Abstract
C18H25CaN3O8, orthorhombic, Cmce (no. 64), a = 15.4480(13) Å, b = 18.4903(11) Å, c = 15.1952(14) Å, V = 4,340.3(6) Å3, Z = 8, R gt (F) = 0.0670, wR ref (F2) = 0.11410, T = 219(10) K.
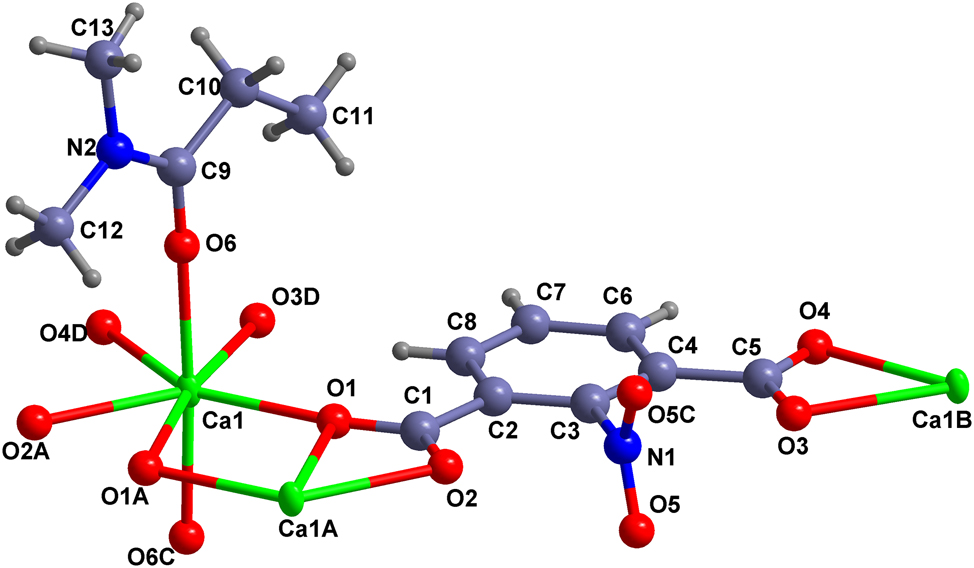
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.23 × 0.16 × 0.11 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.34 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Rigaku SuperNova, ω scan 29.8°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7386, 2678, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1987 |
| N(param)refined: | 160 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
An amount of 1 mmol of hexahydrate of the calcium nitrate and 1 mmol of 2-nitro-1,3-phenylenedicarboxylic acid were dissolved in 10 mL of N,N-dimethylpropionamide. After stirring the mixture was transferred to a 25 mL reaction vessel lined with polytetrafluoroethylene inlay and placed in a 393 K oven for 72 h. After that, the reaction mixture was removed and allowed to cool naturally, and a large amount of colorless crystals were obtained. The crystals were washed three times with 10 mL of anhydrous ethanol and dried naturally, with a yield of 35.3 % (based on 2-nitro-1,3-phenylenedicarboxylic acid).
2 Experimental details
The structure was solved by Direct Methods with the SHELXS-2018 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (Uiso (H) = 1.2 Ueq(C) or 1.5 Ueq(C)) using a riding model with C–H = 0.93 Å, 0.96 and 0.97 Å.
3 Comment
Calcium ions can coordinate well with 1,3-benzenedicarboxylic acid ligands. So far, many single crystal structures of complexes based on calcium ions and 1,3-benzenedicarboxylic acid ligands in the presence of amides (as solvents or solvents and ligands) have been reported, including aminoisophthalate and N,N-dimethylacetamide, 5 (1-oxo-1,3-dihydro-2H-isoindol-2-yl)benzene-1,3-dicarboxylate and N,N-dimethylformamide, 6 isophthalate and 1-methylpyrrolidin-2-one or pyrrolidin-2-one, 7 (1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)isophthalate and N,N-dimethylformamide, 8 isophthalate and imidazolidin-2-one, 9 {[(anthracen-9-yl)methyl]amino}benzene-1,3-dicarboxylate and N,N-dimethylformamide, 10 {[(pyren-1-yl)methyl]amino}benzene-1,3-dicarboxylate and N,N-dimethylformamide. 11 The title compound crystallizes in the orthorhombic crystal system, with the Cmce (no. 64) space group. There are half a calcium ion, one coordinated N, N-dimethylformamide molecule, and half fully deprotonated 2-nitro-1,3-phenyldicarboxylic acid ligand in its asymmetric unit. All atoms in the 2-nitro-1,3-phenyldicarboxylate anion, except for the two oxygen atoms on the nitro group, are in the crystallographically imposed mirror plane, and the entire nitro group is almost perpendicular to this plane. As shown in the figure, the calcium ions are seven-coordinated using a pentagonal bipyramidal coordination mode, coordinating with oxygen atoms from two N, N-dimethylformamide anions and five oxygen atoms from three carboxyl groups on three 2-nitro-1,3-phenyldicarboxylates (code A: 1−x, 1−y, 1−z, code B: 1−x, 1.5 −y, −0.5+z, code C: 1 −x, y, z, code D: 1 −x, 1.5 −y, −0.5+z). The two N, N-dimethylformamide are almost in the axial position of the pentagonal bipyramid. All bond lengths are normal and consistent with the similar results reported in the literature. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
References
1. CrysAlisPRO 1.171.38.43f; Rigaku OD: Oxfordshire, England, 2015.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. Using Phases to Determine the Space Group. Acta Crystallogr. 2018, A74, A353; https://doi.org/10.1107/s0108767318096472.Suche in Google Scholar
5. Lin, J.-D.; Wu, S.-T.; Li, Z.-H.; Du, S.-W. Syntheses, Topological Analyses, and NLO-Active Properties of New Cd(II)/M(II) (M = Ca, Sr) Metal-Organic Frameworks Based on R-Isophthalic Acids (R = H, OH, and t–Bu). Dalton Trans. 2010, 39, 10719–10728; https://doi.org/10.1039/c0dt00390e.Suche in Google Scholar PubMed
6. Singh, A. R.; Rawat, N. S.; Devi, P. S.; Lonibala, R. Hydrothermal Synthesis of a Coordination polymer[CaII(5-AIP)(DMA)]n and Its Application in Epoxidation of Various Organic Substrates. Polyhedron 2017, 137, 52–59; https://doi.org/10.1016/j.poly.2017.07.027.Suche in Google Scholar
7. Yao, X. Q.; Tang, Y. Synthesis, Crystal Structure, and Luminescent Property of a New Heterometallic Compound Based on a Large π-Conjugated Dicarboxylate Ligand. J. Struct. Chem. 2022, 63, 1887–1893; https://doi.org/10.1134/s0022476622110191.Suche in Google Scholar
8. Kang, M.; Luo, D.; Deng, Y.; Li, R.; Lin, Z. Solvothermal Synthesis and Characterization of New Calcium Carboxylates Based on Cluster- and Rod-like Building Blocks. Inorg. Chem. Commun. 2014, 47, 52–55; https://doi.org/10.1016/j.inoche.2014.07.015.Suche in Google Scholar
9. Reger, D. L.; Leitner, A. P.; Smith, M. D. Supramolecular Metal-Organic Frameworks of S- and F-Block Metals: Impact of 1,8-naphthalimide Functional Group. Cryst. Growth Des. 2016, 16, 527–536; https://doi.org/10.1021/acs.cgd.5b01575.Suche in Google Scholar
10. Teixeira, M.; Maia, R. A.; Karmazin, L.; Louis, B.; Baudron, S. A. Ionothermal Synthesis of Calcium-Based Metal-Organic Frameworks in a Deep Eutectic Solvent. CrystEngComm 2022, 24, 601–608; https://doi.org/10.1039/d1ce01497h.Suche in Google Scholar
11. Lu, J.; Wu, H.-F.; Wang, W.-F.; Xu, J.-G.; Zheng, F.-K.; Guo, G.-C. Calcium-based Efficient Cathode-Ray Scintillating Metal-Organic Frameworks Constructed from π-Conjugated Luminescent Motifs. Chem. Commun. 2019, 55, 13816–13819; https://doi.org/10.1039/c9cc06760d.Suche in Google Scholar PubMed
12. Bi, W.-J.; Xie, D.; Hu, X.-M.; Chen, H.; Bian, H.-D. The Crystal Structure of Catena-poly[aqua-μ2-2-nitro-benzene-1,3-dicarboxylato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)zinc(II)]. Z. Kristallogr. - N. Cryst. Struct. 2025, 240, 11. https://doi.org/10.1515/ncrs-2024-0286.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

