Recent development in the formation and surface modification of cellulose-bead nanocomposites as adsorbents for water purification: a comprehensive review
Abstract
Water pollution is an issue of global concern that demands effective and sustainable solutions through water purification. Adsorption is a popular method for water treatment because it is inexpensive and has a high performance rate. Although commercial activated carbon is the generally preferred adsorbent for adsorption, its widespread use is affected by the high cost and challenges encountered during column adsorption. Biopolymers like cellulose and its derivatives have the potential to replace expensive adsorbents due to their unique characteristics. In recent years, cellulose-bead nanocomposites have gained significant attention as promising adsorbents due to their ability to circumvent the challenges encountered when using powdered adsorbents. To fabricate cellulose beads, cellulose fiber is separated from its source, dissolved in appropriate solvents, shaped into spherical particles and subsequently modified (via esterification, oxidation, crosslinking agents, etc.) to improve its adsorption capacity. This comprehensive review paper presents a detailed analysis of the recent development in the formation and surface modification of 3-D structured cellulose nanocomposites. The outcome of this review on modified cellulose-bead demonstrates their successful fabrication and high adsorption capacities for different contaminants. It is anticipated that cellulose beads, as a bio-adsorbent in industrial settings, will be a low-cost alternative to the more expensive adsorbents shortly.
1 Introduction
Water is the world’s most copious natural resource. However, freshwater makes up just approximately 3 % of the existing water sources and less than one-third of it is suitable for various household, agricultural and industrial activities [1, 2]. As global water consumption increases due to rapid population growth and development of the industrial sector and other environmental factors, the limited freshwater supplies are further depleted. In recent times, water-stressed conditions have been worsened by the contamination of available water resources [3]. Industrial effluents (e.g. textile fibre, rubber and leather wastewater, pharmaceutical, dye and plastic industries effluent), agricultural activities (e.g. pesticides application), municipal effluents and other environmental activities are the main sources of water contamination [4]. The water crisis is now an issue of global concern. Wastewater treatment has a vital role in the global augmentation of water supply. However, reclaiming contaminated water for re-use will require some form of effective treatment to remove contaminants [4, 5]. There have been reports of several removal techniques, comprising coagulation/flocculation, floatation, chemical precipitation, biological processes, advanced oxidation processes, membrane filtration and ion exchange resin [6], [7], [8], [9]. However, these methods are not frequently employed because of a number of limitations, such as their high cost, poor selectivity and sensitivity, inadequate removal, delicate working conditions, high energy demand, generation of secondary pollutants and costly disposal processes [6, 9–13]. Table 1 lists the benefits and limitations of the main conventional wastewater treatment methods.
Benefits and limitations of the major conventional methods used for the treatment of contaminated wastewater [6].
| Techniques | Main features | Merits | Limitations |
|---|---|---|---|
| Ion exchange |
|
|
|
| Solvent extraction (liquid-liquid extracion) |
|
|
|
| Chemical precipitation |
|
|
|
| Coagulation/flocculation |
|
|
|
| Electro-coagulation |
|
|
|
| Biological methods |
|
|
|
| Membrane filtration (ultrafilteration, nanofileration, reverse osmosis) |
|
|
|
| Advanced chemical oxidation |
|
|
|
Adsorption treatment technology is the preferred option for water treatment because of the main advantages of the technique which include high selectivity and performance, low cost, simplicity of operation, insensitivity to side effects of toxic pollutants, high regeneration potentials and availability of a wide range of adsorbents [9, 10]. Furthermore, adsorption may be utilized to remove diverse inorganic and organic contaminants without the formation of harmful intermediates or by-products [8, 14–16]. A significant factor in attaining optimal contaminant removal requires choosing an appropriate adsorbent based on the adsorbent type and characteristics of the adsorbate [17]. Many different natural materials including clay minerals, activated carbon (AC) and zeolite, have been explored as promising adsorbents for the removal of pollutants. AC is an extensively employed adsorbent with a wide specific surface area, large porosity and a high degree of surface reactions, which makes it unique [18]. However, the application of commercial AC is usually affected by the high cost of production, its non-selectivivity and scarcity of raw materials. The drawbacks associated with the other natural adsorbents include high manufacturing cost and low adsorption efficiency towards selected toxic compounds which limits their wider application [19], [20], [21].
Several researchers have in recent times, concentrated on the manufacture of adsorbents from sustainable and less expensive precursors such as agricultural wastes [22]. Agricultural residues are lignocellulosic materials that are superior to other adsorbents because they may be used without chemical modification or with minimal processing, thereby, significantly reducing production costs [23, 24]. They are low in ash composition and rich in carbon, which makes them useful precursors for the manufacture of activated carbon and as components of composite materials for the adsorption of organic and inorganic pollutants [25]. For instance, Olorundare et al. [26] chemically modified and converted maize tassels to activate carbon and therafter, utilized the AC for the elimination of phenolic chemicals [bisphenol A (BPA), ortho-nitrophenol (o-NTP) and parachlorophenol (PCP)]. The results obtained showed that the maize tassel-activated carbon (MTAC) was able to effectively remove the phenolic compounds with removal efficiencies of 90.84–98.49 %, 80.75–97.11 %, and 78.27–97.08 % for BPA, o-NTP and PCP respectively). In a similar report, Omo-Okoro et al. [27] created nanocomposite-activated carbon, known as physically activated maize tassel silver (PAMTAg) nanocomposites and chemically activated maize tassel silver (CAMTAg) nanocomposites to remove per and polyfluorinated alkyl substances (PFASs) from aqueous solutions. Omo-okoro and colleagues observed that the maximum adsorption capacity of the CAMTAg adsorbent for PFOS and PFOA were 454.1 mg/g and 321.2 mg/g respectively.
Unfortunately, studies have revealed some limitations in the conversion of plant biomass to AC. This includes the low carbonization energy of plant biomass which could be problematic, especially during thermal treatment. Unlike wood which undergoes thermal treatment at high temperatures, the surface morphology of plant biomass may be destroyed during pyrolysis because they easily burn off at low temperatures (160–200 °C) and generate soft ash. Furthermore, there is a risk of organic matter seeping from the biochar, which might lead to re-contamination of the final treated water (secondary pollution) [28]. To resolve this issue, the active component of the plant biomass could be recovered and directly modified for use. Most often, the total mass of the biomaterial is chemically modified with little consideration to isolate the active component responsible for the adsorption properties. The isolation of the most active component from the plant biomass is deemed crucial since this will eliminate components that may suppress or interfere with the adsorption properties, and yield cleaner, functional materials, resulting in increasing adsorption performance [29]. Cellulose is an important constituent of plant biomass which has been recognized as the most available and sustainable biopolymer on earth with distinctive characteristics that make it ideal for a variety of industrial applications [30, 31]. It is biodegradable, non-toxic with good mechanical capabilities, thus, it is regarded as environmentally friendly. Cellulose can be isolated from its source, chemically modified, and utilized as a nanocomposite for adsorption purposes [32–34]. The synthesized nanocomposites would be in a purer form due to the extraction of the cellulose from the plant biomass before subsequent modification. Studies have shown that the cellulose framework contains many valuable functional groups that facilitate its modification or chemical grafting onto different materials to form products that are ideal for a wide range of applications [29–31, 35, 36]. It has a significant amount of carbon and a proven ability for sorption. Cellulose has been successfully applied as nanocomposites for the potential removal of contaminants from water. Abou-Zeid et al. [37] created nanocomposites from cane pulp-derived cellulose through TEMPO oxidation and magnetic amino-modification and evaluated their effectiveness for removing lead ions from water fibers (FM-NPs). They reported that the nanocomposite had an 80 % effectiveness in eliminating lead ions from water. In a related work, Wang et al. [38] produced carboxymethylated cellulose fiber (CMF) adsorbent from bleached softwood kraft pulp fiber (SKF) using a controlled carboxymethylation modification. According to Wang and colleagues, the CMF adsorbent had good adsorption capacity as an adsorption capacity of 16.90 mg/g and 11.63 mg/g was recorded for Cu (II) and Ni (II), respectively. Similarly, Bawaani et al. [39] investigated the adsorption performance of nanocrystalline cellulose isolated from oil palm empty fruit bunch for the removal of dye from textile effluent. Bawaani and colleagues reported a 50.91 mg/g colour removal at an adsorbent dose as low as 0.066 mg/ml, confirming the efficiency of the NCC adsorbent.
Although the functionalization of cellulose and other powdered biomaterials is known to improve their compatibility and adsorption capacity for pollutants, the soft texture of the adsorbents in water can clog columns and significantly reduce the flow of eluent used to remove adsorbate from the adsorbent surface in adsorption/desorption studies. Cellulose beads/hydrogels are an advanced form of cellulose that has aroused more interest in recent times, due to their distinctive high porosity, functional surface and consequently numerous potential applications in filtration, catalysis, energy storage and drug or cell delivery among others. Furthermore, its large pores provide a wide surface area for interactions with target molecules, allowing for significant adsorption [40]. However, there are limited works in the literature on the fabrication, modification and application of cellulose beads as adsorbents. This article extensively explores the progress made in the development of cellulose beads and their application as adsorbents in water purification. The current study can offer a fundamental framework and a more in-depth perspective into modification techniques and fabrication procedures required for the development of cellulose bead nanocomposite. It provides an overview of cellulose; its composition, nano-forms and derivatives, making it a high-performing natural biopolymer of interest especially when utilized in the form of 3-D adsorbent. The review article further presents an in-depth discussion on the preparation procedures of cellulose beads, the recent modification strategies applied for enhanced adsorption performance of the beads as well as their potential applications/performance as adsorbents for the removal of contaminants from aqueous solutions.
2 The adsorption process and types of adsorbents for contaminants removal
Adsorption is a separation process that occurs when the constituents of a fluid, liquid or gas adhere to the exterior and interior surfaces of a solid substance [41]. The basis for the separation is the selective adsorption (thermodynamic and/or kinetic selectivity) of the contaminants by an adsorbent due to specific interactions between the surface of the adsorbent material and the adsorbed contaminants [41]. The contaminant of interest is the adsorbate present in the fluid phase and the solid phase is any material suitable as an adsorbent. In general, adsorbents exhibit distinct spatial and electrical features, as well as a wide range of activation sites that aids in the binding of the adsorbate and the general adsorption process [15]. Three stages make up the adsorption process: (1) adsorbate transport in the fluid phase to the adsorbent’s surface (film diffusion), (2) adsorbate migration into the pores of the adsorbent (diffusion inside particles) and (3) adsorbate adhesion to the adsorbent’s charged surface (surface bonding) [42]. Some of the parameters that influence the adsorption of molecules onto an adsorbent include the initial contaminant concentration, solution pH, temperature, interfering chemicals, adsorbate characteristics, adsorbent dosage, adsorbent surface type and adsorbent surface area [22].
2.1 Adsorption process in water treatment
The adsorption process is generally influenced by two main types of mechanisms: physical adsorption and chemical adsorption mechanisms. In the presence of interacting forces between the surfaces, the processes may take place at various interfaces, such as solid-liquid and/or solid–gas interfaces [43, 44]. However, the type of contaminants and the structural/chemical characteristics of the adsorbent surface are key factors that determine the adsorption mechanism for removing pollutants [22]. Physical adsorption is a reversible process which is the outcome of the physical contact between the adsorbed chemicals and the solid surface caused by weak interacting forces. Physical adsorption, also referred to as physisorption involves the formation of outer-sphere surface complexes when an adsorbate adheres to the surface of an adsorbent via weak electrostatic forces such as London forces, Van der Waals forces, polarity, steric interactions, hydrogen bonds, hydrophobicity and dipole induced-dipole interactions [45]. Physisorption can occur in either multiple layers or in a single layer because the adsorbate, which is mostly detached from the interacting active plane surface, remains confined by the binding energy [46]. It takes less heat to complete the desorption process in adsorbents involving physiosorption mechanisms, due to the weak binding energy. The majority of adsorbents involved in this type of mechanism have significant potential for regeneration of adsorption capacity, while the effluents discharged have low pollution potential and thus, are acceptable for disposal [47]. The activation energy in the physisorption process often falls between the 20–40 kJ/mol range, indicating a strong tendency for the active sorbent to dissolve in an aqueous media [48]. Though the mechanism correlates with the treatment variables, notably pH, dose, particle size, temperature, contact duration and agitation speed, this low activation energy might undermine the overall adsorption performance [46]. On the other hand, chemical adsorption, also known as chemisorption, is a process in which the adsorbate is permanently bonded to the adsorbent surface via chemical bonds or surface coordination compounds (i.e. inner-sphere surface complexes) between the adsorbate and the adsorbent surface [14, 49, 50]. In chemical sorption, a new bond is formed when the active plane surface of the sorbent and adsorbate breaks as a result of the chemical bonding in the chemisorption process [46]. This means that more intense adsorption energy and temperature; typically between 200 and 400 kJ/mol, are needed. The chemical bonding occurs through electron transfer or pairing, such as ion/ligand exchange, Lewis acid–base interaction and reduction/oxidation, etc. [14, 49, 50]. Single-layer adsorption occurs during chemisorption, and the mechanism is controlled by the treatment variables applicable in physisorption [47]. For a particular water treatment process, multiple adsorption interactions may take place simultaneously. These mechanisms aid in the binding of the contaminants to the surface of the adsorbent. The physisorption and chemisorption mechanisms are depicted in Figure 1.
![Figure 1:
Adsorption mechanisms, (A) physisorption and (B) chemisorption [47].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_001.jpg)
Adsorption mechanisms, (A) physisorption and (B) chemisorption [47].
2.2 Composite materials used for contaminants removal
Adsorbent selection is fundamental for a successful adsorption process development, performance and operation. A viable adsorbent should have a high adsorption interaction and removal efficiency toward the target contaminants. Adsorbents may be synthetic or from natural sources. Some of the common adsorbents used for removing contaminants from water include metal-organic frameworks, graphene-based materials, activated carbons (ACs), biomass, carbon nanotubes and polymeric materials [19, 51–54].
2.2.1 Porous organic polymers (co-ordinate polymers)
Co-ordination polymers, which include covalent organic frameworks (COFs) and metal-organic frameworks (MOFs), are a novel class of synthetic porous crystalline material [54]. MOFs are a kind of hybrid organic-inorganic crystalline materials that are connected by metal ions or clusters and organic ligands, giving them the properties of organic polymers and the stability of inorganic substances [54, 55]. In addition to having unsaturated sites and variable pore size; MOFs possess an immense specific surface area and thus, have great prospects as materials for the adsorption of diverse contaminants [55]. Liu et al. [56] created two stable metal-organic frameworks (MOFs) of amine-functionalized MIL-101 based on trivalent metal aluminium and iron [NH2-MIL-101(Al)] and [NH2-MIL-101(Fe)] by simple solvothermal reactions. According to Liu and colleagues, the two MOFs had a high adsorption capacity (>79.414 mg/g at 298 K) for phosphates. Likrwise, Ouyang et al. [57] synthesized a bismuth-based metal-organic framework (Bi-MOF, CAU-17) for the removal of selenite (SeO3 2−) anion from aqueous solution. They reported an ultra-high adsorption capacity of 255.3 mg/g. Furthermore, the adsorbent exhibited fast kinetics and a broad adsorption range of pH 4–11. However, MOF materials have poor mechanical characteristics but they can be mixed with a stable matrix material to enhance their mechanical characteristics [54].
Covalent organic frameworks are large, porous crystalline molecules formed entirely from covalently bonded basic elements (O, B, N, H and C) and organic building blocks. These substances are more extensive in structure and less dense than inorganic substances [53]. COFs have a large surface area of about 6450 m2/g and a stiff, strong structure that is resilient at temperatures as high as 600 °C [53, 58]. Xiong et al. [59] prepared a COF [NH4] +[COF–SO3 −] adsorbent for the selective extraction of thorium (Th IV) from uranium (U), and rare earth elements. Their findings demonstrate that the COF adsorbent had a maximum adsorption capacity of 395 mg/g for Th (IV) and excellent Th(IV) selectivity over uranium and rare earth elements.
2.2.2 Carbon naotubes (CNT)
Carbon nanotubes or one-dimensional carbon nanomaterials are hollow graphitic nanomaterials with well-ordered rolled-up structures, composed entirely of carbon [52]. To comprehend the structure of a carbon nanotube, consider it as a rolled-up sheet of graphene with a planar-hexagonal arrangement of carbon atoms arranged in a honeycomb lattice [60]. CNTs are classified based on their rolling layers of graphene sheets, as single-walled carbon nanotubes (SWCNTs), dual-walled carbon nanotubes (DWCNTs) and multi-walled carbon nanotubes (MWCNTs) [60–62]. They are distinguished from each other by their ability to transport water, specific surface area, chemical inertness and mechanical strength [62]. The SWCNTs are produced from a single graphene sheet that has been folded upon itself with a diameter of 1–2 nm and length determined by varying the production method. The DWCNT are produced from two concentric CNTs, with the outer tube encasing the inner tube. They offer a unique combination of SWNT qualities that are superior to those of SWNT in terms of better stability and stiffness [61]. MWNTs are made up of numerous layers of graphene wrapped up on themselves, with widths ranging from 2 to 50 nm, depending on the number of graphene tubes [63]. CNTs can be produced in large quantities using a variety of techniques, which are typically classified into five types: carbon arc discharge technique, laser ablation, sonochemical or hydrothermal methods, electrolysis and chemical vapour deposition (CVD) [60, 63].
The CNTs are materials of interest in analytical and material sciences due to their various appealing properties which include their strong mechanical and physiochemical stability, larger surface area, high tensile strength, resistance to temperature changes, non-corrosive and good adsorption capacity [52, 64]. Thus, CNTs have been used as adsorbents to remove pollutants from gaseous or aqueous environments, either in their natural form or after some modification treatment. Al-Saidi et al. [65] successfully used pristine MWCNTs as a solid phase for the removal of 95.6–98.91 % bismuth [Bi(III)] ions @ pH 0.1, from aqueous media. Similarly, Ehyaee et al. [66] synthesized magnetic multi-walled carbon nanotube nanocomposites (m-MWCNT) for the adsorptive removal of methyl violet (MV) from aqueous solutions. They reported a MB removal efficiency of 99.51 %. Unfortunately, the application of CNTs has some limitations due to some issues which include (1) the high cost of production, (2) a lack of solubility in most solvents suitable for natural (aqueous) environments, (3) manufacturing batches of CNTs with the same properties and repeatable chemical structure and (4) difficulty in maintaining high quality and low impurity levels during CNTs production [67].
2.2.3 Graphene-based materials
Graphene is a 2D layered sheet composed of single-atom sp2 hybridized carbon that has received notable attention from researchers globally. To create graphene oxide (GO) and reduced graphene oxide (RGO), several oxygen-containing functional groups are incorporated into graphene [54, 68]. Graphene has emerged as a useful nano-adsorbent due to (1) its significant surface area (theoretical value 2630 m2/g (2) its 2D honeycomb lattice structure, which may substantially adsorb contaminants with both sides of a planar sheet; and (3) the hexagonally arranged sp2 carbon atoms which contains a large p-electron delocalized structure that can develop a strong p-stacking interaction and hydrophobic interaction with the contaminants. Graphene may combine with different advanced materials to yield unique composite adsorbents with high adsorption efficiency [54]. A drawback in the use of these adsorbents is the high cost involved when sophisticated methods like chemical vapour deposition are used to produce grapheme-based materials of high quality and quantity while the inexpensive methods produce low-quality materials in lower quantities [69]. Moreover, graphene-based materials develop irreversible aggregates of layers and possess poor bonding affinity for anionic molecules [54, 70]. These disadvantages lower the efficiency of graphene-based adsorbents and limit their applications [19].
2.2.4 Silica-based adsorbents
Silica dioxide, sometimes known as silica, is a key component of sand. Silica has some adsorptive properties such as high surface area and porosity, a large specific surface area, excellent mechanical stability and a high adsorption capacity for dyes and heavy metals [71, 72]. Due to the presence of silanol groups, it exhibits hydrophilic qualities. However, because silica has a low resistance to alkali, it must be utilized in a solution with a pH lower than 8. Furthermore, nonspecific and irreversible adsorption will occur on the surface of siliceous materials containing acidic silanol groups [71]. Silica can be modified by various functional groups (e.g. an amino functional group) to lessen the undesirable properties and improve their adsorption capacities. Silicon alkoxide precursors, most notably tetraethyl orthosilicate (TEOS), are frequently employed as silica sources [73]. Studies on modified silica and mesoporous silica nanoparticles and their efficiency in eliminating environmental toxins from water and wastewater have been reported in the literature [71, 72]. Mesoporous silica (MS), which has pores between 2 and 50 nm in size, is a highly effective synthetic material for adsorption because of its high specific surface areas (as high as 2370 m2/g) and large pore sizes (about 50 nm) [73]. The main benefits of MS materials over other adsorbents with high adsorption capabilities for contaminants are their highly functionable structure and surface chemistry as well as their inexpensive production costs [73]. Organic ligands-based materials with unique physicochemical characteristics can be synthesised and anchored on a silico-based framework to produce adsorbents. For instance, Awual et al. [74] prepared an organic-inorganic based nano-conjugate adsorbent by directly immobilizing organic ligand N,N(octane-1,8-diylidene)di(2-hydroxy-3,5- dimethylaniline) (DHDM) onto mesoporous silica. According to them, the nano-conjugate adsorbent could preferentially adsorb palladium [Pd(II)] under optimal situations. Related studies involving the use of silica-based adsorbents have been reported elsewhere in the literature [21, 75, 76].
2.2.5 Commercial activated carbon (AC)
AC is a highly porous, refined, crushed coal or amorphous solid composed of micro-crystallites with a graphite structure, usually in the form of small pellets or powder [14]. It is the most widely used adsorbent for the adsorption of a broad range of emerging contaminants due to its special properties which include its large specific surface area, highly porous structure and high surface contact [18]. AC can be activated physically or chemically to provide multidimensional application, which substantially increases its adsorption surface area. The large surface area ranges from 500 to 1500 m2/g [14]. The types of AC, according to their particle size classification, are pulverized activated carbon (PAC) and granular activated carbon (GAC). AC can be further grouped according to their pore size as macroporous-structured AC (≥50 nm), mesoporous-structured AC (2–50 nm) and microporous-structured AC (2 ≥ 0.8 nm) [77]. AC has substantial adsorptive capabilities because of its high internal porous network, which provides a wide surface area for adsorption [16, 23, 78]. Ge et al. [79] modified coal-based activated carbon (CAC) with iron nanoparticles via microwave radiation and assessed its performance for the removal of polycyclic aromatic hydrocarbons from aqueous solutions. According to their findings, the modified CAC (0.05Fe-MCAC) had high adsorption capacities of 160.88 mg/g 181.99 mg/g and 199.07 mg/g for naphthalene, phenanthrene and pyrene, respectively. Similarly, Jawad et al. [80] reported that AC derived from Malaysian coal had a high adsorption capacity of 200 mg/g for cationic dye (MB) from an aqueous solution. The use of commercial activated carbon as an adsorbent has some disadvantages. Commercial activated carbon is nonselective and costly (the better the grade, the higher the cost), while the raw materials required for their production are scarce. There are still issues with their rapid saturation and thus regeneration as well as the disposal of used activated carbons. The regeneration of saturated carbon is likewise costly and difficult to regenerate, with the product regenerated having a lower adsorption capacity than the initial activated carbon and results in adsorbent loss [22, 41, 45, 81]. For these reasons, their practical usage is limited. AC derived from wood and plant wastes like coconut shells, nutshells or carbonized plant debris offers a cost-effective approach as a replacement for the petroleum-based AC [81]. Unfortunately, like all powdered adsorbents, they are difficult to use in columns for adsorption due to their clogging tendencies.
2.2.6 Bopolymers
The widespread industrial use of several commercial adsorbents is limited because they are expensive. In addition, these materials have lower mass and heat transfer, long settling time, difficulty in recycling, high-pressure drop, agglomeration at high doses, etc. which limits their wider adsorption applications [41]. Cost is an important parameter for comparing adsorbent materials. A sorbent can be considered low-cost if it requires little processing, is abundant in nature, or is a by-product or waste material from industries [51]. As such, alternative non-conventional adsorbents, mainly products and by-products of biological, industrial and agricultural origin (green adsorbents) were proposed, studied and employed as inexpensive and efficient adsorbents. These include algae, bacteria, fungi, yeasts, sawdust, fruit peels, husks, plant stems and polysaccharides [41]. These are also known as biopolymers since they are from plant and animal-based materials [82, 83]. Natural biopolymers are renewable, degradable and eco-friendly and produce a smaller volume of sludge [84]. Polysaccharides, e.g., starch, cellulose and chitin, are much preferred and researched because they are renewable and naturally abundant [83, 85]. They are an intriguing and appealing alternative as adsorbents because of their unique structure, chemical stability, high reactivity and superior preference towards aromatic compounds and metals. Their unique properties are attributed to the presence of chemically reactive groups (hydroxyl, acetamido or amino functionalities) in their polymer chains [51]. Cellulose is the most abundant, sustainable biopolymer in existence, with a unique composition that enhances its suitability for several industrial purposes [30]. The application of cellulose for making greener and more substantial industrial products is attributed to its no-carbon footprint and unique properties that allow for chemical modification [30].
3 Cellulose, an important biopolymer
3.1 Constituent of agricultural residue
Large amounts of unprocessed refuse are burnt or poorly disposed by the agricultural and manufacturing sectors in many developing nations, thereby polluting the surroundings and endangering the ecosystems. Improper treatment of these wastes before proper disposal may result in similar issues. Therefore, effective agricultural waste management systems via pollution regulation are absolutely important to avoid or decrease the spread of harmful waste to other locations [86, 87]. Many options for the proper disposal of these wastes have been offered in recent years, such as their reuse as precursors for the fabrication of adsorbents for pollution removal [24, 25]. Agricultural wastes are composed of large portions of lignocellulose biomass which consists of mainly three types of polymers namely: cellulose [C6(H2O)5]n, hemicellulose [C5(H2O)4]n and lignin [C10H12O3]n. They are strongly intermeshed and chemically bonded by both non-covalent forces and covalent cross-linkages [16].
The amount of chemical components in any plant residue varies and depends on the type of crop [88, 89]. Lignin biomaterial is a prevalent global natural compound, accounting for around 30 % of lignocellulosic biomass and exclusively occurring within the cell wall structure of plants. It is a complex phenolic and dynamic polymer made from lignin monomers that are primarily known as the three classical monolignols: coniferyl, sinapyl alcohols and p-coumaryl, each with a different degree of methoxylation [89, 90]. Lignin maintains water balance in fibers, protects against biological attack and functions as a stiffener in plant stems, thereby protecting them against gravity and wind. Lignin is a chemical polymer with a complicated three-dimensional structure and molecular mass of above 10,000 units. It is a hydrocarbon with both aliphatic and aromatic properties and it is completely insoluble in most solvents and does not easily decompose into monomeric components. The main phenylpropane repeating components of the lignin structure bind together via a series of linkages to create a complex matrix that includes propyl-phenolic compounds comprising of carbonyl, phenolic, hydroxyl, methoxy and aldehyde functional groups [16].
Cellulose and hemicelluloses are produced in plants through the polymerization of monosaccharides and condensation-dehydration mechanism, which has glucose as its main constituent and consists of other smaller monosaccharides. Cellulose (40–50 %) is a homopolymer consisting of glucose linked by b-1,4-glucosidic linkages while hemicelluloses is a group of polysaccharides made up of a variety of highly branched polymers (monosaccharides) [91]. Hemicellulose is the second most abundant biomolecule in lignocellulosic biomass, accounting for 20–35 % of total biomass. It is composed of different types of uronic acid groups, which are a highly branched heteropolymer of C-5 and C-6 carbon ring sugars, formed from D-glucuronic acid, D-xylose, L-arabinose, D-galactose and D-glucose. These monomers are connected by C=O=C and C=O linkages [92, 93]. Hemicellulose may be distinguished from cellulose in three ways; (1) multiple distinct sugar units (2) several chains branching with about 10 to 100-fold greater degree of polymerization [33] and (3) easily degraded or hydrolysed by alkali, unlike cellulose. However, both hemicellulose and cellulose contain oxygenated functional moieties such as ether, hydroxyl and carbonyl, which are found in lignocellulosic materials and may contribute to the adsorbent production process [94]. Although lignin is the most utilized component in the production of activated carbons, the choice of a precursor as an adsorbent is largely determined by a combination of factors that include its availability and abundance, cost and purity, ease of regeneration or safe disposal, production methodology and intended purpose of the developed adsorbent [25, 95]. Cellulose, therefore, is a suitable precursor for the synthesis of adsorbents since it is widely accessible, renewable and has a substantial amount of carbon as well as an established capability for sorption. The numerous hydroxyl moieties on cellulose can be used as active sites for functionalization, which are essential in the synthesis of adsorbents [16, 96]. The main polymer components of agricultural wastes are depicted in Figure 2.
![Figure 2:
Schematic diagram of lignocellulosic biomass and its constituent: lignin, cellulose and hemicellulose. Adapted from [97].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_002.jpg)
Schematic diagram of lignocellulosic biomass and its constituent: lignin, cellulose and hemicellulose. Adapted from [97].
3.2 Cellulose
The word “cellulose,” which means “a living cell,” was derived from the French words “cellule” and “glucose”. Anselme Payen, a French scientist, first used the term ‘cellule’ to describe cellulose in 1838, after discovering that all tender plants have a fibrous material with a homogeneous chemical content. He thereafter determined the chemical formula of the cellule (C6H10O5) through elemental analysis [98]. A year later, the French Academy coined the name “cellulose” to describe the fibrous cellule material of Payen’s work [99].
3.2.1 Chemical (molecular) composition of cellulose
Cellulose in plant fiber is a well-studied precursor for the production of nanocellulose, which can be applied for diverse industrial purposes. Plant fiber is frequently chosen over tunicate and bacteria-based cellulose because it produces thinner nanofibers and is available in larger amounts [100]. Cellulose is a linear isotactic homopolymer which consists of D-anhydrogluco-pyranose units (AGU), having the chemical formula (C6H10O5)n. The glucose-molecular units are held together by b-l-4-glycosidic linkages to form a dimer known as cellobiose, which is a basic unit of cellulose [101–103]. Cellulose is formed when the OH group at the C-4 axis of one glucose unit at the equatorial position forms a covalent bond with the C-1 atom of the closest glucose unit as shown in Figure 3. The hydroxyl functional groups that occur at both ends of the cellulose chain behave differently. For example, the OH moieties of the C-1 located at the end of the chain has reducing properties, composed of a D-glucopyranose unit in resonance with an aldehyde functional group, whereas the OH groups of the C-4 located on the same chain are non-reducing and consists of numeric C atoms bonded by glycosidic linkages. In addition, the interior rings joined at C1 and C-4 are also non-reducing. Each internal AGU has hydroxyl functional groups at C6, C3 and C2 positions that determine the chemical behaviour (reactivity) of the cellulose fiber. The hydroxyl groups at C6 are more reactive than the others and are known as the main alcohols, whilst those at other positions are known as secondary alcohols [102]. The reactive hydroxyl groups on the cellulose surface allow for cellulose functionalization [101, 104]. In addition, the interaction within the oxygen atoms of the glucopyranose ring, the hydroxyl groups of AGU and the glycosidic linkages inside the cellulose structure or the neighbouring chain facilitates the generation of strong intra- and inter-molecular hydrogen bonds which provides rigidity to the molecular chain [32, 34]. Cellulose molecular structure is responsible for its essential properties such as degradability, chirality, solubility and various chemical components [105].
![Figure 3:
Cellulose molecular structure [106].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_003.jpg)
Cellulose molecular structure [106].
3.2.2 Order of structural arrangement of cellulose
Cellulose fibers contain long chains of single molecules that may be transformed into fiber agglomerates and in a hierarchical sequence during production in plants’ cell wall. Several separate cellulose chains combine to create elementary fibrils (protofibrils), which occur in a variety of packing orientations, based on the conditions that regulate its biosynthesis [107]. The diameter of the elementary fibrils is influenced by their source and ranges between 2 and 20 nm. A sequence of helically wrapped cellular microfibrils makes up the intermediate layer. The microfibrils are generated through the clustering of elementary fibers by coalescence, which is a process for lowering the free energy of surfaces. These microfibrils, which are composed of 30–100 cellulose molecules in extended chain conformation and provide mechanical strength to the fiber, have around 3.5–30 nm width and length of roughly 7 m [108, 109]. The microfibrils created might merge into larger forms known as macro fibrils that are 60–300 nm wide, which may further rearrange to form cellulose fibers [110].
Intermolecular interactions such as Van der Waals forces, intra-and intermolecular hydrogen bonds, as well as the hydroxyl groups’ high spatial regularity, contribute to the agglomeration of the elementary fibrils. The cellulose molecules that combine to produce micro-fibrillated aggregates are composed of firmly arranged cellulose chains which form crystallites (depending on the source), held by strong, complex, hydrogen bonds, as well as some less-ordered chains that produce amorphous areas [111]. As a result, both amorphous and highly crystalline sections exist in cellulose fibers, which are bound together by complex and rigid hydrogen networks. The hydrogen bond within the C6–OH and C3–OH is recognized to be a very important constituent of cellulose’s structure and regular fiber alignment [110, 112]. The crystalline region, reactive O–H moieties and solubility characteristics of cellulose are all influenced by its strong hydrogen bonding systems. Although documented report shows the crystalline cellulose chains in an extended, flat, two-fold helical conformation, small variations in this conformation still exist. The different cellulose chain arrangements within the crystal result in a variety of crystalline polymorphs that can be altered to various forms by diverse treatment methods [113]. The schematic illustration of the types of cellulose fibrils and their hierarchical arrangement is presented in Figure 4.
![Figure 4:
Schematic hierarchical structure showing the elementary, micro and macro fibers [114].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_004.jpg)
Schematic hierarchical structure showing the elementary, micro and macro fibers [114].
3.2.3 Cellulose polymorphs
Cellulose can exist in several polymorphs or allomorphs because of the various inter- and intra-molecular arrangements. It can be categorized into four types of polymorph: cellulose I, II, III and IV. The physicochemical parameters, which include solubility, density, melting temperature, crystal forms and optical and electrical properties vary between these polymorphs [115]. Cellulose I is made up of parallel chains with 1,4-glycosidic linkages pointing in the same direction throughout the microfibrils. This parallel packing is necessitated by the kind of biosynthesis involved in the cellulose production. Investigations using 1H NMR by Atalla and VanderHart in 1984 revealed that natural cellulose has two allomorphs: 1𝛼 and I𝛽, with varying numbers and orientations of glucose units in a unit cell [116]. Cellulose 1𝛼 and I𝛽, also referred to as native cellulose, are the prevailing crystalline forms of cellulose that exist naturally. Cellulose 1𝛼 is composed of a triclinic P 1 crystal that contains one residual cellobiose per unit cell and could be synthesized by bacteria and fungi. The cellulosic chains are aligned in a parallel pattern, as one would anticipate in a single-chain unit cell. In diverse mediums, cellulose 1𝛼 is transformed to a more stable I𝛽 form by annealing at 270 °C. Cellulose I𝛽 form is mostly found in higher plants and bacteria [32, 34, 111]. The unit cell of the cellulose I𝛽 crystal is monoclinic with two cellobiose moieties per unit cell as seen in higher plant species. Furthermore, the ratio of the quantity of cellulose 1𝛼 and I𝛽 in cells is determined by the source of cellulose [103]. Although cellulose linkages are layered in parallel bundles in these polymorph forms, sheets of cellulose are directly layered on top of each other in 1𝛼 form, while layered sheets in cellulose I𝛽 form are staggered between alternate layers. The packing pattern in the lattice differs because different hydrogen bond strength exists between the chains. Hydrogen bonds are generated between intra-layer chains in both allomorphs, while van der Waals forces serve as the only force within sheets that are similar to each other [117]. Cellulose II is made by recrystallizing native cellulose and has an antiparallel chain configuration. It is the main polymorph obtained from industrially processed cellulose. Mercerization via alkali treatment or regeneration (solubilization and subsequent re-crystallization) of cellulose I have been proposed as two possible methods for cellulose II formation [34]. In comparison to Cellulose I, they are tightly packed and strongly bonded, indicating their high thermodynamic stability and low reactivity [118]. Cellulose II is more susceptible to structural alterations, although its structure’s nature is still ambiguous. Cellulose amorphous regions are believed to be one of the main contributors to the shape of cellulose. When water permeates the hydrogen bonds, the internal pressure of the amorphous portion of cellulose I is reduced and the structural chain is converted into a more regular pattern. The inclusion of water molecules can disrupt hydrogen bonds in the crystalline domain, thereby causing the unaligned structures in cellulose II to relax and assume a more enlarged layout of cellulose II [119, 120]. Cellulose type III can be produced by treating cellulose I or cellulose II with an ammonia solution, yielding either cellulose III1 or cellulose III2. The equivalent kind of cellulose type III (III1 and III2) may be converted into cellulose IV1 and IV2 by heat treatment in glycerol solution [103].
3.3 Mechanical properties of cellulose
Many factors including type, climate, yield, age and soil conditions, influence the properties of cellulose fibers. These qualities are further affected by cellulose fiber extraction techniques, such as fermentation, heating, bleaching and spinning, since different mechanical properties may be integrated into natural fibers during processing [115, 121]. Cellulose fibers are stiff and strong, with a low density [108]. The tensile strength of plant cell walls is provided by the cluster of cellulose molecules contained in the micro-fibrils. In experimental conditions, the tensile strength of cellulose microfibrils may reach 110 kg/mm2, which is around 2.5 times stronger than the strongest steel [122]. Conversely, the moistening of cellulose, as it occurs in cell walls, significantly decreases its tensile strength and capacity to offer structural support. However, it occurs within a matrix of hemicellulose, pectin and lignin in biological systems which serve as waterproofing and reinforcing material. Because of its homogenous dispersion, cellulose’s mechanical characteristics are largely determined by both its crystalline and amorphous domains [122, 123]. The amorphous and crystalline regions of nanocellulose induce distinct characteristics, with the crystalline area defining stiffness and elasticity, while flexibility and plasticity are determined by the amorphous area. However, when compared to other nanostructures, the crystalline area is greater, leading to increased stiffness [32, 124]. The Young modulus of cellulose with high crystallinity was reported to range between 100 and 200 GPa, while bacterial cellulose has a modulus of 114 GPa. As a result, nanocellulose is employed as a load-bearing material in a variety of applications [124].
4 Production of cellulose derivatives
Nanocellulose is a cellulose particle with at least one measurement in the nanometre scale (1–100 nm) [85]. Nanocellulose may be generally produced by two methods: top–down (plant fiber-breakdown) or bottom–up (biosynthetic pathway) [125, 126]. Bacteria from the Acetobacter genus are used to digest low-molecular-weight polysaccharides in the bottom-up biosynthesis method. The top-down strategy, on the other hand, entails chemically producing nanocelluloses by reducing or removing the amorphous region [126]. The strategies for creating nanocelluloses, which include mechanical, biological and chemical processes (or a combination of these approaches), will result in natural cellulose with reduced dimensions.
4.1 Classification of nanocellulose derived from cellulose biopolymer
Although multiple cellulose nanomaterials have been produced, the inconsistent and disorganized evaluation of these nanomaterials by numerous research organizations has culminated in a nomenclature of nanoparticles that is contradictory [127]. This has resulted in a plethora of names with no clear definition: nanocellulose, nanowhiskers, cellulose nano-whiskers, cellulose nanocrystals, nanocrystalline cellulose, cellulose microfibrils, cellulose nanofibrils, microcrystalline cellulose, cellulose microfibers, cellulose nanofibers, nanofibrillated cellulose, bacterial cellulose and other variations. Because of these ambiguities in naming nanocelluloses, developing a standard language via rationalization of the diverse name-type based on their size, morphology and synthesis became essential [127, 128]. The nanotechnology department of the Technical Association of Pulp and Paper Industry (TAPPI) was founded a few years ago, to regulate cellulose nanomaterial nomenclature. They created a drafted standard version of “The TAPPI WI: Basic Terms and Their Definition for Cellulose Nanomaterials” for the proper naming of nanocelluloses. Thus, nanocellulose materials may be categorized as nano-objects and nanostructures as shown in Figure 5 [128, 129].
![Figure 5:
Standard nomenclature for cellulose nanomaterials (TAPPIW13021) [130].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_005.jpg)
Standard nomenclature for cellulose nanomaterials (TAPPIW13021) [130].
The fundamental disparity between nano-object cellulose and nanostructured is the nanocellulose size. In this review, nanocellulose materials will be classified based on size, into four nomenclature groups: cellulose microfibers (CMF), cellulose microcrystals (CMC), cellulose nanofibers (CNF) and cellulose nanocrystals (CNC). A high mechanical strength, large surface area, unique optical characteristics, high crystallinity and stiffness are only a few of the outstanding qualities of nanocellulose [131, 132].
Cellulose microfibrils (CMF), also known as micro-fibrillated cellulose (MFC), are mainly composed of cellulosic materials that have been mechanically refined from sources such as highly crystalline wood fiber (WF) and plant fiber (PF). The aspect ratio of these particles is quite high; about 20–100 nm in width, a length of 500–2000 nm and a minimum wavelength of 5–50 µm [128]. CMF is prepared by dispersing wood pulp with strong shear pressures, leading to the release of substructural fibrils. Strong mechanical fibrillar networks and gels produced from heavily entangled fibrils can be used as a viable reinforcing material because of their small dimensions and broad surface area and aspect ratio [132, 133].
Cellulose nanofibrils (CNF) are another type of nano-sized cellulose. The terms CNF and CMF are frequently interchanged in literature, which can lead to misunderstandings about organizational structure. Both can be distinguished by their sizes and structure as cellulose nanofibrils (CNF) are smaller and smoother than MFCs in size, due to the longer defibrillation process time during production [134]. When compared to natural cellulose fibers, CNFs become gel in water at low concentrations (2 wt%) due to the considerable expansion in the specific surface area [128]. Nano-fibrils consist of overlaying long thin fiber structures, with a three-dimensional network of disordered (amorphous) and ordered (crystalline) areas. It has a diameter ranging between 5–30 nm and 500–2000 nm long, based on the intensity of disintegration. Cellulose nanofibers (CNF) are produced by dispersing softwood pulp mechanically in homogenizers without any pre-treatment, or little chemical/enzymatic processing [135]. When macro fibers are mechanically peeled off, the inter-fibrillary linkages between cellulose molecules are easily broken, resulting in nanofibrils with a nano-dimensional diameter and a nano-micrometre-to-micrometre fiber length [126]. Therefore, NFC encompasses both crystalline and amorphous regions within its flexible fibrillary chains. Multiple cycles through the homogenizers yield a significant improvement in viscosity. However, the massive amount of energy needed to disintegrate macro-fibers into nanofibers, which requires many defibrillation cycles in the disintegration device, is a major impediment to CNF commercialization. Therefore, the chemical pre-treatment is applied to lower the energy required for subsequent mechanical procedures [136, 137].
Another nanoform of cellulose is the cellulose nanocrystal (CNC), also known as nanocrystalline cellulose (NCC). CNC can be distinguished from CNF by the ratio of amorphous to crystalline components found in each of the nanocelluloses, with CNCs having little or no amorphous areas. Furthermore, CNCs have nano-scale length and breadth dimensions, whereas CNFs have micro and nano-scale lengths and diameters, respectively [138]. CNC is formed when the crystalline and amorphous domains of microfibrils are exposed to a range of enzymatic and chemical hydrolysis, resulting in fiber disintegration. This isolates the highly crystalline domains as rod-like nanocrystals and degrades the unaligned amorphous area. Thus, the production of rod-like or whisker-shaped cellulose nanocrystals is the outcome of strong chemical (acid) hydrolysis or enzymatic treatment of different nano and microfibrils [132, 139]. The tapering of the long edges of the crystals, which is due to the hydrolysis process, gives CNC its ‘whiskers’ shape. The majority of cellulose nanocrystals (CNCs) are elongated, cylindrical, less flexible and rod-like nanoparticles with a diameter of 3–5 nm, a length between 50 and 500 nm and a crystallinity index of 54–88 %. Furthermore, the size (length/diameter) and crystallinity of CNCs are influenced by the cellulose source and extraction procedures [139, 140]. Due to the presence of amorphous areas in microfibrils, the aspect ratios of CNC are substantially lower than MFC while CNC with more crystalline areas, on the other hand, has higher stiffness and modulus than MFC [85, 131]. When employed in composites, cellulose whiskers and other nanocellulose materials have proven to have good characteristics. Processed fibrillated wood pulp in micro-crystal dimensions is the main source of cellulose microcrystals (CMC) or microcrystalline cellulose (MCC). It is a commercially available substance that is made by reacting wood fiber (pulp) with acid and thereafter, back-neutralization with alkali and spray-drying, for usage in the pharmaceutical and food sectors [139]. The porous particles have a diameter within the range of 10–50 µm with a large cellulosic constituent, larger crystallinity and aggregate microfibril bundles of multi-sized cellulose that are tightly hydrogen bonded to each other. Before being incorporated into composites, MCC macromolecules are usually disintegrated into smaller micron-sized rod-like particles (1–10 mm in length) [139].
4.2 Cellulose extraction from plant biomass
Cellulose is highly hygroscopic in its interaction with water because of the presence of a large number of hydroxyl groups on its surface, although its crystallinity and supramolecular structure makes it insoluble in most solvent [110]. Furthermore, cellulose’s crystallinity renders it resistant to acid and alkali-catalyzed hydrolysis, making chemical treatment of the material difficult. However, it can be hydrolyzed by strong acid to water-soluble sugars [33, 34]. Various mechanical and chemical techniques can be used to extract cellulose from plant fibers, but alkali and enzymatic treatments can be applied as pre-treatment, along with mechanical extraction. These pre-treatment procedures enhance the mechanical extraction process by increasing the inner surface area, accelerating the reactivity of the hydroxyl groups to reagents and disrupting the hydrogen bonds of the cellulose fiber as well [141]. The treatment of lignocellulosic biomass like maize tassels by alkali and acid-chlorite treatment is a common method used for cellulose extraction. Alkali reagents, such as NaOH solution, are a cost-effective reagent used for the hydrolysis of the plant residues during cellulose extraction [89, 142, 143]. Although cellulose’s supramolecular structure makes it resistant to hydrolysis, alkali can activate it and destroy its inner hydrogen bonds, causing it to swell. Alkalization aids in the dissolution and isolation of wax, pectin, lipids and other non-cellulosic materials from the plants’ surface, thereby accelerating the fibrillation of cellulose, as in the case of the extraction of cellulose from maize tassels residues. It also increases the crystallization potential and promotes interfacial bonding. Therefore, alkalization contributes to the improvement of fiber mechanical characteristics [144–146]. To eliminate any hemicellulose or lignin residues, the produced pulp may be bleached using a mixed solution of glacial acetic acid and sodium chlorite or a solution of hydrogen peroxide and NaOH. Thereafter, the bleached pulp fibers are rinsed with NaOH solution and washed with deionized water to obtain a near-neutral pH. The bleached residue is collected and dried in an oven @ 50 °C, resulting in holocellulose devoid of lignin [142]. Figure 6 depicts the morphology of the maize tassel fibers and cellulose fibrils extracted from maize tassels via chemical treatment.
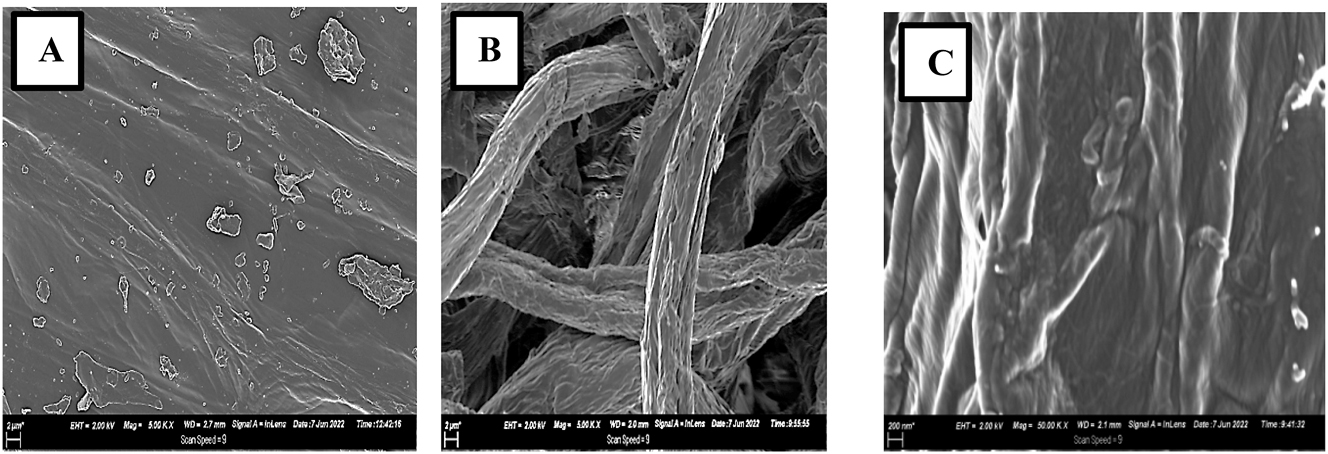
SEM micrographs of (A) maize tassels and (B–C) celluose fibers extracted from maize tassels after chemical treatment at 2000x and 50,000x magnification, respectively.
4.3 Derivatives of cellulose
Pure cellulose is more resistant to chemical treatment due to its reduced solubility in common organic solvents, caused by strong intramolecular and intermolecular interactions. The insolubility of cellulose is also influenced by the length of polymeric chains. According to research, the solubility of celooligomers declines to zero at polymerization degrees greater than celloheptaose [147, 148]. Cellulose is entirely insoluble below 300 °C and rapidly degrades beyond this temperature. Thus, there is no temperature range associated with increasing its solubility. Cellulose derivatives are suitable alternatives to pristine cellulose, due to their unique characteristics and dissolving capabilities of some cellulose derivatives. The most well-known cellulose derivatives include cellulose ethers (e.g., carboxymethyl cellulose, methylcellulose, ethyl cellulose etc.), cellulose esters such as cellulose acetate, cellulose sulphate or sulphated cellulose and cellulose nitrate [149]. A cellulose derivative’s overall chemical structure can be altered by the type of R’ group incorporated via mono-, di- or tri-substitution as shown in Table 2.
Cellulose derivative’s structure with a variety of R’ groups.
| Cellulose derivatives | R’groups |
|---|---|
| Cellulose acetate | H–C2H3O |
| Cellulose nitrate | H–NO2 |
| Carboxymethyl cellulose (CMS) | H–CH2CO2H |
| Ethylcellulose | H–CH2CH3 |
| Methylcellulose | H–CH3 |
| Hydroxypropyl-methylcellulose (HPMC) | H–CH2OCH3 |
| Cellulose sulphate | H–SO3H |
4.3.1 Cellulose acetate (CA)
Various CA preparation procedures, which include ring-opening esterification, trans-esterification and esterification through the use of iminium chloride or N,N-carbonyldiimidazole, have been established in recent years [150]. Paul Schützenberger was the first to synthesise cellulose acetate (CA) from wood pulp treated with acetic anhydride in 1865. Cellulose acetate has also been synthesized via the treatment of cellulose with acetic acid under acidic catalytic conditions [149]. To guarantee uniform acetylation, cellulose is first treated with acetic acid, then dehydrated and reacted with acetic anhydride using a suitable catalyst-solvent system, such as sulphuric acid-anhydrous acetic acid. This produces cellulose triacetate, which may be acid-hydrolyzed to a reduced degree of substitution to obtain cellulose di-acetate and then cellulose acetate. The degree of substitution (DS) of this cellulose ester is crucial since it affects its solubility and biodegradability [151]. The mechanical qualities of cellulose acetate, as well as its hydrophobicity and hydrolytic stability, are noteworthy [149, 152].
Contaminants removal along with the various modified cellulose-based nanocomposites and adsorption performance.
| Adsorbent | Modifying solvent/reagents | Modification method | Features | Contaminant (adsorbate) | Removal/adsorption capacity (mg/g) | References |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
[153] |
|
|
|
|
|
|
[154] |
Functionalized nanostructured cellulose:
|
|
|
|
|
|
[155] |
|
|
|
|
|
|
[156] |
|
|
|
|
|
|
[157] |
|
|
|
|
|
|
[158] |
|
|
|
|
|
|
[159] |
|
|
|
|
|
|
[160] |
|
|
|
|
|
|
[161] |
|
|
|
|
|
|
[162] |
|
|
|
|
|
|
[40] |
|
|
|
|
|
|
[163] |
|
|
|
|
|
|
[164] |
4.3.2 Cellulose nitrate (CN)
Cellulose nitrate (nitrocellulose), often referred to as guncotton, is the essential element in smokeless gunpowder, due to its explosive decomposition properties [165]. The nitration of cellulose produced from wood pulp or cotton fibers with a strong nitrating reagent (e.g., nitric acid), produces CN. The electrophilic reaction of NO2+ ions on the OH moieties cause the hydroxyl groups on the surface of cellulose to be replaced with nitrate esters during the nitration process. This method was initially studied in the context of nitration of alcohols and amines, but further research revealed that it was also relevant to cellulose [166]. According to research, sulphuric acid is an effective additive for stabilizing the nitration solution and preventing inadvertent detonations [167]. In industry, the H2SO4/HNO3 combination is often used for the nitration of a variety of substrates, including glycerol and cellulose. A total nitrogen concentration of 13.5 % or above is considered adequate for the complete nitration of cellulose [166]. The reaction’s success is controlled by factors like the nitrating mixture’s composition, the substrate’s source and the preparatory treatments used for the synthesis of CN [149].
4.3.3 Cellulose ethers
Cellulose ether is a group of cellulose derivatives formed when alkaline cellulose reacts with an etherifying agent under certain conditions. It is the result of ether groups replacing hydroxyl groups on cellulose macromolecules partially or completely [168]. The derivatization reactions of ethers are heterogeneous and require the diffusion of reactants into the mercerized cellulose structure [169]. The etherification of the three hydroxyl moieties of cellulose anhydroglucose units has been used to synthesize water-soluble derivatives, which include methylcellulose (MC), carboxyl methyl cellulose (CMC), ethyl cellulose (EC), hydroxypropyl cellulose (HPC), hydroxypropyl methylcellulose (HPMC) (also known as hypromellose), ethyl hydroxyethyl cellulose (EHEC) and hydroxyethyl cellulose (HEC) [170, 171]. The commonly used ether derivatives are further explained.
4.3.3.1 Carboxymethyl cellulose (CMC)
Carboxymethyl cellulose (CMC) is an important cellulose ether made by treating raw cellulose with an aqueous sodium hydroxide solution, and thereafter, filtering the alkali cellulose and reacting it with monochloroacetic acid or sodium monochloroacetate in an alcohol-supporting medium [43]. Materials containing cellulose such as wood waste, paper sludge, cotton linters, rice or maize husks, have been utilized for the production of CMC. Accordingly, CMC can be categorized as technical, partially purified, or fully purified, depending on its degree of purity [44, 149]. A linear long polysaccharide backbone structure with both carboxyl and hydroxyl surface groups is formed during the etherification process. Chemical reactivity and water solubility are facilitated by CMC’s unique chemical structure [172]. Hydrogels made from Na-CMC with a greater degree of substitution completely dissolve in water, but those made from Na-CMC with a lower DS have a stronger stiffness. The presence of additional COO- groups at greater carboxymethylation DS was thought to produce repulsion between adjacent negatively charged polymeric chains, thereby preventing the development of crosslinks and improving the dissolution properties. Conversely, a lower DS facilitates the formation of covalent bonds between functional groups [173].
4.3.3.2 Ethyl cellulose (EC)
Ethyl cellulose is a cellulosic ether that has been partly ethylated by the reaction between alkali-treated cellulose and ethyl chloride. It is a tasteless, odourless and chemically inert compound, stable in a pH range between 3 and 11, with a glass transition temperature of 120 °C. Although EC is insoluble in water, it may be dissolved in a range of organic solvents/solvent mixtures such as ethanol, methanol, toluene, chloroform, or ethyl acetate [174]. However, EC becomes very impermeable when the degree of substitution exceeds 2.8 [149, 175]. EthocelTM is a brand name for many forms of EC that are characterized by their viscosity, molecular weight and degree of substitution [176].
4.3.3.3 Methylcellulose (MC)
Methylcellulose and ethyl cellulose are both cellulose derivatives with relatively similar chemical composition. Methylcellulose, in its pure state, is a hydrophilic white powder that dissolves in cold water to form a clear viscous liquid or gel. It is indigestible, non-lethal and without allergies, just like cellulose [174]. In aqueous solutions, MC displays thermo-reversible gelation capabilities, gelling between 60 °C and 80 °C and forming a liquid again at lower temperatures [177, 178]. In the methyl ether form, MC consists of long-chain modified cellulose with about 27–32 % of hydroxyl groups. For different grades of MC, the degree of polymerization ranges from 50 to 1000-Da, with average numerical values of molecular weights ranging from 10,000 to 220,000 Da. In addition, the degree of substitution (DS) is an important factor that influences the properties of MC, such as its solubility. The average substituted number of hydroxyl groups per glucose is defined as the degree of substitution of a certain type of methylcellulose. Thus, a DS of 3.0 is the theoretical maximum number required; nevertheless, more frequent values of 1.3–2.6 may be produced. Synthesized MC may occur in two forms; one with a uniform distribution of substituents while the other, with a random distribution of substituents along the chains [179]. On an industrial scale, MC is made by mechanically mixing cellulose with sodium hydroxide and methyl chloride solutions, with the methylation reaction happening more quickly in NaOH-rich and/or higher temperatures, resulting in non-uniform methyl group distribution along the chain. It contains “hydrophobic zones” that are extensively substituted and “hydrophilic zones” that are less substituted [170]. Methoxide can substitute 27–31 % of the hydroxyl moieties in anhydroglucose units after the etherification process (−OCH3 groups). Polymer structure of diverse methyl cellulose formulations can also vary. The MC that has been evenly replaced does attain the DS required for water solubility. As a result, only the heterogeneous kind, which is water-soluble when the DS is between 1.3 and 2.6, is commercially utilized [170, 174, 180].
4.3.3.4 Hydroxypropyl-methylcellulose (HPMC)
HPMC is a derivative of cellulose that has been partially O-methylated and O-(2-hydroxypropylated) [170]. Like MC, the presence of methoxy residues in HPMC causes gelation and the inclusion of hydroxypropyl residues is known to drastically affect the gelation features in a temperature-dependent approach [181]. In addition, HPMC gelation is thought to be caused by the high hydrophobic reactions and the exclusion of water (syneresis) from extensively methoxylated polymer areas [170]. The DS, or the number (average) of hydroxyl groups substituted, which is a maximum of three, is another characteristic of HPMC. The molar substitution or MS (i.e., the reaction between the average amount of reagent molecules like propylene oxide and each anhydroglucose unit), which may be more than 3, determines the number of hydroxypropyl groups attached to the cellulose structure.
4.3.4 Cellulose sulphate (CS)
Cellulose sulphate (CS) is a cellulose ester produced by heterogeneous, homogeneous, or partial sulphation reactions. Compared to pristine cellulose, CS has various benefits, such as improved water solubility and antibacterial characteristics at significant concentrations [182]. During heterogenous sulphation, pure cellulose is treated with a sulphating agent (e.g., sulphuric acid) in a suitable reaction medium, most often isopropyl alcohol which causes the hydroxyl groups on the surface to be replaced with sulphate groups after this reaction. Schweiger R.G. first reported homogeneous sulphation in a study in 1979, where cellulose in SO3-pyridine complex with N,N-dimethylformamide as co-solvent, yielded CS with a relatively high substituted degree (SD) and a reaction intermediate known as cellulose nitrate [149]. The partial-homogeneous procedure is based on aceto-sulphation, which is identified as a gradual dissolution of cellulose in N,N-dimethylformamide combined with a mixture of sulphating and acetylating reagents (e.g. chlorosulphuric acid/acetic anhydride) and subsequent cleaving of acetyl groups upon precipitation. This results in the conversion of cellulose acetate-sulphate into CS. Regioselective sulphation can be achieved depending on the method utilized. For instance, the OH groups at the C2 and C3 locations of the anhydroglucose unit are shielded by acetosulphation, while a larger substitution group is attained at the C6 point. Conversely, C2 and C3 positions can also be influenced by homogenous sulphation [183].
5 Production of cellulose beads
5.1 Physical forms of cellulose adsorbent materials
The physical form in which an adsorbent material exists plays an essential role in removing pollutants from wastewater. Cellulose may be converted into well-defined structures like films or membranes, different-geometry fibers, sponges, powder or granules and round particles (cellulose beads/hydrogels) [184, 185]. The microspheres (aerogel, hydrogel and beads) made from polysaccharide-based materials are the most complex form of adsorbent among all the other forms. Due to the availability, renewability, biocompatibility, biodegradability, specific functionality and great mechanical and chemical resistance of cellulose, well-designed porous cellulose beads are viable candidates for industrial application [158, 186]. Cellulose beads (CB) are tiny spherical particles (in the micro-to millimetre range) that can be porous or nonporous. CBs with a low density and broad surface area are known as porous CBs. They are sometimes referred to as microspheres, pellets, beaded cellulose or pearl cellulose. CBs are more favourable than films or other irregularly shaped particles in flow process because they minimize backpressure and agglomeration during column experiments [187]. In addition, separation in aqueous media becomes difficult when cellulose derivatives are used in the powdery form as absorbents or ion exchangers. Therefore, cellulose beads can circumvent the above-mentioned limitation while the porous surface improves cellulose hydroxyl group availability. The broad surface area to volume ratio of beads facilitates the integration of chemical moieties, thereby improving the contaminants’ binding effectiveness to the adsorbent [185, 188, 189]. Therefore, functionalized porous beads are attracting increasing attention in recent times, due to their distinctive high porosity, functionizable surface and numerous potential applications in filtration, purification, catalysis, energy storage, drug or cell delivery etc. [190, 191].
5.2 Preparation of cellulose beads
A common cellulose bead-making approach consists of the following steps: (1) application of appropriate solvents for dissolving cellulose or its derivatives, (2) polysaccharide solution gelation and shaping into spherical particles and, (3) sol–gel transition and pore adjustment and (4) gel drying [192, 193].
5.2.1 Dissolution of cellulose
The insolubility of cellulose has been attributed to a variety of reasons surrounding the chemical structure of the substance. To start with, native cellulose is a large macropolymer with a high degree of polymerization (DP) that is inherently difficult to dissolve, owing to thermodynamics influence (reduced entropic gain during dissolution for macromolecules, compared to smaller molecules). The presence of five oxygen molecules in the hydroxyl groups, in addition to the ring- and bridge oxygen, results in the formation of complicated hydrogen bond patterns. Moreover, cellulose’s H and C atoms may combine via hydrophobic interactions, indicating the amphiphilic nature of cellulose. Overall, this places a great demand for a cellulose solvent that is effective in disintegrating the bonds and dissolving cellulose [194, 195]. A solvent that is appropriate for dissolving cellulose must be able to permeate through cellulosic linkages and isolate amorphous from crystalline sections. The solvent must target the strongly bonded hydrophobic-hydrogen linkages inside the cellulose chain. Acidic inorganic reagents (e.g., HCl) or a combination of acids at significant concentrations can dissolve cellulose in acidic media [132, 196]. Many possible solvent systems for cellulose dissolution can be categorized into two: derivatizing and non-derivatizing aqueous solvents. Thus, “derivatizing” solvents are those that chemically modify the cellulose during the dissolution process. These solutions react with one or more of the three reactive hydroxyl groups in the cellulose chains, resulting in the formation of “unstable” acetal, ester and ether derivatives that will improve the solubility property of the cellulose in conventional systems [192, 196, 197]. By cleaving the intermediate derivatives, which may be triggered by adding water or changing the pH or temperature, cellulose can be regenerated and moulded into solids such as membranes or beads. Derivatizing solvents transforms cellulose into derivatives that are only metastable under soluble conditions in most situations. Some carboxylic acids (e.g. CF3COOH) and dinitrogen tetroxide (N2O4) (in combination with dimethylsulfoxide (DMSO) or N,N-dimethylformamide) are examples of derivatizing solvents [198]. The term “non-derivatizing” refers to situations in which the polymer dissolves purely through intermolecular interactions instead of chemical conversion of its hydroxyl groups [192, 193]. Non-derivatizing solvents disintegrate cellulose linkages by breaking the forces that keep them intact, without causing any chemical change. They are not just for cellulose dissolution purposes or regeneration procedures, but can also be used to make certain important and highly designed cellulose derivatives. Thus, the non-derivatizing solvents can be further grouped into two categories: aqueous and non-aqueous solvents. A solution of N,N-dimethylacetamide (DMAc) and lithium chloride (LiCl) is a popular non-aqueous cellulose solvent. It is commonly utilized for polysaccharide analytical evaluation and as a reaction medium for uniform cellulose conversion [132]. Conversely, LiOH, NaOH and some inorganic metal complexes that are mainly composed of ions of transition elements and nitrogen ligands like cuprammonium hydroxide (Cuam), which produce brightly coloured solutions, are examples of aqueous solvents [195]. The molten inorganic hydrated salts and concentrated inorganic salt solutions (e.g., magnesium chloride (MgCl2·6H2O), lithium chloride (LiCl·5H2O), lithium perchlorate (LiClO4·3H2O) and zinc chloride (ZnCl2·4H2O) are further examples of aqueous solvents that have been utilized for the homogenous modification of cellulose [132].
5.2.2 Common aqueous solvent for cellulose dissolution
To guarantee the homogeneity of cellulose throughout the solution preparation, different mixed solvent systems will be required for its dissolution. Thus, aqueous NaOH solutions in conjunction with various chemicals that inhibit gelation, such as urea, thiourea or ZnO, have attracted a lot of interest. These reagents are inexpensive, ecologically friendly and nontoxic and they have vast potential applications [193, 199, 200]. Cellulose may be dissolved in NaOH/urea at cold temperatures and in a particular concentration range. According to Chen and his colleagues [201], the reaction occurs when cellulose forms hydrogen-bond-induced complexes in the form of a linked network, after treatment with alkali and urea. The aqueous solvents promptly break down cellulose at lower temperatures of about 10 °C, producing clear solutions from which cellulose may be recovered by agglomeration in diluted acids. The hydrogen link between the cellulose polymer chains is broken by the OH− anion in NaOH, while the Na+ enhances the simultaneous breaking of bonds and modifying of the surrounding water, thereby preventing the re-aggregation of chains [202]. When NaOH/water solvent is employed without additives, the dissolving process is considerably impeded due to the gelation of cellulose solutions. Gelation occurs when hydrogen bonding between cellulose-cellulose molecules is formed, resulting in the agglomeration of cellulose chains and the generation of cellulose-rich areas. The gel formed becomes opaque, suggesting entities that scatter visible light. This heterogeneous structure, with wide pores and dense pore walls, might explain why aerogels formed from gelled solutions have a low specific surface area of 200–250 m2/g. Therefore, a combination of solutions, such as NaOH/urea is preferred [203].
Another environmentally friendly solvent is the NaOH/thiourea solvent system. Separating cellulose chains from each other can also be accomplished by combining NaOH and thiourea hydrates. The hydrates prevent the surface intermolecular hydrogen bond from being regenerated by the cellulose [196, 204]. Complex solvents formed by the combination of NaOH, thiourea and water with the cellulose substrate will likely disrupt the intramolecular hydrogen link in cellulose at low temperatures, unlike NaOH hydrates (NaOH and water), which readily adhere to cellulose linkages to establish a new hydrogen bond [205].
Ionic liquids (ILs) have emerged as a new important solvent for cellulose dissolution because of their superior dissolving capabilities and ability to dissolve cellulose without derivatization [206]. They are chemical salts that have distinctive characteristics such as nonflammability, non-corrosivity, lower melting point (<100 °C), low vapour pressure and low viscosity. These liquids have been used as an alternate method for nanocellulose extraction because they can serve as both a solvent and a catalyst for cellulose hydrolysis [141]. Their stable, polar, non-volatile, non-flammable and recyclable characteristics make ILs eco-friendly solvents. ILs improve cellulose solubility properties by establishing hydrogen bonds with hydroxyl and ether oxygen in cellulose and have been used to fabricate cellulose beads via dispersion and dropping procedures [199]. ILs like imidazolium are capable of dissolving cellulose because of their broad physicochemical qualities, such as low vapour pressure, large concentration range for liquid dissolution, high thermal capacity and ability to become solvents for numerous chemical conversions. Unlike solvents composed mainly of NaOH/water, ionic liquids allow cellulose to be dissolved at several varying concentrations and molecular weights. DMSO or DMF (dimethylformamide) can also be used as co-solvents to change the viscosity of a solution [147, 203]. The relatively high viscosity of cellulose/IL solutions is, however, one of its major disadvantages. In addition, the production of ILs may require sophisticated technological equipment such as an “underwater pelletizer.” Furthermore, the usage of ILs in cellulose dissolution is costly owing to the high production expense and time required. Therefore, ILs frequently require combinations with other chemical compounds as an alternative, thereby reducing consumption [207, 208]. The bead preparation process can be made lucrative and consistent through the use of effective recycling measures while the total IL residue removal from the regenerated cellulose must be accomplished [193, 207, 209].
5.3 Shaping into spherical particles
The process used for producing spherical particles in liquid systems has a large influence on the size of cellulose beads. The transformation from dissolved polysaccharides to solid particles and, thereafter, the bead shape, may be managed by changing the process variables [184]. For example, beads made with higher cellulose concentrations will be less porous, while the morphology, pore size and internal surface area can be controlled by altering the temperature and content of the coagulation medium. Nitric acid, ethanol, acetic acid, hydrochloric acid, sulphuric acid and water have been used as solvents for the coagulation of cellulose solution [193, 210, 211]. It is feasible to fabricate beads for specific applications if the relationship between bead characteristics and individual process factors is well-researched. Thus, the coagulation process (regeneration) determines the physical bead qualities such as density, specific area and pore size structure. The density of cellulose beads has been enhanced up to 2.4 g/cm3 from approximately 1 g/cm3 due to the material’s high porosity [212]. Inorganic compounds can be added to cellulose beads to alter their physical characteristics. The inorganic chemicals are directly added to the cellulose (or cellulose derivative) solution before the shaping process. The particles are confined within the beads when the polysaccharide is regenerated because of their enormous size in comparison to the mean pore diameter. This was achieved by introducing dense particles of TiO2, tungsten carbide, nickel or stainless-steel powder. Although the polysaccharides, solvents and regeneration processes that are used to make cellulose beads may differ, all the procedures have one common factor: the shaping of the beads using either dropping or dispersion techniques. The formation process is appropriate since it is dependent not only on technological considerations but also on a rough macroscopic regeneration of cellulose into beads with a size of 250–500 µm (dropping methods) or less (dispersion). Figure 7 shows a diagram of the preparation methods used in producing spherical beads.

Diagram illustrating the preparation process of cellulose bead.
5.3.1 Dropping technique
Beads can be made by forming spherical droplets from a polysaccharide solution and solidifying these droplets in a non-solvent coagulation bath. Dropping techniques employs various types of processes which include traditional method (conventional dropping), spraying/atomization, jet cutting, electrostatic etc. The conventional dropping procedures include the use of syringes as well as other dropping devices that create droplets at the needle tip (orifice) which fall freely into a gelation or coagulation bath under gravitational attraction [213]. Since forces of gravity and pressure utilized for ejection are the primary force that causes a droplet to form from an orifice, these devices are able to create large droplets that are a few millimetres in diameter, which is generally larger than the nozzle diameter. Various sizes and forms, ranging from extremely flat plates to spheres, may be created by adjusting solution viscosity, orifice diameter, ejection speed, solution distance between the syringe tip and the coagulation bath and bath temperature [184, 214]. To create droplets of a specific size and shape, a variety of standard technological instruments can be used. The automated approach which utilizes spraying/atomization, is a process in which atomizer nozzles completely fragmentize a stream or jet of a non-compressible liquid, resulting in the creation of poly-or monodispersed droplets in the air or a vacuum. Examples of atomizer nozzle devices are vibration atomization and pressure jet atomization. Vibrating nozzles or air jets aimed at the tip/outlet from which the liquid protrudes, can be used to force the formation of smaller droplets under capillary force. The use of automated devices that operate at higher speeds is advantageous, especially for large-scale manufacturing [213].
“Spinning disc atomization” is another possible method for droplet production whereby ejecting cellulose solutions through a spinning cylindrical vessel with tiny openings (spinning drop atomization) forms a large number of droplets within a short period. Droplets with diameters of 500 µm or smaller may be produced due to the strong forces applied to the cellulose solution [203, 215]. Conversely, the jet-cutting approach expels cellulose solutions instead of droplets, through a narrow orifice at a high velocity, resulting in a continual liquid flow. This liquid stream of cellulose may be chopped into spherical particles by running it via a spinning knife instrument (jet cutting), which then falls into a coagulation bath. Ejection and cutting of the cellulose stream into the coagulation media can also be done by underwater pelletizing. The size and form of the beads produced may be controlled by adjusting the knife geometry and rotation speed, in addition to the ejection speed and jet nozzle diameter adjustments [193, 216].
Due to the size of droplets that may be generated, the diameter of cellulose beads produced via dropping procedures is generally limited to a range of roughly 0.5–3 mm [193, 217]. Only a few studies have been published on cellulose aerogel beads and the vast majority are created by syringe-dropping of cellulose dissolved in alkali liquids. Thus, aerogel beads have been developed via cellulose dissolution in 7 % NaOH/12 % urea/water solution and dropping in an aqueous non-solvent and their size and shape varied by changing the coagulation conditions (i.e., bath temperature between 5 and 50 °C and HNO3 conc., from 0.5 to 1 M) [203]. For example, ZnO was stirred into the same solvent in various concentrations (ranging from 0 to 2 %) and beads were created by dropping them into 2 M HCl. Their diameter ranged from 2 to 2.5 mm and expanded as the ZnO concentration increased [218, 219]. According to the authors, a greater ZnO content prevents beads from shrinking. Particle volume ranged from 8 to 20 mm3 and circular shape was primarily influenced by bath temperature, with more deformed particles obtained at lower temperatures.
5.3.2 Dispersion technique
When a solution of cellulose or a cellulose derivative is dispersed in an immiscible solvent of opposite polarity at a high rotating speed, emulsions, which can be stabilized with the aid of surfactants, are produced. These emulsions include dissolved polysaccharide droplet particles that may be consolidated into beads of equal size. The mixing speed, type and amount of surfactant, the ratio of hydrophobic to hydrophilic solvent, and the viscosity of the dispersion medium and cellulose solution affect the droplet’s width inside the dispersion medium [220, 221]. As a result, cellulose beads produced through the dispersion technique are nearly ten times smaller than those made using dropping procedures. Unlike methods that rely on dropping techniques, no specific equipment is required to produce beads with consistent features in this method. Thus, dispersion-made beads of various diameters, ranging from 10 to 250 µm, are obtainable in this method [193]. Figure 8 shows a schematic representation of the common methods used for producing spherical beads [213].
![Figure 8:
Schematic illustrations of various methods for producing cellulose beads: (A) dropping, (B) jet cutting, (C) spinning disc atomization, (D) spinning drop atomization and (E) dispersion [193].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_008.jpg)
Schematic illustrations of various methods for producing cellulose beads: (A) dropping, (B) jet cutting, (C) spinning disc atomization, (D) spinning drop atomization and (E) dispersion [193].
6 Drying techniques used for cellulose micro-beads
Maintaining the porous framework of nanocellulose-based solids during solvent removal remains a key issue. The water evaporation process that occurs on a porous structure causes capillary pressure-induced stress, which can distort the pores and cause them to warp, collapse, or fracture [222]. Water, ethanol or acetone is commonly used to remove the solvent from cellulose solutions and gels, while isopropanol is used less frequently. If water is utilized during bead production, it is then substituted with ethanol or acetone combined with CO2. All exchanges are diffusion-controlled and consequently, take a long time. The time it takes for cellulose solvent to diffuse out and non-solvent to percolate into the structure is determined by the concentration of cellulose, the shape of the sample and the bath temperature. As proposed, the diffusion coefficient increases as bath temperature increases, leading to a decrease in cellulose concentration. Because the diffusion coefficient correlates with the sample’s thickness in the order of power 2, it takes days to rinse cellulose solvent out of a dense three-dimensional sample. Size difference owing to a “wet” network shrinkage during solvent exchange should be taken into consideration when calculating the solvent diffusion coefficient [223]. To preserve the surface characteristics as much as possible, drying is necessary. The typical drying approach for the creation of lightweight and porous solids is supercritical drying. The creation of a liquid/vapour interface is prevented by replacing the initial solvent (typically water) with a supercritical fluid, which eliminates the formation of a meniscus or capillary pressure during solvent extraction. Drying surface pores with supercritical liquids with no meniscus formation preserves mesoporosity and avoids unnecessary additional chemical treatments to improve the contact angle. The diffusivity of a supercritical fluid is equivalent to that of gas and it has a density that is halfway between gas and liquid. It also has a high solvation power. Supercritical fluids were initially discovered in the first half of the nineteenth century and are presently employed in a variety of applications [203]. Carbon (iv) oxide (CO2) is frequently used during supercritical drying, with a critical temperature and pressure of 31.3 °C and 72.9 atm, which can be easily attained [222]. The use of supercritical fluids necessitates the application of high-pressure technologies, which has significant disadvantages. However, the low viscosity, high diffusivity and solvation properties of these fluids can overcome the high-pressure disadvantage. CO2 is the simplest option for drying aerogels due to its low critical point, temperature and pressure. In addition, it is chemically inert, non-flammable, non-toxic and inexpensive. Beads can also be dried via lyophilisation (freeze-drying) and ambient pressure drying (low vacuum drying). Freeze-dried gels are technically referred to as “cryogels,” a word that applies to a substance that gels when frozen, or a process whereby water is sublimated from a frozen aqueous system, which is also known as ice-templating [203, 224].
Aerogels fabricated from nanocellulose and its derivatives are usually freeze-dried, although, the self-agglomeration of nanocellulose can reduce the specific surface area of the aerogel. Tert-butyl alcohol is another solvent exchange that can preserve the gel structure of nanocellulose and its derivatives better than water, thereby preventing the cellulose aerogel structure from collapsing. Tert-butyl alcohol has a low interfacial tension due to its single hydroxyl group that may create a hydrogen bond with the hydroxyl or carboxyl group on nanocellulose surfaces. Simultaneously, the steric hindrance generated by a large number of butyl groups can effectively impede nanocellulose aggregation [225]. Conversely, “xerogel” which technically refers to a “dry gel” is a term that has long been used to describe meso- and micro-porous systems with porous structures of up to 50 % that are dried at room temperature or low vacuum. Only a few studies have been published on low-density cellulose “xerogels,” and most of them have a poor specific surface area due to capillary pressure forming during drying via epuration, along with the formation of hydrogen bonding between polysaccharide chains. This generally results in network collapse and the formation of a non-porous substance. Using fluids with surface tension lower than water (0.073 N/m) such as ethanol (0.022 N/m), acetone (0.0237 N/m), hexane (0.0184 N/m), methanol (0.0226 N/m) or pentane (0.0158 N/m) might help to reduce pore closure during drying [203]. Figure 9 elucidates the morphology of aerocellulose bead at two scales, using scanning electron microscope (SEM).
![Figure 9:
SEM micrographs of cellulose bead @ (A) 1 mm and (B) 5 µm magnification [192].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_009.jpg)
SEM micrographs of cellulose bead @ (A) 1 mm and (B) 5 µm magnification [192].
7 Surface modification of cellulosic materials for enhanced adsorption
In order to generate a renewable and sustainable adsorbent with high sorption capacity and selectivity, the OH moieties of the surface of cellulose fiber must be chemically modified [136, 226]. CB can be modified or crosslinked with additional functional groups or coupling agents or employed as a solid framework for the immobilization of particles and fillers in pharmaceutical applications for controlled release [186, 227]. Although surface modification can be used to attain other objectives such as the degradation of cellulose into its nanoforms, conversion of cellulose to derivatives with unique properties and the modification of the surface charge density of cellulose for improved electrostatic interaction with other molecular species, as well as its usage for the introduction of functional groups and charge onto cellulose seems to be the most prevalent. Chemical modification of beads involves the introduction of a chemical functionality to the material’s surface that improves its interactions with the environment without compromising its surface morphology. Although cellulose could be modified before bead shaping, heterogeneous modification of cellulose beads after shape formation is by far the most often used method for the production of functional cellulose beads due to its simplicity and efficiency. Moreover, the challenge of cellulose dissolution can be overcome by chemical modification into its derivatives, such as cellulose esters (e.g., CA) or ethers (e.g., CMC, methylcellulose (MC), EC and hydroxyethyl cellulose (HEC), which are soluble in water and common organic solvents [228]. Cellulose functionalization is intended to impart an anionic, cationic or neutral functionality on cellulose, alter its hydrophobic properties to hydrophilic and improve its mechanical strength and chemical interaction. The common modification strategies which involve the use of acids alkalis, organic/inorganic compounds, and oxidizing agents, include oxidation, esterification, etherification, amidation, silylation, cationization and polymer grafting reactions [143, 229–231]. Others include irradiation technique and the application of crosslinking agents. The schematic illustration of strategies commonly employed for the modification of nanocelluloses is presented in Figure 10.
![Figure 10:
Schematic representation of strategies for nanocellulose modification [101].](/document/doi/10.1515/polyeng-2023-0056/asset/graphic/j_polyeng-2023-0056_fig_010.jpg)
Schematic representation of strategies for nanocellulose modification [101].
7.1 Oxidation
The term “cellulose oxidation” refers to a process whereby cellulose functional moieties interact chemically with oxidizing reagents, thereby altering their functionality. The OH moieties at the C6 position of the cellulose chemical structure can be converted to COOH via the oxidation reaction, while the secondary OH groups located at C2 and C3 can be oxidized to CHO and –COOH, respectively. Superoxide anion (O2 −) and hydrogen peroxide are the two most often utilized oxidants. In addition, sodium periodate (NaIO4) can be used to convert the hydroxyl groups of cellulose to dialdehyde groups during oxidation [232]. Thereafter, sodium chlorite (NaClO2) can further oxidize the aldehyde groups to carboxyl groups under heterogeneous conditions [232, 233]. This process makes the C2–C3-linkage of the anhydroglucose ring in the cellulose repeating unit to be specifically cleaved, producing two reactive carbonyl moieties. Some researchers have opted to use a catalyst such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and azide modification to introduce carboxylate functional groups onto cellulose [234, 235]. Tempo oxidation is a method that produces a high yield of the carboxyl group, hence its prevalent use in the oxidation of nanocelluloses.
7.2 Esterification
The interaction of OH functionalities on cellulose with one or more acids results in the production of cellulose esters, which are trivalent polymeric alcohols [230, 236]. The ester functional groups (O–C=O) are bonded to the hydroxyl groups of cellulose during esterification through condensation of the cellulosic material [136]. Although anhydrides (cyclic), such as succinic anhydride are more typically employed, other esterifying agents such as citric acid (CA), EDTA dianhydride, acyl halide and maleic anhydride have also been used [236]. The esterification of cellulose beads with inorganic acid derivatives like sulphate or phosphate groups, may produce charged functionalities, that can be employed in affinity or ion-exchange adsorption chromatography [193]. Tosylchloride and p-Iodobenzoyl chloride has also been used to modify cellulose beads, making them more convenient for nucleophilic displacement processes [237]. The tosyl moiety is a good leaving group that may be replaced by amines and ammonia. However, there are few studies in the literature about the synthesis of functional cellulose beads via the esterification process with carboxylic acid derivatives, which could be ascribed to the poor stability of the ester linkages under aqueous alkaline conditions. Further processing might strengthen cellulose adsorptive properties for metallic contaminants after esterification. Therefore, the cellulose obtained after esterification is frequently treated with a saturated sodium bicarbonate solution because the carboxylate functional group have better chelating capacities than the carboxylic groups [230].
7.3 Etherification
Etherification is a procedure that produces cellulose ethers by partially or fully replacing the C-2, C-3, and C-6 OH groups on cellulose macromolecules with ether groups, as previously stated in this article. Hydroxypropyl methylcellulose (HPMC), hydroxyethyl methyl cellulose (HEMC) and hydroxyethyl cellulose (HEC) are only a few examples of the products from the treatment of cellulose with ethylene oxide or other epoxides [230]. A typical etherification process employed in the production of carboxymethyl cellulose (CMC) involves 2 reaction conditions. The first is the mercerization stage, in which cellulose reacts with an alkali to form alkali-cellulose and water. The etherification process comes next, whereby the alkali-cellulose mixture is converted into CMC by using sodium chloroacetate or chloroacetic acid [238]. Under alkaline conditions, cellulose could also be etherified by reactioning with halogen, vinyl compounds (alkylation) or oxiranes (hydroxy alkylation). Etherification can also be achieved by the use of other chemical reagents like acrylonitrile, triphenyl chloromethane and (trityl chloride) among others [239, 240].
7.4 Amidation
The amidation method involves a mild and efficient modification process that utilizes biomolecules containing amine groups to functionalize the surface of nanocellulose. The reaction occurs during the conjugation of two molecules, each consisting of an amine and a carboxyl group. However, since these functionalities are absent on the nanocellulose surface, they must be incorporated onto the nanocellulose surface via appropriate modification methods (e.g. TEMPO oxidation) before amidation modification [144, 241]. The amidation process could be performed in aqueous solutions or organic solvents such as dimethylformamide (DMF). During the process, N-hydroxy succinimidyl (NHS) esters are formed, which activate the Tempo-oxidized carboxylated nanocellulose in an aqueous reaction system. This activation increases the reaction between the carboxyl molecules of the nanocellulose and the generated amide groups. The second stage in the amidation process involves the combination of amide-molecules and NHS-activated carboxylated nanocellulose which are then conjugated to the nanocellulose surface through amide bonds [144].
7.5 Silylation
Silane surface functionalization is a straightforward approach to increase the hydrophobicity of the hydrophilic cellulosic surface. Alkyl-dimethylchlorosilanes are frequently employed in the silylation process. After considerable silylation of microfibrillated cellulose with isopropyl dimethylchlorosilane (C3H8O–CH3)2SiHCl), Gousse et al. [242] reported the rheological characteristics of the MFCs These silylated MFCs demonstrated exceptional flexible and rheological characteristics while suspended in methyl oleate (C19H36O2), resulting in a shear thinning effect [243].
7.6 Cationic modification
Weak or highly charged ammonium-containing compounds, such as EPTMAC (2,3,epoxypropyl-trimethylammonium chloride), can be grafted onto the surfaces of CNCs because positive charges can be easily incorporated into CNCs. Cationization of CNCs occurs by reacting OH groups of alkali pre-treated cellulose with the charged EPTMAC. Thus, well-dispersed aqueous CNCs suspensions are formed by the attachment of EPTMAC to the surface of CNCs by nucleophilic addition. The nanocrystal properties are preserved during modification while the grafting process decreases the overall surface charge density with a reversal of the initial negative surface charge of CNCs to cations [244]. However, due to the high viscosity of the solution, shear birefringence develops in the cationic-modified CNCs, preventing the formation of the chiral crystalline liquid phase.
7.7 Grafting polymerization
Graft polymerisation combines two or more polymers into a single physical unit [245]. Some examples of polymerization techniques employed for the modification of cellulose include ring-opening polymerisation, free radical polymerisation, nitroxide-mediated polymerisation, reversible addition-fragmentation chain transfer (RAFT) and atom transfer radical polymerisation (ATRP) [185, 246]. There are few studies on using ring-opening polymerization on cellulose grafting. The first direct ROP of cyclic monomers e.g.-caprolactone (–CL), with cellulosic paper material and cotton fibers as initiators were reported by Hafren and Cordova [247]. According to a recent study by Loennberg et al. [248], e-caprolactone and L-lactic acid were successfully grafted onto cellulose fibers via ring-opening polymerization. The filter paper was treated with xyloglucan-bis(methylol)-2-methyl propanamide and 2,20-bis (methylol)propionic acid (bis-MPA) for the ROP of e-caprolactone and L-lactic acid, respectively, in order to incorporate more readily accessible hydroxyl groups to the cellulose surface. The ratio of the additional free initiator to the monomer was what determined how quickly the graft polymerized. According to their findings, bis-MPA-modified cellulose samples demonstrated better grafting effectiveness. The fibers that were produced demonstrated strong resistance to enzymatic breakdown.
The most often utilized radical polymerization technology for modifying the surface characteristics of cellulose and cellulose derivatives is ATRP. In the ATRP method, an appropriate alkyl halide experiences a reversible redox reaction that is catalyzed by a transition metal complex (activator, Mt n −Y/ligand, where Y may be a counter ion or a different ligand), resulting in the formation of an active radical and a metal halide complex by the oxidative addition of the halide (X) [249]. The active radicals can interact with one kind of monomer to multiply further with new monomers or they can remove the halide atom from the oxidized metal complex (deactivator, X–Mt n+1–Y/ligand), resulting in the formation of a dormant alkyl halide species and the activator. The metal complex activator then reactivates the alkyl halide species, which then replicates. ATRP was used for the first time by Carlmark and Malmstrom to graft monomers onto cellulose fibers at room temperature [250]. In order to create initiators at the cellulose surface, they functionalized the hydroxyl groups of a cellulose filter paper using 2-bromoisobutyryl bromide. In the presence of an expendable initiator, methyl acrylate (MA) was grafted on the surface. The final PMA-grafted paper obtained demonstrated exceptional hydrophobicity.
7.8 Irradiation technique
UV-irradiation is a simple method for incorporating metallic nanoparticles (NP) onto cellulose surface through the photo-activation of the cellulose fibrous surface by photons, and thereafter, by chemical reduction of metal ion species [251]. The mechanism is based on the quantity of reducing active sites on the cellulose surface, which can be adjusted by light intensity and irradiation time, resulting in different metal NP sizes and morphology. Light intensity and period of ultraviolet irradiation, for example, can influence both the distribution and quantity of silver metal NPs in cellulose/silver nanocomposites [252].
7.9 Use of cross-linking agents
Crosslinkers are necessary during bead fabrication to prevent the hydrophilic polymer chains of the cellulose beads from swelling or dissolving into the aqueous phase [193]. Crosslinking improves the cellulose bead’s chemical stability, mechanical strength and surface area [253, 254]. Cellulose beads can be cross-linked by physical crosslinking, chemical crosslinking or a combination of both physical and chemical interactions [255, 256]. Physical crosslinking is a simple and effective method that involves hydrogen bonding, ionic crosslinking, and electrostatic contact. In chemical crosslinking, a covalent bond is formed between the polymer chains [253]. Natural crosslinkers include genipin, enzymes, citric acid and tannic acid while glutaric dialdehyde, glycol diglycidyl ether, glutaraldehyde (GA), epichlorohydrin, carbodiimide, phosphate groups, boric acid (BA), acrylamides and aldehydes are examples of synthetic crosslinkers [253]. GA is the most often utilized crosslinker but its toxicity may be an issue unlike the other synthetic crosslinkers which present moderate or low toxicity [254, 256] Synthetic crosslinkers reduce the solubility of cellulose beads in acidic aqueous solutions while increasing their chemical stability [257].
8 Cellulose bead nanocomposite: application as an adsorbent in water purification and challenges encountered
8.1 Application of cellulose bead nanocomposites in water purification
Cellulose beads are useful materials for a variety of applications, especially when chemically treated to improve their performance [258]. Because of the spherical form of CBs and their ability to resist high flow rates, they are utilized as suitable filler materials for chromatographic columns and as stationary phases in size exclusion chromatography [217]. A summary of the performance of cellulose-based nanocomposite in water purification is presented in Table 3. Nanocellulose, when used as spherical adsorbents can be beneficial and of tremendous interest in wastewater treatment. It primarily depends on the presence of active sites on the sorbent, surface chemistry, chemical properties (e.g. sorbate pKa, basicity or acidity of the sorbent, etc.) physical properties (e.g. the sorbate molecular size, sorbent pore density, contact area, etc.) and specific interactions between adsorbent-adsorbate. According to scientific literature, chemically modified cellulose beads outperformed untreated cellulose in their ability to remove pollutants from aqueous solutions. For instance, Liu et al. [259] investigated the use of TEMPO-mediated oxidation and Fe3+ crosslinking for the modification of cellulose beads formed from cotton linter pulp (95 % α-cellulose), to produce Fe(III)-carboxylated cellulose beads (Fe-CCBs) adsorbents. The functionalized CBs were used to recover low levels of bromide in pharmaceutical effluents. During the oxidation process, 3 mmol sodium bromide and TEMPO reagent (0.0975 mmol) were added to 5 g of CBs immersed in 500 mL deionized water. Then, in phases, 10 % (w/w) NaClO (100 mL) was added to the mixture at pH 10. Thereafter 5 g of the wet carboxylated cellulose bead (5 g) were immersed in 10 mM FeCl3 metal salt solutions under magnetic stirring which produce Fe(III)-carboxylated cellulose beads (Fe-CCBs) by complexation mechanism. Liu and co-workers reported that an adsorption efficiency of 82.33 % was achieved for the elimination of bromide ions.
Microcrystalline cellulose (MC) produced from bamboo, was used by Qiao et al. [163] to create unique porous spherical beads with high adsorption capacity for heavy metal ions. During the procedure, MC was dissolved in 45 mL of an aqueous solution containing 12 wt% NaOH and 8 wt% thiourea. Subsequently, 900 mL ethylene glycol diglycidyl ether (ETH) was added to the solution while being stirred at 500 rpm in an ice bath. After that, a reactor containing oil phase (120 mL paraffin oil, 1.0 g Tween-80, and 5.0 g Span-80) was used to emulsify 10 mL of cellulose solution with some ETH to form spheres The cellulose beads were then repeatedly rinsed in ethanol and distilled water before being regenerated and solidified using 50 mL of sulfuric acid (5 wt%) aqueous solution. The beads were modified by dispersing 100 mg of the CB and 5 mmol of NaOH in a 100 mL solution of DMSO and water at a volume ratio of 2:1. Epichlorohydrin (10 mL) was added dropwise after the liquid had been heated to 40 °C in a thermostatic shaker spinning at 200 rpm. After that, 50 mL of a mixture containing 5 mmol glutamic acid (GA), 2 mmol Na2CO3 and 0.01 mmol NaBH4 was mixed with the epoxylated cellulose beads and the mixture was agitated at 100 rpm for 3 h. Qiao and colleagues reported that the adsorbent exhibited exceptional adsorption capacities toward the heavy metal ions (98.86 mg/g for Cu2+ and 102.29 mg/g for Co2+, respectively).
In a related study, Yang et al. [162] prepared cellulose nanocomposite beads from cotton linter pulp (95 % cellulose). These beads were functionalized with Mg–Al layered double hydroxide (LDH@CB) and employed as adsorbents for amoxicillin elimination in the aqueous medium. In order to make the cellulose beads, 6 g of cellulose were dissolved in 200 g of a solution of NaOH, urea and water (7/12/81, by weight), which had been pre-cooled to 12.5 °C. This mixture was vigorously stirred for 2 min. Using a syringe pump and a continuous flow rate of 5 mL/min, the resultant solution was added dropwise into a sodium chloride solution (250 mL, 10 wt%). Thereafter, in a three-neck round-bottom flask containing 10 mM Magnesium nitrate, 5 mM Aluminum nitrate, and 35 mM Urea, 20 g of the CB was added to create the functionalized cellulose (Mg–Al LDH@CB). According to Yang and colleagues, the LDH@CB had a maximum adsorption capacity of 138.3 mg/g for the elimination of amoxicillin. Meng et al. [40], fabricated carboxylated cellulose beads with a highly porous structure from discarded cotton (approx.93.6 wt% cellulose, DP *790) and the beads produced were subsequently carboxylated using citric acid and trisodium citrate. The adsorption performance of the carboxylated cellulose beads was investigated for the removal of methylene blue (MB). Cellulose beads nanocomposites were prepared by dissolving cotton cellulose into 7 wt% NaOH, 12 wt% urea solution and dripping the cellulose solution into a coagulation bath containing 1.5 M H2SO4 and 80 g/L Na2SO4 with the aid of a 0.7 mm needle-diameter peristaltic pump, at 30 °C. A constant distance (1 cm) between the needle tip and the bath surface was kept. Carboxylation of the cellulose beads was performed by dispersing wet cellulose beads (10 g) into a mixed solution of 4 g of citric acid and 1.5 g of trisodium citrate as catalysts with ultrasonic treatment for 30 min and heating at 110–120 °C for between 2 and 6 h. Meng and colleagues reported that the carboxylated porous cellulose beads (CCB) exhibited remarkable adsorption performance with a maximum adsorption capacity of 288.81 mg/g, about 8 times higher than unmodified cellulose beads. Their adsorption capacity for MB still retained 250.78 mg/g, after ten adsorption–desorption cycles.
In a related study, Li et al. [164], used ionic liquid to dissolve cellulose and, thereafter, synthesized CB from it via acid precipitation and surface-grafting with aminoguanidine hydrochloride using glutaric anhydride as a coupling agent. About 0.2 g microcrystalline cellulose (MCCs) was immersed in 10 mL of 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) ionic liquid and stirred at 100 °C for approximately 2 h until completely dissolved. Thereafter, nano-sized calcium carbonate (CaCO3) and 30 % wt of MCC were added and the mixtures introduced dropwise into an acidic solution (1 M HCl) to promote the formation of cellulose beads. The pore structure of the beads (2–3 mm) was generated when CaCO3 reacted with HCl. Accordingly, aminoguanidine hydrochloride was grafted on the beads via the use of a coupling agent (glutaric anhydride (0.5 g) after washing and freeze-drying. Micro cellulose powder (MCP) was also prepared. The maximum adsorption capacity of MCBs towards Hg (II) and Cu (II) removals were 581.4 and 94.88 mg/g respectively. XPS analysis showed –NH– and –NH2 as the main functional groups involved in the Hg (II) and Cu (II) adsorption on the adsorbents.
8.2 Challenges in the use of plant-based nanocomposites for water treatment
The challenges encountered in the production and usage of pulverized adsorbents have been identified. Columns could be clogged by the soft plant biomass thereby restricting the flow of eluent used to remove the adsorbate from the adsorbent surface in desorption experiments. Likewise, plant biomass is more likely to undergo degradation of surface morphology during pyrolysis as a result of their low carbonization energy while the separation and recovery of pulverized adsorbent from water could be challenging. In addition, the literatures reviewed in this work suggest that cellulose adsorbents may not be adopted immediately in industries and businesses since the majority of the studies have concentrated on the adsorption from laboratory-produced synthetic wastewater that contains contaminants and replicate industrial wastewater.
The problems can be resolved by first separating the cellulose from the plant biomass and then converting it into beads. Cellulose beads are easy to separate from aqueous media for re-use (unlike activated carbon) and can reduce backpressure and agglomeration during column experiments, The immobilization of cellulose in the form of beads is believed to increase the strength and stiffness of its structure. The porous surface of the beads also enhances the availability of the cellulose hydroxyl group while their high surface area to volume ratio simplifies the process of incorporating chemical moieties, thereby increasing the efficiency with which pollutants attach to the adsorbent. Moreover, the use of cellulose in the beaded form will prevent any potential damage to the surface characteristics since high pyrolytic temperatures are not required.
9 Conclusion and future perspective
Cellulose is the most prevalent, renewable biopolymer that can be readily integrated and chemically modified with other compounds and macromolecules to provide a plethora of commercial applications. Its unique attributes, such as low cost, renewable extraction source, low toxicity and excellent mechanical properties, have piqued the curiosity of both the industrial and scientific communities. This article strives to provide a comprehensive overview of the development of cellulose bead nanocomposites from cellulose biopolymer over the past few decades and their prospects for environmental remediation. Furthermore, different cellulose solvent systems (such as LiCl/DMAc, ionic liquids, organic-base aqueous solutions, alkali/urea solutions, etc.) and shaping techniques are discussed for the “bottom-up” process for producing cellulose beads. It has been established in the literature that cellulose is a viable precursor for the creation of cellulose beads for adsorption systems. Current modification strategies which could be applied for the conversion of cellulosic adsorbents include oxidative, etherification, esterification, amidation, silylation and polymer grafting. Although the application of cellulose beads for water purification is still an ongoing research in the scientific community, the outcome of the literature reviewed in this work showed their remarkably high adsorption performance for the removal of diverse contaminants. Their extraordinary adsorption performance could be attributed to the functionable surface of the beads, the functional moieties grafted to their surface during modification, along with the interplay of mechanisms like the electrostatic forces and complexation mechanism. A knowledge gap identified in the literatures reviewed shows that the development and application of 3-D nanocomposites for adsorption is currently done on a laboratory scale using synthetic wastewater and contaminants, since the up-scaling process is quite expensive. Thus, global research into the development of sustainable cellulose beads, suitable for the removal of contaminants from real effluents and on a larger scale is recommended. The formation of cellulose bead nanocomposites offers a procedure of choice for diverse modification processes and thus, a lot of promising benefits for commercial purpose in the future. Therefore, the authors suggest that more investigation should be carried out on the fabrication of cellulose beads with a focus on modification and preparation techniques in order to produce beads with strong structural forms, porous morphology and thus, high adsorption capacity. This will enable their widespread adoption as a cleaner, suitable replacement for powdered adsorbents like activated carbon and thus, the production prospects will be brighter. It is therefore anticipated that the need for cellulose and cellulose-bead nanocomposites in the field of wastewater treatment will be more appealing and widely adopted globally in the near future.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicting financial/personal interest regarding this article.
-
Research funding: No funding declared.
References
1. Akter, M., Bhattacharjee, M., Dhar, A. K., Rahman, F. B. A., Haque, S., Ur Rashid, T. U., Kabir, S. M. F. Cellulose-based hydrogels for wastewater treatment: a concise review. Gels 2021, 7, 1–28; https://doi.org/10.3390/gels7010030.Search in Google Scholar PubMed PubMed Central
2. Amin, M. T., Alazba, A. A., Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. https://doi.org/10.1155/2014/825910.Search in Google Scholar
3. Du Plessis, A. Current and future water scarcity and stress. In Water as an Inescapable Risk: Current Global Water Availability, Quality and Risks with a Specific Focus on South Africa; Springer: Cham, 11, 2019; pp. 13–25.10.1007/978-3-030-03186-2_2Search in Google Scholar
4. Djilani, C., Zaghdoudi, R., Modarressi, A., Rogalski, M., Djazi, F., Lallam, A. Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chem. Eng. J. 2012, 189–190, 203–212; https://doi.org/10.1016/j.cej.2012.02.059.Search in Google Scholar
5. Godfrey, J. M., Chalmers, K. Water Accounting: International Approaches to Policy and Decision-Making; Edward Elgar Publishing, Inc.: Northampton Massachusetts, USA, 2012.10.4337/9781849807500Search in Google Scholar
6. Crini, G., Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155; https://doi.org/10.1007/s10311-018-0785-9.Search in Google Scholar
7. Omar, M. A. B., Zin, N. S. B. M., Salleh, S. N. A. B. M. A review on performance of chemical, natural and composite coagulant. Int. J. Eng. Technol. 2018, 7, 56–60.Search in Google Scholar
8. Santhosh, C., Velmurugan, V., Jacob, G., Jeong, S. K., Grace, A. N., Bhatnagar, A. Role of nanomaterials in water treatment applications: a review. Chem. Eng. J. 2016, 306, 1116–1137; https://doi.org/10.1016/j.cej.2016.08.053.Search in Google Scholar
9. Awual, M. R. A. Facile composite material for enhanced cadmium(II) ion capturing from wastewater. J. Environ. Chem. Eng. 2019, 7, 103378; https://doi.org/10.1016/j.jece.2019.103378.Search in Google Scholar
10. Awual, M. R., Hasan, M. M., Asiri, A. M., Rahman, M. M. Cleaning the arsenic(V) contaminated water for safe-guarding the public health using novel composite material. Compos. B Eng. 2019, 171, 294–301; https://doi.org/10.1016/j.compositesb.2019.05.078.Search in Google Scholar
11. Salman, M. S., Sheikh, M. C., Hasan, M. M., Hasan, M. N., Kubra, K. T., Rehan, A. I., Awual, M. E., Rasee, A. I., Waliullah, R. M., Hossain, M. S., Khaleque, M. A., Alsukaibi, A. K. D., Alshammari, H. M., Awual, M. R. Chitosan-coated cotton fiber composite for efficient toxic dye encapsulation from aqueous media. Appl. Surf. Sci. 2023, 622, 157008; https://doi.org/10.1016/j.apsusc.2023.157008.Search in Google Scholar
12. Chai, W. S., Cheun, J. Y., Kumar, P. S., Mubashir, M., Majeed, Z., Banat, F., Ho, S. H., Show, P. L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589; https://doi.org/10.1016/j.jclepro.2021.126589.Search in Google Scholar
13. Chakraborty, R., Asthana, A., Singh, A. K., Jain, B., Susan, A. B. H. Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379; https://doi.org/10.1080/03067319.2020.1722811.Search in Google Scholar
14. Rathi, B. S., Kumar, P. S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995; https://doi.org/10.1016/j.envpol.2021.116995.Search in Google Scholar PubMed
15. Lobato-Peralta, D. R., Duque-Brito, E., Ayala-Cortés, A., Arias, D. M., Longoria, A., Cuentas-Gallegos, A. K., Sebastian, P. J., Okoye, P. U. Advances in activated carbon modification, surface heteroatom configuration, reactor strategies, and regeneration methods for enhanced wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 1–23; https://doi.org/10.1016/j.jece.2021.105626.Search in Google Scholar
16. Zhou, Y., Zhang, L., Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: a review. J. Mol. Liq. 2015, 212, 739–762; https://doi.org/10.1016/j.molliq.2015.10.023.Search in Google Scholar
17. Rathi, B. S., Kumar, P. S., Show, P. A review on effective removal of emerging contaminants from aquatic systems: current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413; https://doi.org/10.1016/j.jhazmat.2020.124413.Search in Google Scholar PubMed
18. Kosheleva, R. I., Mitropoulos, A. C., Kyzas, G. Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438; https://doi.org/10.1007/s10311-018-0817-5.Search in Google Scholar
19. Solangi, N. H., Kumar, J., Mazari, S. A., Ahmed, S., Fatima, N., Mubarak, N. M. Development of fruit waste derived bio-adsorbents for wastewater treatment: a review. J. Hazard. Mater. 2021, 416, 125848; https://doi.org/10.1016/j.jhazmat.2021.125848.Search in Google Scholar
20. Hsu, Y. C., Sil, M. C., Lin, C. H., Chen, C. M. Modification of covalent organic framework by hydrolysis for efficient and selective removal of organic dye. Appl. Surf. Sci. 2023, 612, 155890; https://doi.org/10.1016/j.apsusc.2022.155890.Search in Google Scholar
21. Hasan, M. M., Kubra, K. T., Hasan, M. N., Awual, M. E., Salman, M. S., Sheikh, M. C., Rehan, A. I., Rasee, A. I., Waliullah, R. M., Islam, M. S., Khandaker, S., Islam, A., Hossain, M. S., Alsukaibi, A. K. D., Alshammari, H. M., Awual, M. R. Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater. J. Mol. Liq. 2023, 371, 121125; https://doi.org/10.1016/j.molliq.2022.121125.Search in Google Scholar
22. Tapia-orozco, N., Ibarra-cabrera, R., Tecante, A., Gimeno, M., Parra, R., Garcia-arrazola, R. Removal strategies for endocrine disrupting chemicals using cellulose-based materials as adsorbents: a review. Biochem. Pharmacol. 2016, 4, 3122–3142; https://doi.org/10.1016/j.jece.2016.06.025.Search in Google Scholar
23. Mahmoud, D. K., Salleh, M. A. M., Abdul Karim, W. A. Langmuir model application on solid-liquid adsorption using agricultural wastes: environmental application review. J. Purity, Util. React. Environ. 2012, 1, 170–199.Search in Google Scholar
24. Noeline, B. F., Manohar, D. M., Anirudhan, T. S. Kinetic and equilibrium modelling of lead (II) sorption from water and wastewater by polymerized banana stem in a batch reactor. Sep. Purif. Technol. 2005, 45, 131–140; https://doi.org/10.1016/j.seppur.2005.03.004.Search in Google Scholar
25. Ahmed, M. J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: review. Biochem. Pharmacol. 2016, 4, 89–99; https://doi.org/10.1016/j.jece.2015.10.027.Search in Google Scholar
26. Olorundare, O. F., Msagati, T. A. M., Krause, R. W. M., Okonkwo, J. O., Mamba, B. B. Preparation and use of maize tassels’ activated carbon for the adsorption of phenolic compounds in environmental waste water samples. Environ. Sci. Pollut. Res. 2015, 22, 5780–5792; https://doi.org/10.1007/s11356-014-3742-6.Search in Google Scholar PubMed
27. Omo-Okoro, P. N., Curtis, C. J., Marco, A. M., Melymuk, L., Okonkwo, J. O. Removal of per- and polyfluoroalkyl substances from aqueous media using synthesized silver nanocomposite-activated carbons. J. Environ. Heal. Sci. Eng. 2021, 19, 217–236; https://doi.org/10.1007/s40201-020-00597-3.Search in Google Scholar PubMed PubMed Central
28. Yahya, M. D., Agie, J. O., Obayomi, K. S., Olugbenga, A. G., Afolabi, E. A. Immobilization of maize tassel in polyvinyl alcohol for the removal of phosphoric compounds from surface water near farmland. Cogent Eng. 2021, 8, 1–25; https://doi.org/10.1080/23311916.2021.1924940.Search in Google Scholar
29. Youssef, A. M., El-Naggar, M. E., Malhat, F. M., El Sharkawi, H. M. Efficient removal of pesticides and heavy metals from wastewater and the antimicrobial activity of F-MWCNTs/PVA nanocomposite film. J. Clean. Prod. 2019, 206, 315–325; https://doi.org/10.1016/j.jclepro.2018.09.163.Search in Google Scholar
30. Vazquez, A., Foresti, M. L., Moran, J. I., Cyras, V. P. Extraction and production of cellulose nanofibers. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Pandey, J., Takagi, H., Nakagaito, A., Kim, H., Eds. Springer: Berlin/Heidelberg, 2015; pp. 81–118.10.1007/978-3-642-45232-1_57Search in Google Scholar
31. Tanase, E. E., Rapa, M., Popa, O. Biopolymers based on renewable resources: a review. Open Chem. Eng. J. 2014, XVIII, 188–195.Search in Google Scholar
32. Joseph, B., Sagarika, V. K., Sabu, C., Kalarikkal, N., Thomas, S. Cellulose nanocomposites: fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237; https://doi.org/10.1016/j.jobab.2020.10.001.Search in Google Scholar
33. Motaung, T. E., Linganiso, L. Z. Critical review on agrowaste cellulose applications for biopolymers. Int. J. Plast. Technol. 2018, 22, 185–216; https://doi.org/10.1007/s12588-018-9219-6.Search in Google Scholar
34. Suhas, Gupta, V. K., Carrott, P. J. M., Singh, R., Chaudhary, M., Kushwaha, S. Cellulose: a review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076; https://doi.org/10.1016/j.biortech.2016.05.106.Search in Google Scholar PubMed
35. Badawy, A. A., Ghanem, A. F., Yassin, M. A., Youssef, A. M., Abdel Rehim, M. H. Utilization and characterization of cellulose nanocrystals decorated with silver and zinc oxide nanoparticles for removal of lead ion from wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100501; https://doi.org/10.1016/j.enmm.2021.100501.Search in Google Scholar
36. Fallah, Z., Nasr, H., Mahmood, I., Hamed, T., Amouei, A. TiO2-grafted cellulose via click reaction: an efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2018, 25, 639–660; https://doi.org/10.1007/s10570-017-1563-8.Search in Google Scholar
37. Abou-zeid, R. E., Kamal, K. H., El-aziz, M. E. A., Morsi, S. M., Kamel, S. Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int. J. Biol. Macromol. 2021, 167, 1091–1101; https://doi.org/10.1016/j.ijbiomac.2020.11.063.Search in Google Scholar PubMed
38. Wang, J., Liu, M., Duan, C., Sun, J., Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843; https://doi.org/10.1016/j.carbpol.2018.11.059.Search in Google Scholar PubMed
39. Shanmugarajah, B., Meileng, I., Mujawar, N., Sheanyaw, T., Yoo, C., Tan, K. Valorization of palm oil agro-waste into cellulose biosorbents for highly effective textile ef fl uent remediation. J. Clean. Prod. 2019, 210, 697–709; https://doi.org/10.1016/j.jclepro.2018.10.342.Search in Google Scholar
40. Meng, R., Liu, L., Jin, Y., Luo, Z., Gao, H., Yao, J. Recyclable carboxylated cellulose beads with tunable pore structure and size for highly efficient dye removal. Cellulose 2019, 26, 8963–8969; https://doi.org/10.1007/s10570-019-02733-1.Search in Google Scholar
41. Crini, G., Lichtfouse, E., Wilson, L. D., Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213; https://doi.org/10.1007/s10311-018-0786-8.Search in Google Scholar
42. Adewuyi, A. Chemically modified biosorbents and their role in the removal of emerging pharmaceuticalwaste in the water system. Water 2020, 12, 1–31; https://doi.org/10.3390/w12061551.Search in Google Scholar
43. Altunina, L. K., Tikhonova, L. D., Yarmukhametova, E. G. Method for deriving carboxymethyl cellulose. Eurasian Chem. J. 2016, 3, 49; https://doi.org/10.18321/ectj416.Search in Google Scholar
44. Alatawi, F. S., Monier, M., Elsayed, N. H. Amino functionalization of carboxymethyl cellulose for efficient immobilization of urease. Int. J. Biol. Macromol. 2018, 114, 1018–1025; https://doi.org/10.1016/j.ijbiomac.2018.03.142.Search in Google Scholar PubMed
45. Carmalin, A. S., Eder, L. C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17; https://doi.org/10.1016/j.ecoenv.2017.12.026.Search in Google Scholar PubMed
46. Malhotra, M., Suresh, S., Garg, A. Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 2018, 25, 32210–32220; https://doi.org/10.1007/s11356-018-3148-y.Search in Google Scholar PubMed
47. Katibi, K. K., Yunos, K. F., Man, H. C., Aris, A. Z., Nor, M. Z. M., Azis, R. S., Umar, A. M. Contemporary techniques for remediating endocrine-disrupting compounds in various water sources: advances in treatment methods and their limitations. Polymers 2021, 13, 1–46.10.3390/polym13193229Search in Google Scholar PubMed PubMed Central
48. Dickenson, E. R. V., Drewes, J. E. Quantitative structure property relationships for the adsorption of pharmaceuticals onto activated carbon. Water Sci. Technol. 2010, 62, 2270–2276; https://doi.org/10.2166/wst.2010.497.Search in Google Scholar PubMed
49. Patel, H. Fixed - bed column adsorption study: a comprehensive review. Appl. Water Sci. 2019, 9, 1–17; https://doi.org/10.1007/s13201-019-0927-7.Search in Google Scholar
50. Al-Ghouti, M. A., Da’ana, D. A. Guidelines for the use and interpretation of adsorption isotherm models: a review. J. Hazard. Mater. 2020, 393, 122383; https://doi.org/10.1016/j.jhazmat.2020.122383.Search in Google Scholar PubMed
51. Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70; https://doi.org/10.1016/j.progpolymsci.2004.11.002.Search in Google Scholar
52. Zhou, T., Che, G., Ding, L., Sun, D., Li, Y. Recent progress of selective adsorbents: from preparation to complex sample pretreatment. TrAC – Trends Anal. Chem. 2019, 121, 1–18; https://doi.org/10.1016/j.trac.2019.115678.Search in Google Scholar
53. Khakbaz, M., Ghaemi, A., Mir Mohamad Sadeghi, G. Synthesis methods of microporous organic polymeric adsorbents: a review. Polym. Chem. 2021, 12, 6962–6997; https://doi.org/10.1039/d1py01145f.Search in Google Scholar
54. Chen, Z., Li, Z., Chen, J., Kallem, P., Banat, F., Qiu, H. Recent advances in selective separation technologies of rare earth elements: a review. J. Environ. Chem. Eng. 2022, 10, 1–16; https://doi.org/10.1016/j.jece.2021.107104.Search in Google Scholar
55. Wang, Y., Rui, M., Lu, G. Recent applications of metal–organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194; https://doi.org/10.1002/jssc.201700401.Search in Google Scholar PubMed
56. Liu, R., Chi, L., Wang, X., Wang, Y., Sui, Y., Xie, T., Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168; https://doi.org/10.1016/j.cej.2018.09.122.Search in Google Scholar
57. Ouyang, H., Chen, N., Chang, G., Zhao, X., Sun, Y., Chen, S., Zhang, H., Yang, D. Metal – organic frameworks selective capture of toxic selenite anions by bismuth-based metal – organic frameworks angewandte. Communications 2018, 266590, 13197–13201; https://doi.org/10.1002/anie.201807891.Search in Google Scholar PubMed
58. Wang, L., Wang, L., Zhao, J., Yan, T. Adsorption of selected gases on metal-organic frameworks and covalent organic frameworks: a comparative grand canonical Monte Carlo simulation adsorption of selected gases on metal-organic frameworks and covalent organic frameworks: a comparative grand. J. Appl. Phys. 2012, 111, 1–6.10.1063/1.4726255Search in Google Scholar
59. Xiong, X. H., Tao, Y., Yu, Z. W., Yang, L. X., Sun, L. J., Fan, Y. L., Luo, F. Selective extraction of thorium from uranium and rare earth elements using sulfonated covalent organic framework and its membrane derivate. Chem. Eng. J. 2020, 384, 1–7; https://doi.org/10.1016/j.cej.2019.123240.Search in Google Scholar
60. Chen, L., Xie, H., Yu, W., Gui, Z. H., Hou, C., Fan, L. L., Wang, Y., Wang, T. Functionalization methods of carbon nanotubes and its applications. In Carbon Nanotubes Applications on Electron Devices; Intech: Shanghai, 41, 2011; pp. 215–222.10.5772/18547Search in Google Scholar
61. Ibrahim, K. S. Carbon nanotubes – properties and applications: a review. Carbon Lett. 2013, 14, 131–144; https://doi.org/10.5714/cl.2013.14.3.131.Search in Google Scholar
62. Dutt, M. A., Hanif, M. A., Nadeem, F., Bhatti, H. N. A review of advances in engineered composite materials popular for wastewater treatment. J. Environ. Chem. Eng. 2020, 8; https://doi.org/10.1016/j.jece.2020.104073.Search in Google Scholar
63. Sabzehmeidani, M. M., Mahnaee, S., Ghaedi, M., Heidari, H., Roy, V. A. L. Carbon based materials: a review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627; https://doi.org/10.1039/d0ma00087f.Search in Google Scholar
64. Hashemi, B., Zohrabi, P., Shamsipur, M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337–347; https://doi.org/10.1016/j.talanta.2018.05.053.Search in Google Scholar PubMed
65. Al-saidi, H. M., Abdel-fadeel, M. A., El-sonbati, A. Z., El-bindary, A. A. Multi-walled carbon nanotubes as an adsorbent material for the solid phase extraction of bismuth from aqueous media: kinetic and thermodynamic studies and analytical applications. J. Mol. Liq. 2016, 216, 693–698; https://doi.org/10.1016/j.molliq.2016.01.086.Search in Google Scholar
66. Ehyaee, M., Safa, F., Shariati, S. Magnetic nanocomposite of multi-walled carbon nanotube as effective adsorbent for methyl violet removal from aqueous solutions: response surface modeling and kinetic study. Korean J. Chem. Eng. 2017, 34, 1051–1061; https://doi.org/10.1007/s11814-016-0353-6.Search in Google Scholar
67. Pitroda, J. A. Critical review on carbon nanotubes. Int. J. Constr. Res. Civ. Eng. 2016, 2, 36–42.Search in Google Scholar
68. Sharma, P., Singh, A. K., Shahi, V. K. Selective adsorption of Pb(II) from aqueous medium by cross- linked chitosan-functionalized graphene oxide adsorbent. ACS Sustain. Chem. Eng. 2019, 7, 1427–1436; https://doi.org/10.1021/acssuschemeng.8b05138.Search in Google Scholar
69. Sui, Z.-Y., Zhou, D., Han, B.-H. Fabrication of graphene-based porous materials and their applications in environmental fields. In Graphene Science Handbook. Applications and Industrialization; Aliofkhazraei, M., Ali, N., Milne, W. I., Ozkan, C. S., Mitura, S., Gervasoni, J. L. E., Eds.; CRC press: Boca Raton, Florida, 2016; pp. 399–418.Search in Google Scholar
70. Ali, I., Arsh, A., Mbianda, X. Y., Burakov, A., Galunin, E., Burakova, I., Mkrtchyan, E., Tkachev, A., Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180; https://doi.org/10.1016/j.envint.2019.03.029.Search in Google Scholar PubMed
71. Mo, J., Yang, Q., Zhang, N., Zhang, W., Zheng, Y., Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405; https://doi.org/10.1016/j.jenvman.2018.08.069.Search in Google Scholar PubMed
72. Oladoye, P. O. Natural, low-cost adsorbents for toxic Pb (II) ion sequestration from (waste) water: a state-of-the-art review. Chemosphere 2022, 287, 132130; https://doi.org/10.1016/j.chemosphere.2021.132130.Search in Google Scholar PubMed
73. Cashin, V. B., Eldridge, D. S., Yu, A., Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: a review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128; https://doi.org/10.1039/c7ew00322f.Search in Google Scholar
74. Awual, M. R., Hasan, M. M., Znad, H. Organic-inorganic based nano-conjugate adsorbent for selective palladium(II) detection, separation and recovery. Chem. Eng. J. 2015, 259, 611–619; https://doi.org/10.1016/j.cej.2014.08.028.Search in Google Scholar
75. Awual, M. R., Yaita, T. Rapid sensing and recovery of palladium(II) using N,N-Bis(Salicylidene)1,2- bis(2-Aminophenylthio)Ethane modified sensor ensemble adsorbent. Sens. Actuators, B Chem. 2013, 183, 332–341; https://doi.org/10.1016/j.snb.2013.04.009.Search in Google Scholar
76. Salman, M. S., Hasan, M. N., Hasan, M. M., Kubra, K. T., Sheikh, M. C., Rehan, A. I., Waliullah, R. M.; Rasee, A. I., Awual, M. E., Hossain, M. S., Alsukaibi, A. K. D., Alshammari, H. M., Awual, M. R. Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J. Mol. Struct. 2023, 1282, 1–10; https://doi.org/10.1016/j.molstruc.2023.135259.Search in Google Scholar
77. Rout, P. R., Zhang, T. C., Bhunia, P., Surampalli, R. Y. Treatment technologies for emerging contaminants in wastewater treatment plants: a review. Sci. Total Environ. 2021, 753, 1–17; https://doi.org/10.1016/j.scitotenv.2020.141990.Search in Google Scholar PubMed
78. Hoseinzadeh, R., Mohd, W., Wan, A., Sahu, J. N., Arami-niya, A. Journal of analytical and applied pyrolysis the effects of a microwave heating method on the production of activated carbon from agricultural waste: a review. J. Anal. Appl. Pyrol. 2013, 100, 1–11.10.1016/j.jaap.2012.12.019Search in Google Scholar
79. Ge, X., Wu, Z., Wu, Z., Yan, Y., Cravotto, G., Ye, B. C. Enhanced PAHs adsorption using iron-modified coal-based activated carbon via microwave radiation. J. Taiwan Inst. Chem. Eng. 2016, 64, 235–243; https://doi.org/10.1016/j.jtice.2016.03.050.Search in Google Scholar
80. Jawad, A. H., Mehdi, Z. S., Ishak, M. A. M., Ismail, K. Large surface area activated carbon from low-rank coal via microwave-assisted KOH activation for methylene blue adsorption. Desalin. Water Treat. 2018, 110, 239–249; https://doi.org/10.5004/dwt.2018.22226.Search in Google Scholar
81. El-hendawy, A. A., Samra, S. E., Girgis, B. S. Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surf. 2001, 180, 209–221; https://doi.org/10.1016/s0927-7757(00)00682-8.Search in Google Scholar
82. Nandini, G. K. M., Sheba, M. C. Emanating trends in the usage of bio-coagulants in potable water treatment: a review. Int. Res. J. Eng. Technol. 2016, 3, 970–974.Search in Google Scholar
83. Choy, S. Y., Prasad, K. M. N., Wu, T. Y., Ramanan, R. N. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367–390; https://doi.org/10.1007/s13762-013-0446-2.Search in Google Scholar
84. Kiew, P. L., Chong, K. H. Development of fruit-based waste material as bioflocculant for water clarification. J. Mech. Eng. 2017, 4, 1–10.Search in Google Scholar
85. Trache, D. Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater. Sci. 2018, 5, 201–205; https://doi.org/10.3934/matersci.2018.2.201.Search in Google Scholar
86. Abdel-shafy, H. I., Mansour, M. S. M. Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290; https://doi.org/10.1016/j.ejpe.2018.07.003.Search in Google Scholar
87. Elbasiouny, H., Elbanna, B. A., Al-najoli, E., Alsherief, A. Agricultural waste management for climate change mitigation: some implications to Egypt. In Waste Management in MENA Regions; Negm, A. M., Shareef, N., Eds. Springer Nature: Egypt, 2020.10.1007/978-3-030-18350-9_8Search in Google Scholar
88. Testa, M. L., Tummino, M. L. Lignocellulose biomass as a multifunctional tool for sustainable catalysis and chemicals: an overview. Catalysts 2021, 11, 1–27; https://doi.org/10.3390/catal11010125.Search in Google Scholar
89. Duarah, P., Haldar, D., Purkait, M. K. Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: a review. Int. J. Biol. Macromol. 2020, 163, 1828–1843; https://doi.org/10.1016/j.ijbiomac.2020.09.076.Search in Google Scholar PubMed
90. Davin, L. B., Lewis, N. G. Lignin primary structures and dirigent sites. Curr. Opin. Biotechnol. 2005, 16, 407–415; https://doi.org/10.1016/j.copbio.2005.06.011.Search in Google Scholar PubMed
91. Yoon, S. Y., Han, S. H., Shin, S. J. The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy 2014, 77, 19–24; https://doi.org/10.1016/j.energy.2014.01.104.Search in Google Scholar
92. Fernandes, E. M., Pires, R. A., Mano, J. F., Reis, R. L. Bionanocomposites from lignocellulosic resources: properties , applications and future trends for their use in the biomedical field. Prog. Polym. Sci. 2013, 38, 1415–1441; https://doi.org/10.1016/j.progpolymsci.2013.05.013.Search in Google Scholar
93. Jayapal, N., Samanta, A. K., Kolte, A. P., Senani, S., Sridhar, M., Suresh, K. P., Sampath, K. T. Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind. Crop. Prod. 2013, 42, 14–24; https://doi.org/10.1016/j.indcrop.2012.05.019.Search in Google Scholar
94. Collard, F., Blin, J. A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose , hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608; https://doi.org/10.1016/j.rser.2014.06.013.Search in Google Scholar
95. Ali, I., Asim, M., Khan, T. A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183; https://doi.org/10.1016/j.jenvman.2012.08.028.Search in Google Scholar PubMed
96. Wu, Z., Cheng, Z., Ma, W. Bioresource technology adsorption of Pb (II) from glucose solution on thiol-functionalized cellulosic biomass. Bioresour. Technol. 2012, 104, 807–809; https://doi.org/10.1016/j.biortech.2011.10.100.Search in Google Scholar PubMed
97. Zhang, X., Lei, H., Chen, S., Wu, J. Catalytic Co-pyrolysis of lignocellulosic biomass with polymers: a critical review. Green Chem. 2016, 18, 4145–4169; https://doi.org/10.1039/c6gc00911e.Search in Google Scholar
98. Harris, D. M. Molecular and Chemical Dissection of Cellulose Biosynthesis in Plants; University of Kentucky: Kentucky, 2011.Search in Google Scholar
99. Credou, J., Thomas, B. C. From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788.10.1039/C4TB00431KSearch in Google Scholar PubMed
100. Chirayil, C. J., Mathew, L., Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28.Search in Google Scholar
101. Tavakolian, M., Jafari, S. M., van de Ven, T. G. M. A review on surface-functionalized cellulosic nanostructures as biocompatible antibacterial materials. Nano-Micro Lett. 2020, 12, 1–23; https://doi.org/10.1007/s40820-020-0408-4.Search in Google Scholar PubMed PubMed Central
102. Börjesson, M., Westman, G. Crystalline Nanocellulose — Preparation, Modification, and Properties; Intech Open, Vol. 32, 2015; pp. 137–144.10.5772/61899Search in Google Scholar
103. Huber, T., Müssig, J., Curnow, O., Pang, S., Bickerton, S., Staiger, M. P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186; https://doi.org/10.1007/s10853-011-5774-3.Search in Google Scholar
104. Sunasee, R., Hemraz, U. D. Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals. Fibers 2018, 6, 1–19; https://doi.org/10.3390/fib6010015.Search in Google Scholar
105. Gupta, P. K., Raghunath, S. S., Prasanna, D. V., Shree, V. An update on overview of cellulose, its structure and applications. In Cellulose; IntechOpen: France, 2019; pp. 1–23.Search in Google Scholar
106. Esteban, U. B. Cellulose Nanocrystals Properties and Applications in Renewable Nanocomposites; Clemson University: New york, 2011.Search in Google Scholar
107. Jonoobi, M., Oladi, R., Davoudpour, Y., Oksman, K., Dufresne, A., Hamzeh, Y., Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose 2015, 22, 935–969; https://doi.org/10.1007/s10570-015-0551-0.Search in Google Scholar
108. Kalia, S., Dufresne, A., Cherian, B. M., Kaith, B. S., Avérous, L., Njuguna, J., Nassiopoulos, E. Cellulose-based bio- and nanocomposites: a review. Int. J. Polym. Sci. 2011, 2011, 1–15; https://doi.org/10.1155/2011/837875.Search in Google Scholar
109. Bergh, M. Absorbent Cellulose Based Fibers Investigation of Carboxylation and Sulfonation of Cellulose; Chalmers University of Technology: Göteborg, Sweden, 2011.Search in Google Scholar
110. Lindman, B., Medronho, B., Alves, L., Costa, C., Edlund, H., Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718; https://doi.org/10.1039/c7cp02409f.Search in Google Scholar PubMed
111. George, J., Sabapathi, S. N. Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54; https://doi.org/10.2147/nsa.s64386.Search in Google Scholar
112. Zugenmaier, P. Order in cellulosics: historical review of crystal structure research on cellulose. Carbohydr. Polym. 2021, 254, 117417; https://doi.org/10.1016/j.carbpol.2020.117417.Search in Google Scholar PubMed
113. Makarem, M., Lee, C. M., Kafle, K., Huang, S., Chae, I., Yang, H., Kubicki, J. D., Kim, S. H. Probing cellulose structures with vibrational spectroscopy. Cellulose 2019, 26, 35–79; https://doi.org/10.1007/s10570-018-2199-z.Search in Google Scholar
114. Sharma, P. R., Sharma, S. K., Lindström, T., Hsiao, B. S. Nanocellulose-enabled membranes for water purification: perspectives. Adv. Sustain. Syst. 2020, 1900114, 1–28; https://doi.org/10.1002/adsu.201900114.Search in Google Scholar
115. Wyman, C., Decker, S., Himmel, M., Brady, J., Skopec, C., Viikari, L. Hydrolysis of cellulose and hemicellulose. In Polysaccharide: Structural Diversity and Functional Versatility; Marcel Dekker Inc: New york, 2004; pp. 1023–1062.10.1201/9781420030822.ch43Search in Google Scholar
116. Atalla, R. H., VanderHart, D. L. Native cellulose: a composite of two distinct crystalline forms. Adv. Sci. 2011, 223, 283–285; https://doi.org/10.1126/science.223.4633.283.Search in Google Scholar PubMed
117. Hangasky, J., Detomasi, T. C., Lemon, C. Glycosidic bond oxidation: the structure, function, and mechanism of polysaccharide monooxygenases. In Comprehensive Natural Products III, 3rd ed.; Elsevier: Amsterdam, 5, 2020; pp. 1–34.10.1016/B978-0-12-409547-2.14859-0Search in Google Scholar
118. Ioelovich, M., Leykin, A. Accessibility and supermolecular structure of cellulose. Cellul. Chem. Technol. 2009, 43, 379–385.Search in Google Scholar
119. Kargarzadeh, H., Mariano, M., Gopakumar, D., Ahmad, I., Thomas, S., Dufresne, A., Huang, J., Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189; https://doi.org/10.1007/s10570-018-1723-5.Search in Google Scholar
120. Sun, S., Mitchell, J. R., Macnaughtan, W., Foster, T. J., Harabagiu, V., Song, Y., Zheng, Q. Comparison of the mechanical properties of cellulose and starch films. Biomacromolecules 2010, 11, 126–132; https://doi.org/10.1021/bm900981t.Search in Google Scholar PubMed
121. Baghaei, B., Skrifvars, M. All-cellulose composites: a review of recent studies on structure, properties and applications. Molecules 2020, 25, 1–19; https://doi.org/10.3390/molecules25122836.Search in Google Scholar PubMed PubMed Central
122. Duchesne, L. C., Larson, D. W. Cellulose and the evolution of plant life. Bioscience 1989, 39, 238–241; https://doi.org/10.2307/1311160.Search in Google Scholar
123. Fathi, M., Karim, M., Ahmadi, N. Nanostructures of cellulose for encapsulation of food ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Jafari, S. M., Ed.; Elsevier: Amsterdam, 2019; pp. 493–519.10.1016/B978-0-12-815663-6.00017-3Search in Google Scholar
124. Lin, N., Dufresne, A. Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 2014, 59, 302–325.10.1016/j.eurpolymj.2014.07.025Search in Google Scholar
125. Yang, Y., Chen, Z., Zhang, J., Wang, G., Zhang, R., Suo, D. Preparation and applications of the cellulose nanocrystal. Int. J. Polym. Sci. 2019, 2019, 1–11; https://doi.org/10.1155/2019/1767028.Search in Google Scholar
126. Shak, K. P. Y., Pang, Y. L., Mah, S. K. Nanocellulose: recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498; https://doi.org/10.3762/bjnano.9.232.Search in Google Scholar PubMed PubMed Central
127. Trache, D., Hussin, M. H., Haafiz, M. K. M., Thakur, V. K. Recent progress in cellulose nanocrystals: sources and production. Nanoscale 2017, 9, 1763–1786; https://doi.org/10.1039/c6nr09494e.Search in Google Scholar PubMed
128. Kargarzadeh, H., Ioelovich, M., Ahmed, I., Thomas, S., Dufresne, A. Methods for extraction of nanocellulose from various sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds., 2017; pp. 1–49.10.1002/9783527689972.ch1Search in Google Scholar
129. Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co KG: Berlin, 2017.10.1515/9783110480412Search in Google Scholar
130. Mariano, M. Applications of Cellulose Nanocrystals: Thermal , Rheological and Mechanical Properties of New Materials; Université Grenoble Alpes: France, 2017.Search in Google Scholar
131. Hassan, S. H., Voon, L. H., Velayutham, T. S., Zhai, L., Kim, H. C., Kim, J. Review of cellulose smart material: biomass conversion process and progress on cellulose-based electroactive paper. J. Renew. Mater. 2018, 6, 1–25; https://doi.org/10.7569/jrm.2017.634173.Search in Google Scholar
132. Jedvert, K., Heinze, T. Cellulose modification and shaping – a review. J. Polym. Eng. 2017, 37, 845–860; https://doi.org/10.1515/polyeng-2016-0272.Search in Google Scholar
133. Priya, S., Khan, G., Uddin, M., Haque, M., Islam, M., Abdullah-Al-Mamun, M., Gafur, M., Alam, M. Characterization of micro-fibrillated cellulose produced from sawmill wastage: crystallinity and thermal properties. Am. Chem. Sci. J. 2015, 9, 1–8; https://doi.org/10.9734/acsj/2015/19752.Search in Google Scholar
134. Hamad, W. Y. Cellulose Nanocrystals: Properties, Production and Applications; John Wiley & Sons: West Sussex, UK, 2017.10.1002/9781118675601Search in Google Scholar
135. Davoudpour, Y., Hossain, S., Khalil, H. A., Haafiz, M. M., Ishak, Z. M., Hassan, A., Sarker, Z. I. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crop. Prod. 2015, 74, 381–387; https://doi.org/10.1016/j.indcrop.2015.05.029.Search in Google Scholar
136. Missoum, K., Belgacem, M. N., Bras, J. Nanofibrillated cellulose surface modification: a review. Materials 2013, 6, 1745–1766; https://doi.org/10.3390/ma6051745.Search in Google Scholar PubMed PubMed Central
137. Ioelovich, M. Peculiarities of cellulose nanoparticles. TAPPI J. 2014, 13, 45–52; https://doi.org/10.32964/tj13.5.45.Search in Google Scholar
138. Gopakumar, D. A. Nanocellulose Based Functional Constructs for Clean Water and Microwave Suppression; Université de Bretagne Sud: Lorient, France, 2017.Search in Google Scholar
139. Moon, R. J., Martini, A., Nairn, J., Simonsen, J., Youngblood, J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994; https://doi.org/10.1039/c0cs00108b.Search in Google Scholar PubMed
140. Habibi, Y., Lucia, L. A., Rojas, O. J. Cellulose nanocrystals: chemistry , self-assembly , and applications. Am. Chem. Soc. 2010, 110, 3479–3500; https://doi.org/10.1021/cr900339w.Search in Google Scholar PubMed
141. Rojas, J., Bedoya, M., Cho, Y. Current trends in the production of cellulose nanoparticles and nanocomposites for biomedical applications. In Cellulose-fundamental Aspects and Current Trends; InTechOpen, Vol. 32, 2015; pp. 193–228.10.5772/61334Search in Google Scholar
142. Sharma, A., Thakur, M., Bhattacharya, M., Mandal, T., Goswami, S. Commercial application of cellulose nano-composites – a review. Biotechnol. Rep. 2019, 21, e00316; https://doi.org/10.1016/j.btre.2019.e00316.Search in Google Scholar PubMed PubMed Central
143. Mohanty, A. K., Misra, M., Drzal, L. T. Surface modifications of natural fibers and performance of the resulting biocomposites: an overview. Compos. Interfaces 2012, 8, 313–343; https://doi.org/10.1163/156855401753255422.Search in Google Scholar
144. Huang, J., Ma, X., Yang, G., Alain, D. Introduction to nanocellulose. Nanocellulose Fundam. Adv. Mater. 2019, 1, 1–20.10.1002/9783527807437.ch1Search in Google Scholar
145. Rahman, N. S. A., Yhaya, M. F., Azahari, B., Ismail, W. R. Utilisation of natural cellulose fibres in wastewater treatment. Cellulose 2018, 25, 4887–4903; https://doi.org/10.1007/s10570-018-1935-8.Search in Google Scholar
146. Li, Y., Jiang, L., Xiong, C., Peng, W. Effect of different surface treatment for bamboo fiber on the crystallization behavior and mechanical property of bamboo fiber/nanohydroxyapatite/poly (lactic- Co -glycolic) composite. Ind. Eng. Chem. Res. 2015, 54, 12017−12024, https://doi.org/10.1021/acs.iecr.5b02724.Search in Google Scholar
147. Mohd, N., Draman, S. F. S., Salleh, M. S. N., Yusof, N. B. Dissolution of cellulose in ionic liquid: a review. AIP Conf. Proc. 2017, 1809, 0020035 13.10.1063/1.4975450Search in Google Scholar
148. Bergenstråhle, M., Wohlert, J., Himmel, M. E., Brady, J. W. Simulation studies of the insolubility of cellulose. Carbohydr. Res. 2010, 345, 2060–2066; https://doi.org/10.1016/j.carres.2010.06.017.Search in Google Scholar PubMed
149. Oprea, M., Voicu, S. I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683–116701; https://doi.org/10.1016/j.carbpol.2020.116683.Search in Google Scholar PubMed
150. Biswas, A., Selling, G. S., Shogren, R. L., Willet, J. L., Buchanan, C. M., Cheng, H. N. Iodine-catalyzed esterification of polysaccharides. Chem. Today 2009, 27, 33–35.Search in Google Scholar
151. Cheng, H. N., Dowd, M. K., Selling, G. W., Biswas, A. Synthesis of cellulose acetate from cotton byproducts Q. Carbohydr. Polym. 2010, 80, 449–452; https://doi.org/10.1016/j.carbpol.2009.11.048.Search in Google Scholar
152. Khoshnevisan, K., Maleki, H., Samadian, H., Doostan, M., Reza, M. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nano fi brous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268; https://doi.org/10.1016/j.ijbiomac.2019.08.207.Search in Google Scholar PubMed
153. Jiang, Z., Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114; https://doi.org/10.1016/j.molliq.2018.11.153.Search in Google Scholar
154. Villabona-Ortíz, Á., Figueroa-Lopez, K. J., Ortega-Toro, R. Kinetics and adsorption equilibrium in the removal of azo-anionic dyes by modified cellulose. Sustainability 2022, 14, 1–19; https://doi.org/10.3390/su14063640.Search in Google Scholar
155. Komal, Gupta, K., Kaur, S., Kaur, J., Kaushik, A., Singhal, S. A comparative analysis of source based distinctly functionalized nanostructured cellulose for the adsorptive removal of toxic colorants. Cellulose 2019, 26, 1703–1724; https://doi.org/10.1007/s10570-018-2191-7.Search in Google Scholar
156. Madivoli, E., Kareru, P., Gachanja, A., Mugo, S., Murigi, M., Kairigo, P., Kipyegon, C., Mutembei, J., Njonge, F. Adsorption of selected heavy metals on modified nano cellulose. Int. Res. J. Pure Appl. Chem. 2016, 12, 1–9; https://doi.org/10.9734/irjpac/2016/28548.Search in Google Scholar
157. Liu, X., Yang, R., Xu, M., Ma, C., Li, W., Yin, Y., Huang, Q., Wu, Y., Li, J., Liu, S. Hydrothermal synthesis of cellulose nanocrystal-grafted-acrylic acid aerogels with superabsorbent properties. Polymers 2018, 10, 1–15; https://doi.org/10.3390/polym10101168.Search in Google Scholar PubMed PubMed Central
158. Ding, Y., Song, C., Gong, W., Liu, L., Wu, M., Li, L., Yao, J. Robust, sustainable, hierarchical multi-porous cellulose beads via pre-crosslinking strategy for efficient dye adsorption. Cellulose 2021, 28, 7227–7241; https://doi.org/10.1007/s10570-021-03979-4.Search in Google Scholar
159. Jarrah, K., Hisaindee, S., Al-Sayah, M. H. Preparation of oil sorbents by solvent-free grafting of cellulose cotton fibers. Cellulose 2018, 25, 4093–4106; https://doi.org/10.1007/s10570-018-1846-8.Search in Google Scholar
160. Tian, D., Zhang, X., Lu, C., Yuan, G., Zhang, W., Zhou, Z. Solvent-free synthesis of carboxylate-functionalized cellulose from waste cotton fabrics for the removal of cationic dyes from aqueous solutions. Cellulose 2014, 21, 473–484; https://doi.org/10.1007/s10570-013-0112-3.Search in Google Scholar
161. Gao, X., Zhang, H., Chen, K., Zhou, J., Liu, Q. Removal of heavy metal and sulfate ions by cellulose derivative-based biosorbents. Cellulose 2018, 25, 2531–2545; https://doi.org/10.1007/s10570-018-1690-x.Search in Google Scholar
162. Yang, C., Wang, L., Yu, Y., Wu, P., Wang, F., Liu, S., Luo, X. Highly efficient removal of amoxicillin from water by Mg-Al layered double hydroxide/cellulose nanocomposite beads synthesized through in-situ coprecipitation method. Int. J. Biol. Macromol. 2020, 149, 93–100; https://doi.org/10.1016/j.ijbiomac.2020.01.096.Search in Google Scholar PubMed
163. Qiao, L., Li, S., Li, Y., Liu, Y., Du, K. Fabrication of superporous cellulose beads via enhanced inner cross-linked linkages for high efficient adsorption of heavy metal ions. J. Clean. Prod. 2020, 253, 120017; https://doi.org/10.1016/j.jclepro.2020.120017.Search in Google Scholar
164. Li, B., Pan, Y., Zhang, Q., Huang, Z., Liu, J., Xiao, H. Porous cellulose beads reconstituted from ionic liquid for adsorption of heavy metal ions from aqueous solutions. Cellulose 2019, 26, 9163–9178; https://doi.org/10.1007/s10570-019-02687-4.Search in Google Scholar
165. McKeen, L. Renewable Resource and Biodegradable Polymers. The Effect of Sterilization on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2012.10.1016/B978-1-4557-2598-4.00012-5Search in Google Scholar
166. Nikolsky, S. N., Zlenko, D. V., Melnikov, V. P., Stovbun, S. V., Ras, P. The fibrils untwisting limits the rate of cellulose nitration process ☆. Carbohydr. Polym. 2019, 204, 232–237, https://doi.org/10.1016/j.carbpol.2018.10.019.Search in Google Scholar PubMed
167. Li, L., Frey, M. Preparation and characterization of cellulose nitrate-acetate mixed esterfibers. Polymer 2010, 51, 3774–3783; https://doi.org/10.1016/j.polymer.2010.06.013.Search in Google Scholar
168. Zhang, J., Qi, Y., Shen, Y., Li, H. Research progress on chemical modification and application of cellulose: a review. Mater. Sci. 2022, XX, 1–8; https://doi.org/10.5755/j02.ms.25485.Search in Google Scholar
169. Ibbett, R. N., Philp, K., Price, D. M. 13C n.m.r. studies of the thermal behaviour of aqueous solutions of cellulose ethers. Polymer 1992, 33, 4087–4094; https://doi.org/10.1016/0032-3861(92)90610-9.Search in Google Scholar
170. Jain, S., Sandhu, P. S., Malvi, R., Gupta, B. Cellulose derivatives as thermoresponsive polymer: an overview. J. Appl. Pharm. Sci. 2013, 3, 139–144.Search in Google Scholar
171. Clasen, C., Kulicke, W. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919; https://doi.org/10.1016/s0079-6700(01)00024-7.Search in Google Scholar
172. Singh, V., Joshi, S., Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: an efficient drug carrier. Int. J. Biol. Macromol. 2018, 112, 390–398; https://doi.org/10.1016/j.ijbiomac.2018.01.184.Search in Google Scholar PubMed
173. Capanema, N. S. V., Mansur, A. A. P., de Jesus, A. C., Carvalho, S. M., de Oliveira, L. C., Mansur, H. S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234; https://doi.org/10.1016/j.ijbiomac.2017.08.124.Search in Google Scholar PubMed
174. Lavanya, D., Kulkarni, P. K., Dixit, M., Raavi, P. K., Krishna, L. N. V. Sources of cellulose and their applications – a review. Int. J. Drug Formul. Res. 2011, 2, 19–38.Search in Google Scholar
175. Brady, J., Drig, T., Lee, P. I., Li, J. X. Polymer properties and characterization. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Qiu, Y., Chen, Y., Geoff, G., Zhang, Z., Lawrence, Yu, R. V., Eds., 2nd ed. 2017; pp. 181–223.10.1016/B978-0-12-802447-8.00007-8Search in Google Scholar
176. Adeleke, O. A. Premium ethylcellulose polymer based architectures at work in drug delivery. Int. J. Pharm. X 2019, 1, 100023; https://doi.org/10.1016/j.ijpx.2019.100023.Search in Google Scholar PubMed PubMed Central
177. Li, L., Shan, H., Yue, C. Y., Lam, Y. C., Tam, K. C., Hu, X. Thermally induced association and dissociation of methylcellulose in aqueous solutions. Langmuir 2002, 18, 7291–7298; https://doi.org/10.1021/la020029b.Search in Google Scholar
178. Takahashi, M., Shimazaki, M., Yamamoto, J. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions. J. Polym. Sci. B Polym. Phys. 2001, 39, 91–100; https://doi.org/10.1002/1099-0488(20010101)39:1<91::aid-polb80>3.0.co;2-c.10.1002/1099-0488(20010101)39:1<91::AID-POLB80>3.0.CO;2-CSearch in Google Scholar
179. Ke, H., Zhou, J., Zhang, L. Structure and physical properties of methylcellulose synthesized in NaOH/urea solution. Polym. Bull. 2006, 56, 349–357; https://doi.org/10.1007/s00289-006-0507-5.Search in Google Scholar
180. Schupper, N., Rabin, Y., Rosenbluh, M. Multiple stages in the aging of a physical polymer gel. Macromolecules 2008, 41, 3983–3994; https://doi.org/10.1021/ma702613j.Search in Google Scholar
181. Haque, A., Morris, E. R.Thermogelation of Methylcellulose Part I: molecular structures and processes. Carbohydr. Polym. 1993, 22, 161–173; https://doi.org/10.1016/0144-8617(93)90137-s.Search in Google Scholar
182. Aggarwal, N., Altgärde, N., Svedhem, S., Zhang, K., Fischer, S., Groth, T. Effect of molecular composition of heparin and cellulose sulfate on multilayer formation and cell response. Langmuir 2013, 29, 13853–13864; https://doi.org/10.1021/la4028157.Search in Google Scholar PubMed
183. Strätz, J., Liedmann, A., Heinze, T., Fischer, S., Groth, T. Effect of sulfation route and subsequent oxidation on derivatization degree and biocompatibility of cellulose sulfates. Macromol. Biosci. 2020, 20, 1–11; https://doi.org/10.1002/mabi.201900403.Search in Google Scholar PubMed
184. Trygg, J., Fardim, P., Gericke, M., Mäkilä, E., Salonen, J. Physicochemical design of the morphology and ultrastructure of cellulose beads. Carbohydr. Polym. 2013, 93, 291–299; https://doi.org/10.1016/j.carbpol.2012.03.085.Search in Google Scholar PubMed
185. Syazwani, N., Rahman, A., Firdaus, M., Baharin, Y. Utilisation of natural cellulose fibres in wastewater treatment. Cellulose 2018, 25, 4887–4903; https://doi.org/10.1007/s10570-018-1935-8.Search in Google Scholar
186. Carpenter, A. W., De Lannoy, C. F., Wiesner, M. R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287; https://doi.org/10.1021/es506351r.Search in Google Scholar PubMed PubMed Central
187. Zimmermann, M. V. G., Borsoi, C., Lavoratti, A., Zanini, M., Zattera, A. J., Santana, R. M. C. Drying techniques applied to cellulose nanofibers. J. Reinf. Plast. Compos. 2016, 35, 682–697; https://doi.org/10.1177/0731684415626286.Search in Google Scholar
188. Pan, B., Pan, B., Zhang, W., Lv, L., Zhang, Q., Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29; https://doi.org/10.1016/j.cej.2009.02.036.Search in Google Scholar
189. Tripathy, S., Sahu, S., Kishore, R., Bihari, R., Kar, P. K. Groundwater for sustainable development efficient removal of Cr (VI) by polyaniline modified biochar from date (phoenix dactylifera) seed. Groundw. Sustain. Dev. 2021, 15, 100653; https://doi.org/10.1016/j.gsd.2021.100653.Search in Google Scholar
190. Ding, Y., Song, C., Gong, W., Liu, L., Wu, M., Li, L., Yao, J. Robust, sustainable, hierarchical multi-porous cellulose beads via pre-crosslinking strategy for efficient dye adsorption. Cellulose 2021, 28(11), 7227–7241; https://doi.org/10.1007/s10570-021-03979-4.Search in Google Scholar
191. Cheng, W., Jiang, Y., Xu, X., Wang, Y., Lin, K., Pescarmona, P. P. Easily recoverable titanosilicate zeolite beads with hierarchical porosity: preparation and application as oxidation catalysts. J. Catal. 2016, 333, 139–148; https://doi.org/10.1016/j.jcat.2015.09.017.Search in Google Scholar
192. Zaman, A., Huang, F., Jiang, M., Wei, W., Zhou, Z. Preparation, properties, and applications of natural cellulosic aerogels: a review. Energy Built. Environ. 2020, 1, 60–76; https://doi.org/10.1016/j.enbenv.2019.09.002.Search in Google Scholar
193. Gericke, M., Trygg, J., Fardim, P. Functional cellulose beads: preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836; https://doi.org/10.1021/cr300242j.Search in Google Scholar PubMed
194. Liebert, T., Schiller, F., Jena, D. Cellulose solvents: for analysis, shaping and chemical modification. In Cellulose Solvents – Remarkable History, Bright Future; ACS Symposium Series; American Chemical Society: Washington, DC, 2010; pp. 3–54.10.1021/bk-2010-1033.ch001Search in Google Scholar
195. Heinze, T., Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762; https://doi.org/10.1016/s0079-6700(01)00022-3.Search in Google Scholar
196. Medronho, B., Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508; https://doi.org/10.1016/j.cis.2014.05.004.Search in Google Scholar PubMed
197. Ghasemi, M., Tsianou, M., Alexandridis, P. Assessment of solvents for cellulose dissolution. Bioresour. Technol. 2017, 228, 330–338; https://doi.org/10.1016/j.biortech.2016.12.049.Search in Google Scholar PubMed
198. Heinze, T., Koschella, A. Solvents applied in the field of cellulose chemistry: a mini review. Polímeros 2005, 15, 84–90; https://doi.org/10.1590/s0104-14282005000200005.Search in Google Scholar
199. Zainal, S. H., Mohd, N. H., Suhaili, N., Anuar, F. H., Lazim, A. M., Othaman, R. Preparation of cellulose-based hydrogel: a review. J. Mater. Res. Technol. 2021, 10, 935–952; https://doi.org/10.1016/j.jmrt.2020.12.012.Search in Google Scholar
200. Liu, G., Li, W., Chen, L., Zhang, X., Niu, D., Chen, Y., Yuan, S., Bei, Y., Zhu, Q. Molecular dynamics studies on the aggregating behaviors of cellulose molecules in NaOH/urea aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124663; https://doi.org/10.1016/j.colsurfa.2020.124663.Search in Google Scholar
201. Chen, X., Chen, J., You, T., Wang, K., Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2013, 125, 85–91; https://doi.org/10.1016/j.carbpol.2015.02.054.Search in Google Scholar
202. Gassan, J., Bledzki, A. K. Alkali treatment of jute fibers: relationship between structure and mechanical properties. J. Appl. Polym. Sci. 1999, 71, 623–629; https://doi.org/10.1002/(sici)1097-4628(19990124)71:4<623::aid-app14>3.0.co;2-k.10.1002/(SICI)1097-4628(19990124)71:4<623::AID-APP14>3.0.CO;2-KSearch in Google Scholar
203. Budtova, T. Cellulose II aerogels: a review. Cellulose 2019, 26, 81–121; https://doi.org/10.1007/s10570-018-2189-1.Search in Google Scholar
204. Zhang, S., Li, F. X., Yu, J. y, Hsieh, Y.Lo. Dissolution behaviour and solubility of cellulose in NaOH complex solution. Carbohydr. Polym. 2010, 81, 668–674; https://doi.org/10.1016/j.carbpol.2010.03.029.Search in Google Scholar
205. Morgado, D. L., Frollini, E., Castellan, A., Rosa, D. S., Coma, V. Biobased films prepared from NaOH/thiourea aqueous solution of chitosan and linter cellulose. Cellulose 2011, 18, 699–712; https://doi.org/10.1007/s10570-011-9516-0.Search in Google Scholar
206. Ibrahim, F., Moniruzzaman, M., Yusup, S., Uemura, Y. Dissolution of cellulose with ionic liquid in pressurized cell. J. Mol. Liq. 2015, 211, 370–372; https://doi.org/10.1016/j.molliq.2015.07.041.Search in Google Scholar
207. Sescousse, R., Le, K. A., Ries, M. E., Budtova, T. Viscosity of cellulose-imidazolium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 7222–7228; https://doi.org/10.1021/jp1024203.Search in Google Scholar
208. Shamsuri, A. A., Abdullah, D. K., Rusli, D. Fabrication of agar/biopolymer blend aerogels in ionic liquid and Co-solvent mixture. Cellul. Chem. Technol. 2012, 46, 45–52.Search in Google Scholar
209. Wasserscheid, P., Welton, T. Ionic Liquids in Synthesis; Wiley VCH: Weinheim, Vol. 1, 2008.10.1002/9783527621194Search in Google Scholar
210. De Wever, P., Janssens, J., Fardim, P. Fabrication of cellulose cryogel beads via room temperature dissolution in onium hydroxides. Carbohydr. Polym. Technol. Appl. 2022, 3, 1–19; https://doi.org/10.1016/j.carpta.2022.100206.Search in Google Scholar
211. Sescousse, R., Gavillon, R., Budtova, T. Wet and dry highly porous cellulose beads from cellulose-NaOH-water solutions: influence of the preparation conditions on beads shape and encapsulation of inorganic particles. J. Mater. Sci. 2011, 46, 759–765; https://doi.org/10.1007/s10853-010-4809-5.Search in Google Scholar
212. Stevens, E. S., Ashby, R. D., Solaiman, D. K. Y. Gelatin plasticized with a biodiesel coproduct stream. J. Biobased Mater. Bioenergy 2009, 3, 57–61; https://doi.org/10.1166/jbmb.2009.1007.Search in Google Scholar
213. Ganesan, K., Budtova, T., Ratke, L., Gurikov, P., Baudron, V., Preibisch, I., Niemeyer, P., Smirnova, I., Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 1–37; https://doi.org/10.3390/ma11112144.Search in Google Scholar
214. Liyanage, S., Acharya, S., Parajuli, P., Shamshina, J. L., Abidi, N. Production and surface modification of cellulose bioproducts. Polymers 2021, 13, 3433; https://doi.org/10.3390/polym13193433.Search in Google Scholar
215. Senuma, Y., Hilborn, J. G. High speed imaging of drop formation from low viscosity liquids and polymer melts in spinning disk atomization. Polym. Eng. Sci. 2002, 42, 969–982; https://doi.org/10.1002/pen.11006.Search in Google Scholar
216. Prüße, U., Bruske, F., Breford, J., Vorlop, K. D. Improvement of the jet cutting method for the preparation of spherical particles from viscous polymer solutions. Chem. Eng. Technol. 1998, 21, 153–157; https://doi.org/10.1002/(sici)1521-4125(199802)21:2<153::aid-ceat153>3.0.co;2-8.10.1002/(SICI)1521-4125(199802)21:2<153::AID-CEAT153>3.0.CO;2-8Search in Google Scholar
217. De Oliveira, W., Glasser, W. G. Hydrogels from polysaccharides. I. Cellulose beads for chromatographic support. J. Appl. Polym. Sci. 1996, 60, 63–73; https://doi.org/10.1002/(sici)1097-4628(19960404)60:1<63::aid-app8>3.0.co;2-t.10.1002/(SICI)1097-4628(19960404)60:1<63::AID-APP8>3.0.CO;2-TSearch in Google Scholar
218. Kamal Mohamed, S. M., Ganesan, K., Milow, B., Ratke, L. The effect of zinc oxide (ZnO) addition on the physical and morphological properties of cellulose aerogel beads. RSC Adv. 2015, 5, 90193–90201; https://doi.org/10.1039/c5ra17366c.Search in Google Scholar
219. Ilou, I., Souabi, S., Digua, K. Quantification of pollution discharges from tannery wastewater and pollution reduction by pre-treatment station. Int. J. Sci. Res. 2014, 3, 1706–1715.Search in Google Scholar
220. Maggioris, D., Goulas, A., Alexopoulos, A. H., Chatzi, E. G., Kiparissides, C. Prediction of particle size distribution in suspension polymerization reactors: effect of turbulence nonhomogeneity. Chem. Eng. Sci. 2000, 55, 4611–4627; https://doi.org/10.1016/s0009-2509(00)00100-7.Search in Google Scholar
221. Karbstein, H., Schubert, H. Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem. Eng. Process. Proc. Intensif. 1995, 34, 205–211; https://doi.org/10.1016/0255-2701(94)04005-2.Search in Google Scholar
222. Lavoine, N., Lennart, B. Nanocellulose-based foams and aerogels: processing, properties, and applications. J. Mater. Chem. 2017, 5, 16105–16117; https://doi.org/10.1039/c7ta02807e.Search in Google Scholar
223. Sescousse, R., Budtova, T. Influence of processing parameters on regeneration kinetics and morphology of porous cellulose from cellulose-NaOH-water solutions. Cellulose 2009, 16, 417–426; https://doi.org/10.1007/s10570-009-9287-z.Search in Google Scholar
224. Lozinsky, V. I., Galaev, I. Y., Plieva, F. M., Savina, I. N., Jungvid, H., Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451; https://doi.org/10.1016/j.tibtech.2003.08.002.Search in Google Scholar PubMed
225. Wang, X., Zhang, Y., Jiang, H., Song, Y., Zhou, Z., Zhao, H. Tert-butyl alcohol used to fabricate nano-cellulose aerogels via freeze-drying technology. Mater. Res. Exp. 2017, 4, 65006; https://doi.org/10.1088/2053-1591/aa72bc.Search in Google Scholar
226. Aldaz, B., Figueroa, F., Bravo, I. Cellulose for the effective decontamination of water pollution. Rev. Bionatura 2020, 5, 1150–1155; https://doi.org/10.21931/rb/2020.05.02.13.Search in Google Scholar
227. Grøssereid, I., Lethesh, K. C., Venkatraman, V., Fiksdahl, A. New dual functionalized zwitterions and ionic liquids; synthesis and cellulose dissolution studies. J. Mol. Liq. 2019, 292, 111353; https://doi.org/10.1016/j.molliq.2019.111353.Search in Google Scholar
228. Carvalho, J. P. F., Silva, A. C. Q., Silvestre, A. J. D., Freire, C. S. R., Vilela, C. S. Spherical cellulose micro and nanoparticles: a review of recent developments and applications. Nanomater. Rev. 2021, 11, 1–35; https://doi.org/10.3390/nano11102744.Search in Google Scholar PubMed PubMed Central
229. Belgacem, M. N., Gandini, A. The surface modification of cellulose fibres for use as reinforcing elements in composite materials. Compos. Interface 2012, 12, 41–75; https://doi.org/10.1163/1568554053542188.Search in Google Scholar
230. Hokkanen, S., Bhatnagar, A., Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173; https://doi.org/10.1016/j.watres.2016.01.008.Search in Google Scholar PubMed
231. Jamshaid, A., Hamid, A., Muhammad, N., Naseer, A., Ghauri, M., Iqbal, J., Rafiq, S., Shah, N. S. Cellulose-based materials for the removal of heavy metals from wastewater – an overview. ChemBioEng Rev. 2017, 4, 240–256; https://doi.org/10.1002/cben.201700002.Search in Google Scholar
232. Kim, U. J., Kuga, S., Wada, M., Okano, T., Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492; https://doi.org/10.1021/bm0000337.Search in Google Scholar PubMed
233. Liimatainen, H., Visanko, M., Sirviö, J. A., Hormi, O. E. O., Niinimaki, J. Enhancement of the nanofibrillation of wood cellulose through sequential periodate-chlorite oxidation. Biomacromolecules 2012, 13, 1592–1597; https://doi.org/10.1021/bm300319m.Search in Google Scholar PubMed
234. Saito, T., Isogai, A. TEMPO-mediated oxidation of native cellulose . The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989; https://doi.org/10.1021/bm0497769.Search in Google Scholar PubMed
235. Takaichi, S., Isogai, A. Oxidation of wood cellulose using 2-azaadamantane N-oxyl (AZADO) or 1-methyl-AZADO catalyst in NaBr/NaClO system. Cellulose 2013, 20, 1979–1988; https://doi.org/10.1007/s10570-013-9932-4.Search in Google Scholar
236. Zhang, Z. Surface Modification of Cellulose Nanocrystals by Esterification and ATRP Reactions for Advanced Applications; Université de Bordeaux: Talence, France, 2017.Search in Google Scholar
237. Es-said, A., El Hamdaoui, L., El Moussaouiti, M., Bchitou, R. Esterification optimization of cellulose with p-iodobenzoyl chloride using experimental design method. J. Polym. Res. 2019, 26, 1–9.10.1007/s10965-019-1862-xSearch in Google Scholar
238. Pinto, E., Nkrumah, W., Boakye, P., Amenuvor, G., Sokama-neuyam, Y. A., Kwadwo, M., Karimaie, H., Sarkodie, K., Daniel, C., Erzuah, S., Ama, M., Rockson, D. Cellulose processing from biomass and its derivatization into carboxymethylcellulose: a review. Sci. African 2022, 15, e01078; https://doi.org/10.1016/j.sciaf.2021.e01078.Search in Google Scholar
239. Ishimura, D., Morimoto, Y., Saito, H. Influences of chemical modifications on the mechanical strength of cellulose beads. Cellulose 1998, 5, 135–151; https://doi.org/10.1023/a:1009277216057.10.1023/A:1009277216057Search in Google Scholar
240. Volkert, B., Wolf, B., Fischer, S., Li, N., Lou, C. Application of modified bead cellulose as a carrier of active ingredients. Macromol. Symp. 2009, 280, 130–135; https://doi.org/10.1002/masy.200950615.Search in Google Scholar
241. Rana, A. K., Frollini, E., Thakur, V. K. Cellulose nanocrystals: pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581; https://doi.org/10.1016/j.ijbiomac.2021.05.119.Search in Google Scholar PubMed
242. Goussé, C., Chanzy, H., Cerrada, M. L., Fleury, E. Surface silylation of cellulose microfibrils: preparation and rheological properties. Polymer 2004, 45, 1569–1575; https://doi.org/10.1016/j.polymer.2003.12.028.Search in Google Scholar
243. Thakur, V., Guleria, A., Kumar, S., Sharma, S., Singh, K. Recent advances in nanocellulose processing, functionalization and applications: a review. Mater. Adv. 2021, 2, 1872–1895; https://doi.org/10.1039/d1ma00049g.Search in Google Scholar
244. Lizundia, E., Meaurio, E., Vilas, J. L. Grafting of cellulose nanocrystals. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Elsevier Inc., 2016; pp. 61–113.10.1016/B978-0-323-44248-0.00003-1Search in Google Scholar
245. Roy, D., Semsarilar, M., Guthrie, J. T., Perrier, S., Tada, H. Cellulose modification by polymer grafting: a review. Chem. Soc. Rev. 2009, 38, 2046–2064; https://doi.org/10.1039/b808639g.Search in Google Scholar PubMed
246. Roy, N., Sengupta, R., Bhowmick, A. K. Progress in polymer science modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 2012, 37, 781–819; https://doi.org/10.1016/j.progpolymsci.2012.02.002.Search in Google Scholar
247. Hafren, J., Cordova, A. Direct organocatalytic polymerization from cellulose fibers. Macromol. Rapid Commun. 2005, 26, 82–86; https://doi.org/10.1002/marc.200400470.Search in Google Scholar
248. Lonnberg, H., Zhou, Q., Iii, H. B., Teeri, T. T., Malmstro, E., Hult, A. Grafting of cellulose fibers with poly (E -caprolactone) and poly (L -lactic acid) via ring-opening polymerization. Biomacromolecules 2006, 7, 2178–2185; https://doi.org/10.1021/bm060178z.Search in Google Scholar PubMed
249. Matyjaszewski, K., Davis, T. P. Handbook of Radical Polymerization; Matyjaszewski, K., Davis, T. P., Eds. John Wiley & Sons, Inc., 2002.10.1002/0471220450Search in Google Scholar
250. Carlmark, A., Malmstro, E. Atom transfer radical polymerization from cellulose fibers at ambient temperature. J. Am. Chem. Soc. 2002, 124, 1–2.10.1021/ja016582hSearch in Google Scholar PubMed
251. Omrani, A. A., Taghavinia, N. Photo-induced growth of silver nanoparticles using UV sensitivity of cellulose fibers. Appl. Surf. Sci. 2012, 258, 2373–2377; https://doi.org/10.1016/j.apsusc.2011.10.038.Search in Google Scholar
252. Pinto, R. J. B., Marques, P. A. A. P., Neto, C. P., Trindade, T., Daina, S., Sadocco, P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009, 5, 2279–2289; https://doi.org/10.1016/j.actbio.2009.02.003.Search in Google Scholar PubMed
253. Hamidon, T. S., Adnan, R., Haafiz, M. K. M., Hussin, M. H. Cellulose-based beads for the adsorptive removal of wastewater effluents: a review. Environ. Chem. Lett. 2022, 20, 1965–2017.10.1007/s10311-022-01401-4Search in Google Scholar
254. Alavarse, A. C., Frachini Garcia, E. C., Silva, R. L. C. G. D, Lima, V. H., Shavandi, A., Alavarse, A. C., Frachini, E. C. G., Petri, D. F. S. Crosslinkers for polysaccharides and proteins: synthesis Conditions,Mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2021, 202, 558–596.10.1016/j.ijbiomac.2022.01.029Search in Google Scholar PubMed
255. Hennink, W. E., Nostrum, C. F. V. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236; https://doi.org/10.1016/j.addr.2012.09.009.Search in Google Scholar
256. Patil, S. V., Dhanraj, J. Crosslinking of polysaccharides: methods and applications. Pharm. Rev. 2008, 6, 1–11.Search in Google Scholar
257. Sheth, Y., Dharaskar, S., Khalid, M., Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: a review. Sustain. Energy Technol. Assessments 2021, 43, 100951; https://doi.org/10.1016/j.seta.2020.100951.Search in Google Scholar
258. Grishkewich, N., Li, Y., Liu, K., Chiu, K. Synthesis and characterization of modified cellulose nanofibril organosilica aerogels for the removal of anionic dye. J. Polym. Res. 2022, 29, 1–13; https://doi.org/10.1007/s10965-022-03102-6.Search in Google Scholar
259. Liu, S., Cheng, G., Xiong, Y., Ding, Y., Luo, X. Adsorption of low concentrations of bromide ions from water by cellulose- based beads modified with TEMPO-mediated oxidation and Fe (III) complexation. J. Hazard. Mater. 2020, 384, 121195; https://doi.org/10.1016/j.jhazmat.2019.121195.Search in Google Scholar PubMed
© 2023 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Material Properties
- A brief review on polymer nanocomposites: current trends and prospects
- Recent development in the formation and surface modification of cellulose-bead nanocomposites as adsorbents for water purification: a comprehensive review
- CdSe nanodots to nanorods in PVA films: effect of shape transition and loading on the opto-mechanical and biodegradation properties
- Kinetic and thermodynamic studies of H2S adsorption by lignin-based composite membranes
- Preparation and Assembly
- Fabrication of avian eggshell membrane derived dispersed collagen hydrogels for potential bone regeneration
- Antimicrobially effective protein-loaded metal chelated chitosan composite
- Engineering and Processing
- Highly thermally conductive polyamide 6 composites with favorable mechanical properties, processability and low water absorption using a hybrid filling of short carbon fiber, flake graphite and expanded graphite
- Tribological behavior of ultra-high molecular weight polyethylene (UHMWPE) for acetabular replacement under frictional heat based on molecular dynamics
Articles in the same Issue
- Frontmatter
- Material Properties
- A brief review on polymer nanocomposites: current trends and prospects
- Recent development in the formation and surface modification of cellulose-bead nanocomposites as adsorbents for water purification: a comprehensive review
- CdSe nanodots to nanorods in PVA films: effect of shape transition and loading on the opto-mechanical and biodegradation properties
- Kinetic and thermodynamic studies of H2S adsorption by lignin-based composite membranes
- Preparation and Assembly
- Fabrication of avian eggshell membrane derived dispersed collagen hydrogels for potential bone regeneration
- Antimicrobially effective protein-loaded metal chelated chitosan composite
- Engineering and Processing
- Highly thermally conductive polyamide 6 composites with favorable mechanical properties, processability and low water absorption using a hybrid filling of short carbon fiber, flake graphite and expanded graphite
- Tribological behavior of ultra-high molecular weight polyethylene (UHMWPE) for acetabular replacement under frictional heat based on molecular dynamics

