observed that the aerosol droplets did not drop down completely to the bottom of the organ, but were absorbed into the tissue on the whole surface exposed, including the upper segment of the organ. The only remaining unstained surface was the upper part of the bladder neck (dotted line), an area located above the tight pursuing suture. The organs were clean (no significant bacteriological contamination) and manipulation was simple and hygienic. Costs were minimal (around 5€/organ). All organs provided by the slaughterhouse could be used.
As a further embodiment, a brush electrode could be placed (IonWand®, Alesi Surgical) to induce electrostatic precipitation of the therapeutic aerosol within the experimental system.
In contrast, manipulation of inverted bovine stomachs was cumbersome. It was difficult to dissect reproducible fragments, due to the complex anatomy of the multilobulated stomach in the cow. Extensive running sutures (up to 160 cm) were needed to obtain a closed space. Geometry of the sutured organ was pyramidal (photodocumentation on file), oval volumes could not be easily generated. Complete tightness was difficult to achieve. The final volume of the organ was difficult to predetermine. The organs were contaminated with bacteria, rapid tissue necrosis and degradation was observed. Therefore, the inverted stomach model was rapidly abandoned.
From the occupational health safety perspective, manipulation of toxic therapeutic aerosols containing platin-based chemotherapeutic agents in this ex vivo model was demonstrated to be safe. Environmental air analysis in the laboratory revealed no traces of cisplatin.
Discussion
We propose to use the bovine urinary bladder as an ex vivo model for evaluating homogeneity of distribution of a therapeutic substance using pressurized aerosols. An interesting property of the ex vivo bovine urinary bladder model is that it allows evaluation of the target effect of the therapeutic substance onto the mucosa or alternatively onto the serosa. For this last purpose, the organ can be simply inverted through a small bladder neck incision in order to expose the serosa on the inner surface of the organ.
Potentially, the inverted bladder model might allow evaluation of (1) the target effect on serosa (pharmacological and biological effects) (2) the homogeneity of aerosol distribution (3) the effect various therapeutic substances and (4) optimization of the physico-chemical parameters of the operating environment.
The present e-vivo (inverted) urinary bladder model has several advantages over existing models, as summarized in Table 1. In vivo experiments on the living animal are probably the best model for optimizing therapeutic aerosols [5, 6], as compared to ex vivo experiments [4] or experiments using cadavers [7]. Although the use of live animals continues to be necessary to protect human animal health, it is desirable to replace such procedures by other methods not entailing the use of live animals [8]. Evaluation of the homogeneity of repartition of therapeutic aerosols in the live animals might also have an unexpected limitation: drug absorption through the serosa or the mucosa might depend not only on the quality of the aerosol, but also on the organ examined [9]. For example, small bowel serosa – which permits bacterial translocation [10] – might be much more permeable to macromolecules than the gastric serosa. Thus, evaluation of the influence of technical factors on homogeneity of aerosol distribution in vivo might be biased by organ-specific factors [7]. Moreover, performing animal experiments requires extensive administrative work for obtaining regulatory approval. Finally, animal experiments are expensive.
Comparison of three experimental systems for quality-by-design approaches for optimization of therapeutic (pressurized) aerosols.
| In-vivo large animal model (live experiment) | Tissue fragments in a plastic container | Ex-vivo (inverted) bovine urinary bladder | |
|---|---|---|---|
| Living animal needed | Yes | No | No |
| Animal review board approval needed | Yes | No | No |
| Costs | High | Low | Low |
| Role of anatomical factors | Considered | Not considered | Not considered |
| Role of vascularization | Considered | Not considered | Not considered |
| Evaluation of technical factors | Difficult | Easy | Easy |
| Adequacy of the parietal surfaces | Excellent | Poor | Good |
| Handling | Cumbersome | Moderately easy | Easy |
| Reproducibility | Moderate | High | High |
A further advantage of the e-vivo (inverted) urinary bladder model over the ex vivo model described previously by our group [11] is the superior absorption properties of the exposed serosal or mucosal surfaces in CO2. Within a plastic container, these droplets will sediment earlier than within an organ, since the absorption of the aerosol into the plastic walls will be indeed close to zero and the aerosol droplets will bead rapidly along the (colder) walls down to the floor of the experimental system, due to gravity [7].
The availability of an adequate model is important for pharmaceutical development in order to empower Quality by Design (QbD) approaches [12]. Pharmaceutical QdB is defined as a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management [7]. For example, in pulmonary aerosol medicine, the aerodynamic size of the aerosol inspired largely determines the distribution of this aerosol into an inhalable fraction (below 100 μm aerodynamic size), a thoracic fraction (around 11 μm) and a respirable fraction (around 4 μm) [13]. In pulmonary medicine, this classification of aerosol particle or droplets is typically achieved by using a cascade impactor (a device that classifies particles present in a sample of air or gas into known size ranges).
The (inverted) urinary bladder model is intended to be used for pharmacodynamics studies. For example, it allows easy testing of tissue uptake of various therapeutic substances over time. It also allows taking into consideration the physic-chemical characteristics of the operating environment, e. g. by examining the influence of pressure, temperature or electrostatic charges on tissue uptake. The model also allows to compare homogeneity of drug distribution between different nebulizers or aerosolizing devices.
The framework conditions during PIPAC are indeed much simpler than during respiratory application since the aerosol can be distributed directly to the target tissue without having to proceed through the trachea-bronchial tree and since no collaboration of the patient is needed for inspiring the therapeutic aerosol. However, quality of a therapeutic aerosol distributed with PIPAC cannot fully be guaranteed by only testing the finished solution. PIPAC is a complex multicomponent system, including a nebulizer, a high-pressure injector driving the nebulizer, a formulated therapeutic substance and a solution. This multicomponent system operates within a gaseous environment which physico-chemical characteristics (pressure, temperature, relative humidity, etc.) influence the behavior of the therapeutic aerosol. The performance of PIPAC in delivering an adequate dose to the target location requires a thorough understanding of the above-mentioned variables.
In conclusion, the (inverted) bovine urinary bladder ex vivo model is a versatile tool for optimizing drug delivery with PIPAC and provides experimental conditions close to the clinical situation. It allows evaluating the effect of various substances both on the mucosa and on the serosa. It is better suited for drug distribution studies than a plastic box. It is simple and cost-effective. This model will save a large number of experiments in the large animal. For Quality-by-Design experiments needed to optimize therapeutic aerosols administered as PIPAC, this model might find an acceptance comparable to the Anderson’s impactor in pulmonary medicine.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: Research funding was provided by institutional funds and by Capnomed GmbH, Villingendorf, Germany.
Employment or leadership: MAR is holder of various patents on PIPAC technology and a shareholder of Capnomed GmbH, Villingendorf, Germany. The other authors declare no conflict of interest.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann Surg Oncol 2014;21:553–9.10.1245/s10434-013-3213-1Suche in Google Scholar PubMed PubMed Central
2. Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol 2015;137:223–8.10.1016/j.ygyno.2015.02.009Suche in Google Scholar PubMed
3. Khalili-Harbi N, Herath N, Solass W, Giger-Pabst U, Dutreix M, Reymond MA. Pressurized intraluminal aerosol chemotherapy with Dbait in the distal esophagus of swine. Endoscopy 2016;48:184–7.10.1055/s-0034-1393180Suche in Google Scholar PubMed
4. Khosrawipour V, Khosrawipour T, Diaz-Carballo D, Förster E, Zieren J, Giger-Pabst U. Exploring the spatial drug distribution pattern of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2016;23:1220–4.10.1245/s10434-015-4954-9Suche in Google Scholar PubMed
5. Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012;26:1849–55.10.1007/s00464-012-2148-0Suche in Google Scholar PubMed
6. Kakchekeeva T, Demtröder C, Herath NI, Griffiths D, Torkington J, Solaß W, et al. In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol 2016;23:592–8.10.1245/s10434-016-5108-4Suche in Google Scholar PubMed PubMed Central
7. Khosrawipour V, Khosrawipour T, Kern AJ, Osma A, Kabakci B, Diaz-Carballo D, et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol 2016;142:2275–80.10.1007/s00432-016-2234-0Suche in Google Scholar PubMed
8. Directive 2010/63/EU of the European Parliament an of the Council on the protection of animals used for scientific purposes. Official Journal of the European Union L 276/3, 20.10.2010.Suche in Google Scholar
9. Flessner MF. Small-solute transport across specific peritoneal tissue surfaces in the rat. J Am Soc Nephrol 1996;7:225–33.10.1681/ASN.V72225Suche in Google Scholar PubMed
10. Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol 2017;30:45–53.10.20524/aog.2016.0086Suche in Google Scholar PubMed PubMed Central
11. Solass W, Herbette A, Schwarz T, Hetzel A, Sun JS, Dutreix M, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: Proof of concept. Surg Endosc 2012;26:847–52.10.1007/s00464-011-1964-ySuche in Google Scholar PubMed PubMed Central
12. ICH Expert Working Group. ICH Harmonized tripartite guideline. Pharmaceutical development Q8(R2). International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2009.Suche in Google Scholar
13. ISO 7708:1995. Air quality: Particle size fraction definitions for health-related sampling International Standards Organisation.Suche in Google Scholar
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Climate changes in the peritoneal cavity after CO2 laparoscopy: The risk of desertification
- Review
- Recommendations for pathological diagnosis on biopsy samples from peritoneal dialysis patients
- Opinion Paper
- Changes in the coelomic microclimate during carbon dioxide laparoscopy: morphological and functional implications
- Research Article
- Preserving fertility in pseudomyxoma peritonei, a novel approach
- Short Communication
- A new ex vivo model for optimizing distribution of therapeutic aerosols: the (inverted) bovine urinary bladder
Artikel in diesem Heft
- Frontmatter
- Editorial
- Climate changes in the peritoneal cavity after CO2 laparoscopy: The risk of desertification
- Review
- Recommendations for pathological diagnosis on biopsy samples from peritoneal dialysis patients
- Opinion Paper
- Changes in the coelomic microclimate during carbon dioxide laparoscopy: morphological and functional implications
- Research Article
- Preserving fertility in pseudomyxoma peritonei, a novel approach
- Short Communication
- A new ex vivo model for optimizing distribution of therapeutic aerosols: the (inverted) bovine urinary bladder

