Abstract
C22H32O6, orthorhombic, P212121 (no. 19), a = 8.259(3) Å, b = 21.221(9) Å, c = 23.813(10) Å, V = 4174(3) Å3, Z = 8, R gt (F) = 0.0511, wR ref (F2) = 0.1307, T = 265(2) K.
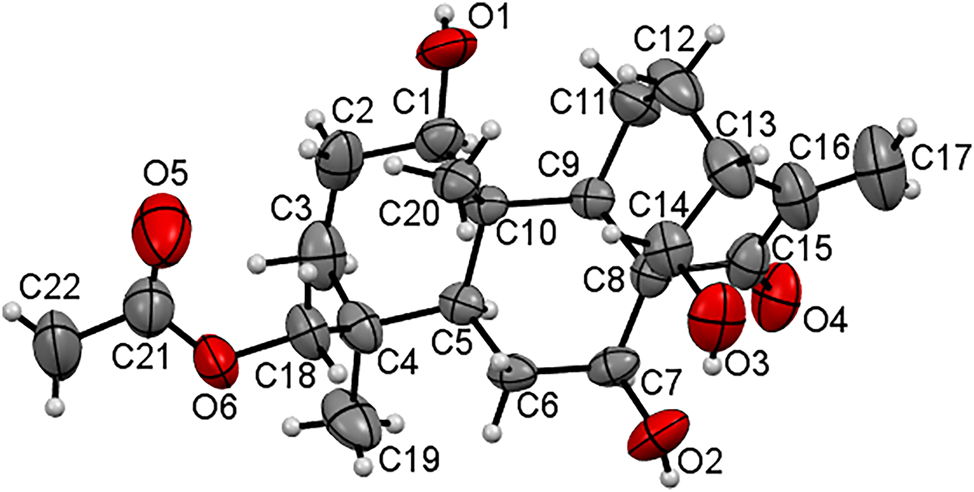
One of two crystallographically independent molecules of the title structure (systematic name: ((1R,4S,6S,6aS,9R,11bS,12S)-1,6,12-trihydroxy-4,11b-dimethyl-8-methylene-7-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalen-4-yl)methyl acetate is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless needle crystal |
| Size: | 0.16 × 0.13 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| θmax, completeness: | 25.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 54,968, 7,324, 0.112 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5,114 |
| N(param)refined: | 514 |
| Programs: | Olex2, 1 Bruker, 2 Shelx, 3 Diamond 4 |
1 Source of material
Isodon latifolius, is a perennial herbaceous plant belonging to the genus Isodon within the family Lamiaceae. According to Flora Reipublicae Popularis Sinicae, this species is primarily distributed in eastern Sichuan Province. 5 Previous phytochemical studies have reported the isolation of numerous structurally novel compounds from Isodon species, including diterpenoids, triterpenoids, organic acids, and, flavonoids, many of which exhibit significant biological activities. 6 , 7 , 8 To facilitate the effective utilization of this plant resource, the aerial parts of Isodon latifolius were dried, pulverized, and subjected to triple methanol extraction. The combined extracts were concentrated under reduced pressure and sequentially partitioned with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate fraction was subjected to column chromatography on macroporous adsorption resin and eluted with a gradient of 30 %, 50 %, 70 %, 90 %, and 100 % methanol in water. The 70 % methanol fraction was further purified using silica gel and Sephadex LH-20 column chromatography, yielding a white solid. Recrystallization from methanol afforded compound Excisanin D (12.6 mg). This compound was identified as an ent-kaurane-type diterpenoid and named Kaur-16-en-15-one,18-(acetyloxy)-1,7,14-trihydroxy-,(1alpha,4beta,7alpha,14R)-(9CI) (systematic name: ((1R,4S,6S,6aS,9R,11bS,12S)-1,6,12-trihydroxy-4,11b-dimethyl-8-methylene-7-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalen-4-yl)methyl acetate).
2 Experimental details
The carbon-bound hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms with d(C–H) = 0.93–0.98 Å, Uiso(H) = 1.5 times Ueq(C) and 1.2 times Ueq(O).
3 Comment
ent–Kaurane-type diterpenoids have attracted extensive research interest due to their intriguing chemical structures and pharmacological activities. These diterpenes belong to a class of tetracyclic diterpenoids with a hydrogenated phenanthrene core and feature an alpha, beta-unsaturated carbonyl unit. Based on their oxidation patterns and ring formation, they can be classified into two major types. 9 First, C-20 non-oxygenated ent-kauranes: in this group, carbon C-20 is always an isolated methyl and C-15 is generally functionalized by a ketone or hydroxyl group. Interestingly, only carbon C-5 and C-9 (except for shikoccidin) have never been functionalized, such as wardiisins A. 10 Second, C-20 oxygenated ent-kauranes. In contrast to the C-20 non-oxygenated ent-kauranes, C-20 of this group is usually an oxymethylene, oxymethine, or carbonyl group, such as oridonin. 11 These compounds exhibit various pharmacological effects including anti-tumor, antibacterial, and anti-inflammatory activities. 12 In terms of structure-activity relationships, 9 ent-kauranes were the representative cytotoxic components, most of the active ent-kauranes share an alpha, beta-unsaturated ketone moiety. The presence of a hydroxyl group on the C ring increases the activity, while the methyl oxidation at C-4 decreases the toxicity. For 7,20-epoxy-ent-kauranes, the 7,20-epoxy moiety and hydroxyls at C-6 and C-7 were beneficial for the cytotoxic activity.
There are two crystallographically independent molecules in the title structure. Both show almost the same geometrical parameters. one of them is shown in the figure. In detail, the C=O distance of the carbonyl group in the molecule shown in the figure is 1.225(6) Å (O4–C15). The double bond of the molecule shown in the figure has a bond length of 1.337(8) Å (C16–C17). Both independent molecules form O–H⋯O hydrogen bonds with neighboring molecules.
Funding source: National natural science foundation of China
Award Identifier / Grant number: 82360840
Acknowledgements
The authors gratefully acknowledge support from the National natural science foundation of China (82360840).
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
2. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. Diamond; Visual Crystal Structure Information System. Ver. 4.0. Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora Reipublicae Popularis Sinicae, Vol. 66; Science Press: M. Beijing, 2004; p. 416.Suche in Google Scholar
6. Ye, J. H.; Deng, R.; Zou, J.; Zhao, C. L.; Zhang, J. J. Study on Chemical Constituents of Isodon phyllostachys. J. Chin. Tradit. Herb. Drugs 2019, 50, 3024–3028.Suche in Google Scholar
7. Li, J. X.; Ye, J. H.; Zou, J.; Pan, L. T.; Zhang, J. J. Study on Diterpenoid Constituents from Bouyei Medicine Isodon lophanthoides. J. China Pharm. 2020, 31, 2458–2461.Suche in Google Scholar
8. Zou, J.; Ye, J. H.; Zhao, C. L.; He, K.; Zhang, J. J.; Pan, L. T. Chemical Constituents of Isodon rubescens. J. Chin. Med. Mater. 2022, 45, 601–605.Suche in Google Scholar
9. Liu, M.; Wang, W. G.; Sun, H. D.; Pu, J. X. Diterpenoids from Isodon Species: An Update. J. Nat. Prod. Rep. 2017, 34, 1090–1140; https://doi.org/10.1039/c7np00027h.Suche in Google Scholar PubMed
10. Tan, Q.; Hu, K.; Li, X. N.; Yang, X. Z.; Sun, H. D.; Puno, P. T. Cytotoxic C-20 Non-Oxygenated ent-kaurane Diterpenoids from Isodon wardii. J. Bioorg. Chem. 2023, 135, 106512; https://doi.org/10.1016/j.bioorg.2023.106512.Suche in Google Scholar PubMed
11. Hu, M.; Xu, S. T.; Xu, J. Y. Research Progress on the Antitumor Mechanism of Oridonin and its Structural Modification. J. Pharmaceut. Clin. Res. 2017, 25, 425–430.Suche in Google Scholar
12. Zang, Y.; Sun, R. N.; Feng, R. Q.; Zhu, H. H.; Li, X. W. Recent Advances of Terpenoids with Intriguing Chemical Skeletons and Biological Activities. J. Chin. J. Chem. 2025, 43, 443–469; https://doi.org/10.1002/cjoc.202400697.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

