Abstract
A new conductometric biosensor was developed and characterized; the biosensor was based on horseradish peroxidase that was deposited in chitosan and chitosan/AuNPs films. The biosensors were characterized by scanning electron microscopy and current-voltage curves. Current-voltage curves in biosensors showed that the electrical conductivity and bistability in biosensors can be modulated by horseradish peroxidase. Horseradish peroxidase catalyzed the reduction of H2 O2 to H2 O with the oxidation of the prosthetic group (Fe3+) in the enzyme to Fe4+=O. Conductometric signal in the biosensors increased with the gradual increase of H2 O2 concentration, and it was due to the H2 O2 reduction. Linear hydrogen peroxide detection was observed for a concentration between 0 and 15 mm. The results proved that these biosensors could have promising industrial applications, due to its rapid and sensitive H2 O2 detection.
1 Introduction
Hydrogen peroxide (H2 O2) is a by-product of several highly selective oxidases, and also, it is an essential mediator of several types of oxidative metabolism in many fields such as, food industry, pharmaceutical, environmental, biological, industry settings, clinical control, and environmental protection [1, 2]. Conventional techniques for H2 O2 quantification, e.g., titrimetry, chemiluminescence, and spectrometry are generally time consuming, highly prone to interferences, difficult for automation detection, and the fact that they cannot be used in food or biological samples, where the samples have several compounds [1].
In recent years, a lot of investigations have been carried out on biosensors for H2 O2 quantification [3–6], according to Malhotra et al. [7] “Biosensors are analytical devices incorporating biological materials such as enzymes, tissues, micro-organism, antibodies, cell receptors or biologically derived materials or biomimic component in intimate contact with a physic-chemical transducer or transducing microsystems’’. Electrochemical biosensors are rapid, selective, sensitive, and can be produced at low cost [2]; in addition, electrochemical biosensors have been demonstrated to be a simple, very efficient, and inexpensive method for H2 O2 quantification [8].
Electrochemical biosensors can be classified as amperometric, potentiometric, and conductometric [9]; among these, the amperometric biosensor has been extensively studied due to its rapid detection, sensitivity, and selectivity [1, 2, 10–15]. However, the selectivity of the amperometric devices is only governed by the redox potential of the electroactive species present. Therefore, the current measured by the instrument can include the contributions of several chemical species [9]. Conductometric biosensors could have several advantages when compared with amperometric biosensors, e.g., easy miniaturization and large-scale production using inexpensive technology [14].
Recently, the use of chitosan and gold nanoparticles (AuNPs) to improve the stability of enzymes in biosensors has been reported [1, 16]. In addition, chitosan has been found to be an interesting biopolymer for biosensor development due to its excellent film-forming ability, high permeability, high mechanical strength, nontoxicity, biocompatibility, and low cost [2].
The aim of this work was to study the construction of a simple conductometric biosensor to determine H2 O2 by using horseradish peroxidase (HRP). The effect of gold nanoparticles in the electrical response of biosensors also was studied.
2 Materials and methods
2.1 Materials
Chitosan (deacetylated grade 85%) was obtained from Polymar (Fortaleza, Ceará, Brazil). Horseradish peroxidase type VI-A (HRP) was purchased from Sigma (Saint Louis, MO, USA). Chloroauric acid (HAuCl4), hydrogen peroxide and other chemicals were of analytical grade and used without further purification.
2.2 Synthesis of gold nanoparticles
Synthesis of AuNPs was performed according to previous reports [17]. Chitosan solution (6.92 mg/ml) was prepared by dispersion of chitosan powder in 1% acetic acid at constant agitation for 30 min. Subsequently, the solution was filtered using a vacuum and a filter paper with 14 μm diameter pores. For AuNPs synthesis, 10 ml of the chitosan solution prepared previously was mixed with 4 ml of HAuCl4 (11 mm). Synthesis of AuNPs was carried out in a water bath at 90°C for 3 h.
2.3 Films construction
Films were prepared by casting method and using filmogenic solutions. Chitosan/AuNPs film was prepared by mixing 5 ml of chitosan solution (20 mg/ml) previously dissolved in acetic acid (1%), with 5 ml of a colloid containing AuNPs at 11 mm. Chitosan films without AuNPs were prepared using 10 ml of chitosan solution (20 mg/ml) [17]. To each 10 ml of filmogenic solution, 0.10 mg of HRP was added and mixed for 15 min at room temperature in a dark room. All solutions obtained were dried at room temperature for 24 h in a SOLAB oven (Piracicaba, São Paulo, Brazil) [17]. Thickness determination was performed at least 10 times in each film; at least three films for each formulation were measured. The thickness was reported as average and standard error, and it was similar for all films (2.2±0.1 μm).
2.4 Enzyme activity of hydrogen peroxide immobilized in films: guaiacol test
Chitosan, chitosan/AuNPs, chitosan/HRP, and chitosan/AuNPs/HRP films were immersed in a solution of hydrogen peroxide (3% v/v); immediately, 3 ml of guaiacol (0.5% v/v) was added. The color change in the solutions was monitored visually for 5 min. All solutions were prepared using a buffer solution (0.1 m and pH=6.8) as solvent [18].
2.5 Film morphology
Surface analyses of chitosan and chitosan/AuNPs films were reported previously [17]. The surface analysis of chitosan/HRP and chitosan/AuNPs/HRP films was performed by means of a field emission scanning electron microscope (Jeol ISM 7500F, Akishima, Tokyo, Japan).
2.6 Electrical characterization of films
Electrical measurements of chitosan, chitosan/AuNPs, chitosan/HRP and chitosan/AuNPs/HRP films of 5 mm diameter were carried out using a Keithley 8009 Resistivity Test Fixture (Cleveland, OH, USA) coupled to a Keithley 2612A System SourceMeter (Cleveland, OH, USA) and using concentric electrodes (Figure 1A). Electrical measurements were carried out at room temperature. The films were stabilized by applying 0 V for 1 min, followed by the application of a controlled voltage cycle consisting of a forward voltage sweep from 0 to 6 V and reverse voltage sweep from 6 to 0 V. For each formulation, the films were studied at least three times.
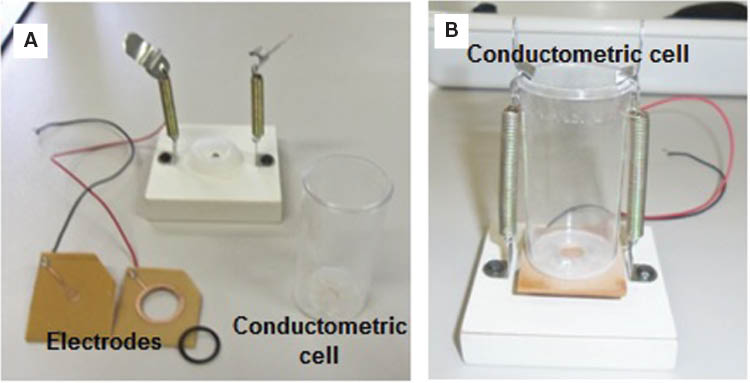
Illustration of the electrodes (A) and conductometric cell (B) used for the electrical analyses (photo taken by an author).
2.7 Electrical characterization of films: response to different concentrations of hydrogen peroxide
Electrical response of chitosan, chitosan/AuNPs, chitosan/HRP, and chitosan/AuNPs/HRP films of 5 mm diameter was assessed in the same equipment (as in section 2.6) and using a conductometric cell coupled to the electrodes (Figure 1B). Initially, films were stabilized by applying 0 V for 1 min; 5 ml of buffer solution (0.1 m and pH=6.8) was added immediately, followed by hydrogen peroxide (3% v/v) in several volumes to the buffer solution to achieve concentrations of hydrogen peroxide between 0 and 35 mm. The solution was stirred by 2 min, and then a voltage ramp between 0 to 6 V was applied. For each formulation, the films were studied at least three times.
3 Results and discussion
3.1 Enzyme activity of hydrogen peroxide immobilized in films: guaiacol test
Chitosan and chitosan/AuNPs films were negative to the guaiacol test (no color change). In contrast, films with HRP were positive to the same test. The solution changed from no-color to dark yellow for chitosan/HRP film and light yellow for chitosan/AuNPs/HRP film. These results indicate that the HRP has enzymatic activity in the films [18]. Differences between color change of the chitosan/HRP and chitosan/AuNPs/HRP films indicate that the AuNPs in films could alter the enzymatic activity of horseradish peroxidase.
3.2 Film morphology
Figure 2A, B shows the morphology of chitosan/HRP and chitosan/AuNPs/HRP films. Films with AuNPs exhibited a granular structure, which was different to the morphology observed for films without AuNPs; this result agrees with those reported previously for chitosan and chitosan/AuNPs films [17]. At the surface of chitosan/HRP and chitosan/AuNPs/HRP, the HRP immobilized in the films was observed, and it showed a globular morphology revealing immobilization (Figure 2C). This image was similar to that observed by Yardimci et al. [2] for an amperometric biosensor based on HRP.
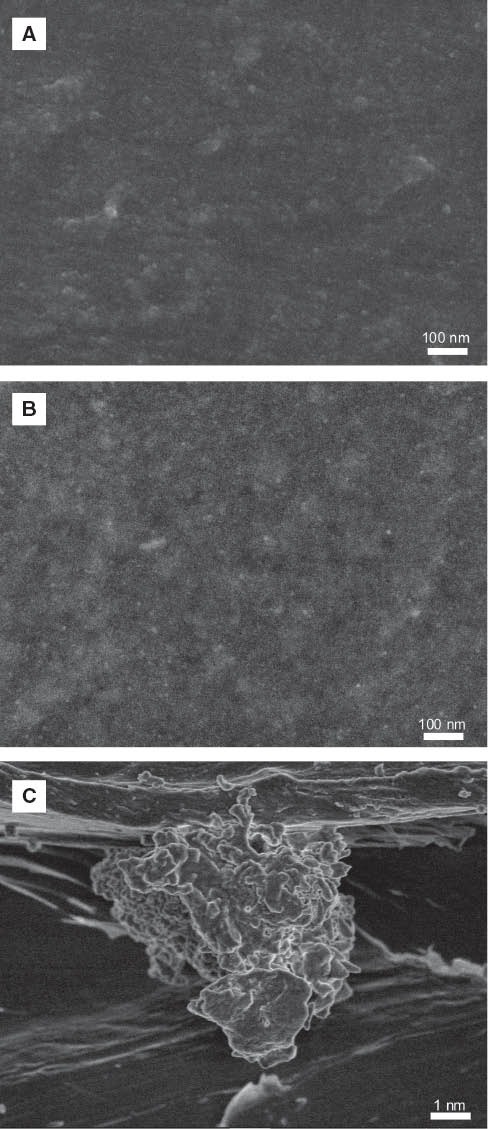
Scanning electron microscopy: chitosan/HRP (A, C) and chitosan/AuNPs/HRP (B) films.
3.3 Electrical characterization of films
According to what was reported previously, the electrical conductivity can be modulated by the AuNP concentration; hence, the AuNPs increase the conductivity and bistability in chitosan films due to the capture of electrons in the AuNPs and its subsequent electron transfers between AuNPs [17]. A similar behavior was observed when the HRP was incorporated in films (Figure 3). This electrical behavior could be due the fact that horseradish peroxidase has as active center iron ions (Fe3+). Iron ions could improve the trapping process, and the electrical transport could be more efficient due to the accumulation of charge in the quantum wells created by Fe3+ in HRP [17]. Hence, chitosan/HRP and chitosan/AuNPs/HRP acted as charge transport matrices that transferred the electrons from iron ions in HRP to others iron ions or to the electrodes [19].
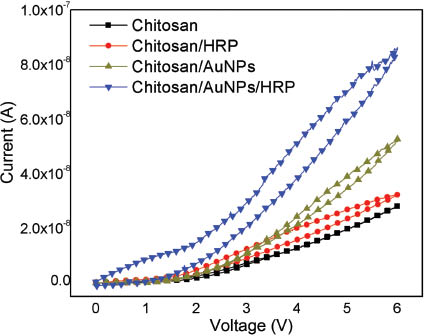
Current-voltage curves for the chitosan, chitosan/AuNPs, chitosan/HRP, and chitosan/AuNPs/HRP films.
3.4 Electrical characterization of films: response to different concentrations of hydrogen peroxide
The current in chitosan and chitosan/AuNPs films was not influenced by H2 O2 concentration (Figure 4), i.e., H2 O2 was not reduced by the contact with the polymer matrix or AuNPs. Current-voltage curves for chitosan/HRP and chitosan/AuNPs/HRP films to successive additions of H2 O2 are shown in Figure 5. These films show a rapid and sensitive response to the addition of H2 O2. Upon addition of an aliquot of H2 O2 to the conductometric cell, the reduction current increases uniformly until a stable value was reached. In comparison with films without HRP (Figure 4), the current in chitosan/HRP and chitosan/AuNPs/HRP films increased with H2 O2 addition; it was attributed to the reduction of hydrogen peroxide into oxygen and water by HRP. The present results reveal that the electron transfer between HRP and chitosan or chitosan/AuNPs films can be easily performed; hence, chitosan/HRP and chitosan/AuNPs/HRP films could be used as biosensors for H2 O2 detection.
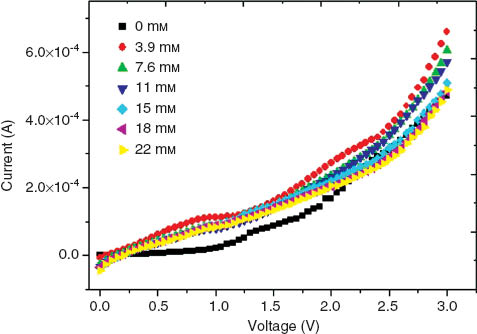
Current-voltage curves for the chitosan film to different H2 O2 concentrations.
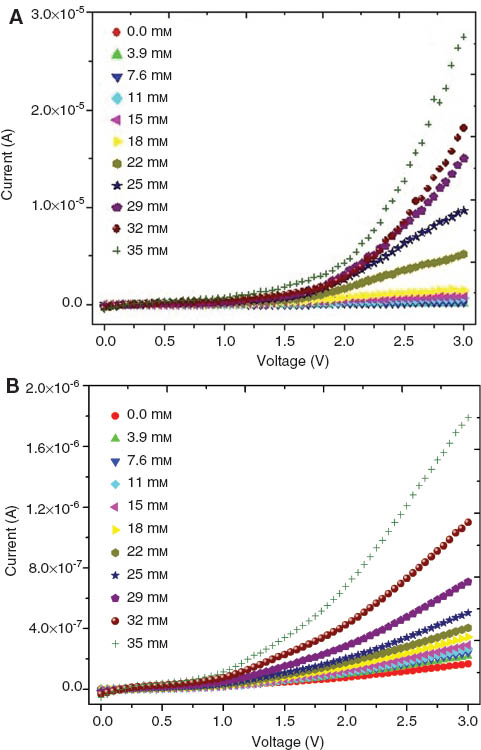
Current-voltage curves for the chitosan/HRP (A) and chitosan/AuNPs/HRP (B) films to different H2 O2 concentrations.
In amperometric systems, the catalytic mechanism of HRP (with oxidation state of Fe3+) for reducing hydrogen peroxide is based on the oxidation of HRP to two-equivalent oxidized forms (Eqs. 1–3), called compound I (with oxidation state of Fe4+=O) and compound II (with oxidation state of Fe5+). In this process, the electrons (e-) and protons (H+) ensure the regeneration of the compound II to HRP [12, 20]. The electron transfer in these reactions is the current that is quantified in an amperometric system [12].
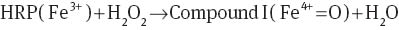
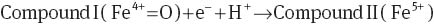
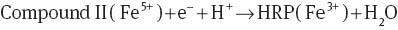
In conductometric systems, the catalytic process does not have proton transfer; therefore, HRP can only oxide to compound I. Compound I is very unstable and can act as a transitory state where electrons move in the films, similar to that reported for AuNPs [17]. Chitosan/HRP film had a higher electrical response than the chitosan/AuNPs/HRP film probably due to the differences between oxidation states of AuNPs and the active site of HRP.
The current response and the concentration of H2 O2 have a polynomial relationship within the concentration range between 0 and 35 mm; the values of these parameters were calculated by nonlinear regression (R2≥0.95) and are reported in Table 1. To 1 V and for a H2 O2 concentration range between 0 and 15 mm, the biosensors have a linear response, such as I=3E–5C–5E–5 and I=3E–6C–8E–8, for chitosan/HRP and chitosan/AuNPs/HRP, respectively. Both fits have an R2=0.94. Linear relationships between current-concentration are very important to develop biosensors with linear calibration [10, 16].
Current (I) fit as function of H2 O2 concentration (C) to the mathematical model: I= aC3+bC2+cC+d for three different voltages.
| Film | Voltage (V) | Polynomial parameters | R2 | |||
|---|---|---|---|---|---|---|
| a | b | c | d | |||
| Chitosan/HRP | 1 | 3.0E-2±1.0E-3 | -7.0E-4±0.5E-4 | 5.0E-6±5.4E-8 | 3.0E-8±1.1E-8 | 0.97–0.99 |
| 2 | -9.2E-2±0.8E-3 | 8.4E-3±1.3E-3 | -8.0E-5±2.6E-7 | 2.0E-7±5.4E-8 | 0.95 | |
| 3 | 7.4E-1±1.4E-2 | -2.9E-3±0.8E-3 | -8.0E-5±1.1E-6 | 4.0E-7±4.2E-8 | 0.98–0.99 | |
| Chitosan/AuNP/HRP | 1 | 6.4E-3±1.8E-3 | -2.0E-4±1.7E-3 | 1.0E-6±1.9E-7 | 2.0E-8±0.7E-8 | 0.99 |
| 2 | 4.4E-2±1E-3 | -1.6E-3±3.4E-4 | 2.0E-5±4.8E-6 | 6.0E-8±1.4E-8 | 0.97–0.99 | |
| 3 | 1.2E-1±2.0E-2 | -4.4E-3±0.3E-3 | 5.0E-5±3.2E-7 | 1.0E-8±3.2E-9 | 0.97–0.99 | |
All values were expressed as mean ± standard error (n=3).
4 Conclusions
In this paper, a new conductometric hydrogen peroxide biosensor was constructed and characterized. Horseradish peroxidase was successfully immobilized in chitosan and chitosan/AuNPs films; these biosensors showed a rapid, sensitive, and linear response to different hydrogen peroxide concentrations. Hence, HRP immobilized in chitosan and chitosan/AuNPs films could be used as biosensors and could have promising industrial applications.
Acknowledgments
The authors gratefully acknowledge the financial support from FAPESP (Fundação de Apoio à Pesquisa do Estado de São Paulo), grant number 2010/50424-3. Field emission scanning electron micrographs were provided by the IQ-UNESP.
References
[1] Kang XB, Pang GC, Liang XY, Wang M, Liu J, Zhu W. Electrochim. Acta 2012, 62, 327–334.10.1016/j.electacta.2011.12.034Search in Google Scholar
[2] Yardimci FS, Senel M, Baykal A. Mater. Sci. Eng. C 2012, 32, 269–275.10.1016/j.msec.2011.10.028Search in Google Scholar
[3] Schmidt TF, Caseli L, dos Santos Jr DS, Oliveira Jr ON. Mater. Sci. Eng. C 2009, 29, 1889–1892.10.1016/j.msec.2009.02.020Search in Google Scholar
[4] Tang J, Wang BQ, Wu ZY, Han XJ, Dong SJ, Wang EK. Biosens. Bioelectron. 2003, 18, 867–872.Search in Google Scholar
[5] Miao Y, Tan SN. Anal. Chim. Acta 2001, 437, 87–93.10.1016/S0003-2670(01)00986-2Search in Google Scholar
[6] Wang G, Xu JJ, Chen HY, Lu ZH. Biosens. Bioelectron. 2003, 18, 335–343.Search in Google Scholar
[7] Malhotra B, Singhal R, Chaubey A, Sharma SK, Kumar A. Recent Trends Biosens. 2005, 5, 92–97.Search in Google Scholar
[8] Wei N, Xin X, Du JY, Li JL. Biosens. Bioelectron. 2011, 26, 3602–3607.Search in Google Scholar
[9] Mello LD, Kubota LT. Food Chem. 2002, 77, 237–256.Search in Google Scholar
[10] Lei CX, Hu SQ, Gao N, Shen GL, Yu RQ. Bioelectrochemistry 2004, 65, 33–39.10.1016/j.bioelechem.2004.06.002Search in Google Scholar PubMed
[11] Krajewska B. Enzyme Microb. Technol. 2004, 35, 126–139.Search in Google Scholar
[12] Lu X, Zhang Q, Zhang L, Li J. Electrochem. Commun. 2006, 8, 874–878.Search in Google Scholar
[13] Lin J, Qu W, Zhang S. Anal. Biochem. 2007, 360, 288–293.Search in Google Scholar
[14] Zazoua A, Hnaien M, Cosnier S, Renault J, Kherrat R. Mater. Sci. Eng. C 2009, 29, 1919–1922.10.1016/j.msec.2009.03.008Search in Google Scholar
[15] Jin X, Xi F, Deshui LV, Lin X. Carbohydr. Polym. 2011, 85, 786–791.Search in Google Scholar
[16] Luo XL, Xu JJ, Zhang Q, Yang GJ, Chen HY. Biosens. Bioelectron. 2005, 21, 190–196.Search in Google Scholar
[17] Valencia GA, Llanos JHR, Vercik LCO, Vercik A. J. Polym. Eng. 2014, 34, 105–111.Search in Google Scholar
[18] Zeraik AE, Souza FSA, Fatibello-Filho O. Quim. Nova 2008, 31, 731–734.10.1590/S0100-40422008000400003Search in Google Scholar
[19] Willner B, Katz E, Willner I. Current Opin. Biotechnol. 2006, 17, 589–596.Search in Google Scholar
[20] Veitch NC. Phytochemistry 2004, 65, 249–259.10.1016/j.phytochem.2003.10.022Search in Google Scholar PubMed
©2014 by De Gruyter
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial improvements at the Journal of Polymer Engineering
- Original articles
- Cationic copolymerization of 1,3-pentadiene with α-pinene
- In vitro degradation of polyglycolic acid synthesized by a one-step reaction
- Synthesis of a novel class of mixed-type surfmers and their properties in water
- Unique viscosity mutation of multi-generation hyperbranched waterborne polyurethane
- Preparation and characterization of oxidized starch-graft-poly(styrene-butyl acrylate) latex via emulsion polymerization
- Peroxide vulcanization of natural rubber. Part I: effect of temperature and peroxide concentration
- The stimuli-response characters of hydrogels prepared using ultrasound
- A new conductometric biosensor based on horseradish peroxidase immobilized on chitosan and chitosan/gold nanoparticle films
- A new approach for the development of textile waste cotton reinforced composites (T-FRP): laminated hybridization vs. coupling agents
- Effects of the surface treatment of wollastonite on the tensile and flow properties for reinforced polypropylene composites
- Effect of the quenching temperature on the mechanical and thermophysical properties of polycarbonate pigmented with titanium dioxide
- Processing and characterization of poly(lactic acid) blended with polycarbonate and chain extender
- Characteristics analysis and mold design for ultrasonic-assisted injection molding
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial improvements at the Journal of Polymer Engineering
- Original articles
- Cationic copolymerization of 1,3-pentadiene with α-pinene
- In vitro degradation of polyglycolic acid synthesized by a one-step reaction
- Synthesis of a novel class of mixed-type surfmers and their properties in water
- Unique viscosity mutation of multi-generation hyperbranched waterborne polyurethane
- Preparation and characterization of oxidized starch-graft-poly(styrene-butyl acrylate) latex via emulsion polymerization
- Peroxide vulcanization of natural rubber. Part I: effect of temperature and peroxide concentration
- The stimuli-response characters of hydrogels prepared using ultrasound
- A new conductometric biosensor based on horseradish peroxidase immobilized on chitosan and chitosan/gold nanoparticle films
- A new approach for the development of textile waste cotton reinforced composites (T-FRP): laminated hybridization vs. coupling agents
- Effects of the surface treatment of wollastonite on the tensile and flow properties for reinforced polypropylene composites
- Effect of the quenching temperature on the mechanical and thermophysical properties of polycarbonate pigmented with titanium dioxide
- Processing and characterization of poly(lactic acid) blended with polycarbonate and chain extender
- Characteristics analysis and mold design for ultrasonic-assisted injection molding

