Abstract
C34H50Cl2N6O10Ni, triclinic,
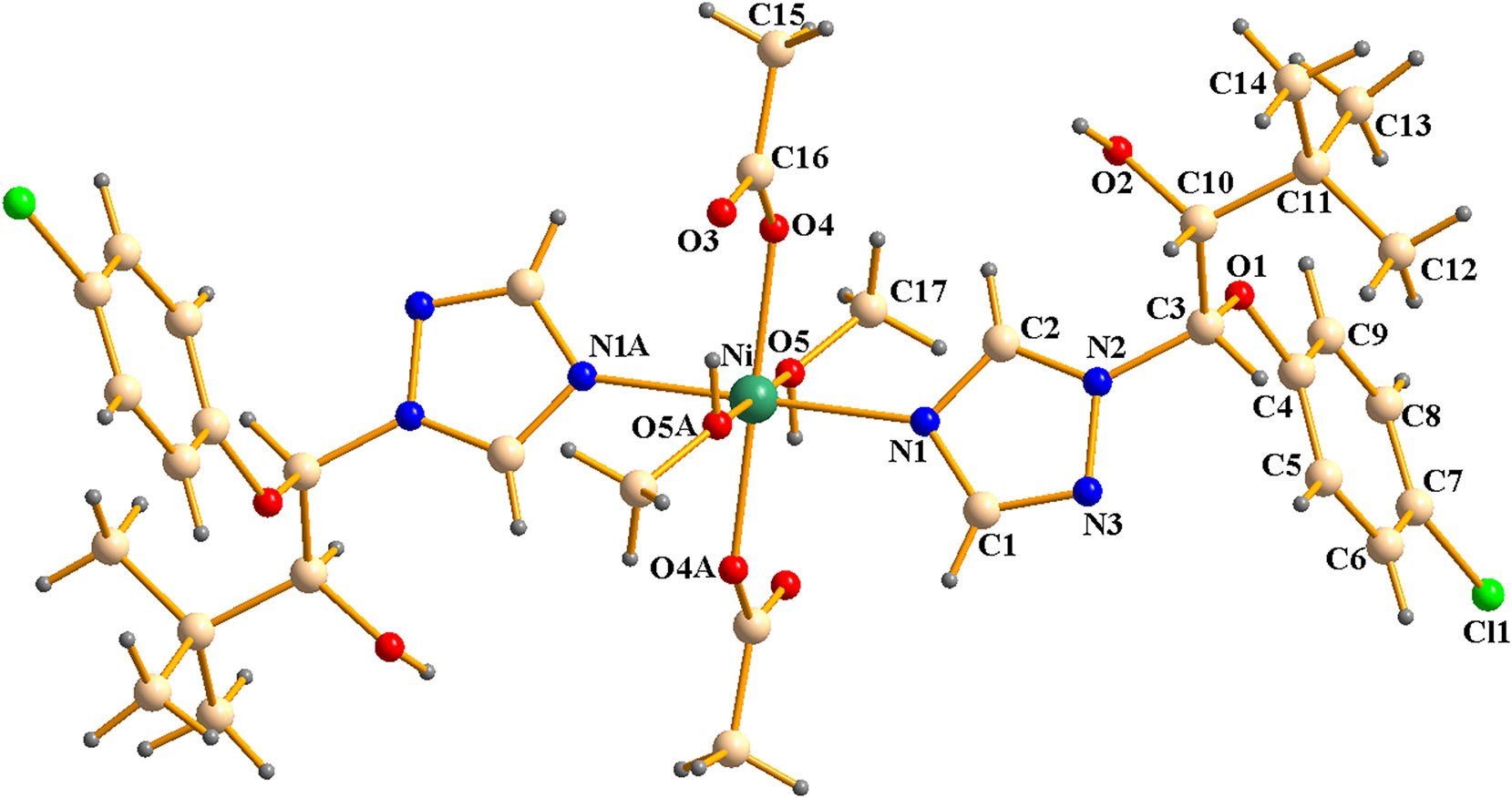
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Blue block |
| Size: | 0.18 × 0.16 × 0.12 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.66 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker APEX-II, φ and ω scans 27.8°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6079, 4,445, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,677 |
| N(param)refined: | 265 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Diamond, 4 Olex2 5 |
1 Source of material
The compound 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl) butan-2-ol (0.2958 g, 1.00 mmol) was dissolved in methanol (10 mL). To this solution was added nickel(II) acetate tetrahydrate (0.1244 g, 0.5 mmol). The mixture was stirred at room temperature for 2 h; blue block crystals of the target complex were subsequently obtained by slow evaporation of the solvent from ethanol.
2 Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms, with C–H = 0.96 Å (methyl), Uiso (H) = 1.5 Ueq(C), C–H = 0.98 Å (methine), Uiso(H) = 1.2 Ueq(C), C–H = 0.93 Å (aromatic and alkenyl), Uiso(H) = 1.2 Ueq(C), and O–H = 0.82 Å (hydroxyl), Uiso(H) = 1.5 Ueq(O).
3 Comment
Fungi are major causative agents of severe infectious diseases in plants worldwide, accounting for the most significant economic losses among crop pathogens. 6 , 7 To combat fungal infections, 1,2,4-triazole fungicides are widely employed. 8 However, their extensive use has led to the rapid development of resistant strains. 9 , 10 In an effort to improve bioactivity and mitigate resistance, strategies such as coordinating these fungicides with metal salts have been investigated. 11 , 12 , 13 Triadimenol (1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol) is a highly effective 1,2,4-triazole fungicide known for its low toxicity, limited residue, and sustained protective activity. It exhibits broad efficacy against powdery mildew, rust, leaf spots, and anthracnose. 13 This paper reports the synthesis and characterization of a novel Ni(II) complex derived from triadimenol.
As shown in the figure, the central Ni(II) ion adopts a six-coordinated octahedral geometry. The coordination sphere comprises two triadimenol ligands, each coordinated through a nitrogen atom (N) from the triazole ring; two coordinated methanol molecules, each bound via an oxygen atom; and two acetate ions, each acting as a monodentate ligand. The triazole rings are coplanar with their respective symmetry planes and are approximately perpendicular to the attached benzene rings, with a dihedral angle of 76.6°. The Ni–N bond length (2.073 Å) and Ni–O bond lengths (2.060 Å, 2.088 Å) are consistent with reported values for similar coordination bonds. 14 In the crystal structure, one type of intermolecular hydrogen bond is observed: atom O2 acts as a hydrogen bond donor to atom O3 of a symmetry-related molecule (symmetry code: (i) −x + 1, −y + 1, −z + 1), forming an O2–H2⋯O3 i interaction. These hydrogen bonds link the molecules into one-dimensional chains. Further stabilization of the crystal architecture is achieved through van der Waals interactions, which connect these chains into a three-dimensional framework.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: The work was supported by National Natural Science Foundation of China (22003055) and Nanhu Scholars Program for Young Scholars of the Xinyang Normal University.
References
1. Bruker. Smart and Saint; Bruker AXS Inc.: Madison, WI, USA, 2003.Suche in Google Scholar
2. Sheldrick, G. M. Shelxtl Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/S2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Brandenburg, K. Diamond; Crystal Impact GbR: Bonn Germany, 2006.Suche in Google Scholar
5. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/S0021889808042726.Suche in Google Scholar
6. Salvatore, M. M.; Andolfi, A. Phytopathogenic Fungi and Toxicity. Toxins 2021, 13, 689; https://doi.org/10.3390/toxins13100689.Suche in Google Scholar PubMed PubMed Central
7. Knogge, W. Fungal Infection of Plants. Plant Cell 1996, 8, 1711–1722; https://doi.org/10.1105/tpc.8.10.1711.Suche in Google Scholar PubMed PubMed Central
8. Song, S. S.; Yu, Q.; Yuan, L. W.; Anwar, W.; Li, Q.; Hao, Q.; Wu, G. L.; Li, Y.; Lai, Y. S. Absorption, Translocation, and Accumulation of the Fungicide Triadimefon in Pak Choi (Brassica rapa var Chinensis), Pepper (Capsicum Annuum), and Cucumber (Cucumis sativus). Environ. Monit. Assess 2023, 195, 1235; https://doi.org/10.1007/s10661-023-11842-1.Suche in Google Scholar PubMed
9. Cui, N.; He, Y. W.; Yao, S. J.; Zhang, H. C.; Ren, J. B.; Fang, H.; Yu, Y. L. Tebuconazole Induces Triazole–Resistance in Aspergillus fumigatus in Liquid Medium and Soil. Sci. Total Environ. 2019, 648, 1237–1243; https://doi.org/10.1016/j.scitotenv.2018.08.247.Suche in Google Scholar PubMed
10. Wang, Y.; Zheng, C.; Qiu, M.; Zhang, L.; Fang, H.; Yu, Y. Tebuconazole Promotes Spread of a Multidrug-Resistant Plasmid into Soil Bacteria to form New Resistant Bacterial Strains. Sci. Total Environ. 2024, 928, 172444; https://doi.org/10.1016/j.scitotenv.2024.172444.Suche in Google Scholar PubMed
11. Li, J.; Ren, G. Y.; Zhang, Y.; Yang, M. Y.; Ma, H. X. Two Cu (II) Complexes of 1, 2, 4-triazole Fungicides with Enhanced Antifungal Activities. Polyhedron 2019, 157, 163–169; https://doi.org/10.1016/j.poly.2018.09.052.Suche in Google Scholar
12. Li, J.; Pei, X. Y. Experimental and Theoretical Study on the Enhanced Antifungal Activities of Tebuconazole After Complexation with Two Zinc Salts. Inorg. Chim. Acta 2025, 574, 122397; https://doi.org/10.1016/j.ica.2024.122397.Suche in Google Scholar
13. Li, J.; Liu, H. Y.; Yang, M. Y.; Song, J. R.; Ma, H. X. Two Cu(II)-triadimenol Complexes as Potential Fungicides: Synergistic Actions and DFT Calculations. RSC Adv. 2018, 8, 2933–2940; https://doi.org/10.1039/c7ra10572j.Suche in Google Scholar PubMed PubMed Central
14. Sanjeev, K.; Jai, D.; Amit, D.; Deepak, K.; Deepak, J.; Sonika, A.; Archana, S. Co(II), Ni(II), Cu(II) and Zn(II) Complexes of Schiff Base Ligands: Synthesis, Characterization, DFT, in Vitro Antimicrobial Activity and Molecular Docking Studies. Res. Chem. Intermed. 2023, 49, 939; https://doi.org/10.1007/s11164-022-04941-0.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

