Abstract
C19H16O6, triclinic, P
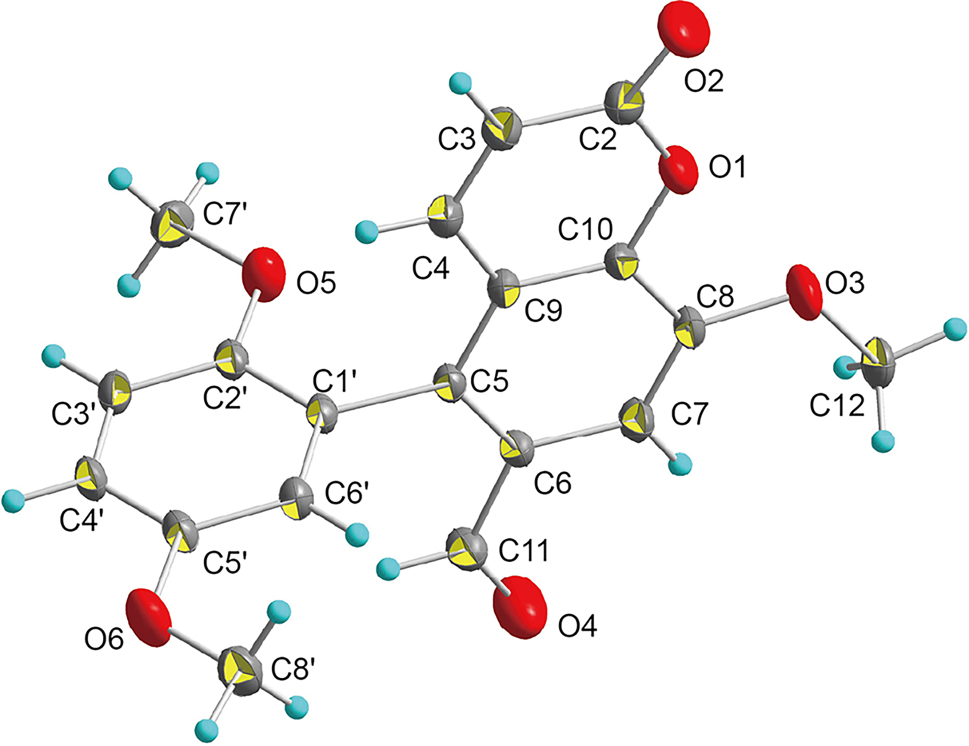
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear whiteish colourless block |
| Size: | 0.12 × 0.10 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.11 mm−1 |
| Diffractometer, scan mode: | Bruker APEX2, φ and ω scans |
| θmax, completeness: | 25.0°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 10386, 2769, 0.064 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1485 |
| N(param)refined: | 229 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of material
In a 250 ml sealed vial, 5-hydroxy-2′,4,5′-trimethoxy[1,1′-biphenyl]-2-carboxaldehyde (8 g, 1 eq), methyl acrylate (4.8 g, 2 eq), palladium(II) acetate (625 mg, 0.1 eq), 1,10-phenanthroline (1.1 g, 0.2 eq), copper(II) acetate (5.1 g, 1 eq), sodium acetate (6.9 g, 3 eq), and 1,2-dichloroethane (80 ml, 10 v/m) were sequentially added. The mixture was stirred at 135 °C for 72 h. After cooling to room temperature, the mixture was filtered, and the filtrate was concentrated. The residue was purified by silica gel column chromatography (petroleum ether:ethyl acetate = 5:1) to afford the desired product (1 g).
2 Experimental details
The hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
3 Comment
Coumarin is a class of natural organic compounds featuring a benzo-α-pyrone core scaffold, where its molecular structure consists of a benzene ring fused to a pyrone ring via a lactone bond, forming a rigid conjugated system. As key intermediates in the synthesis of heterocyclic compounds, the structural plasticity of coumarins enables them to serve as vital building blocks for constructing complex molecules. 5 Modern pharmacological studies have confirmed that coumarin derivatives exhibit various biological activities. These biological activities are closely related to their molecular structures; in particular, the type and position of substituents on the benzene ring significantly affect their pharmacological activities. The title compound, as shown in the figure, is a coumarin substituted with a dimethoxyphenyl group at the C5 and an aldehyde group at the C6.The position of the lactone group was confirmed by the distances d (C2–O1) = 1.381(4) Å and d (C2–O2) = 1.203(4) Å. Meanwhile, the position of the aldehyde group was confirmed by the distance d (C11–O3) = 1.206(4) Å. The structural characteristics of this target compound are similar to those of praecoxin. 9
Funding source: Guizhou University of Traditional Chinese Medicine National and Provincial Science and Technology Innovation Talent Team Cultivation Project
Award Identifier / Grant number: Guizhou University of Traditional Chinese Medicine TD NO.[2022]004
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 82160805
Funding source: Project supported by the Key Laboratory of Guizhou Provincial Education Department
Award Identifier / Grant number: Grant NO:[2023]017
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: Guizhou University of Traditional Chinese Medicine National and Provincial Science and Technology Innovation Talent Team Cultivation Project (TD NO.[2022]004); National Natural Science Foundation of China (82160805); Project supported by the Key Laboratory of Guizhou Provincial Education Department (Grant NO: [2023]017).
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
1. Bruker Smart and Saint; Bruker AXS Inc.: Madison, WI, USA, 2003.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341.10.1107/S0021889808042726Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, A64, 112–122.Suche in Google Scholar
4. Sheldrick, G. M. SHELXT – Integrated Space-group and Crystal. Acta Cryst. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
5. Malamati-Konstantina, K.; Dimitra, H. L. Coumarin Derivatives as Therapeutic Candidates: A Review of their Updated Patents (2017–present). Expert Opin. Ther. Pat. 2024, 34, 1231–1254; https://doi.org/10.1080/13543776.2024.2419827.Suche in Google Scholar PubMed
6. Gupta, D.; Guliani, E.; Bajaj, K. Coumarin-Synthetic Methodologies, Pharmacology, and Application as Natural Fluorophore. Top Curr Chem (Cham) 2024, 382, 16; https://doi.org/10.1007/s41061-024-00462-z.Suche in Google Scholar PubMed
7. Supuran, C. T. Coumarin Carbonic Anhydrase Inhibitors from Natural Sources. J. Enzyme Inhib. Med. Chem. 2020, 35, 1462–1470; https://doi.org/10.1080/14756366.2020.1788009.Suche in Google Scholar PubMed PubMed Central
8. Yadav, A. K.; Maharjan Shrestha, R.; Yadav, P. N. Anticancer Mechanism of Coumarin-Based Derivatives. Eur. J. Med. Chem. 2024, 267, 116179; https://doi.org/10.1016/j.ejmech.2024.116179.Suche in Google Scholar PubMed
9. Zhang, X. D.; Wu, D. W.; Zhang, L. K.; Zhang, H. Y.; Yang, L. P.; Wei, L.; Mei, H. M.; Luo, L. Y.; Jiang, Z.; Huang, C. Predicting the Potential Mechanism of Radix Chimonanthi Pracecocis in Treating Osteoarthritis by Network Pharmacology Analysis Combined with Experimental Validation. J. Ethnopharmacol. 2024, 331, 118231; https://doi.org/10.1016/j.jep.2024.118231.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

