Abstract
Thermal rearrangement of the 1,2-bis(silylene), [{(4-ButPh)C(NDip)2}Si−]2 (Dip = 2,6-diisopropylphenyl, leads to the silyl-silylene, {(4-ButPh)C(NDip)2}Si−Si(H) {N(Dip)C(=NDip)(4-ButPh-H)}, via an intramolecular C−H activation reaction. The X-ray crystal structure, and limited spectroscopic data, for the compound are reported. The structure of the silyl-silylene incorporates an exocyclic imine fragment, which is unprecedented for this compound class.
West’s 1981 synthesis of the first stable disilene, Mes2Si=SiMes2 (Mes = mesityl), via dimerization of a transient silylene, :SiMes2, is considered by many as one of the pivotal results in the development of low oxidation state main group element chemistry (West et al., 1981). It was not until 1994 that the same group reported the first example of an isolable silylene, the two-coordinate silicon center of which was stabilized by incorporation within an N-heterocyclic framework (Denk et al., 1994). Since that time numerous heterocyclic and Lewis base stabilized silylenes have been reported, and their chemistry extensively explored (Asay et al., 2011). In 2012 the first acyclic two-coordinate silylenes were reported in simultaneous papers, one of which described the enhanced reactivity of silylene 1a (Figure 1) towards H-H and C-H bond activations (Protchenko et al., 2012; Rekken et al., 2012). Several two-coordinate acyclic silylenes have followed, including the highly reactive silyl-silylene 1b (Protchenko et al., 2013).

Examples of previously reported acyclic two-coordinate silylenes.
Our interest in low oxidation state silicon chemistry is not restricted to silylenes such as 1, but also includes the stabilization of formally silicon(I) species. In 2011 we were able to prepare such a species, viz. the 1,2-bis(silylene) 2 (Scheme 1), which was stabilized by incorporation of a bulky amidinate ligand developed in the group (Jones et al., 2011). It is noteworthy that Roesky and co-workers had prepared a closely related, but less bulky, amidinate substituted 1,2-bis(silylene), [{PhC(NBut)2}Si−]2 two years earlier (Sen et al., 2009). During the exploration of the further chemistry of 2, we have found that the compound slowly thermally isomerizes to yield a rare example of an amidinate substituted silyl-silylene, the X-ray crystal structure of which we report herein.
The deep blue 1,2-bis(silylene) 2 was dissolved in toluene, and subsequently heated at reflux for 4 days. During this time the initially deep blue solution lightened in color, eventually becoming yellow. Layering of this yellow solution with pentane led to the deposition of yellow crystals of the silyl-silylene 3 in an isolated yield of 34% (Scheme 1). It is of note that attempts to thermally isomerize the 1,2-bis(germylene) and 1,2-bis(stannylene) analogues of 2, led only to deposition of elemental germanium or tin, respectively, and generation of the protonated amidine, (4-ButC6H4)C(NDip){N(H)Dip} (Dip = 2,6-diisopropylphenyl).
With regard to a mechanism for the formation of 3, it seems reasonable that there is an initial de-complexation of one arm of one amidinate ligand from a silicon center to give the transient bis(silylene) intermediate, 4. Rotation of the ligand backbone about its N-C single bond could then bring the tert-butyl phenyl substituent into close proximity with the acyclic two-coordinate silylene center of 4. The phenyl then undergoes an intramolecular C-H activation at one of its ortho-positions to give 3. Such a process is not dissimilar to intramolecular C−H activation processes previously reported for silylenes 1 (Protchenko et al., 2012; Protchenko et al., 2013).
As compound 3 has limited solubility in normal non-coordinating deuterated solvents once crystallized, only its 1H NMR spectrum could be obtained. That spectrum is consistent with the compound retaining its unsymmetrical solid state structure in solution, in that it exhibits two sets of ligand resonances. In addition, a broad signal at δ 6.39 ppm was assigned to the hydride ligand, close to where the hydride resonance for the only other example of an amidinato substituted hydridosilyl-silylene, {PhC(NBut)2}Si−Si(H) {(ButN)2C(H)Ph}, occurs, viz. δ 6.70 ppm (Zhang et al., 2010). It is of note that this silylene was not prepared by thermal rearrangement of a 1,2-bis(silylene), but by reduction of the silicon(IV) precursor compound, {PhC(NBut)2}Si(H)Cl2.
The X-ray crystal structure of compound 3 was obtained, and its molecular structure is depicted in Figure 2 (see also Table 1). This shows it to possess a three-coordinate pyramidal silylene center, Si(2), that is chelated by an amidinate ligand with an electronically delocalised NCN backbone. Further ligation comes from a hydridosilyl fragment that results from the aforementioned intramolecular thermal isomerization. That fragment is a five-membered heterocycle, which encompasses a distorted tetrahedral silicon center, Si(1). The backbone of the five-membered ring is substituted at planar C(1) by an exo-cyclic imino moiety. To the best of our knowledge, this structural motif is unprecedented in silyl-silylene chemistry. The Si−Si distance in 3, 2.394(1) Å, lies in the known range for such interactions, and is comparable to Si−Si distances in related silyl-silylenes, e.g. 2.386(1) Å in 1b, and 2.377(5) Å in {PhC(NBut)2}Si-Si(H){(ButN)2C(H)Ph} (Protchenko et al., 2013; Rodriguez et al., 2015; Yamaguchi and Sekiguchi, 2011; Zhang et al. 2010).
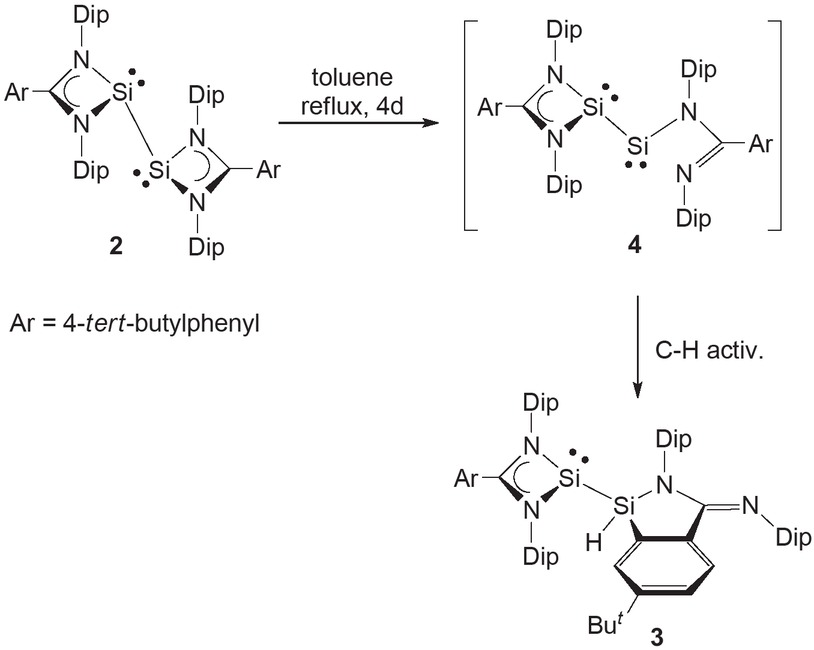
Synthesis of silyl-silylene 3.
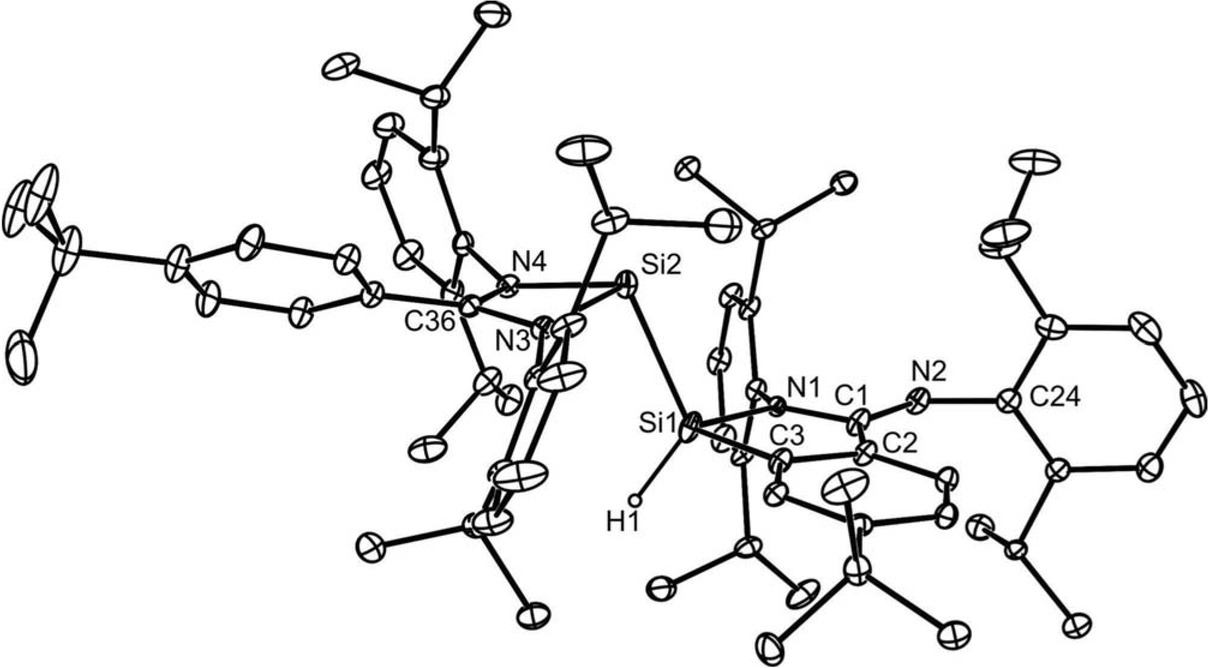
Thermal ellipsoid plot (25% probability surface) of the molecular structure of 3 (hydrogen atoms, except the hydride, omitted). Selected bond lengths (Å) and angles (°): Si(1)-N(1) 1.849(10), Si(1)-C(3) 1.875(2), Si(1)-Si(2) 2.394(1), Si(1)-H(1) 1.43(2), N(1)-C(1) 1.335(11), C(1)-N(2) 1.275(3), C(1)-C(2) 1.494(3), Si(2)-N(3) 1.8662(18), Si(2)-N(4) 1.8836(16), C(2)-C(3) 1.404(3), N(1)-Si(1)-C(3) 89.8(3), Si(2)-Si(1)-H(1) 121.1(9), N(3)-Si(2)-N(4) 69.39(7), N(3)-Si(2)-Si(1) 96.97(6), N(4)-Si(2)-Si(1) 106.08(6), C(1)-N(2)-C(24) 124.56(18).
Summary of crystallographic data for 3.
| 3 | |
|---|---|
| empirical formula | C70H94N4Si2 |
| formula weight | 1047.67 |
| crystal system | triclinic |
| space group | P-1 |
| a (Å) | 12.7450(3) |
| b (Å) | 15.6776(3) |
| c (Å) | 18.5694(4) |
| α (deg.) | 67.456(2) |
| β (deg) | 75.511(2) |
| γ (deg.) | 69.741(2) |
| vol (Å3) | 3185.18(13) |
| Z | 2 |
| ρ (calcd) (g.cm-3) | 1.092 |
| μ (mm-1) | 0.098 |
| F(000) | 1140 |
| reflections collected | 24075 |
| unique reflections | 11461 |
| Rint | 0.0338 |
| R1 indices [I>2σ(I)] | 0.0540 |
| wR2 indices (all data) | 0.1560 |
| largest peak/hole (e/Å3) | 0.58, -0.35 |
| CCDC No. | 1904293 |
In summary, an unusual amidinate coordinated silylsilylene has been prepared by thermal rearrangement of a 1,2-bis(silylene). The mechanism of the rearrangement is thought to include a transient intermediate that possesses a reactive acyclic two-coordinate silylene center. This undergoes an intramolecular C-H activation of one of its phenyl substituents to ultimately yield the isolated silylsilylene. In the solid state this is shown to incorporate a five-membered heterocyclic silyl fragment that is substituted by an imine moiety. We continue to explore the stabilization and reactivity of low oxidation state silicon compounds in our laboratory.
Experimental
General
All manipulations were carried out using standard Schlenk and glove box techniques under an atmosphere of high purity dinitrogen. Toluene was distilled over molten potassium, while pentane was distilled from 1:1 Na/K alloy. The 1H NMR spectrum was recorded on a Bruker AvanceIII 400 spectrometer and was referenced to the residual proton resonance of the solvent used. Compound 2 was prepared by the literature procedure, while all other reagents were used as received (Jones et al., 2011).
Preparation of {(4-ButPh)C(NDip)2}Si-Si(H) {N(Dip)C(=NDip)(4-ButPh-H)} 3
A solution of 2 (0.094 g, 0.090 mmol) was dissolved in toluene (20 mL) and the resultant blue solution heated at reflux for 4 d. During this time, the color of the solution gradually changed to yellow. The solution was then concentrated to ca. 2 mL in vacuo and layered with pentane (ca. 15 mL) in a long Schlenk tube. Yellow crystals of the title compound deposited overnight (0.032 g, 34%). M.p. 231-236°C (decomp.) Data for 3: 1H NMR (400 MHz, 298 K, C6D6): δ 0.96 (s, 9H, C(CH3)3), 1.00 (s, 9H, C(CH3)3), 1.00-1.41 (many overlapping doublets, 48H, CH(CH3)2), 3.34 (sept, J = 6.8 Hz, 2H, CH(CH3)2), 3.58 (sept, J = 6.8 Hz, 2H, CH(CH3)2), 3.62 (sept, J = 6.8 Hz, 2H, CH(CH3)2), 3.72 (sept, J = 6.8 Hz, 2H, CH(CH3)2), 6.39 (br. s, 1H, SiH), 6.76-7.37 (overlapping m, 19H, ArH). A reproducible microanalysis could not be obtained for the compound as it consistently co-crystallized with small amounts of the amidine, (4-ButPh)C(NDip){N(H)Dip}, which could not be separated.
X-ray crystallography
A crystal of 3 suitable for X-ray structural determination was mounted in silicone oil. Crystallographic measurements were carried out at 123(2) K, and were made using an Oxford Gemini diffractometer using a graphite monochromator with Mo Kα radiation (λ = 0.71073 Å). The structure was solved by direct methods and refined on F2 by full matrix least squares (SHELX16) (Sheldrick, 2016) using all unique data. All non-hydrogen atoms are anisotropic with hydrogen atoms included in calculated positions (riding model), except the hydride ligand, the positional parameters of which were freely refined. There is significant disorder in the structure, which involves positional disorder of the methyl groups of one tertbutyl substituent, two isopropyl groups, and one whole NDip fragment. This disorder was successfully modelled. Crystal data, details of the data collection and refinement are given in Table 1. Crystallographic data (excluding structure factors) for the structure have been deposited with the Cambridge Crystallographic Data Centre (CCDC no. 1904293). Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44-1223-336033; email: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
Acknowledgement
This research was supported by the Australian Research Council.
References
Asay M., Jones C., Driess M., N-Heterocyclic carbene analogues with low-valent group 13 and group 14 elements: Syntheses, structures, and reactivities of a new generation of multitalented ligands. Chem. Rev., 2011, 111, 354-396.10.1021/cr100216ySearch in Google Scholar PubMed
Denk M., Lennon R., Hayashi R., West R., Belyakov V., Verne H.P., et al., Synthesis and structure of a stable silylene. J. Am. Chem. Soc., 1994, 116, 2691-2692.10.1021/ja00085a088Search in Google Scholar
Jones C., Bonyhady S.J., Holzmann N., Frenking G., Stasch A., Preparation, characterization, and theoretical analysis of group 14 element(I) dimers: A case study of magnesium(I) compounds as reducing agents in inorganic synthesis. Inorg. Chem., 2011, 50, 12315-12325.10.1021/ic200682pSearch in Google Scholar PubMed
Protchenko A.V., Birjkumar K.H., Dange D., Schwarz A.D., Vidovic D., Jones C., et al., A stable two-coordinate acyclic silylene. J. Am. Chem. Soc., 2012, 134, 6500-6503.10.1021/ja301042uSearch in Google Scholar PubMed
Protchenko A.V., Schwarz A.D., Blake M.P., Jones C., Kaltsoyannis N., Mountford P., et al., A generic one-pot route to acyclic two-coordinate silylenes from silicon(IV) precursors: synthesis and structural characterization of a silylsilylene. Angew. Chem. Int. Ed., 2013, 52, 568-571.10.1002/anie.201208554Search in Google Scholar PubMed
Rekken B.D., Brown T.M., Fettinger J.C., Tuononen H.M., Power P.P., Isolation of a stable, acyclic, two-coordinate silylene. J. Am. Chem. Soc., 2012, 134, 6504-6507.10.1021/ja301091vSearch in Google Scholar PubMed
Rodriguez R., Contie Y., Mao Y., Saffron-Merceron N., Baceiredo A., Branchadell V., et al., Reversible dimerization of phosphine-stabilized silylenes by silylene insertion into SiII−H and SiII−Cl s-bonds at room temperature. Angew. Chem. Int. Ed., 2015, 54, 15276-15279.10.1002/anie.201506951Search in Google Scholar PubMed
Sen S.S., Jana A., Roesky H.W., Schulzke C., A remarkable base-stabilized bis(silylene) with a silicon(I)-silicon(I) bond. Angew. Chem. Int. Ed., 2009, 48, 8536-8538.10.1002/anie.200902995Search in Google Scholar PubMed
Sheldrick G.M., SHELX-16. University of Göttingen, 2016.Search in Google Scholar
West R., Fink M.J., Michl J., Tetramesityldisilene, a stable compound containing a silicon silicon double bond. Science, 1981, 214, 1343-1344.10.1126/science.214.4527.1343Search in Google Scholar PubMed
Yamaguchi Y., Sekiguchi A., Unusual reactivity of a disilyne with 4-dimethylaminopyridine: Formation of an intramolecularly N-coordinated silylene and its isomerization to a zwitterionic silyl anion. J. Am. Chem. Soc., 2011, 133, 7352-7354.10.1021/ja202605xSearch in Google Scholar PubMed
Zhang S.-H., Yeong H.-X., Xi H.-W., Lim K.H., So C.-W., Hydrosilylation of a silicon(II) hydride: Synthesis and characterization of a remarkable silylsilylene. Chem. Eur. J., 2010, 16, 10250-10254.10.1002/chem.201000089Search in Google Scholar PubMed
© 2019 Sidiropoulos et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Article
- Optimization of pH, temperature and carbon source for bioleaching of heavy metals by Aspergillus flavus isolated from contaminated soil
- A new Pb(II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: crystal structure and anti-lung cancer activity
- A new Pb(II)-based coordination polymer constructed by 5-((4-carboxyphenoxy)methyl)benzene-1,3-dioic acid and N-donor co-ligand: crystal structure and anti-glioma activity
- Calcium(II)-mediated resolution of methyl o-chloromandelate with chiral O,O′-dibenzoyltartaric acid in preparative scale
- Sensor application of doped C60 fullerenes in detection of 1-(3-trifluoromethylphenyl)piperazine as an alternative to ecstasy
- Synthesis, spectroscopic characterization and computational studies of Schiff base complexes of tin(IV) chloride
- Synthesis and structural characterization of the complexes of 2-(menthoxycarbonyl)ethyltin chloride
- Bismuth⋅⋅⋅π arene interaction in [Bi{OC6H4(CH2C6H5)-2}3]2
- Synthesis, characterization and electroluminescence properties of a new mixed-ligand diorganotin(IV) complex
- Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb(II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands
- The interaction of morin and morin-5’-sulfonic acid with lead(II): Study of the 1:1 complex formation process in aqueous solution
- Synthesis and biological evaluation of Piroxicam derivative as a lead chelator
- The stabilities and geometries of Re-encapsulated Sin(n=16, 20, 24, 28, 32, 36, and 40) clusters: A computational investigation
- Synthesis, structural characterization, cytotoxicity and encapsulation studies of N,Nʹ-(1, 2-dicyano-1,2-vinylene)-bis(4-hydroxysalicylideneaminato) di(p-chlorobenzyl)tin as potential anticancer drug
- Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD
- Transition metals doped ZnO nanocluster for ethylene oxide detection: A DFT study
- Thermal rearrangement of a 1,2-bis(silylene): Synthesis and crystal structure of a silyl-silylene
- Toxicity of arsenic on isolated human lymphocytes: The key role of cytokines and intracellular calcium enhancement in arsenic-induced cell death
- Theoretical insight of alpha amino acid phenylalanine adsorption on pristine and decorated fullerenes
- DFT/QTAIM analysis of favipiravir adsorption on pristine and silicon doped C20 fullerenes
- New insights into the oxidation of phenoxatellurine with sulfuric acid
Articles in the same Issue
- Research Article
- Optimization of pH, temperature and carbon source for bioleaching of heavy metals by Aspergillus flavus isolated from contaminated soil
- A new Pb(II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: crystal structure and anti-lung cancer activity
- A new Pb(II)-based coordination polymer constructed by 5-((4-carboxyphenoxy)methyl)benzene-1,3-dioic acid and N-donor co-ligand: crystal structure and anti-glioma activity
- Calcium(II)-mediated resolution of methyl o-chloromandelate with chiral O,O′-dibenzoyltartaric acid in preparative scale
- Sensor application of doped C60 fullerenes in detection of 1-(3-trifluoromethylphenyl)piperazine as an alternative to ecstasy
- Synthesis, spectroscopic characterization and computational studies of Schiff base complexes of tin(IV) chloride
- Synthesis and structural characterization of the complexes of 2-(menthoxycarbonyl)ethyltin chloride
- Bismuth⋅⋅⋅π arene interaction in [Bi{OC6H4(CH2C6H5)-2}3]2
- Synthesis, characterization and electroluminescence properties of a new mixed-ligand diorganotin(IV) complex
- Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb(II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands
- The interaction of morin and morin-5’-sulfonic acid with lead(II): Study of the 1:1 complex formation process in aqueous solution
- Synthesis and biological evaluation of Piroxicam derivative as a lead chelator
- The stabilities and geometries of Re-encapsulated Sin(n=16, 20, 24, 28, 32, 36, and 40) clusters: A computational investigation
- Synthesis, structural characterization, cytotoxicity and encapsulation studies of N,Nʹ-(1, 2-dicyano-1,2-vinylene)-bis(4-hydroxysalicylideneaminato) di(p-chlorobenzyl)tin as potential anticancer drug
- Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD
- Transition metals doped ZnO nanocluster for ethylene oxide detection: A DFT study
- Thermal rearrangement of a 1,2-bis(silylene): Synthesis and crystal structure of a silyl-silylene
- Toxicity of arsenic on isolated human lymphocytes: The key role of cytokines and intracellular calcium enhancement in arsenic-induced cell death
- Theoretical insight of alpha amino acid phenylalanine adsorption on pristine and decorated fullerenes
- DFT/QTAIM analysis of favipiravir adsorption on pristine and silicon doped C20 fullerenes
- New insights into the oxidation of phenoxatellurine with sulfuric acid

