Abstract
C11H10ClN5O3, triclinic, P1 (no. 1), a = 4.7400(3) Å, b = 7.3892(4) Å, c = 9.6994(5) Å, α = 77.2610(10)°, β = 77.4300(4)°, γ = 78.6260°, V = 319.42(3) Å3, Z = 1, Rgt(F) = 0.0274, wRref(F2) = 0.0743.
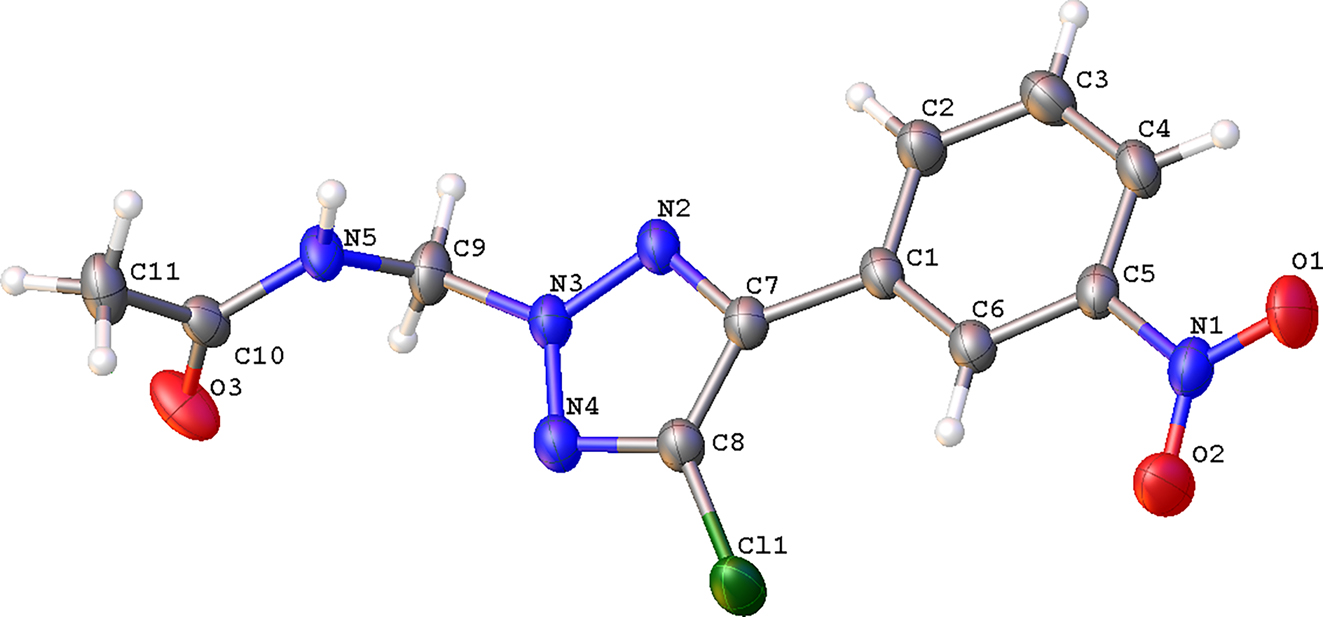
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.13 × 0.12 mm |
| Wavelength: | Ga Kα radiation (1.34139 Å) |
| μ: | 1.86 mm−1 |
| Diffractometer, scan mode: | Bruker PHOTON II, φ and ω scans |
| θmax, completeness: | 57.0°, 97 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6500, 2353, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2343 |
| N(param)refined: | 183 |
| Programs: | Bruker, 1 SHELX, 2 , 3 , 4 Diamond, 5 Olex2 6 |
1 Source of materials
In a 50 mL Schlenk tube, 0.11 g (0.454 mmol) of [4-(3-nitro-phenyl)-[1,2,3]triazol-2-yl]-methanol, 90.7 mg (0.682 mmol) of 1-chloropyrroline-2,5-dione, and 14.89 mg (0.091 mmol) of 2,2-azobisisobutyronitrile were added, followed by 6.0 mL of acetonitrile. The reaction was carried out at 110° for 20 h. The resulted mixture was cooled to room temperature and the solvent was removed under reduced pressure to obtain crude product NTA. 7 The crude product was eluted with a mixed solvent of petroleum ether and ethyl acetate (V/V = 4:1) and subjected to silica gel column chromatography to obtain the titled compound as a white solid (86.1 mg, yield: 55 %). Crystals of the titled compound were grown through slow evaporation of a methanol/dichloromethane mixed solution (V/V = 1:1) at 25 °C for five days.
2 Experimental details
H atoms bonded to C atoms were located in difference maps and subsequently treated as riding, with C–H distances of 0.95 Å (aromatic), 0.99 Å (methylene) and 0.98 Å (methyl), respectively. Uiso(H) = 1.2Ueq (aromatic and methylene C) and 1.5Ueq (methyl C). H atoms bonded to N atoms can be also initially found in difference maps and then restrained to be at their ideal positions with Uiso(H) = 1.2Ueq = (N5).
3 Comments
2,4-disubstituted-1,2,3-triazole derivatives have a wide range of biological activities. 8 , 9 Introducing some certain amide or hydrazide substructures on the 1,2,3-triazole ring is one of the approaches to developing novel 1,2,3-triazole antibacterial agents. As a continued study of one of our previous works, 10 , 11 we have synthesized a new triazole functionalized compound, N-((4-chloro-5-(3-nitrophenyl)-2H-1,2,3-triazol-2-yl)methyl)acetamide (NTA).
In the asymmetric unit of the title structure, there is one discrete NTA molecule. The nitrobenzene ring and the triazole ring are almost coplanar. Among all the atoms constituting the plane, the O1 atom is the farthest from this plane with a distance of merely 0.26 Å. The plane formed by the four atoms of the acetamide (N5/O3/C10/C11) is almost perpendicular to the plane discussed above with a dihedral angle 85.78(1)° between them. This should be attributed to the formation of intermolecular hydrogen bonds in which the acetamide N5 atom acts as a donor being hydrogen-bonded to the O3 at (x−1, y, z) with a N5⋯O3 distance of 2.821(2) Å. Apart from this distinction, the bond lengths and bond angles are comparable to some analogous. 12
In the crystal packing, except for the above mentioned intermolecular N5⋯O3 hydrogen bonds, there is another intermolecular π⋯π stacking interaction, further stablizing the crystal structure. In more details, the interaction exists between the benzene ring and the adjacent triazole ring with the centroid-to-centroid distance being 3.742(2) Å, further forming a one-dimensional array along the [100] direction. 7 , 13
Acknowledgments
This work is supported by the Key Laboratory Project of Huaihua (NO.2023R2206).
References
1. Bruker Smart and Saint; Bruker AXS Inc.: Madison, WI, USA, 2003.Suche in Google Scholar
2. Sheldrick, G. M. Shelxtl Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/S2053273314026370.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Refinement with Shelx. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/S0108767307043930.Suche in Google Scholar PubMed
5. Brandenburg, K. Diamond; Crystal Impact GbR: Bonn Germany, 2006.Suche in Google Scholar
6. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. https://doi.org/10.1107/S0021889808042726.10.1107/S0021889808042726Suche in Google Scholar
7. Deng, X.; Lei, X.; Nie, G.; Jia, L.; Li, Y.; Chen, Y. Copper-Catalyzed Cross-Dehydrogenative N2-Coupling of NH-1,2,3-Triazoles with N,N-Dialkylamides: N-Amidoalkylation of NH-1,2,3-Triazoles. J. Org. Chem. 2017, 82, 6163–6171. https://doi.org/10.1021/acs.joc.7b00752.Suche in Google Scholar PubMed
8. Aruri, H.; Singh, U.; Kumar, M.; Sharma, S.; Aithagani, S. K.; Gupta, V. K.; Mignani, S.; Vishwakarma, R. A.; Singh, P. P. Metal-Free Cross-Dehydrogenative Coupling of HN-Azoles with Alpha-C(sp3)-H Amides via C–H Activation and its Mechanistic and Application Studies. J. Org. Chem. 2017, 82, 1000–1012. https://doi.org/10.1021/acs.joc.6b02448.Suche in Google Scholar PubMed
9. Yan, W.; Wang, Q.; Lin, Q.; Li, M.; Petersen, J. L.; Shi, X. N-2-Aryl-1,2,3-Triazoles: A Novel Class of UV/Blue-Light-Emitting Fluorophores with Tunable Optical Properties. Chem. Eur. J 2011, 17, 5011–5018. https://doi.org/10.1002/chem.201002937.Suche in Google Scholar PubMed
10. Li, Y.; Lei, S.; Liu, Y. Design, Synthesis and Fungicidal Activities of Novel 1,2,3-Triazole Functionalized Strobilurins. Chem. Select 2019, 4, 1015–1018. https://doi.org/10.1002/slct.201803597.Suche in Google Scholar
11. Wang, L.; Lei, S.; Li, Y., The Crystal Structure of 4,4′-dichloro-3,5′-diphenyl-1′H-1,3′-bipyrazole, C18H12Cl2N4. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 249–252. https://doi.org/10.1515/ncrs-2022–0576.10.1515/ncrs-2022-0576Suche in Google Scholar
12. Liu, Y.; Yan, W.; Chen, Y.; Petersen, J. L.; Shi, X. Efficient Synthesis of N-2-aryl-1,2,3-triazole Fluorophores via Post-Triazole Arylation. Org. Lett. 2008, 10, 5389–5392. https://doi.org/10.1021/ol802246q.Suche in Google Scholar PubMed
13. Spek, A. L. Structure Validation in Chemical Crystallography. Acta Crystallogr. 2009, D65, 148–155. https://doi.org/10.1107/S090744490804362X.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

