Stress modulation of fear and extinction in psychopathology and treatment
-
Shira Meir Drexler
, Christian J. Merz
, Valerie L. Jentsch
and Oliver T. Wolf
Abstract
The glucocorticoid cortisol, a major player in the development of stress-related psychopathology, can also be used for the augmentation of extinction-based psychotherapies (e.g., exposure therapy). Substantial evidence supports its beneficial effects in the treatment of post-traumatic stress disorder and specific phobias. In this review, we first present the role of stress and cortisol in the development of maladaptive emotional memories. Then, we describe the mechanisms that may account for the cortisol-induced augmentation of exposure, namely, the enhancement of extinction memory consolidation and the reduction of the contextual dependency of the extinction memory. Finally, we discuss several considerations and limitations for the use of cortisol in psychotherapy, focusing on the possible adverse effects of cortisol in a reconsolidation-based (as opposed to extinction-based) intervention.
Zusammenfassung
Das Glucocorticoid Cortisol ist beteiligt an der Entwicklung von stress-assoziierten Psychopathologien, kann aber auch benutzt werden um die extinktionsbasierte Psychotherapie (z.B. Exposition) zu verbessern. Substanzielle Befunde unterstützen seine vorteilhaften Effekte bei der Behandlung der Posttraumatischen Belastungsstörung und Phobien. Überblicksartig erläutern wir zuerst die Rolle von Stress und Cortisol bei der Entwicklung von maladaptiven emotionalen Erinnerungen. Danach beschreiben wir die Mechanismen, die für die Cortisol-induzierte Verbesserung der Expositionstherapie verantwortlich sein könnten, nämlich die Verstärkung der Konsolidierung und die Reduktion der Kontextabhängigkeit des Extinktionsgedächtnisses. Zuletzt diskutieren wir die Einbindung des Cortisols in die Psychotherapie mit einem Fokus auf mögliche negative Auswirkungen einer Cortisolgabe im Rahmen einer Rekonsolidierungsbasierten (im Gegensatz zu einer extinktionsbasierten) Intervention.
Stress and the strength of emotional memories
Unusually challenging physical or psychological events may lead to stress, a subjective state of tension that is difficult to manage or endure (Colman, 2001). Two neuroendocrine systems come into play to promote an adaptive response to a stressful situation (see Figure 1): the sympathetic nervous system (SNS), mainly through the release of adrenaline and noradrenaline, and the hypothalamus-pituitary-adrenocortical (HPA) axis, mainly through the release of the glucocorticoid (GC) cortisol. The SNS is responsible for the fast and short-term responses occurring in the initial phase of the stressful event (e.g., elevated heart rate and breathing, increased arousal), whereas the HPA axis responds slower and has long-lasting effects (e.g., increase in blood sugar, suppression of the immune system) that promote the response to the stressor and the subsequent return to homeostasis (McCarty, 2016; McEwen, 2019). The effects of the SNS and HPA axis are not limited to responding to present events; through their ability to modulate learning and memory processes, they influence the response to future events as well (Joëls et al., 2006).
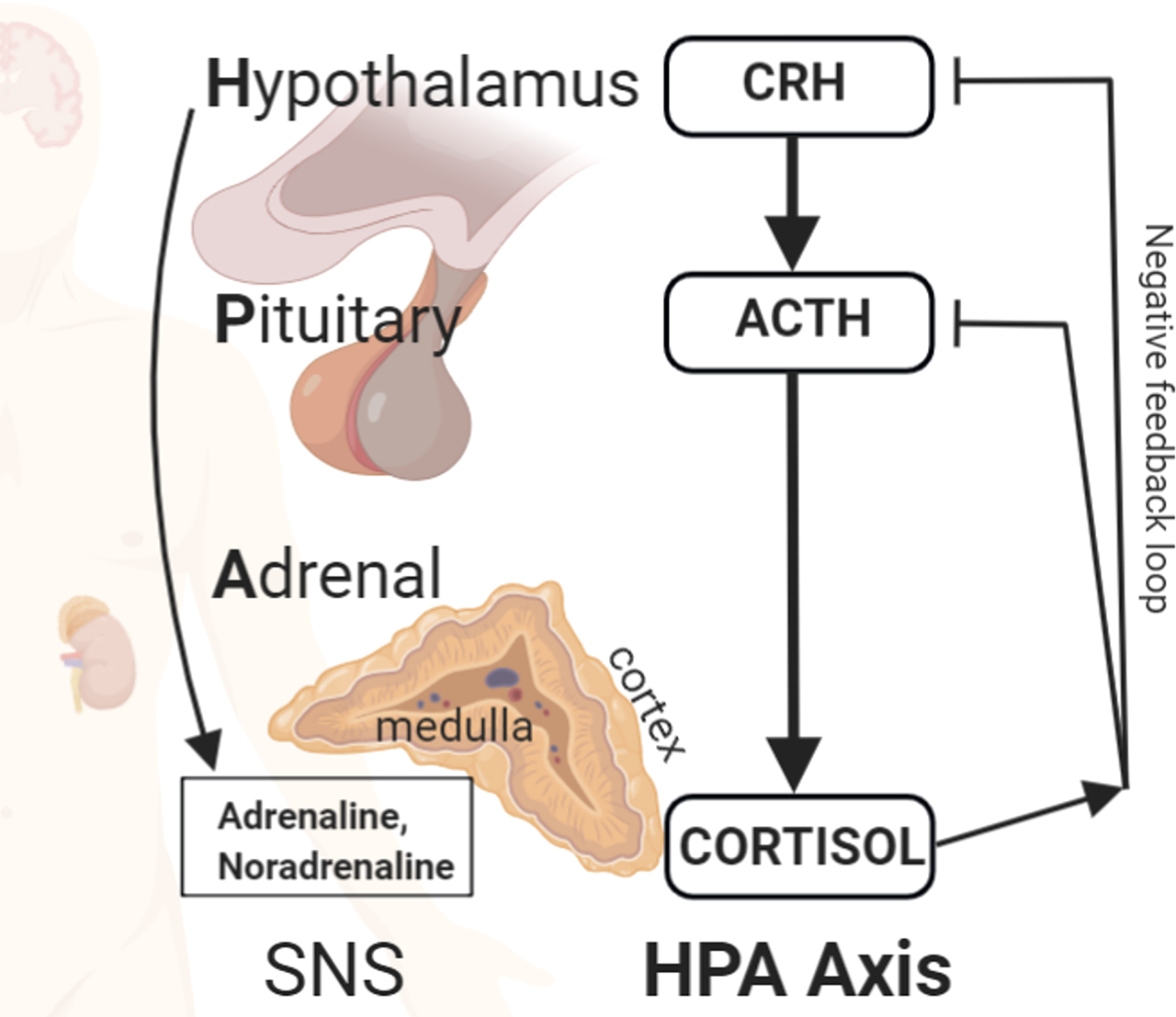
The stress response. Two systems come into play to promote an adaptive response to a stressful situation: the sympathetic nervous system (SNS; mainly through the secretion of adrenaline and noradrenaline) and the hypothalamus-pituitary-adrenocortical (HPA) axis (mainly through the secretion of the glucocorticoid cortisol). Cortisol is also involved in a negative feedback loop, affecting the HPA axis. ACTH: adrenocorticotropic hormone; CRH: corticotrophin-releasing hormone. The figure was created with BioRender.com.
The effects of stress of learning and memory processes depend on various modulating factors, such as intensity and duration of stress, the characteristics of the learning task, and individual differences, such as age, sex, and personality traits (Meir Drexler and Wolf, 2017a; Shields et al., 2017). Another significant modulating factor is the timing of stress in relation to the task: Did learning occur before stress or after it has subsided or did an older or unrelated memory have to be recalled during or after the stressful episode itself? In general, cortisol (through interaction with noradrenaline) promotes the consolidation of emotional or arousing memories but at the same time impairs the retrieval of previously consolidated memories (de Quervain et al., 2017; Roozendaal, 2002). For this reason, a stressed student might have difficulties recalling the learning material during an exam, but the memory of the stressful exam experience itself might be easily recalled later.
In addition to enhancing emotional memory consolidation, stress also affects the contextualization of memories. Because stress can disrupt the context dependency of memories (Schwabe et al., 2009), emotional memories are often not only stronger but also more easily generalized from the original learning context to other contexts. In extreme cases, strong and generalized emotional memories will not fade over time and might become overpowering and maladaptive. Strong maladaptive memories underlie post-traumatic stress disorder (PTSD), phobias (Merz et al., 2016), and chronic pain (Elsenbruch and Wolf, 2015).
Exposure therapy and the problem of relapse
Exposure therapy is a type of cognitive behavioral treatment, which is often used for the treatment of PTSD and phobias (Craske et al., 2018). One of the possible underlying mechanisms of exposure therapy is extinction learning, which involves repeated confrontation with the conditioned stimulus (CS; e.g., dog) in the absence of the unconditioned stimulus (UCS; e.g., dog bite), typically resulting in a decrement of conditioned responses (e.g., fear). Extinction learning depends on the formation of a new inhibitory (i.e., safety) memory and does not erase the original (i.e., fear) memory (Bouton, 2014). Following extinction, the original and the extinction memory will compete against one another for the control over behavior. The challenge for exposure therapy stems from the differences in the strength and context dependency of both memories. The original memory is often robust, is not bound to a specific context, and is thus more easily generalized (e.g., generalizing the fear of dogs from the park, where a dog attack had happened, to other places). Extinction memory, in contrast, is not the first association that is learned about a stimulus and as such is encoded as a conditional (e.g., context-dependent) exception to the rule (for instance, feeling safe despite the presence of a dog but only while being at the clinic).
As a result, relapse (or “return of fear”) may occur under various conditions: after an exposure to an aversive stimulus (“reinstatement”), after a change in context (“renewal”), or just by the passage of time (“spontaneous recovery”; Bouton, 2014). This significant challenge to the long-term success of exposure therapy has led many research groups to investigate various (e.g., cognitive, pharmacological) methods of extinction augmentation (Craske et al., 2018; Ressler et al., 2004). Growing knowledge on the role of stress and cortisol in learning and memory has shown that cortisol can act as an adjuvant in extinction-based therapy (de Quervain et al., 2017).
What is the mechanism of cortisol-induced extinction augmentation?
Recently, we suggested the STaR model (the initials of which stand for “Stress Timing affects Relapse”; see Figure 2) to illustrate the consequences of stress timing on the strength and context dependency of extinction memories, resulting in either relapse or not (Meir Drexler et al., 2019a). These findings are based on several studies in which we used exposure to laboratory stress or a pharmacological cortisol administration at different times: before extinction learning (i.e., to affect the encoding and consolidation of the extinction memory), after extinction learning (i.e., to affect extinction memory consolidation only), or before extinction retrieval.
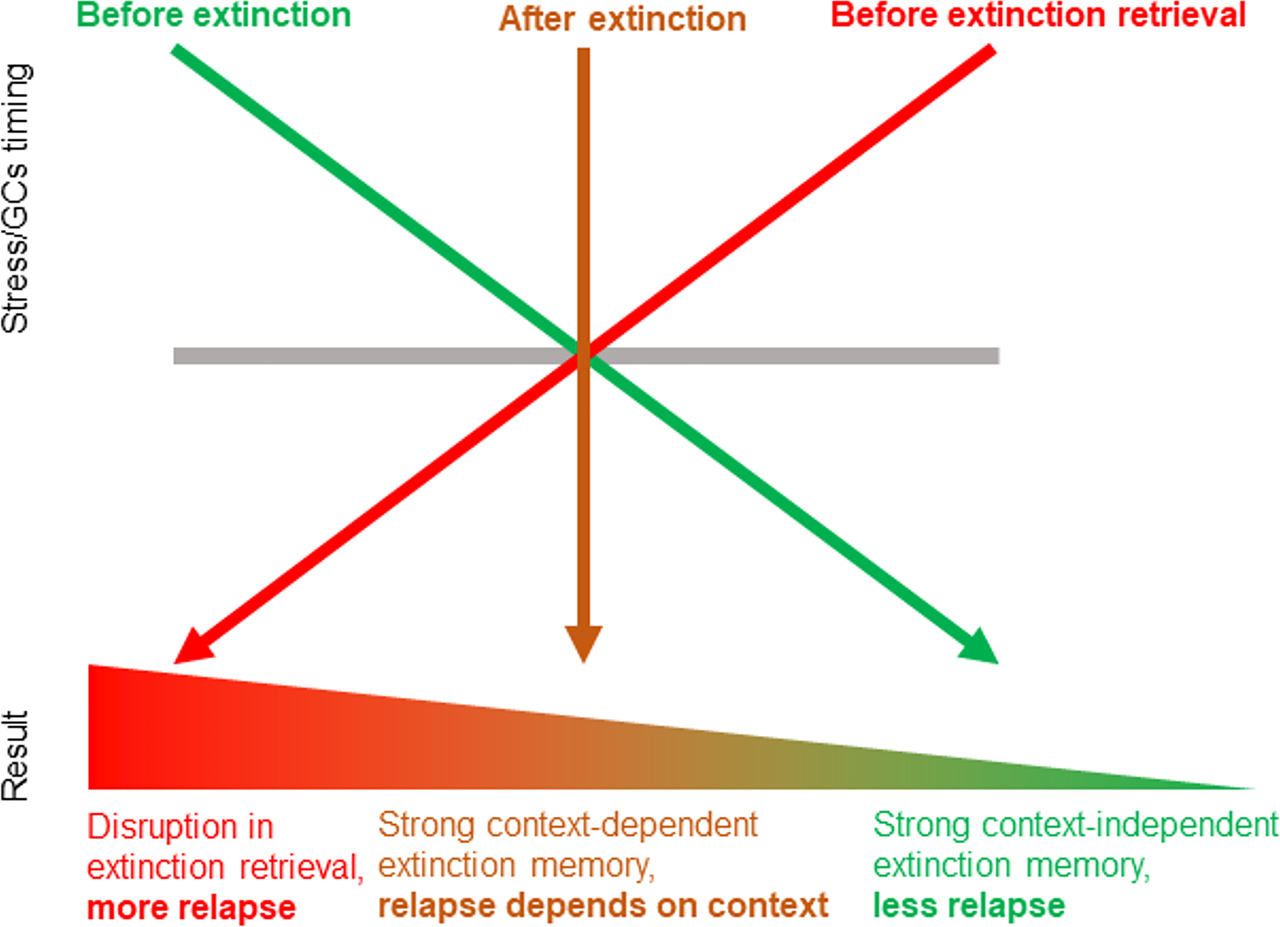
The STaR (Stress Timing affects Relapse) model represents the timing-dependent modulation of extinction and relapse by stress/glucocorticoids (GCs). Stress or GCs before extinction promote memory consolidation in a context-independent manner, making extinction memory more generalized and thus resistant to relapse after context change. Stress or GCs after extinction also enhance extinction consolidation, but in a context-bound manner, thus making extinction retrieval more likely only in the context in which it had been learned. In contrast, stress or GCs before an extinction retrieval test impair extinction retrieval and promote relapse. Reprinted from Meir Drexler et al. (2019a), Copyright (2019), with permission from Elsevier.
We found that exposure to stress or cortisol administration before extinction learning promotes extinction memory consolidation in a context-independent way (Meir Drexler et al., 2017, 2018; but see: Merz et al., 2018), making extinction memory more resistant to relapse after context change (see similar GCs-related contextual impairments in other tasks: McGlade et al., 2019; Schwabe et al., 2009; van Ast et al., 2013). In contrast, exposure to stress/cortisol after extinction leads to an enhanced, but context-dependent, extinction memory trace (Hamacher-Dang et al., 2013, 2015), making extinction retrieval more likely, but only in the context in which it had been learned (see GCs-related context-dependency in other tasks: van Ast et al., 2013). Finally, we found that when stress or cortisol is given before a retrieval test, extinction retrieval is impaired (Hamacher-Dang et al., 2013; Kinner et al., 2016, 2018; but see: Merz et al., 2014), making relapse more likely to occur (see GC-related retrieval deficit in other tasks: Shields et al., 2017).
At the neural level (as illustrated in Figure 3), we could show that the timing-dependent effects of stress/cortisol on extinction memories are modulated by alterations in the amygdala, the hippocampal complex, the prefrontal cortex, and their communication with other brain regions (Meir Drexler et al., 2019a). In line with evidence from previous works on animals and humans (Milad and Quirk, 2012), our findings suggest that the ventromedial prefrontal cortex (vmPFC) and the hippocampus are activated and determine, based on the given context, whether or not extinction memory is expressed under nonstressful conditions. However, if cortisol was administered before extinction learning, activity of the hippocampus and its functional connectivity to the vmPFC increases in a later retrieval task, leading to enhanced extinction retrieval and thus reduced fear (Merz et al., 2018). In contrast, exposure to cortisol before the retrieval task itself suppresses vmPFC activation and its connectivity with the parahippocampal gyrus, enhances activation of the amygdala, and leads to impaired extinction retrieval and thus enhanced fear (Kinner et al., 2016, 2018).
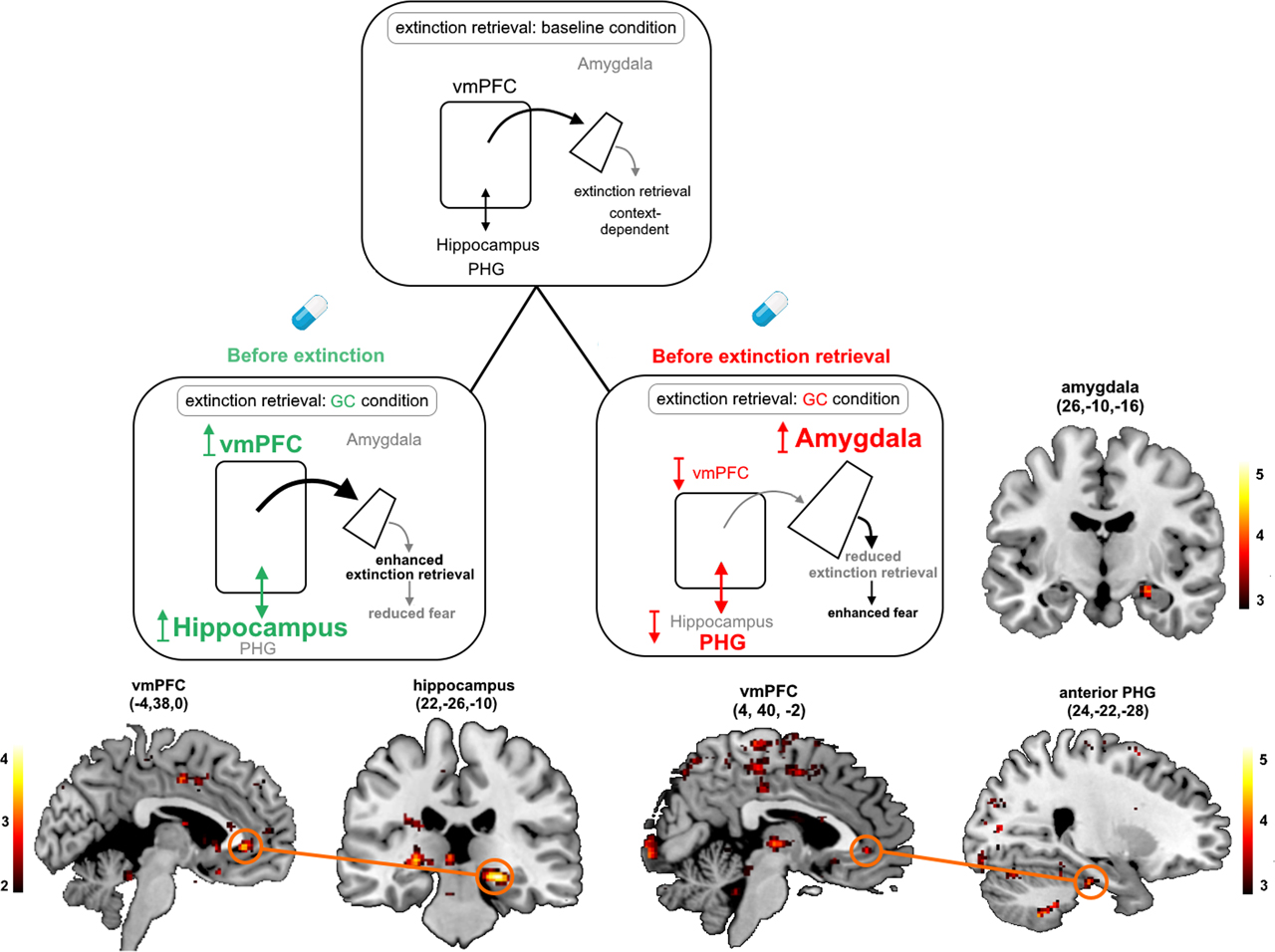
Simplified scheme of the neural network mediating extinction retrieval under baseline conditions (upper panel) and the proposed neural mechanisms underlying the effects of glucocorticoids (GCs) on this network, when administered before extinction learning (lower left panel) or before extinction retrieval (lower right panel). Neural activation and functional connectivity are additionally shown for the comparison between conditioned stimuli in the respective brain regions. The ventromedial prefrontal cortex (vmPFC) and the hippocampus are activated and determine, based on the given context, whether or not extinction memory is expressed under nonstressful (baseline) conditions. However, if cortisol was administered before extinction learning, activity of the hippocampus and its functional connectivity to the vmPFC increases in a later retrieval task, leading to enhanced extinction retrieval and thus reduced fear. In contrast, exposure to cortisol before the retrieval task itself suppresses vmPFC activation and its connectivity with the parahippocampal gyrus (PHG), enhances activation of the amygdala, and leads to impaired extinction retrieval and thus enhanced fear. The size of the structures indicates activation dominance. The colors of the arrows depict the proposed modulating influence (black = modulation; gray = reduced modulation by GCs; green = enhancing GC effects; red = impairing GC effects). Reprinted from Meir Drexler et al. (2019), Copyright (2019), with permission from Elsevier.
Our findings provide additional support for the beneficial (time-specific) use of cortisol in psychotherapy (de Quervain et al., 2017). By focusing on the contextual factor, which is a crucial element not only in renewal but also in other relapse phenomena (Bouton, 2014), these findings may help in developing more efficient interventions. In particular, our data suggest that the use of cortisol or stress should be promoted shortly before and avoided after extinction-based psychotherapy, taking into account possible factors that can affect cortisol concentrations (e.g., sex, sex hormones, and medication including hormonal contraceptives; see Kudielka et al., 2009; Merz and Wolf, 2017; Raeder et al., 2019). In cases wherein cortisol administration is not feasible, behavioral interventions might promote the desired moderate and time-limited cortisol response (Lass-Hennemann and Michael, 2014; Meir Drexler et al., 2017, 2018).
Cortisol and memory reconsolidation
While repeated presentations of conditioned cues usually lead to the formation of a new memory trace (i.e., extinction learning, as discussed previously), a single brief presentation of the CS triggers a reconsolidation process, resulting in an alteration or update of the original memory itself (Merlo et al., 2014). It was suggested that the strength of the prediction error (i.e., the discrepancy between a predicted and an actual outcome) in each case leads to either an update of the old memory when the prediction error is moderate or a formation of a new memory altogether when a stronger prediction error occurs (Gershman et al., 2017).
Much like newly acquired memories, reactivated memories are sensitive to various (e.g., behavioral, pharmacological) manipulations that can be designed to weaken or strengthen the memory until its reconsolidation is complete (Josselyn and Tonegawa, 2020). Among these are stress and cortisol manipulations (Akirav and Maroun, 2013), yet the direction of the effect is still debated (Meir Drexler and Wolf, 2017c, 2018; Shields et al., 2017). These conflicting findings may result from methodological differences that are common in the general reconsolidation field (Meir Drexler and Wolf, 2017c), such as variations in the type or age of the memory, study sample, or the manipulation itself. For instance, although we previously found an enhancing effect of cortisol on fear memory reconsolidation in men (Meir Drexler et al., 2015), we found no effect in women (possibly owing to interactions with female sex hormones: Meir Drexler et al., 2016). In contrast, exposure to mild stress led to a fear memory impairment in men (possibly through an interruption of memory reconsolidation: Meir Drexler and Wolf, 2017b). Moreover, while the previous studies used the commonly used CS-based reconsolidation paradigm (i.e., reactivation by a single unreinforced CS presentation), in an alternative UCS-based reconsolidation paradigm (i.e., through a single weaker UCS presentation), the reactivation method itself prevented the return of fear regardless of the pharmacological (cortisol or placebo) treatment (Meir Drexler et al., 2019b).
As these findings demonstrate, one has to bear in mind that a given behavioral or pharmacological manipulation can lead to different behavioral outcomes when paired with either extinction (e.g., less fear: Meir Drexler et al., 2019a) or reconsolidation (e.g., more fear, at least in men: Meir Drexler et al., 2015). Unlike cortisol, more promising targets for future reconsolidation-based therapies may include the GC receptor antagonist mifepristone (Nikzad et al., 2011; Pitman et al., 2011), the noradrenergic β-blocker propranolol (Soeter and Kindt, 2015), or cognitive techniques for memory updating (Josselyn and Tonegawa, 2020; Meir Drexler and Wolf, 2017b, 2018), but more evidence in clinical populations is needed.
Conclusion
Cortisol, a GC involved in the development of maladaptive memories, can also be used as a pharmacological agent for the augmentation of exposure therapy. We suggest that the beneficial effect of cortisol in exposure-based psychotherapy results from its modulation of extinction processes, in particular the enhancement of extinction memory consolidation and the reduction of its contextual dependence. When used in a reconsolidation paradigm, however, cortisol may lead to an enhancement of the original fear memory and, thus, to adverse effects. The current findings encourage further investigation of the clinical use of cortisol in extinction-based (but not reconsolidation-based) interventions.
Funding source: Deutsche Forschungsgemeinschaft
About the authors

Shira Meir Drexler is a postdoctoral researcher in the Department of Cognitive Psychology at the Ruhr University Bochum. She studied psychology and psychobiology in the University of Haifa, Israel, and obtained her PhD in Neuroscience from the International Graduate School of Neuroscience (IGSN) at the Ruhr University Bochum. Her current research focuses on the effects of stress and cortisol in classical and instrumental conditioning paradigms, and she is particularly interested in extinction and reconsolidation of memories as these processes are relevant to the understanding and treatment of stress-related disorders. In addition, she is involved in several meta-research projects in the field of fear conditioning.

Christian J. Merz is a principal investigator and assistant professor in the Department of Cognitive Psychology at the Ruhr University Bochum. He received his diploma in psychology from Justus Liebig University Giessen and his PhD and habilitation from the Ruhr University Bochum. He conducted his postdoctoral work in Giessen, Bochum, and Trier, where he was also an interim professor for Biological and Clinical Psychology. His special interests concern the modulation of declarative and fear memories by stress and sex hormones and its clinical implications. In addition, he is involved in several meta-research projects in the field of fear conditioning.

Valerie L. Jentsch is a postdoctoral researcher in the Department of Cognitive Psychology at the Ruhr University Bochum. She studied psychology at the Ruhr University Bochum, where she also obtained her PhD in 2017, investigating the effects of the stress hormone cortisol on the neural mechanisms of extinction learning and its retrieval. In her current position, she continues working on the neuroendocrine regulation of fear and extinction memories and their multidimensional assessment via behavioral, psychophysiological, and neural indices. She is also involved in several meta-research projects in the field of fear conditioning. Furthermore, she has a special interest in the bidirectional effects of stress and emotion regulation processes, which has become her second major line of research.

Oliver T. Wolf is a full professor and head of the Department of Cognitive Psychology at the Ruhr University Bochum. He received his diploma in psychology from Trier University, where he also conducted his PhD thesis. After a postdoc at The Rockefeller University and New York University, he moved to Heinrich Heine University Düsseldorf, where he obtained his habilitation. In 2005, he became a full professor at Bielefeld University before moving to the Ruhr University Bochum in 2007. For more than 20 years, he has investigated the impact of acute and chronic stress on cognitive processes taking a psychoneuroendocrine perspective. Although his focus has been on learning and memory, additional processes of interest such as decision-making, social cognition, and emotion regulation have emerged over the years.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through the research unit FOR 1581 (P5) and the collaborative research center SFB 1280 (project number 316803389; A09).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Akirav, I. and Maroun, M. (2013). Stress modulation of reconsolidation. Psychopharmacology 226, 747–761, https://doi.org/10.1007/s00213-012-2887-6.Search in Google Scholar PubMed
Bouton, M.E. (2014). Why behavior change is difficult to sustain. Prev. Med. 68, 29–36, https://doi.org/10.1016/j.ypmed.2014.06.010.Search in Google Scholar PubMed PubMed Central
Colman, A.M. (2001). Oxford Dictionary of Psychology (Oxford, UK: Oxford University Press).Search in Google Scholar
Craske, M.G., Hermans, D., and Vervliet, B. (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170, https://doi.org/10.1098/rstb.2017.0025.Search in Google Scholar PubMed PubMed Central
de Quervain, D.J.F., Schwabe, L., and Roozendaal, B. (2017). Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 18, 7–19, https://doi.org/10.1038/nrn.2016.155.Search in Google Scholar PubMed
Elsenbruch, S., and Wolf, O.T. (2015). Could stress contribute to pain-related fear in chronic pain?. Front. Behav. Neurosci. 9, 1–8, https://doi.org/10.3389/fnbeh.2015.00340.Search in Google Scholar PubMed PubMed Central
Gershman, S.J., Monfils, M.H., Norman, K.A., and Niv, Y. (2017). The computational nature of memory modification. ELife 6, 1–48, https://doi.org/10.7554/eLife.23763.Search in Google Scholar PubMed PubMed Central
Hamacher-Dang, T.C., Engler, H., Schedlowski, M., and Wolf, O. T. (2013). Stress enhances the consolidation of extinction memory in a predictive learning task. Front. Behav. Neurosci. 7, 1–8, https://doi.org/10.3389/fnbeh.2013.00108.Search in Google Scholar PubMed PubMed Central
Hamacher-Dang, T.C., Üngör, M., and Wolf, O.T. (2013). Stress impairs retrieval of extinguished and unextinguished associations in a predictive learning task. Neurobiol. Learn. Mem. 104, 1–8, https://doi.org/10.1016/j.nlm.2013.04.007.Search in Google Scholar PubMed
Hamacher-Dang, T.C., Merz, C.J., and Wolf, O.T. (2015). Stress following extinction learning leads to a context-dependent return of fear. Psychophysiology 52, 489–498, https://doi.org/10.1111/psyp.12384.Search in Google Scholar PubMed
Joëls, M., Pu, Z., Wiegert, O., Oitzl, M.S., and Krugers, H.J. (2006). Learning under stress: how does it work?. Trends Cognit. Sci. 10, 152–158, https://doi.org/10.1016/j.tics.2006.02.002.Search in Google Scholar PubMed
Josselyn, S.A., and Tonegawa, S. (2020). Memory engrams: Recalling the past and imagining the future. Science 367, eaaw4325, https://doi.org/10.1126/science.aaw4325.Search in Google Scholar PubMed PubMed Central
Kinner, V.L., Merz, C.J., Lissek, S., and Wolf, O.T. (2016). Cortisol disrupts the neural correlates of extinction recall. NeuroImage 133, 233–243, https://doi.org/10.1016/j.neuroimage.2016.03.005.Search in Google Scholar PubMed
Kinner, V.L., Wolf, O.T., and Merz, C.J. (2018). Cortisol increases the return of fear by strengthening amygdala signaling in men. Psychoneuroendocrinology 91, 79–85, https://doi.org/10.1016/j.psyneuen.2018.02.020.Search in Google Scholar PubMed
Kudielka, B.M., Hellhammer, D.H., and Wüst, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34, 2–18, https://doi.org/10.1016/j.psyneuen.2008.10.004.Search in Google Scholar PubMed
Lass-Hennemann, J., and Michael, T. (2014). Endogenous cortisol levels influence exposure therapy in spider phobia. Behav. Res. Ther. 60, 39–45, https://doi.org/10.1016/j.brat.2014.06.009.Search in Google Scholar PubMed
McCarty, R. (2016). The fight-or-flight response: A cornerstone of stress research. Stress: Concepts, Cognition, Emotion, and Behavior: Handbook of Stress (Elsevier Inc), https://doi.org/10.1016/B978-0-12-800951-2.00004-2.Search in Google Scholar
McEwen, B.S. (2019). What is the confusion with cortisol?. Chronic Stress 3, 1–3, https://doi.org/10.1177/2470547019833647.Search in Google Scholar PubMed PubMed Central
McGlade, A., Zbozinek, T.D., Treanor, M., and Craske, M.G. (2019). Pilot for novel context generalization paradigm. J. Behav. Ther. Exp. Psychiatr. 62, 49–56, https://doi.org/10.1016/j.jbtep.2018.08.009.Search in Google Scholar PubMed
Meir Drexler, S., and Wolf, O.T. (2017a). Stress and memory consolidation. In Cognitive Neuroscience of Memory Consolidation. N. Axmacher and B. Rasch, eds. (Cham, Switzerland: Springer), pp. 285–300, https://doi.org/10.1007/978-3-319-45066-7_17.Search in Google Scholar
Meir Drexler, S., and Wolf, O.T. (2017b). Stress disrupts the reconsolidation of fear memories in men. Psychoneuroendocrinology 77, 95–104, https://doi.org/10.1016/j.psyneuen.2016.11.027.Search in Google Scholar PubMed
Meir Drexler, S., and Wolf, O.T. (2017c). The role of glucocorticoids in emotional memory reconsolidation. Neurobiol. Learn. Mem. 142, 126–134, https://doi.org/10.1016/j.nlm.2016.11.008.Search in Google Scholar PubMed
Meir Drexler, S., and Wolf, O.T. (2018). Behavioral disruption of memory reconsolidation: From bench to bedside and back again. Behav. Neurosci. 132, 13–22, https://doi.org/10.1037/bne0000231.Search in Google Scholar PubMed
Meir Drexler, S., Merz, C.J., Hamacher-Dang, T.C., Tegenthoff, M., and Wolf, O.T. (2015). Effects of cortisol on reconsolidation of reactivated fear memories. Neuropsychopharmacology 40, 3036–3043, https://doi.org/10.1038/npp.2015.160.Search in Google Scholar PubMed PubMed Central
Meir Drexler, S., Merz, C.J., Hamacher-Dang, T.C., and Wolf, O.T. (2016). Cortisol effects on fear memory reconsolidation in women. Psychopharmacology 233, 2687–2697, https://doi.org/10.1007/s00213-016-4314-x.Search in Google Scholar PubMed
Meir Drexler, S., Hamacher-Dang, T.C., and Wolf, O.T. (2017). Stress before extinction learning enhances and generalizes extinction memory in a predictive learning task. Neurobiol. Learn. Mem. 141, 143–149, https://doi.org/10.1016/j.nlm.2017.04.002.Search in Google Scholar PubMed
Meir Drexler, S., Merz, C.J., and Wolf, O.T. (2018). Preextinction stress prevents context-related renewal of fear. Behav. Ther. 49, 1008–1019, https://doi.org/10.1016/j.beth.2018.03.001.Search in Google Scholar PubMed
Meir Drexler, S., Merz, C.J., Jentsch, V.L., and Wolf, O.T. (2019a). How stress and glucocorticoids timing-dependently affect extinction and relapse. Neurosci. Biobehav. Rev. 98, 145–153, https://doi.org/10.1016/J.NEUBIOREV.2018.12.029.Search in Google Scholar PubMed
Meir Drexler, S., Merz, C.J., Lissek, S., Tegenthoff, M., and Wolf, O.T. (2019b). Reactivation of the unconditioned stimulus inhibits the return of fear independent of cortisol. Front. Behav. Neurosci. 13, https://doi.org/10.3389/fnbeh.2019.00254.Search in Google Scholar PubMed PubMed Central
Merlo, E., Milton, A.L., Goozee, Z.Y., Theobald, D.E., and Everitt, B.J. (2014). Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J. Neurosci. 34, 2422–2431, https://doi.org/10.1523/JNEUROSCI.4001-13.2014.Search in Google Scholar PubMed PubMed Central
Merz, C.J., and Wolf, O.T. (2017). Sex differences in stress effects on emotional learning. J. Neurosci. Res. 95, 93–105, https://doi.org/10.1002/jnr.23811.Search in Google Scholar PubMed
Merz, C.J., Hamacher-Dang, T.C., and Wolf, O.T. (2014). Exposure to stress attenuates fear retrieval in healthy men. Psychoneuroendocrinology 41, 89–96, https://doi.org/10.1016/j.psyneuen.2013.12.009.Search in Google Scholar PubMed
Merz, C.J., Elzinga, B.M., and Schwabe, L. (2016). Stress, fear, and memory in healthy individuals. In Post-traumatic Stress Disorder: From Neurobiology to Treatment. J. D. Bremner, ed. (Malden, MA: Wiley-Blackwell), https://doi.org/10.1002/9781118356142.ch8.Search in Google Scholar
Merz, C.J., Hamacher-Dang, T.C., Stark, R., Wolf, O.T., and Hermann, A. (2018). Neural underpinnings of cortisol effects on fear extinction. Neuropsychopharmacology 43, 384–392, https://doi.org/10.1038/npp.2017.227.Search in Google Scholar PubMed PubMed Central
Milad, M.R., and Quirk, G.J. (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 63, 129–151, https://doi.org/10.1146/annurev.psych.121208.131631.Search in Google Scholar PubMed PubMed Central
Nikzad, S., Vafaei, A.A., Rashidy-Pour, A., and Haghighi, S. (2011). Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress-Int. J. Biol. Stress 14, 459–464, https://doi.org/10.3109/10253890.2010.548171.Search in Google Scholar PubMed
Pitman, R.K., Milad, M.R., Igoe, S.A., Vangel, M.G., Orr, S.P., Tsareva, A., Gamache, K., Nader, K. (2011). Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behav. Neurosci. 125, 632–638, https://doi.org/10.1037/a0024364.Search in Google Scholar PubMed
Raeder, F., Heidemann, F., Schedlowski, M., Margraf, J., and Zlomuzica, A. (2019). No pills, more skills: The adverse effect of hormonal contraceptive use on exposure therapy benefit. J. Psychiatr. Res. 119, 95–101, https://doi.org/10.1016/j.jpsychires.2019.09.016.Search in Google Scholar PubMed
Ressler, K.J., Rothbaum, B.O., Tannenbaum, L., Anderson, P., Graap, K., Zimand, E., Hodges, L., and Davis, M. (2004). Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatr. 61, 1136–1144, https://doi.org/10.1001/archpsyc.61.11.1136.Search in Google Scholar PubMed
Roozendaal, B. (2002). Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 78, 578–595, https://doi.org/10.1006/nlme.2002.4080.Search in Google Scholar PubMed
Schwabe, L., Böhringer, A., and Wolf, O.T. (2009). Stress disrupts context-dependent memory. Learn. Mem. 16, 110–113, https://doi.org/10.1101/lm.1257509.Search in Google Scholar PubMed
Shields, G.S.S., Sazma, M.A.A., McCullough, A.M.M., and Yonelinas, A.P.P. (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull. 143, 636–675, https://doi.org/10.1037/bul0000100.Search in Google Scholar PubMed PubMed Central
Soeter, M., and Kindt, M. (2015). An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol. Psychiatr. 78, 880–886, https://doi.org/10.1016/j.biopsych.2015.04.006.Search in Google Scholar PubMed
van Ast, V.A., Cornelisse, S., Meeter, M., Joëls, M., and Kindt, M. (2013). Time-dependent effects of cortisol on the contextualization of emotional memories. Biol. Psychiatr. 74, 809–816, https://doi.org/10.1016/j.biopsych.2013.06.022.Search in Google Scholar PubMed
© 2020 Shira Meir Drexler et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Stress modulation of fear and extinction in psychopathology and treatment
- Clinical implications of fear extinction in anxiety disorders
- From gut feelings to memories of visceral pain
- Principles of extinction learning of nonaversive experience
- Beyond the classic extinction network: a wider, comparative view
- How learning shapes immunity
- Presentation of scientific institutions
- Forschungskolleg “NeurodegX”
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Stress modulation of fear and extinction in psychopathology and treatment
- Clinical implications of fear extinction in anxiety disorders
- From gut feelings to memories of visceral pain
- Principles of extinction learning of nonaversive experience
- Beyond the classic extinction network: a wider, comparative view
- How learning shapes immunity
- Presentation of scientific institutions
- Forschungskolleg “NeurodegX”
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft

