Abstract
C19H15FFeO, orthorhombic, P212121 (no. 19), a = 5.9299(10) Å, b = 11.0947(16) Å, c = 22.793(4) Å, V = 1499.5(4) Å3, Z = 4, R gt(F) = 0.0337 wR ref (F 2) = 0.0822, T = 260 K.
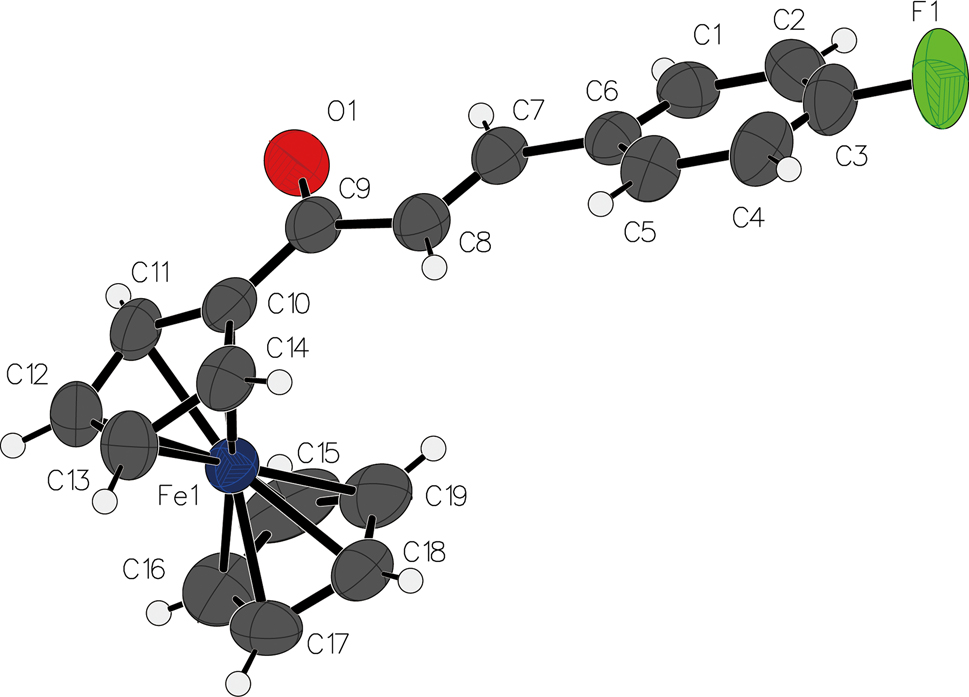
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Darkred block |
| Size: | 0.16 × 0.15 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.01 mm−1 |
| Diffractometer, scan mode: | Bruker APEX2, φ and ω scans |
| θ max, completeness: | 28.4°, 100 % |
| N(hkl)measured , N(hkl)unique, R int: | 44139, 3741, 0.127 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3048 |
| N(param)refined: | 200 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
1 Source of materials
Single crystals suitable for X-ray diffraction analysis were obtained by slow evaporation of a solution of (E)-(3-(4-fluorophenyl)acryloyl)ferrocene, which was purchased from Aladdin, in a mixture of dichloromethane and n-hexane (approximately 1:2 v/v) at room temperature over a period of one week. Over a period of some days, well-formed, dark red, block crystals suitable for single-crystal X-ray diffraction analysis had deposited at the interface of the two solvents.
2 Experimental details
The single-crystal X-ray diffraction experiment was conducted at room temperature using a Bruker D8 Venture diffractometer with MoKα radiation. 1 The structure was solved with ShelXT 2 and refined in ShelXL, 3 both implemented within the Olex2 interface. 4 Hydrogen atoms were placed in idealized positions and refined using a riding model with isotropic displacement parameters.
3 Comment
Ferrocene-based compounds have attracted sustained research interest due to their unique electrochemical properties, structural versatility, and widespread applications in catalysis, materials science, and medicinal chemistry. 5 , 6 The introduction of a conjugated enone bridge functionalized with an electron-withdrawing group, such as a 4-fluorophenyl moiety, is known to enhance molecular polarization and offers potential for non-linear optical behavior. Determining the precise molecular and supramolecular structure through single-crystal X-ray diffraction is crucial for understanding the relationship between the structure and physicochemical properties.
The molecular structure of (E)-(3-(4-fluorophenyl)acryloyl)ferrocene reveals a nearly planar conformation of the enone bridge [O=C–C=C], which adopts a typical trans (E) configuration. The dihedral angle of O1–C9–C8–C7 is only 4.5°. The ferrocene unit exhibits slightly tilted cyclopentadienyl (Cp) rings, with the substituted Cp ring rotated to maximize conjugation with the acryloyl chain. 7 , 8 , 9 , 10 The dihedral angle between this Cp ring and the fluorophenyl ring is minimal, indicating effective electronic communication across the entire π-system, a feature critical for its electronic and redox properties. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
In the crystal lattice, molecules are primarily connected through weak intermolecular vdW interactions. These supramolecular features, combined with the inherent dipole moment from the polarized C=O bond and electron-withdrawing fluorine atom, facilitate a well-organized and stable crystal packing.
Funding source: Natural Science Foundation of Shaanxi Province
Award Identifier / Grant number: 2025JC–YBMS-888
Award Identifier / Grant number: 2025JC–YBMS-1084
Funding source: Shaanxi Provincial Department of Education
Award Identifier / Grant number: 24JS004
Funding source: Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city
Award Identifier / Grant number: 2021QXNL–PT-0008
Funding source: School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules
Award Identifier / Grant number: 2024KCTD04
Acknowledgments
This work was financially supported by the projects of Natural Science Foundation of Shaanxi Province (2025JC–YBMS-888, 2025JC–YBMS-1084); The 2024 Key Scientific Research Program Projects of the Shaanxi Provincial Department of Education (Key Laboratory Projects, 24JS004); Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL–PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. Bruker. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2012.Suche in Google Scholar
2. Sheldrick, G. M. Shelxt-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Verma, R. K.; Verma, G. K.; Shukla, G.; Singh, M. S. InCl3 Catalyzed Domino Route to 2H-Chromene-2-ones via [4+2] Annulation of 2-Hydroxyarylaldehydes with α-Oxoketene Dithioacetal Under Solvent-Free Conditions. RSC Adv. 2012, 2, 2413–2421; https://doi.org/10.1039/c2ra00987k.Suche in Google Scholar
6. Allison, M.; Wilson, D.; Pask, C. M.; McGowan, P. C.; Lord, R. M. β-Diketonate Versus β-Ketoiminate: The Importance of a Ferrocenyl Moiety in Improving the Anticancer Potency. ChemBioChem 2020, 21, 1988–1996; https://doi.org/10.1002/cbic.202000028.Suche in Google Scholar PubMed PubMed Central
7. Celedón, S.; Fuentealba, M.; Roisnel, T.; Ledoux-Rak, I.; Hamon, J.-R.; Carrillo, D.; Manzur, C. Side-Chain Metallopolymers Containing Second-Order NLO-active Bimetallic NiII and PdII Schiff-Base Complexes: Syntheses, Structures, Electrochemical and Computational Studies. Eur. J. Inorg. Chem. 2016, 2016, 3012–3023; https://doi.org/10.1002/ejic.201600236.Suche in Google Scholar
8. Halder, B.; Dewangan, S.; Barik, T.; Mishra, A.; Dhiman, R.; Chatterjee, S. Solid Supported Synthesis of Unsymmetrical Bi-Functionalized Ferrocenyl-Rhodaminyl Molecular System to Explore Phosgene, Heavy Metal Ion Sensing, and Cell Imaging Properties. J. Organomet. Chem. 2022, 972, 122369; https://doi.org/10.1016/j.jorganchem.2022.122369.Suche in Google Scholar
9. Artigas, V.; González, D.; Fuentealba, M. Syntheses, Characterisation and Crystal Structures of Ferrocenyl β-Diketones and Their Schiff Base NNO Ligand Derivatives with 2-picolylamine. J. Mol. Struct. 2017, 1129, 325–332; https://doi.org/10.1016/j.molstruc.2016.09.009.Suche in Google Scholar
10. Jin, Z.; Huo, A.; Liu, T.; Hu, Y.; Liu, J.; Fang, J. Synthesis, Structures and Biological Activity Research of Novel Ferrocenyl-Containing 1H-1,2,4-Triazole Derivatives. J. Organomet. Chem. 2005, 690, 1226–1232; https://doi.org/10.1016/j.jorganchem.2004.11.028.Suche in Google Scholar
11. Novoa, N.; Roisnel, T.; Dorcet, V.; Hamon, J.-R.; Carrillo, D.; Manzur, C.; Robin-Le Guen, F.; Cabon, N. Anisyl and Ferrocenyl Adducts of Methylenepyran-Containing β-Diketone: Synthesis, Spectral, Structural, and Redox Properties. J. Organomet. Chem. 2014, 762, 19–28; https://doi.org/10.1016/j.jorganchem.2014.03.029.Suche in Google Scholar
12. Allison, M.; Caramés-Méndez, P.; Pask, C. M.; Phillips, R. M.; Lord, R. M.; McGowan, P. C. Bis(Bipyridine)Ruthenium(II) Ferrocenyl β-Diketonate Complexes: Exhibiting Nanomolar Potency Against Human Cancer Cell Lines. Chem.-Eur. J. 2021, 27, 3737–3744; https://doi.org/10.1002/chem.202004024.Suche in Google Scholar PubMed
13. Zhang, J.; Hu, X.; Liu, X.; He, Y. The Crystal Structure of (E)-1-Ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 437–439; https://doi.org/10.1515/ncrs-2022-0063.Suche in Google Scholar
14. Zhang, L.; Xie, M. The Crystal Structure of (E)-3-(2-Chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 531–533; https://doi.org/10.1515/ncrs-2022-0042.Suche in Google Scholar
15. Long, S.-J.; Liu, X.-L.; Liu, Y.-H. (E)-1-Ferrocenyl-3-(4-methoxyphenyl) Prop-2-en-1-One. Acta Crystallogr. E 2008, 64, m1164; https://doi.org/10.1107/s1600536808025518.Suche in Google Scholar
16. Celedón, S.; Hamon, P.; Artigas, V.; Fuentealba, M.; Kahlal, S.; Carrillo, D.; Saillard, J.-Y.; Hamon, J.-R.; Manzur, C. Ferrocene Functionalized Enantiomerically Pure Schiff Bases and Their Zn(II) and Pd(II) Complexes: A Spectroscopic, Crystallographic, Electrochemical and Computational Investigation. New J. Chem. 2022, 46, 3948–3960; https://doi.org/10.1039/d1nj06106b.Suche in Google Scholar
17. Delavaux-Nicot, B.; Maynadié, J.; Lavabre, D.; Lepetit, C.; Donnadieu, B. The First X-ray Characterized Monosubstituted Ferrocenyl Azacrown Chalcone: Focus on its Calcium Interaction/Electrochemical Detection Studies. Eur. J. Inorg. Chem. 2005, 2005, 2493–2505; https://doi.org/10.1002/ejic.200400781.Suche in Google Scholar
18. Zhang, S.; Gao, J.; Wen, J.; Gao, Y.; Liu, B. Crystal Structure of (E)-(3-(2,4-Dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO. Z. Kristallogr. - N. Cryst. Struct. 2025, 240, 535. https://doi.org/10.1515/ncrs-2025–0121.10.1515/ncrs-2025-0121Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

