The delivery of N-myc downstream-regulated gene 2 (NDRG2) self-amplifying mRNA via modified lipid nanoparticles as a potential treatment for drug-resistant and metastatic cancers
Abstract
The protein, N-myc downstream-regulated gene 2 (NDRG2), a tumor suppressor, is significantly decreased or absent in many types of cancer. There is a significant negative correlation between the levels of NDRG2 and the development and progression of cancer tumor recurrence and tumor invasion, in different cancers. In contrast, the in vitro and in vivo overexpression of the NDRG2 protein decreases the proliferation, growth, adhesion and migration of many types of cancer cells. The in vitro overexpression of NDRG2 increases the efficacy of certain anticancer drugs in specific types of cancer cells. We hypothesize that the delivery of the mRNA of the NDRG2 protein, encapsulated by lipid nanoparticles, could represent a potential treatment of metastatic and drug-resistant cancers. This would be accomplished using a self-amplifying mRNA that encodes the NDRG2 protein and an RNA-dependent-RNA polymerase, obtained from an in vitrotranscribed (IVT) mRNA. The IVT mRNA would be encapsulated in a lipid nanoformulation. The efficacy of the nanoformulation would be determined in cultured cancer cells and if the results are positive, nude mice transplanted with either drug-resistant or metastatic drug-resistant cancer cells, would be treated with the nano- formulation and monitored for efficacy and adverse effects. If the appropriate preclinical studies indicate this formulation is efficacious and safe, it is possible it could be evaluated in clinical trials.
The protein, N-myc downstream-regulated gene 2 (NDRG2), is a 41 kDa protein encoded on human chromosome 14q11.2 [1]. The ndrg2 gene is a member of the ndrg family, which includes ndrg1, ndrg3 and ndrg4 and the ndrg2 gene was cloned from glioblastoma, using polymerase chain reaction-based subtractive hybridization [1]. ndrg2 mRNA is present in the brain, heart, muscle, cartilage, liver and kidney, albeit at lower levels [2]. The NDRG2 protein belongs to the alpha/beta-hydrolase (ABH) superfamily but it lacks enzymatic activity [3]. The NDRG2 protein has two domains: a large canonical ABH fold and a small, cap-like domain [3]. The NDRG2 protein is present predominantly in the cytoplasm but it can also be present in the cell nucleus [2]. Based on structural data, NDRG2’s alpha-6 helix of NDRG2 extends from its main body, interacting with certain cellular molecules that contribute to its biological effects [3].
Studies indicate that nrdg2 is a tumor suppressor gene, is involved in the cell stress response and is expressed at a relatively low level in many types of cancer [4]. Indeed, clinical studies have shown a significant decrease in the levels of NDRG2 protein and/or mRNA in patients diagnosed with gastrointestinal, genitourinary, neurologic, breast, lung, and oral squamous cell carcinoma, fibrosarcoma, thyroid cancer and myeloid leukemia [4]. A downregulation of NDRG2 levels is significantly correlated with the development and progression of cancer, tumor lymph node metastasis and tumor-node-metastasis stages, tumor recurrence and tumor invasion, in different types of cancer [4]. In NDRG2-deficient mice, lymphoma, hepatocellular carcinoma, bronchoalveolar carcinoma and malignant lymphomas develop twice as frequently as in wild-type mice [5].
Numerous in vitro and in vivo studies have reported that the overexpression of the NDRG2 protein significantly decreases the growth, proliferation, adhesion, migration and metastasis of various types of cancer. NDRG2 overexpression produces its anticancer efficacy by (1) decreasing cellular proliferation (decreasing cyclin D1 levels and suppressor of cytokine signaling 1 (SOC1), decreasing the phosphorylation of p38 MAPK, decreasing the levels of activated AKT (p-AKT)); (2) reprogramming or altering cellular biometabolism and bioenergetics (decreasing the levels of proteins involved in glycolysis, including glucose transporter 1, hexokinase 2, pyruvate kinase isomer M2, lactate dehydrogenase A – this decreases glucose availability) and glutamine biosynthesis (decreasing levels of the glutamine transporter, ASC amino acid transporter 2 (ASCT2) and the enzyme, glutaminase 1 (GLS1) – this decreases glutamine, which cancer cells use as a biometabolic source) and (3) decreasing the levels of proteins involved in the epithelial-to-mesenchymal transition and metastasis – beta-catenin, signal transducer and activator of transcription 3 (STAT3), E-cadherin, extracellular signal-related kinases 1 and 2 (ERK-1/2), nuclear factor kappa B (NF-κB), tumor growth factor – beta, certain metallomatrix proteinases, myeloid cell leukemia sequence 1 (Mcl-1) (Figure 1) [4], [5], [6].
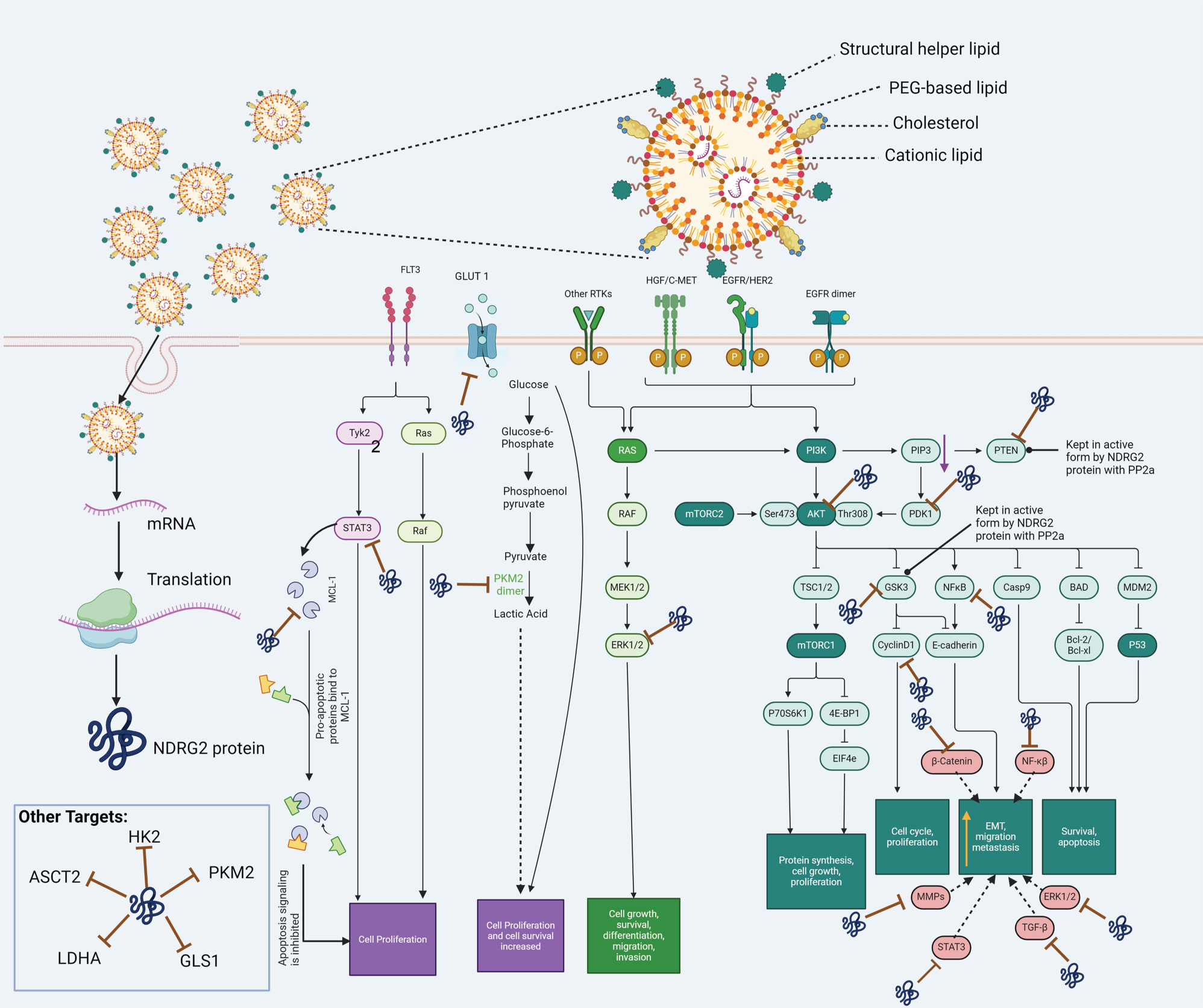
The delivery of N-myc downstream-regulated gene 2 (NDRG2) self-amplifying mRNA via modified lipid nanoparticles: On its surface, the lipid nanoparticle consists of cationic lipid, structural helper lipid, PRG-based lipid, and cholesterol. Once the formulation has entered into the cell, the mRNA is released and translated into the NDRG2 protein. Data suggest that the NDRG2 protein could produce anticancer efficacy by (1) decreasing the expression of proteins involved in bioenergetic and biometabolism of cancer cells, including GLUT1, hexokinase 2 (HK2), pyruvate kinase isomer M2 (PKM2), glutaminase 1 (GLS1), lactate dehydrogenase A (LDHA) and ASC amino acid transporter 2 (ASCT2); (2) interacting with protein phosphatase 2a (PP2a), which maintains PTEN and glycogen synthase kinase – 3β (GSK-3β) in an active state, decreasing cancer cell proliferation and growth; (3) decreasing the expression of STAT3, cyclin D1, NF-kB and ERK1/2, decreasing cancer cell growth (4) decreasing metastasis by decreasing the EMT and migration of cancer cells by decreasing the expresison of certain MMPs, TGF-β, β-catenin, NF-kB, Mcl-1 and ERK1/2 and (5) decreasing the expression of Mcl. ERK1/2, extracellular signal-regulated protein kinases 1 and 2; GLUT-1, glucose transporter 1; Mcl-1, myeloid leukemia cell sequence-1; MMPs, matrix metalloproteinases; mRNA, messenger RNA; NF-κB, nuclear factor kappa B; PTEN, phosphatase and tensin homolog; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor-beta
Based on in vitro and in vivo experiments, it has been postulated that the anticancer efficacy of NDRG2 is due to its modulating the expression and phosphorylation of specific proteins. In cancer cells expressing low or undetectable levels of NDRG2, the activity of phosphorylated phosphatase and tensin homolog deleted on chromosome 10 (PTEN) [5] and the glycogen synthase kinase 3β (GSK-3β) [7], which are inactive states of these enzymes, are significantly increased, which increases the likelihood of the development of cancer. In contrast, in cancer cells overexpressing NDRG2 via forced induction, the interaction of NDRG2 with the enzyme, protein phosphatase 2a (PP2a), dephosphorylates PTEN[5] and GSK-3β[7], converting them to an active state, producing anticancer efficacy.
The expression of the nrdg2 gene can be repressed or decreased by the (1) protein and transcription factor, myelocytomatosis (Myc, an oncogene), in combination with the protein, c-Myc-interacting zinc finger protein-1 (Miz-1) and possibly other proteins[8]; (2) hypermethylation of the promoter region of the ndrg2 gene [9] and (3) mutations in the ndrg2 promoter or coding regions [9].
The overexpression of NDRG2 has been reported to increase the efficacy of pazopanib (in SKOV-3 cancer cells)[10], adriamycin (adriamycin-resistant MCF-7 breast cancer cells)[11] and cisplatin (U937 histiocytic lymphoma cells) [12]. NDRG2 overexpression significantly decreased the in vitro growth of castration-resistant prostate cancer (CRPC) cells [13]. Furthermore, the overexpression of NDRG2 decreased the growth of CRPC tumors in a nude mouse xenograft model [13].
Based on the known studies with NRDG2 and cancer, we hypothesize that a therapeutic approach to treating metastatic and drug-resistant cancers could be the delivery of the mRNA of NRDG2 encapsulated by lipid nanoparticles, a technology that was used to develop mRNA vaccines for COVID-19 [14]. A self-amplifying mRNA, which encodes for NDRG2 and an RNA-dependent RNA polymerase, derived from an in vitro-transcribed (IVT) mRNA [15], would be used. The mRNA construct for NDRG2 would contain: (1) a 7-methylguanosine (m7G-ppp-N-p) cap at the 5′-end; (2) optimized 5′-UTR; (3) an open reading frame and the a) mRNA sequence that encodes the amino acids for the NDRG2 protein and b) an alphavirus nsP1-4 RNA-dependent RNA polymerase located downstream of the 5′-UTR, allowing for use of low doses and a longer duration of action; (4) optimized 3′-UTR and (5) a polyadenylate 3′-tail containing at least 200 units in length. The uridine nucleosides in the mRNA would be replaced post-translationally with N1-methylpseudouridine, which avoids mRNA detection by the innate immune system [14]. The IVT mRNA preparation would be purified using high-pressure liquid chromatography or fast protein liquid chromatography. It is important to note that the use of NDRG2 mRNA avoids the hypermethylation and genomic alterations that would decrease the transcription of ndrg2. However, NDRG2 mRNA translation could be decreased or repressed by certain microRNAs (miRNAs), most likely by binding primarily to a complementary sequence in the NDRG2 mRNA 5-UTR, producing translational repression.
The resulting purified IVT mRNA would be encapsulated in a lipid nanoformulation, protecting the mRNA from biodegradation and significantly increasing the cellular uptake and expression of the mRNA [14]. The four lipid constituents that will be used to encapsulate the mRNA, yielding the nanoparticles, are: (1) cationic/ionizable lipid (SM102 – used in the production of the COVID-19 vaccines, mRNA-1273 and BNT162b2); (2) structural helper lipid (1,2-distearoyl-sn-glycero-3-phosphochline (DSPC)); (3) cholesterol and (4) PEG-based lipid (PEG2000-DMG) [14]. At least three different cancer cell lines would be incubated with the lipid nanoparticles to determine if they penetrate into the cells and that the mRNA is transcribed to NDRG2. If these results are positive, the in vivo experiments would use male and female nude mice that have been subcutaneously (under a shoulder pad) transplanted with either non-metastatic drug-resistant cancer cells or metastatic drug resistant cells. The mice would be given low doses (1, 3 or 10 μg) of the NDRG2 mRNA lipid nanoparticles or a non-mRNA lipid nanoparticle formulation (control group) intramuscularly and the levels of NDRG2 and the weight and volume of the tumors would be determined. The effects of multiple NDRG2 mRNA administrations would also be determined but self-amplifying mRNA would minimize the number of administrations needed for efficacy. All mice would be monitored for adverse and/or toxic effects during and after treatment. If the NDRG2 mRNA nanoformulation is safe and effective after appropriate preclinical testing, it is possible that it could undergo the appropriate evaluation in clinical trials.
In conclusion, we hypothesize that a self-amplifying mRNA NDRG2 nanoformulation could be used as a treatment for drug-resistant and metastatic cancers, alone or in combination with other therapies.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Qu, X, Zhai, Y, Wei, C, Zhang, C, Xing, G, Yu, Y, et al.. Characterization and expression of three novel differentiation-related genes belong to the human NDRG family. Mol Cell Biochem 2002;229:35–44. https://doi.org/10.1023/a:1017934810825.10.1023/A:1017934810825Suche in Google Scholar
2. Yao, L, Zhang, J, Liu, X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophy Sci 2008;40:625–35. https://doi.org/10.1111/j.1745-7270.2008.00434.x.Suche in Google Scholar PubMed
3. Hwang, J, Kim, Y, Kang, HB, Jaroszewski, L, Deacon, AM, Lee, H, et al.. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J Biol Chem 2011;286:12450–60. https://doi.org/10.1074/jbc.m110.170803.Suche in Google Scholar PubMed PubMed Central
4. Hu, W, Fan, C, Jiang, P, Ma, Z, Yan, X, Di, S, et al.. Emerging role of N-myc downstream-regulated gene 2 in cancer. Oncotarget 2015;7:209–23. https://doi.org/10.18632/oncotarget.6228.Suche in Google Scholar PubMed PubMed Central
5. Nakahata, S, Ichikawa, T, Maneesaay, P, Saito, Y, Nagai, K, Tamura, T, et al.. Loss of NRDG2 expression activates PI3K-AKT signaling via PTEN phosphorylation in ATLL and other cancers. Nat Commun 2014;5:3393. https://doi.org/10.1038/ncomms4393.Suche in Google Scholar PubMed PubMed Central
6. Lee, KW, Lim, S, Kim, KD. The function of N-myc downstream-regulated gene 2 (NDRG2) as a negative regulator in tumor cell metastasis. Int J Mol Sci 2022;23:9365. https://doi.org/10.3390/ijms23169365.Suche in Google Scholar PubMed PubMed Central
7. Park, S, Han, HT, Oh, SS, Kim, DH, Jeong, JW, Lee, KW, et al.. NDRG2 sensitizes myeloid leukemia cells to arsenic trioxide via GSK3β-NDRG2-PP2A complex formation. Cells 2019;8:495. https://doi.org/10.3390/cells8050495.Suche in Google Scholar PubMed PubMed Central
8. Zhang, J, Li, F, Liu, X, Shen, L, Liu, J, Su, J, et al.. The repression of human differentiation gene NDRG2 expression by Myc via Miz-dependent interaction with the NDRG2 core promoter. J Biol Chem 2006;281:39159–68. https://doi.org/10.1074/jbc.m605820200.Suche in Google Scholar
9. Liu, N, Wang, L, Liu, X, Yang, Q, Zhang, J, Zhang, W, et al.. Promoter methylation, mutation, and genomic deletion are involved in the decreased NDRG2 expression levels in several cancer cells lines. Biochem Biophys Res Commun 2007;358:164–9. https://doi.org/10.1016/j.bbrc.2007.04.089.Suche in Google Scholar PubMed
10. Cui, Y, Shen, G, Ma, L, Lv, Q. Overexpression of NDRG2 promotes the therapeutic effect of pazopanib on ovarian cancer. J Recept Signal Transduct Res 2021;41:546–52. https://doi.org/10.1080/10799893.2020.1831536.Suche in Google Scholar PubMed
11. Wei, Y, Yu, S, Zhang, YP, Zhang, Y, Zhao, H, Xiao, Z, et al.. NDRG2 promotes Adriamycin sensitivity through a Bad/p53 complex at the mitochondria in breast cancer. Oncotarget 2017;8:29038–47. https://doi.org/10.18632/oncotarget.16035.Suche in Google Scholar PubMed PubMed Central
12. Park, S, Oh, SS, Lee, KW, Lee, YK, Kim, NY, Kim, JH, et al.. NDRG2 contributes to cisplatin sensitivity through modulation of BAK-to-Mcl-1 ratio. Cell Death Dis 2018;9:30. https://doi.org/10.1038/s41419-017-0184-3.Suche in Google Scholar PubMed PubMed Central
13. Yu, C, Wu, G, Li, R, Gao, L, Yang, F, Zhao, Y, et al.. NDRG2 acts as a negative regulator downstream of androgen receptor and inhibits the growth of androgen-dependent and castration-resistant prostate cancer. Cancer Biol Ther 2015;16:287–96. https://doi.org/10.1080/15384047.2014.1002348.Suche in Google Scholar PubMed PubMed Central
14. Granados-Riveron, JT, Aquino-Jarquin, G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother 2021;142:111953. https://doi.org/10.1016/j.biopha.2021.111953.Suche in Google Scholar PubMed PubMed Central
15. Schmidt, C, Schnierle, BS. Self-amplifying RNA vaccine candidates: alternative platforms for mRNA vaccine development. Pathogens 2023;12:138. https://doi.org/10.3390/pathogens12010138.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- The multiple faces of cGAS-STING in antitumor immunity: prospects and challenges
- The molecular and cellular choreography of early mammalian lung development
- DNA-based nanostructures for RNA delivery
- A critical review of diagnostic and prognostic markers of chronic hepatitis B infection
- Perspectives
- The delivery of N-myc downstream-regulated gene 2 (NDRG2) self-amplifying mRNA via modified lipid nanoparticles as a potential treatment for drug-resistant and metastatic cancers
- Unleashing the potential: super-resolution microscopy as the key to advanced mitochondrial research
- Original Article
- Genomic characterization and risk stratification of esophageal squamous dysplasia
Artikel in diesem Heft
- Frontmatter
- Reviews
- The multiple faces of cGAS-STING in antitumor immunity: prospects and challenges
- The molecular and cellular choreography of early mammalian lung development
- DNA-based nanostructures for RNA delivery
- A critical review of diagnostic and prognostic markers of chronic hepatitis B infection
- Perspectives
- The delivery of N-myc downstream-regulated gene 2 (NDRG2) self-amplifying mRNA via modified lipid nanoparticles as a potential treatment for drug-resistant and metastatic cancers
- Unleashing the potential: super-resolution microscopy as the key to advanced mitochondrial research
- Original Article
- Genomic characterization and risk stratification of esophageal squamous dysplasia

