Abstract
C9H9BrO3, monoclinic, C2/c (no. 15), a = 19.8576(5) Å, b = 4.1080(2) Å, c = 23.1284(8) Å, β = 94.983(3)°, V = 1879.57(12) Å3, Z = 8, Rgt(F) = 0.0416, wRref(F2) = 0.1155, T = 297 K.
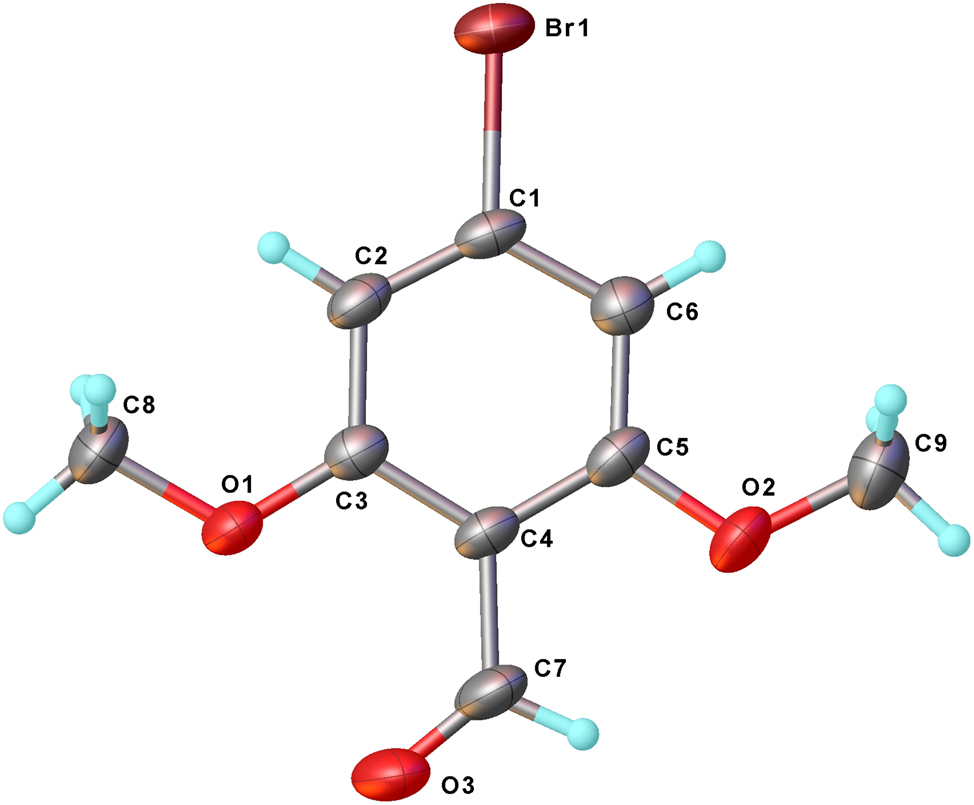
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Needle, colourless |
| Size: | 0.20 × 0.05 × 0.05 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 5.76 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω-scans |
| θmax, completeness: | 76.6°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7846, 1842, 0.087 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ (Iobs), 1,535 |
| N(param)refined: | 120 |
| Programs: | Rigaku 1 , OLEX2 2 , SHELX 3 , 4 |
1 Source of materials
The title compound was bought from Shanghai Bide Pharmatech Co., Ltd. About 20 mg powder was placed into a 10 ml penicillin bottle, followed by addition of 3 ml ethanol. After all the powder was disolved in ethanol, the penicillin bottle was left open and placed in a fume hood. After 5 days, needle-shaped crystals were harvested.
2 Experimental details
The positions of hydrogen atoms were calculated geometrically and refined using the riding model. Their coordinates and displacement parameters were constrained to ride on the carrier atom.
3 Comment
The title compound is a key intermediate in the synthesis of many bioactive molecules. 5 , 6 , 7
The titled compound was crystallized in C2/c space group with one molecule in its asymmetric unit. The bond lengths and angles within these moieties are in the expected ranges. 8 All non-hydrogen atoms are essentially coplanar. Only a weak hydrogen bond between C8–H8C and O31−x,1−y,1−z is observed with D⋯A length of 3.325(5) Å. Some π–π interaction exists between adjacent aromatic rings with centroid-to-centroid distance of 4.108(2) Å and interplanar distance of 3.5429(15) Å.
Funding source: Discipline construction of Institute of Chemical Drugs, NIFDC
Award Identifier / Grant number: 2025HYZX11
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: Discipline construction of Institute of Chemical Drugs, NIFDC (No. 2025HYZX11).
References
1. Rigaku Oxford Diffraction. CrysAlisPRO Software System, Version 1.171.42.92a; Rigaku Corporation: Oxford, UK, 2023.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Eakkaphon, R.; Jakkrit, S.; Sirikan, D.; Jakapun, S.; Sumrit, W.; Tirayut, V.; Thomayant, P. Development of In-Water Scalable Process for the Preparation of [2-(3-Bromo-2-methylphenyl)-7-chloro-1,3-benzoxazol-5-yl]methanol, A Key Intermediate in the Synthesis of Potent PD-1/PD-L1. Synlett 2025, 36, 363–368. https://doi.org/10.1055/s-0043–1774944.10.1055/s-0043-1774944Suche in Google Scholar
6. Zhang, F.; Lin, Y.; Min, W.; Hou, Y.; Yuan, K.; Wang, J.; Yang, P. Computational Discovery, Structural Optimization and Biological Evaluation of Novel Inhibitors Targeting Transient Receptor Potential Vanilloid Type 3 (TRPV3). Bioorg. Chem. 2021, 114, 105093. https://doi.org/10.1016/j.bioorg.2021.105093.Suche in Google Scholar PubMed
7. Huang, Q.; Pu, C.; Tan, L.; Wang, S.; Zhang, H.; Yu, S.; Deng, R.; Luo, D.; Ma, X.; Li, R. Synthesis and Biological Evaluation of a Novel c-Myc Inhibitor Against Colorectal Cancer via Blocking c-Myc/Max Heterodimerization and Disturbing its DNA Binding. Eur. J. Med. Chem. 2022, 243, 114779. https://doi.org/10.1016/j.ejmech.2022.114779.Suche in Google Scholar PubMed
8. Pramanik, S.; Mondal, P. P.; Maity, S. Organo-Photoredox-Catalyzed Selective Mono- and Bis-C-H Alkylation of Electron-Rich (Hetero)Arenes. Org. Chem. 2023, 88 (21), 15256–15269. https://doi.org/10.1021/acs.joc.3c01757.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

