Abstract
C19H16I2O3, triclinic, P1̄ (no. 2), a = 8.9049(1) Å, b = 9.1514(1) Å, c = 12.0282(2) Å, α = 101.511(1)°, β = 101.691(1)°, γ = 93.386(1)°, V = 935.49(2) Å3, Z = 2, R gt (F) = 0.0454, wRref(F2) = 0.1289, T = 297 K. CCDC no.: 2471389
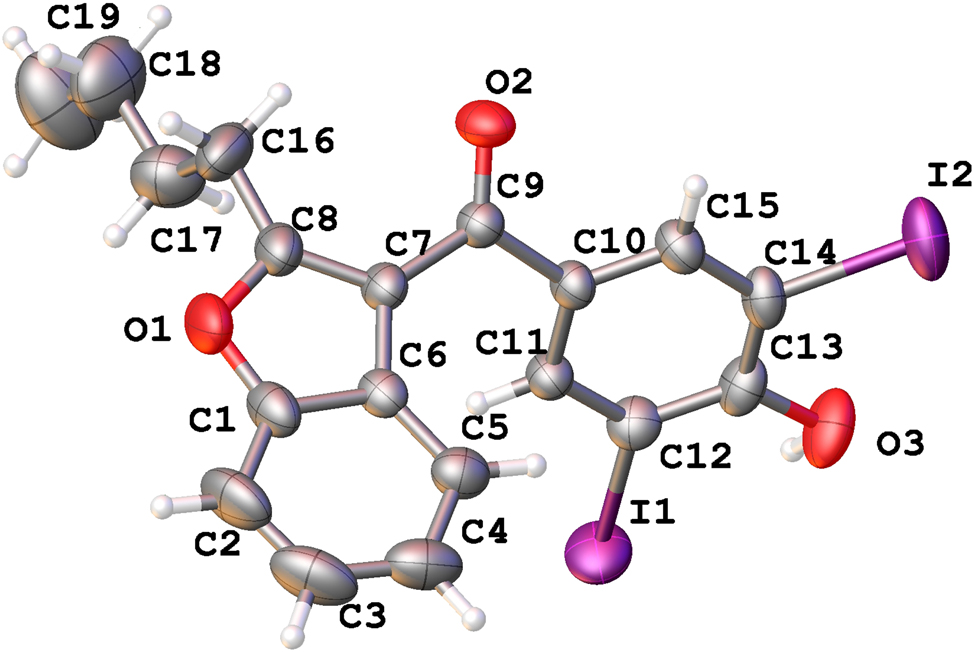
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Prism, colourless |
| Size: | 0.5 × 0.2 × 0.1 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 26.52 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω-scans |
| θmax, completeness: | 76.4°, > 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 17784, 3781, 0.046 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3,483 |
| N(param)refined: | 222 |
| Programs: | CrysAlis PRO , 1 Olex2, 2 Shelx 3 , 4 |
1 Source of materials
The title compound was bought from Guangzhou Dmstandards Co., Ltd. About 50 mg powder was placed into a 10 ml penicillin bottle, followed by addition of 5 ml ethanol. After all the powder was dissolved, the penicillin bottle was left open and placed in a fume hood. After 5 days, prism-shaped crystals were harvested.
2 Experimental details
The positions of hydrogen atoms on carbon atoms were calculated geometrically and refined using the riding model. Their coordinates and displacement parameters were constrained to ride on the carrier atom.
The hydrogen atom on oxygen atom was located from difference Fourier map inspection and refined with Uiso(H) = 1.5Ueq(O). The distance between the hydrogen atom and the oxygen atom was refined freely. A DFIX restrain was used to get a better model for the hydrogen atom.
3 Comment
Amiodarone has become an important drug for the treatment of supraventricular and ventricular arrhythmias, in short-term inpatient and outpatient settings. 5 The title compound is an impurity of the synthesis of amiodarone, 6 and this compound is usually controlled as a process impurity. 7 A certain inhibition effect of thyroxine deiodination is observed in this compound. 8
The titled compound was crystallized in P1̄ space group with one molecule in its asymmetric unit. The bond lengths and angles within these moieties are in the expected ranges. 9 The four atoms, including C7 and C10 attached to the carbonyl group, are coplanar. However, the benzofuran ring and benzene ring connected to the carbonyl group are not in the same plane. The dihedral angle between the benzofuran ring and the benzene ring can be represented by the torsion angle C6–C7–C9–C10, with a value of 39.4(6)°. The butyl group attached to the benzofuran ring adopts a typical all-trans conformation. The only hydroxyl group in the molecule forms an O2–H2⋯O31−x,1−y,1−z hydrogen bond with the carbonyl group of an adjacent molecule, and the donor acceptor length of this hydrogen bond is 3.027(6) Å. In addition to hydrogen bonding, π–π stacking interactions also could be observed, with centroid-centroid distance of 3.58 Å, and shift distance of 0.63 Å.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Discipline construction of Institute of Chemical Drugs, NIFDC (No.2025HYZX11).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction CrysAlisPRO Software System, Version 171.42.92a; Rigaku Corporation: Oxford, UK, 2023.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Lacrok, P. M.; Curran, N. M.; Sy, W. W.; Goreck, D. K.; Thibault, P.; Blay, P. K. Liquid Chromatographic Determination of Amiodarone Hydrochloride and Related Compounds in Raw Materials and Tablet. J. AOAC Int. 1994, 77, 1447–1453. https://doi.org/10.1093/jaoac/77.6.1447.Suche in Google Scholar
6. Karmarkar, S.; Yang, X.; Garber, R.; Szajkovics, A.; Koberda, M. Quality by Design (QbD) Based Development and Validation of an HPLC Method for Amiodarone Hydrochloride and its Impurities in the Drug Substance. J. Pharmaceut. Biomed. Anal. 2014, 100, 167–174. https://doi.org/10.1016/j.jpba.2014.07.002.Suche in Google Scholar PubMed
7. Ha, H.; Stieger, B.; Grassi, G.; Altorfer, H. R.; Follath, F. Structure-Effect Relationships of Amiodarone Analogues on the Inhibition of Thyroxine Deiodination. E. J. Clin. Pharmacol. 2000, 55, 807–814. https://doi.org/10.1007/s002280050701.Suche in Google Scholar PubMed
8. Goldschlager, N.; Epstein, A. E.; Naccarelli, G.; Olshansky, B.; Singh, B. Practical Guidelines for Clinicians who Treat Patients with Amiodarone. Arch. Intern. Med. 2000, 160, 1741–1748. https://doi.org/10.1001/archinte.160.12.1741.Suche in Google Scholar PubMed
9. Cody, V.; Luft, J. Structure-Activity Relationships of Antiarrhythmic Agents: Crystal Structure of Amiodarone Hydrochloride and Two Derivatives, and their Conformational Comparison with Thyroxine. Acta Crystallogr., Sect. B: Struct. Sci. 1989, 45, 172; https://doi.org/10.1107/S0108768188013126.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

