Abstract
C20H23NO2, monoclinic, P21/n (no. 14), a = 9.58280(10) Å, b = 23.1183(2) Å, c = 23.4140(2) Å, β = 91.9840(10)°, V = 1522.93(11) Å3, Z = 12, R gt (F) = 0.0468, wR ref (F2) = 0.1207, T = 150 K.
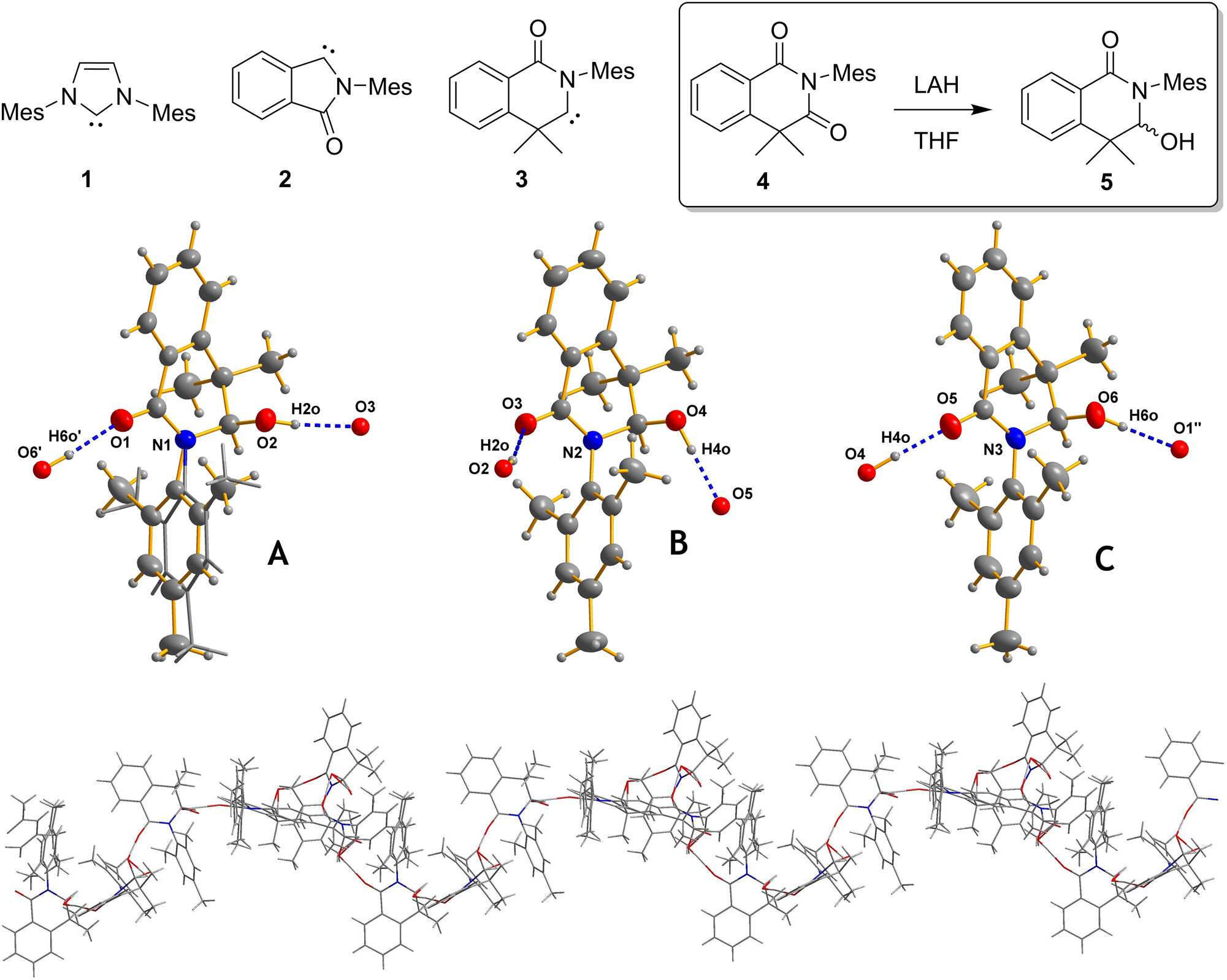
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless stick-shaped |
| Size: | 0.45 × 0.18 × 0.14 mm |
| Wavelength: μ: |
CuKα radiation (1.54184 Å) 0.60 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Excalibur, ω scan 75.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 59290, 10394, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 9,125 |
| N(param)refined: | 709 |
| Programs: | CrysAlis PRO , 1 SHELX, 2 , 3 Diamond 4 |
1 Source of materials
Under nitrogen, a solution of 2-mesityl-4,4-dimethylisoquinoline-1,3(2H,4H)-dione 4 (2.00 g, 6.51 mmol) in 20 mL tetrahydrofuran was added dropwise to a stirred suspension of lithium aluminium hydride (493 mg, 12.99 mmol) in 20 mL of tetrahydrofuran at 0 °C over a period of 5 min. The cooling bath was removed and the reaction mixture was stirred at ambient temperature for 5 h. The mixture was again cooled to 0 °C and quenched by cautious dropwise addition of 0.5 mL water, followed by 0.5 mL 15 % aqueous NaOH solution, and finally 1.5 mL of water, all while maintaining the temperature of 0 °C. The mixture was stirred for additional 15 min at ambient temperature. 5.0 g of anhydrous magnesium sulfate was then added, and stirring was continued for another 15 min. The resulting suspension was filtered through a G3 frit and the solid was washed two times with 10 mL of THF each. Removal of the solvent in vacuo yielded the reduction product 5 (1.6 g, 5.21 mmol, 80 %) as a colorless solid.
2-Mesityl-4,4-dimethylisoquinoline-1,3(2H,4H)-dione (4): 1 H NMR (300 MHz, CDCl3) δ 8.29 (dd, J = 7.9, 1.5 Hz, 1H), 7.70 (td, J = 7.6, 7.6, 1.5 Hz, 1H), 7.55 (dd, J = 8.0, 1.5 Hz, 1H), 7.48 (td, J = 7.6, 7.6, 1.2 Hz,1H), 6.98 (s, 2H), 2.32 (s, 3H), 2.03 (s, 6H), 1.75 (s, 6H) ppm. 13 C{1H} NMR (75.48 MHz, CDCl 3 ) δ 160.7, 147.3, 138.3, 136.3, 134.3, 134.2, 129.6, 129.5, 127.6, 125.7, 123.9, 50.4, 35.0, 21.3, 17.6 ppm. Elemental analysis calculated for C20H21NO2: C 78.15 %, H 6.89 %, N 4.56 %, found: C 77.83 %, H 6.79 %, N 4.63 %.
3-Hydroxy-2-mesityl-4,4-dimethyl-3,4-dihydroisoquinolin-1(2H)-one (5): 1 H NMR (600 MHz, CDCl 3 ): δ 8.19 (d, J = 7.7 Hz, 1H), 7.56 (td: J = 7.6, 7.6, 1.5 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 7.40 (td, J = 7.5; 7.5; 1.2 Hz, 1H), 6.94 (s, 1H), 4.86 (s,1H), 2.47 (s, 1H) 2.30 (s, 3H), 2.26 (s, 3H), 2.18 (s, 3H,), 1.53 (s, 3H), 1.52 (s, 3H) ppm. 13 C{1H}-NMR (151 MHz, CDCl 3 ): δ 163.17, 144.40, 137.86, 137.77, 135.69, 135.46, 132.91, 130.02, 129.96, 129.09, 127.65, 127.25, 124.58, 89.59, 40.25, 30.67, 23.48, 21.05, 19.52, 19.31 ppm. Elemental analysis calculated for C20H23NO2: C 77.64 %, H 7.49 %, N 4.53 %, found: C 77.76 %, H 7.56 %, N 4.51 %.
2 Experimental details
The diffraction data were collected using a standard schedule on a RIGAKU Synergy four-circle diffractometer. 1 The structure solution and refinement were performed using the SHELX program system. 2 , 3 Hydrogen atoms were introduced using AFIX constraints of the SHELX system. Their Uiso values were coupled with those of the corresponding parent atoms. The figure was created using the DIAMOND software. 4 There are three crystallographically independent molecules in the title structure (see the figure).
A disorder refinement of the mesityl moiety of the molecule A (N1, O1, O2; left side of the figure) was necessary because of the disorder situation. The refined ratio of this orientational disorder shows a ratio of 0.571(9)/0.429(9). This molecule faces no additional disorder. To stabilize the refinement of the displacement parameters of this mesityl group, the SHELX cards ISOR and RIGU as well as an AFIX 66 card for the six-membered rings were applied. 3
For the two other crystallographically independent molecules B and C (middle and right part of the figure) a disorder of the hydroxy group needs to be assumed based on the difference electron density maps, geometric parameters and the refinement result (the minor occupation is not shown for clarity). In both cases of B and C the disorder creates an inverted configuration at the responsible carbon site. In the centrosymmetric space group of the title structure the racemic situation doesn’t change. For molecule B a ratio of 0.731(2)/0.269(2) and for molecule C a ratio of 0.889(2)/0.111(2) was obtained in the least-squares refinement process.
Alternatively an inclusion of some keto-starting material 4 has been discussed. Because the NMR-spectra didn’t show any signals of the starting material 4 after the reaction this possibility was ruled out, even though the structure refinement didn’t show significant differences to this refinement with a disorder of the C-OH moiety in molecule B and C. Finally the electron density map shows only small peaks with a maximum of 0.27 e/Å3 and a minimum of −0.24 e/Å3 documenting the success of the structure refinement.
3 Introduction
N-heterocyclic carbenes (NHCs) have long played a pivotal role in chemical research, particularly as ligands for metal organic catalysis, where their unique electronic properties enable a variety of transformations. Imidazolidinylidenes represent by far the most common structural motif among NHCs with IMes 1 being an iconic example. 5 Structural modifications include the ring size of the heterocycle, the nature of N-substituents and the introduction of functional groups. 6 For example, in the NHC 2 the carbene carbon atom is connected to an aryl moiety and an electron withdrawing amide group. 7 In the course of our investigations of NHCs with enhanced electrophilicity, 8 , 9 we identified NHC 3 as an interesting synthetic target. A key step on the way to 3 is the reduction of one keto group in the isoquinoline-1,3-dione 4 with lithiumaluminiumhydride to the hydroxy compound 5. We report here about the attempted crystal structure determination of the hydroxy compound 5.
4 Structural description and discussion
The title crystal structure is a Z′ = 3 structure with three molecules that face different disorder situations in each of the three independent molecules A–C. All bond lengths and angles as well as other geometric parameters are in the expected ranges. 10 , 11 As depicted in the figure, all crystallographically independent molecules are very similar. Finally it should be mentioned that the mean planes of the mesityl moieties are in all cases almost perpendicular to a plane defined by the nitrogen atom and the neighboring carbon atoms within the five-membered ring of each molecule.
The molecules of 3-hydroxy-2-mesityl-4,4-dimethyl-3,4-dihydroisoquinolin-1(2H)-one (5) form hydrogen-bonded curved chains along (10–1) with a repeated molecular sequence ⋯A–B–C–A′⋯ (see the lower part of the figure). Even though the molecular shapes of all thee crystallographically independent 3-hydroxy-2-mesityl-4,4-dimethyl-3,4-dihydroisoquinolin-1(2H)-one molecules is very similar, their connection to neighboring molecules is obviously different in order to fulfil the needs of sterical hindrance, hydrogen bonding and packing in general (see lower an middle part of the figure). The O⋯O distances within the O–H⋯O hydrogen bonds range from 2.6749(18) Å to 2.7469(17) Å. Therefore they can be classified as medium strong. In detail the donor-acceptor distances are: O2–H2O⋯O3 = 2.7208(16) Å, O4–H4O⋯O5 2.6748(18) Å, O6′–H6O′⋯O1: 2.7470(17) Å (−1/2 + x, 1/2 − x, 1/2 + z) (see the figure; ″ = x + 1/2, −y + 1/2, z − 1/2′).
5 Summary
We present here a rare structural scenario of a Z′ = 3 structure, which faces additional crystallographic issues for each of the three crystallographically independent molecules in the centrosymmetric space group P21/n, which enlarges the number of geometrically different molecules in the title crystal structure. In a related structure with a very high Z′, conformational flexible molecules have been reported, along with a systematic disorder that further increases the number of geometrically distinct species. 12
Acknowledgments
We gratefully acknowledge support by the Ministry of Innovation, Science and Research of North–Rhine Westphalia and the German Research Foundation (DFG) for financial support (Rigaku Synergy diffractometer; Grant 440366605).
References
1. Oxford Diffraction Ltd, CrysAlisPRO: Abingdon, Oxfordshire, England, 2024.Suche in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Hopkinson, M. N. C.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-heterocyclic Carbenes. Nature 2014, 510, 485–496; https://doi.org/10.1038/nature13384.Suche in Google Scholar PubMed
6. Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; Cesar, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733; https://doi.org/10.1021/cr100328e.Suche in Google Scholar PubMed
7. Sultane, P. R.; Ahumada, G.; Janssen-Müller, D.; Bielawski, C. W. Cyclic (Aryl)(Amido)Carbenes: NHCs with Triplet-like Reactivity. Angew. Chem., Int. Ed. 2019, 58, 16320–16325; https://doi.org/10.1002/anie.201910350.Suche in Google Scholar PubMed
8. Karl, L.; Deißenbeck, D.; Meisner, J.; Ganter, C. β-Lactam Ylidenes: An Overlooked Class of N-Heterocyclic Carbenes. Chem. Eur. J. 2025, 31, e202501320; https://doi.org/10.1002/chem.202501320.Suche in Google Scholar PubMed PubMed Central
9. Puütz, J. M.; Hauer, S. T.; Nellesen, J.; Deißenbeck, D.; Muüller, T. J. J.; Meisner, J.; Ganter, C. Stable N-Heterocyclic Carbenes with the N,N′-Diarylquinazolin-4-one Backbone: Improved Synthesis, Electronic Properties, and Reactivity. Organometallics 2024, 43, 141–163; https://doi.org/10.1021/acs.organomet.3c00466.Suche in Google Scholar
10. Brüggemann, P.; Mzyk, K.; Molter, M.; Nellesen, J.; Schaper, K.; Ganter, C. Synthesis, Reactivity and Electronic Properties of Quinazolin-2-one-Based N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2022, 2022, e202100894.10.1002/ejic.202100894Suche in Google Scholar
11. Fung, J.; Duong, T.-V.; Braceros, K. C. A.; Brooks, M. L.; Schloesser-Lingscheit, K.; Tagawa, T. K. S.; Wilson, J. M.; Jones, K. K.; Valente, E. J. Arylpyran Pseudoacid Racemization: Rate Estimation and Structural Influences. J. Chem. Crystallogr. 2021, 51, 14–41; https://doi.org/10.1007/s10870-020-00829-2.Suche in Google Scholar
12. Heimgert, J.; Morsbach, F.; Kleinschmidt, M.; Reiss, G. J. Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System. Solids 2022, 3, 385–396. https://doi.org/10.3390/solids3030027.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

