Abstract
C24H21NO3S, monoclinic, P21/c (no. 14), a = 13.5232(6) Å, b = 8.5187(5) Å, c = 19.3029(10) Å, β = 107.174(2)°, V = 2124.55(19) Å3, Z = 4, Rgt(F) = 0.0480 wRref(F2) = 0.1348, T = 297 K.
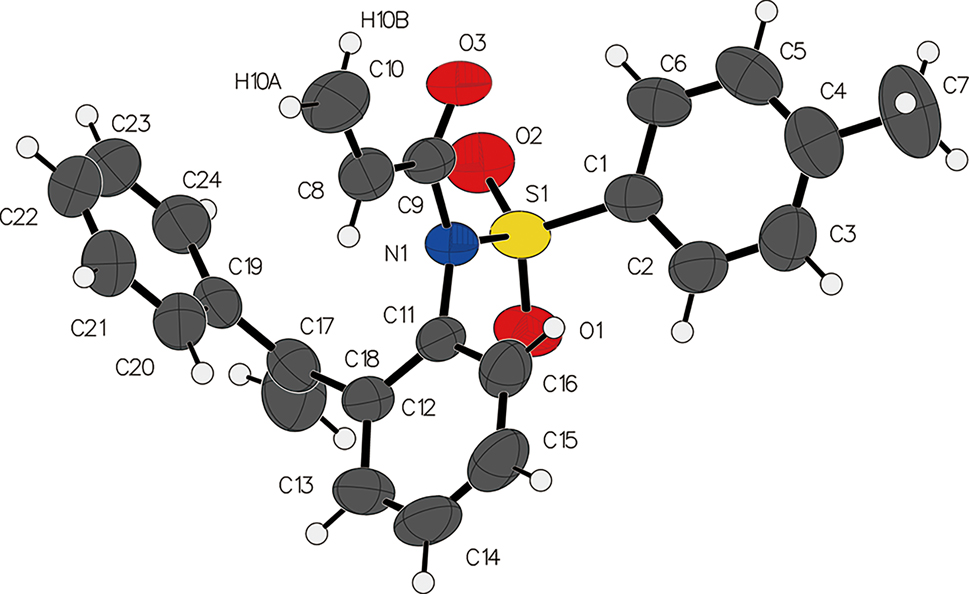
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.33 × 0.25 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.18 mm−1 |
| Diffractometer, scan mode: | Rigaku Synergy, ω scans |
| θmax, completeness: | 28.3°, 97 % |
| N(hkl)measured, N(hkl)unique, Rint: | 83045, 5160, 0.099 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4,036 |
| N(param)refined: | 279 |
| Programs: | Rigaku, 1 SHELX, 2 , 3 Olex2 4 |
1 Source of materials
The synthetic route to the title compound, N-(2-(1-phenylethenyl)phenyl)-N-(tosyl)acrylamide, is outlined herein. Commercial (2-aminophenyl)(phenyl) methanone was converted to 2-(1-phenylethenyl)aniline via a catalytic dehydration reaction. This amine intermediate was then subjected to N-tosylation using p-toluenesulfonyl chloride and triethylamine in dichloromethane to yield N-(2-(1-phenylethenyl)phenyl)-4-methylbenzenesulfonamide. The final acrylation was achieved by treating the sulfonamide with acryloyl chloride in the presence of sodium hydride in tetrahydrofuran. The crude product was purified by column chromatography. Single crystals of the title compound, which were obtained by slow evaporation from a dichloromethane/n-hexane solution, were subjected to X-ray crystallography for definitive structural confirmation. 5
2 Experimental details
The structure was solved (SHELXT 2 ) and refined (SHELXL 3 within Olex2 4 ), respectively, with anisotropic displacement parameters for all non-hydrogen atoms.
3 Comment
The construction of nitrogen-containing heterocycles, particularly medium-sized benzazepinones, is of paramount importance in organic and medicinal chemistry due to their prevalence in pharmacologically active compounds. 6 , 7 Among modern methodologies, radical-mediated cyclizations of alkenes stand out for their mild conditions and atom economy. 8 Within this framework, N-allyl-N-tosyl aniline derivatives serve as privileged substrates for intramolecular sulfonyl radical additions. The title compound, N-[2-(1-phenylethenyl)phenyl]-N-[(4-methylphenyl)sulfonyl]prop-2-enamide, was designed as an advanced analogue featuring two distinct alkenes to probe chemoselective radical cyclizations. Preliminary studies confirm an exceptional regioselectivity favoring cyclization across the acrylamide moiety. 5 , 9 To unravel the structural basis for this selectivity, we report herein its unambiguous solid-state molecular structure determined by single-crystal X-ray diffraction, providing crucial conformational insight into its reactivity.
The molecular structure of the title compound was unequivocally confirmed by single-crystal X-ray diffraction. The asymmetric unit comprises one discrete molecule. The molecular conformation is defined by key interplanar angles, revealing a near-orthogonal relationship (86.20°) between the 1-phenylethenyl substituent and the aniline moiety, whereas the p-toluenesulfonyl (tosyl) phenyl group and the aniline are nearly coplanar (dihedral angle = 23.82°). The amide functionality exhibits a C–N bond length of 1.405 Å, indicative of partial double-bond character consistent with electron delocalization within the N–C(=O) fragment. However, a significant twist of 39.55° between the acryloyl group and the aniline plane disrupts extended π-conjugation across the molecule.
In the crystal, molecules are assembled into a one-dimensional supramolecular chain via a pivotal C10–H10B⋯O1 hydrogen bond (H⋯O = 2.49 Å, D–H⋯A = 136°). These primary chains are further extended into a cohesive three-dimensional architecture through a combination of van der Waals forces and likely C–H⋯π interactions, which is commonly observed in similar tosylamide-containing compounds. 10 , 11 , 12 , 13
Acknowledgments
This work was financially supported by the projects of Natural Science Foundation of Shaanxi Province (2024JC-YBMS-733, 2025JC-YBMS-888); The 2024 Key Scientific Research Program Projects of the Shaanxi Provincial Department of Education (Key Laboratory Projects, 24JS004); Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL-PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. Shelx-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Zhang, Z.; Tan, P.; Wang, S.; Wang, H.; Xie, L.; Chen, Y.; Han, L.; Yang, S.; Sun, K. Visible-Light-Promoted Selective Sulfonylation and Selenylation of Dienes to Access Sulfonyl-/Seleno-Benzazepine Derivatives. Org. Lett. 2023, 25, 4208–4213; https://doi.org/10.1021/acs.orglett.3c01550.Suche in Google Scholar PubMed
6. Feng, J.; Geng, W. C.; Jiang, H.; Wu, B. Recent Advances in Biocatalysis of Nitrogen-Containing Heterocycles. Biotechnol. Adv. 2022, 54, 107813; https://doi.org/10.1016/j.biotechadv.2021.107813.Suche in Google Scholar PubMed
7. Amin, A.; Qadir, T.; Sharma, P. K.; Jeelani, I.; Abe, H. A Review on the Medicinal and Industrial Applications of N-Containing Heterocycles. Open Med. Chem. J. 2022, 16; https://doi.org/10.2174/18741045-v16-e2209010.Suche in Google Scholar
8. Zhi, S.; Ma, X.; Zhang, W. Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes. Molecules 2024, 29, 2559; https://doi.org/10.3390/molecules29112559.Suche in Google Scholar PubMed PubMed Central
9. Zhou, N.; Kuang, K.; Wu, M.; Wu, S.; Xu, Q.; Xia, Z.; Zhang, M. Tert-Butyl Hydroperoxide-Initiated Radical Cyclization of 1-(Allyloxy)-2-(1-Arylvinyl) Benzenes with Sulfinic Acids to Access Sulfonated Benzoxepines. Adv. Synth. Catal. 2021, 363, 3491–3495; https://doi.org/10.1002/adsc.202100466.Suche in Google Scholar
10. Tanpure, S. D.; Kale, B. S.; Liu, R. S. Gold(I)-Catalyzed Oxidative 1, 4-Additions of 3–En-1-Ynamide with Nitrones via Carbon-Versus Nitrogen-Addition Chemoselectivity. Org. Lett. 2021, 23, 1394–1399; https://doi.org/10.1021/acs.orglett.1c00055.Suche in Google Scholar PubMed
11. Zhang, C.; Chen, L.; Chen, K.; Wang, C.; Xu, Z.; Jiang, H.; Zhu, S. Cu(I)-Catalyzed Stereoselective Synthesis of Trisubstituted Z-Enol Esters via Interrupting the 1,3-O-Transposition Reaction. Org. Chem. Front. 2018, 5, 2510–2514; https://doi.org/10.1039/c8qo00664d.Suche in Google Scholar
12. Liu, J.; Chen, M.; Zhang, L.; Liu, Y. Gold(I)-Catalyzed 1,2-Acyloxy Migration/[3+2] Cycloaddition of 1,6-Diynes with an Ynamide Propargyl Ester Moiety: Highly Efficient Synthesis of Functionalized Cyclopenta[b]Indoles. Chemistry 2015, 21, 1009–1013; https://doi.org/10.1002/chem.201405965.Suche in Google Scholar PubMed
13. Zhang, X. Z.; Gan, K. J. I.; Liu, X. X.; Deng, Y. H.; Wang, F. X.; Yu, K. Y.; Zhang, J.; Fan, C. A. N. Enantioselective Synthesis of Functionalized 4-Aryl Hydrocoumarins and 4-Aryl Hydroquinolin-2-ones via Intramolecular Vinylogous Rauhut-Currier Reaction of Para-Quinone Methides. Org. Lett. 2017, 19, 3207; https://doi.org/10.1021/acs.orglett.7b01331.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

