Abstract
The complexes of 2-(menthoxycarbonyl)ethyltin chloride, MenOCOCH2CH2SnCl3⋅L (Men = Menthyl, L = benzyl phenyl sulfoxide (bpSO), 1; 2,2’-bipyridine (bpy), 2; 1,10-phenanthroline (phen), 3) and [MenOCOCH2CH2SnCl2(OCH3)]2 (4), have been synthesized and characterized by means of elemental analysis, FT-IR, NMR (1H, 13C and 119Sn) spectra. The crystal structures of 1, 3 and 4 have been determined by single crystal X-ray diffraction. The tin atoms in 1-4 are all hexa-coordinated. The tin atom in 1 adopts a distorted [CSnCl3O2] octahedral geometry with an oxygen atom of the ligand and an intramolecular coordination of the oxygen atom from the carbonyl group to the tin atom. Complex 3 possesses a distorted [CSnCl3N2] octahedral geometry with two nitrogen atoms of a chelating phen ligand. The carbonyl oxygen atom of the ester moiety is not coordinating. Compound 4 is a centrosymmetric dimer with a four-membered Sn2O2 ring, and the tin atom has a distorted [CSnCl2O3] octahedral geometry with an intramolecular C=O→Sn coordination and intermolecular methoxy bridging.
1 Introduction
The Lewis acid strengths of the organotin chlorides RnSnCl(4-n) (n = 1-3) decrease as n increases. When n = 2 or 1, five- or six-coordinate complexes with Lewis bases, such as N- and O-donor ligands, are usually formed via both inter- and intramolecular interactions (Davies, 2004). 2-Alkoxycarbonylethyltin trichlorides, ROCOCH2CH2SnCl3, are functionally substituted alkyltin chlorides, and are readily available from the reactions of CH2=CHCOOR with SnCl2 and HCl (Hutton et al., 1978). These compounds have received considerable attention because of the variety of coordination geometries regarding the tin atom (de Morais et al., 2017; Harrison et al., 1979; Howie and Wardell, 2002; Lima et al., 2009; Tian et al., 2005; Tian et al., 2016). Crystal structure determinations and spectra data (Balasubramanian et al., 1997; Buchanan, et al., 1996; Harrison et al., 1979; Howie and Wardell, 2002; Lima et al., 2009; Liu et al., 2015; Maughan et al., 1981; Tian et al., 2005) have shown that the ROCOCH2CH2 unit is a C,O-chelating ligand by the intramolecular coordination of the carbonyl oxygen atom to the tin atom. However, the coordination can be broken and replaced by the other N and O donors (Balasubramanian et al., 1997; Buchanan, et al., 1996; de Morais et al., 2017; Maughan et al., 1981; Tian et al., 2016). In order to continue to expand the structural chemistry of functional alkyltin chlorides, we recently reported the crystal structure of the complexes of 2-(methoxycarbonyl)ethyltin trichloride with HMPA (hexamethylphosphoryltriamide) and phen (Guo et al., 2017). 2-(Menthoxycarbonyl)ethyltin trichloride containing an optically active menthyl group is a potential Lewis acid chiral catalyst, and the reaction with the Lewis base has been not investigated. Herein, we report the synthesis and characterization of the complexes formed by the reaction of MenOCOCH2CH2SnCl3 with bpSO, bipy, phen, or py (pyridine) in the methanol.
2 Results and discussion
2.1 Synthesis
Complexes 1-4 were prepared by the reactions of 2-(menthoxycarbonyl)ethyltin trichloride with the ligand bpSO, bpy, phen or py in 1:1 molar ratio in methanol.
When py was used, the expected complex MenOCOCH2CH2SnCl3⋅py was not obtained, and compound 4, a methoxide of 2-(menthoxycarbonyl)ethyltin trichloride, was generated (Scheme 1). The complexes are colorless crystalline, and can be dissolved in common organic solvents such as chloroform, methanol, and acetone.
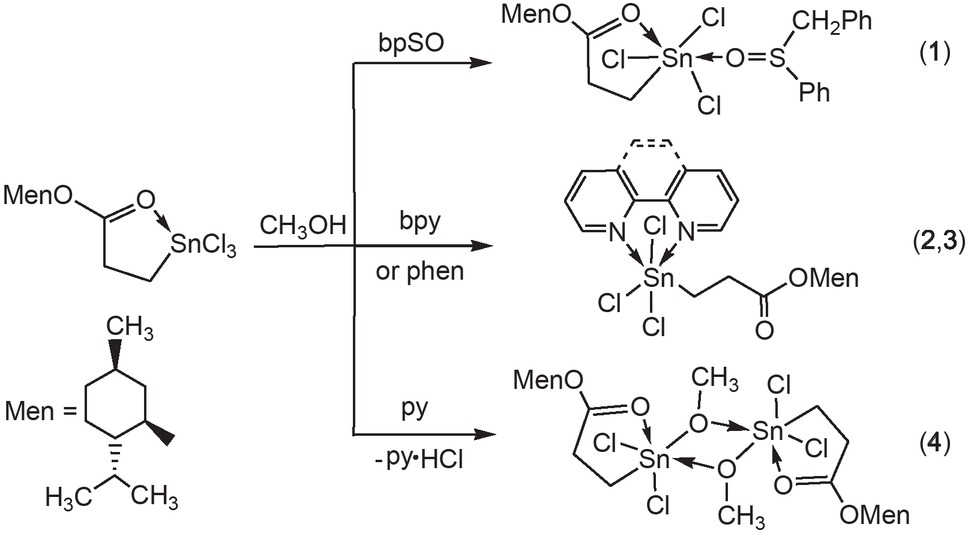
Synthesis of the complexes 1 - 4.
2.2 Spectroscopic analysis
In the IR of 2-(menthoxycarbonyl)ethyltin trichloride, the stretching vibration absorption of intramolecularly coordinated carbonyl is at 1645 cm-1. In 1-4, this band appears at 1645, 1719, 1730, and 1648 cm-1, respectively, indicating that the C=O→Sn coordination is maintained in 1 and 4 and is broken to accommodate two nitrogen atoms of the chelate ligands in 2 and 3. Considerable changes are observed for the ligand vibrations in 1-3. In 1, the ν(S=O) at 1035 cm-1 in the free bpSO is shifted to 955 cm-1, and the decreased value can be attributed to the complex formation with tin (Howie et al., 2012; Maughan et al., 1981). In free bpy molecule, the ν(C=N) and ν(C=C) (ring stretching vibrations) are observed at 1570 and 1420 cm-1. New bands are observed at 1601 and 1446 cm-1 in 2 as a result of complex formation. The out-of-plane C-H bending vibration at 750 cm-1 in free ligand appears at 778 and 729 cm-1 in 2, indicating the bidentate nature of the ligand (Garad et al., 1981). Similar behavior is observed in phen complex 3.
In CHCl3 solution (ca. 0.02 mol/L), IR absorption frequency of carbonyl (C=O) in 1-4 is almost the same as in the solid, and is respectively observed at 1649, 1720, 1724, and 1652 cm-1, indicating that in the solution the intramolecular coordination of carbonyl to tin atom still exists in 1 and 4, and the carbonyl is free in 2 and 3.
The assignment of NMR for 1-4 was achieved by examining their chemical shift values, integration values and multiplicity patterns and also by careful comparison of NMR data with the related compounds reported earlier, such as MenOCOCH2CH2SnCl3 (Tian et al., 2005), CH3COOMen (Lu et al., 2016), bpSO (Wang et al., 2018), [Ru(bpy)2{4,4′-bpy(COOH)2}](PF6)2 (Wang et al., 1999), and (p-CH3C6H4CH2)2SnCl2⋅phen (Chandrasekar et al., 2015) (see experimental section). The 1H and 13C NMR data also support the presence of C=O→Sn coordination in 1 and 4. The proton resonance of -OCH- (H-4) in the substrate, 1 and 4 that appeared at ∼5.0 ppm shows a downfield shift by ∼0.3 ppm compared with that of 2 and 3, and the δ (13C) values of C=O (C-3) and -OCH- (C-4) in the substrate, 1 and 4 are deshielded by ∼8 ppm (C-3) and ∼5 ppm (C-4), respectively, relative to those of 2 and 3. The C=O→Sn coordination in the substrate, 1 and 4 causes the deshielding of the 1H and 13C nuclei of the -CHOC=O moiety. However, when the coordination is broken by an external ligand (bpy and phen), the shielding of -CHOC=O is recovered again. In complex 2, the chemical environment of two pyridine rings from the ligand bpy is different due to the rigid chelate ring formed by bpy coordination to the tin atom, so that the pyridine ring displays two sets of 1H and 13C NMR signals (Tian et al., 2018; Van Koten and Noltes, 1976; Wang et al., 1999). The same behavior is also found in phen complex 3. The formation of the complexes is further confirmed by the changes of chemical shifts of the ligands. For example, the δ (1H) value of H-6′ (H-6′′) at 8.59 ppm in free bpy shifts to 9.24 (H-6′) and 9.90 (H-6′′) ppm in 2, and the δ (1H) value of H-2a (H-2b) at 9.18 ppm in free phen shifts to 9.61 (H-2a) and 10.22 (H-2b) ppm in 3.
The 119Sn chemical shifts (δ) primarily depend on the coordination number and the nature of the donor atom directly bound to the central tin atom. It has been reported that the δ values from +200 to -60 ppm for four-coordinated, -90 to -190 ppm for five-coordinated and -210 to -400 ppm for six-coordinated tin atoms in solution (Holecek et al., 1986). The complexes 1-4 exhibit a single 119Sn resonance at -332.6, -278.3, -293.7 and -364.5 ppm, respectively, which fall well within the range proposed for six-coordinate tin centres (Airapetyan et al., 2015; Holecek et al., 1986). Thus, the tin atoms in these complexes have six-coordinate environments in CDCl3 solution, and it is suggested that compound 4 is an oxygen-bridged dimer in solution (see below X-ray analysis).
2.3 Crystal structure analysis
The molecular structures of 1, 3, and 4 are shown in Figures 1-4. The selected bond lengths and bond angles are listed in Table 1. Complex 1 crystallizes in chiral space group P212121 and is a discrete molecule with no close intermolecular contacts (Figure 1). The complex contains a five-membered chelate ring formed via intramolecular C=O→Sn coordination. The tin atom is hexa-coordinated with the coordinating atoms C(1), Cl(1), C1(2), Cl(3), O(1) and O(2) in a distorted octahedral arrangement. The distortion from ideal octahedral geometry is expressed by C(1)-Sn(1)-Cl(3), O(2)-Sn(1)-Cl(2) and O(1)-Sn(1)-C1(1) angles of 156.89(19), 172.72(11) and 174.43(12)°, respectively, deviating from 180°. The interatomic Sn(l)-O(2) distance of 2.403(4) Å) is similar to 2.397(7) Å reported for MenOCOCH2CH2SnCl3 (Tian et al., 2005). The Sn(1)-O(1) distance of 2.259(4) Å is obviously shorter than Sn(1)-O(2) (2.403(4) Å), indicating a stronger S=O→Sn than C=O→Sn interaction (Maughan et al., 1981). In addition, the Sn(1)-O(1) distance is also in agreement with that observed in other organotin sulfoxide complexes, such as Ph2SnCl2[(PhCH2)2SO]2 (Sn-O 2.258(4) Å) (Sousa et al., 2009) and [2-(Me2NCH2)C6H4]SnCl3[(CH3)2SO] (Sn-O 2.253(2) Å) (Varga et al., 2005). Compared with the S(1)-O(1) (1.501(4) Å) in free bpSO (Fuller et al. 2009), the bond in 1 becomes longer (1.513(4) Å), further confirming the formation of S=O→Sn coordination. The Sn-Cl distance lies in the range of 2.3320(18)-2.4310(19) Å, and the Sn(1)-Cl(1) bond involving the ligand O(l) atom trans to Cl(1) atom is the longest. The features are comparable with those found in CH3OCOCH2CH2SnCl3⋅O=P[N(CH3)2]3 (Guo et al., 2017), H2NCOCH2CH2SnCl3⋅O=S(CH3)2 (Howie et al., 2012).
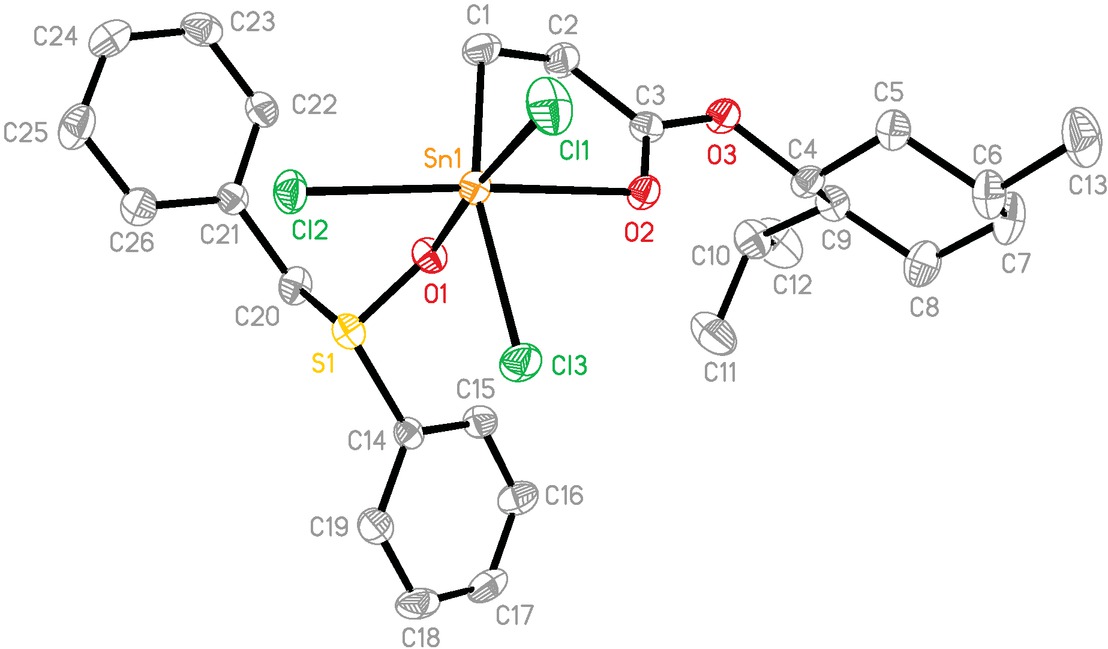
The molecular structure of 1. Ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity.
Selected bond lengths (Å) and angles (°) for 1,3 and 4.
| 1 | |||||
|---|---|---|---|---|---|
| Sn(1)-O(1) | 2.259(4) | Sn(1)-C(1) | 2.135(7) | Sn(1)-Cl(2) | 2.3855(15) |
| Sn(1)-O(2) | 2.403(4) | Sn(1)-Cl(1) | 2.4310(19) | Sn(1)-Cl(3) | 2.3320(18) |
| C(1)-Sn(1)-O(1) | 82.7(2) | Cl(3)-Sn(1)-Cl(2) | 97.26(7) | C(1)-Sn(1)-Cl(1) | 96.3(2) |
| C(1)-Sn(1)-Cl(3) | 156.89(19) | C(1)-Sn(1)-O(2) | 76.2(2) | O(1)-Sn(1)-Cl(1) | 174.43(12) |
| O(1)-Sn(1)-Cl(3) | 86.11(12) | O(1)-Sn(1)-O(2) | 83.00(14) | Cl(3)-Sn(1)-Cl(1) | 92.80(9) |
| C(1)-Sn(1)-Cl(2) | 102.9(2) | Cl(3)-Sn(1)-O(2) | 82.37(11) | Cl(2)-Sn(1)-Cl(1) | 95.83(7) |
| O(1)-Sn(1)-Cl(2) | 89.73(11) | Cl(2)-Sn(1)-O(2) | 172.72(11) | O(2)-Sn(1)-Cl(1) | 91.44(11) |
| 3⋅CHCl3 | |||||
| 2.122(8) | Sn(1)-Cl(2) | 2.4111(19) | Sn(2)-N(4) | 2.291(6) | |
| Sn(1)-C(1) | 2.286(5) | Sn(1)-Cl(3) | 2.467(2) | Sn(2)-Cl(5) | 2.4195(17) |
| Sn(1)-N(1) | 2.231(6) | Sn(2)-C(26) | 2.157(7) | Sn(2)-Cl(4) | 2.4493(19) |
| Sn(1)-N(2) | 2.450(2) | Sn(2)-N(3) | 2.256(6) | Sn(2)-Cl(6) | 2.466(2) |
| Sn(1)-Cl(1) | 165.4(3) | C(1)-Sn(1)-Cl(3) | 94.0(3) | N(4)-Sn(2)-Cl(5) | 160.22(17) |
| C(1)-Sn(1)-N(2) | 92.9(3) | N(2)-Sn(1)-Cl(3) | 84.51(15) | C(26)-Sn(2)-Cl(4) | 95.5(2) |
| C(1)-Sn(1)-N(1) | 72.5(2) | N(1)-Sn(1)-Cl(3) | 85.35(16) | N(3)-Sn(2)-Cl(4) | 83.76(16) |
| N(2)-Sn(1)-N(1) | 104.6(2) | Cl(2)-Sn(1)-Cl(3) | 90.29(8) | N(4)-Sn(2)-Cl(4) | 89.31(17) |
| C(1)-Sn(1)-Cl(2) | 89.99(15) | Cl(1)-Sn(1)-Cl(3) | 168.56(8) | Cl(5)-Sn(2)-Cl(4) | 94.71(7) |
| N(2)-Sn(1)-Cl(2) | 162.26(18) | C(26)-Sn(2)-N(3) | 173.5(3) | C(26)-Sn(2)-Cl(6) | 94.2(2) |
| N(1)-Sn(1)-Cl(2) | 95.6(3) | C(26)-Sn(2)-N(4) | 100.5(3) | N(3)-Sn(2)-Cl(6) | 85.75(16) |
| C(1)-Sn(1)-Cl(1) | 84.64(15) | N(3)-Sn(2)-N(4) | 73.0(2) | N(4)-Sn(2)-Cl(6) | 81.77(17) |
| N(2)-Sn(1)-Cl(1) | 87.96(16) | C(26)-Sn(2)-Cl(5) | 98.4(2) | Cl(5)-Sn(2)-Cl(6) | 91.04(8) |
| N(1)-Sn(1)-Cl(1) | 93.30(8) | N(3)-Sn(2)-Cl(5) | 88.12(15) | Cl(4)-Sn(2)-Cl(6) | 167.86(7) |
| Cl(2)-Sn(1)-Cl(1) | |||||
| 4 | |||||
| Sn(1)-O(1) | 2.367(3) | Sn(1)-O(3)#1 | 2.181(3) | Sn(1)-Cl(2) | 2.3742(11) |
| Sn(1)-O(3) | 2.048(3) | Sn(1)-C(1) | 2.147(4) | Sn(1)-Cl(3) | 2.4013(12) |
| O(3)-Sn(1)-C(1) | 152.16(15) | O(3)#1-Sn(1)-O(1) | 88.84(10) | O(3)-Sn(1)-Cl(2) | 92.74(8) |
| O(3)-Sn(1)-O(3)#1 | 70.59(11) | O(3)-Sn(1)-Cl(1) | 97.47(8) | C(1)-Sn(1)-Cl(2) | 99.54(14) |
| C(1)-Sn(1)-O(3)#1 | 94.35(15) | C(1)-Sn(1)-Cl(1) | 106.10(12) | O(3)#1-Sn(1)-Cl(2) | 163.04(8) |
| O(3)-Sn(1)-O(1) | 80.27(10) | O(3)#1-Sn(1)-Cl(1) | 90.20(8) | O(1)-Sn(1)-Cl(2) | 85.16(8) |
| C(1)-Sn(1)-O(1) | 76.02(13) | O(1)-Sn(1)-Cl(1) | 177.73(7) | Cl(1)-Sn(1)-Cl(2) | 95.20(5) |
Symmetry code #1: -x, -y+1, -z+1.
Complex 3 crystallizes in the monoclinic space group P21, and the asymmetric unit contains two independent molecules which do not differ from each other significantly (Figure 2). The central Sn atom exists in a
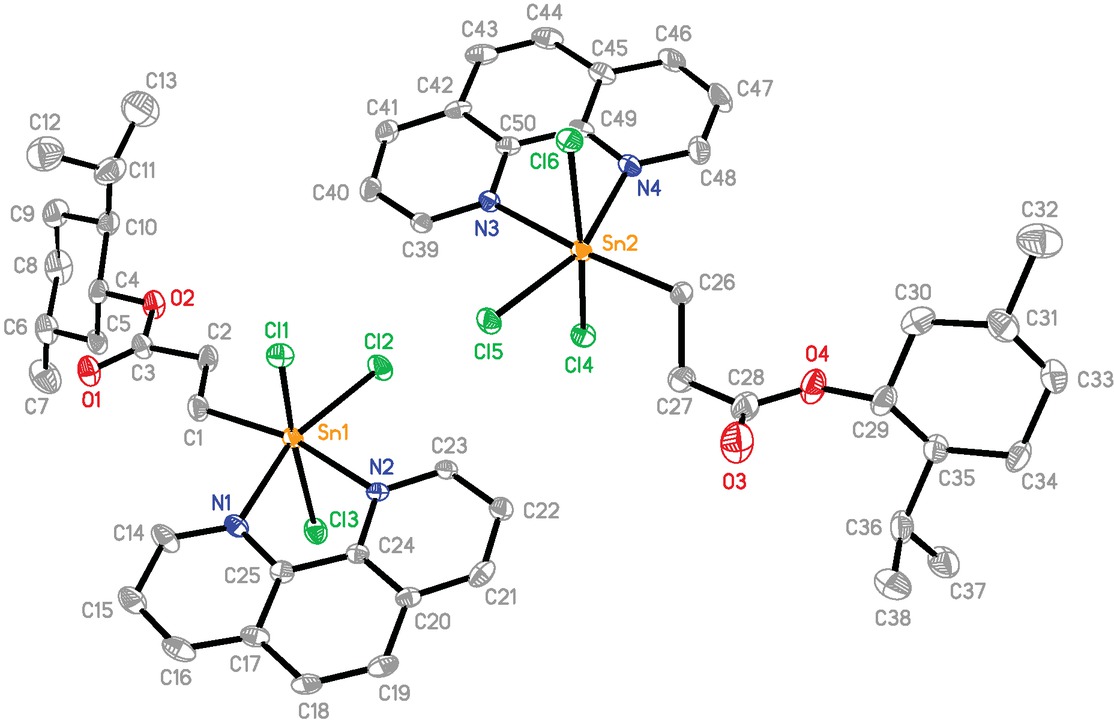
The molecular structure of 3. Ellipsoids are drawn at the 30% probability level. Chloroform and hydrogen atoms are omitted for clarity.
distorted octahedral geometry defined by a carbon atom of menthoxycarbonylethyl group, three Cl atoms and the two N atoms from a chelating phen ligand with the axial bond angles of 160.22(17)-173.5(3)°. The distortions from the ideal geometry may be rationalized by the restricted bite angle N(1)-Sn(1)-N(2) (72.5(2)°) and N(3)-Sn(2)-N(4) (73.0(2)°) of the chelate ligand (Guo et al., 2017; Tian et al., 2016,). The five-membered chelate ring formed by the intramolecular C=O→Sn coordination in the substrate MenOCOCH2CH2SnCl3 (Tian et al., 2005) has been broken and the carbonyl oxygen atom of the ester moiety is not coordinating (Sn(1)···O(1) 4.905 Å and Sn(2)···O(3) 5.249(6) Å). The new five-membered chelate ring consisting of N(1), Sn(1), N(2), C(24) and C(25) or N(3), Sn(2), N(4), C(49) and C(50) is essentially planar, and the maximum deviation from the mean plane is 0.049(3) Å for the Sn(1) atom and 0.033(3) Å for the Sn(2) atom, respectively. The Sn(1)-N(2) (2.231(6) Å) and Sn(2)-N(3)
(2.256(6) Å) interatomic distances involving the N atom trans to C atom are shorter than the Sn(1)-N(1) (2.286(5) Å) and Sn(2)-N(4) bonds (2.291(6) Å) involving the N atom trans to Cl atom. The distances of six bonds around the tin atom are similar to those found in a closely related complex, CH3OCOCH2CH2SnCl3⋅phen (Guo et al., 2017). An arrangement of the Cl3N2C donor atoms in complex 3 is not different from another analogue C6H5CH2SnCl3·phen (Hall and Tiekink, 1996). The tin-bound organic group is trans to a nitrogen atom in 3, while it is trans to a chlorine atom in C6H5CH2SnCl3·phen. Complex 3 is linked into a one-dimensional double-chain supramolecular structure by intermolecular C-H⋅⋅⋅Cl hydrogen bonds (C(16)⋅⋅⋅Cl(2)#1 3.61(1) Å, C(16)-H(16)⋅⋅⋅Cl(2)#1 157.3° and C(46)⋅⋅⋅Cl(5)#2 3.65(2) Å, C(46)-H(46)⋅⋅⋅Cl(5)#1 154.4°, symmetry code #1: x+1, y, z+1; #2: x-1, y, z-1) and π-π stacking interactions between phenyl rings (dihedral angle = 1.14°) from phen ligand with the centroid-centroid separations of 3.540(2) Å (Glowka et al., 1999) (Figure 3).
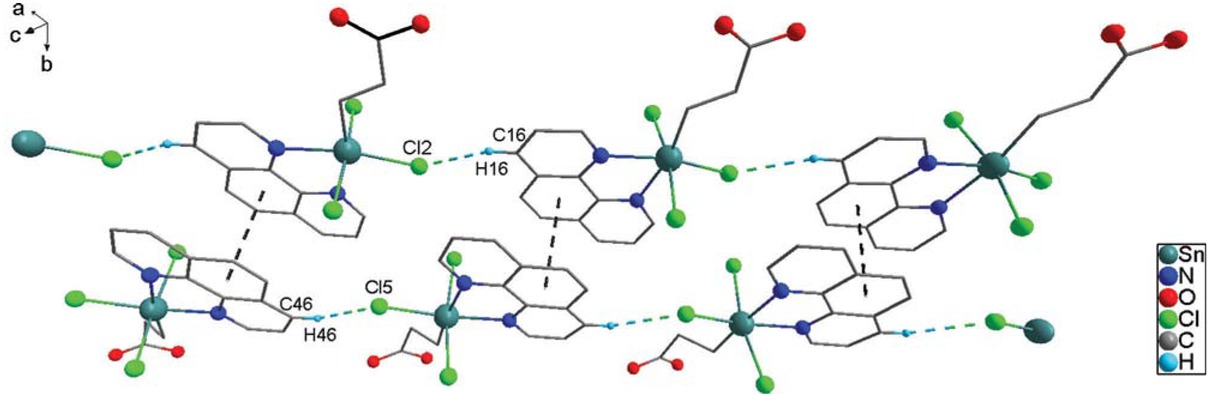
The 1D double-chain supramolecular architecture formed by intermolecular C-H⋅⋅⋅Cl and π-π stacking interactions. Menthyl and hydrogen atoms except H16 and H46 are omitted for clarity.
Compound 4 exists as a centrosymmetric hexa-coordinated dimer with bridging methoxy groups (Figure 4). Each tin atom is coordinated by two chlorine atoms occupying mutually cis-positions, a carbon atom and a carbonyl oxygen atom from the chelating COCH2CH2 moiety. The remaining positions in the distorted octahedral coordination geometry about each tin atom are occupied by two μ2-methoxy groups. The major distortions in the tin atom geometries may be traced to the acute angles induced by the Sn2O2 ring (O(3)-Sn(1)-O(3A) 70.59(11)°) and five-membered chelate CSnO ring (C(1)-Sn(1)-O(1) 76.02(13)°). The Sn-O distances within the Sn2O2 four-membered ring are not symmetrical, with the bond trans to a carbon atom being shorter (Sn(1)-O(3) 2.048(3) Å) than that trans to a chlorine atom (Sn(1A)-O(3) 2.181(3) Å, symmetry code A: -x, -y+1, -z+1), which is comparable with that found in [H2NCOCH2CH2SnCl2(OCH3)]2 (Howie et al., 2011). The Sn(1)-O(1) distance in the five-membered chelate ring is 2.367(3) Å, which is shorter than that (2.403(4) Å) of the hexa-coordinated 1, indicating that the central tin atom in 4 has a stronger Lewis acidity.
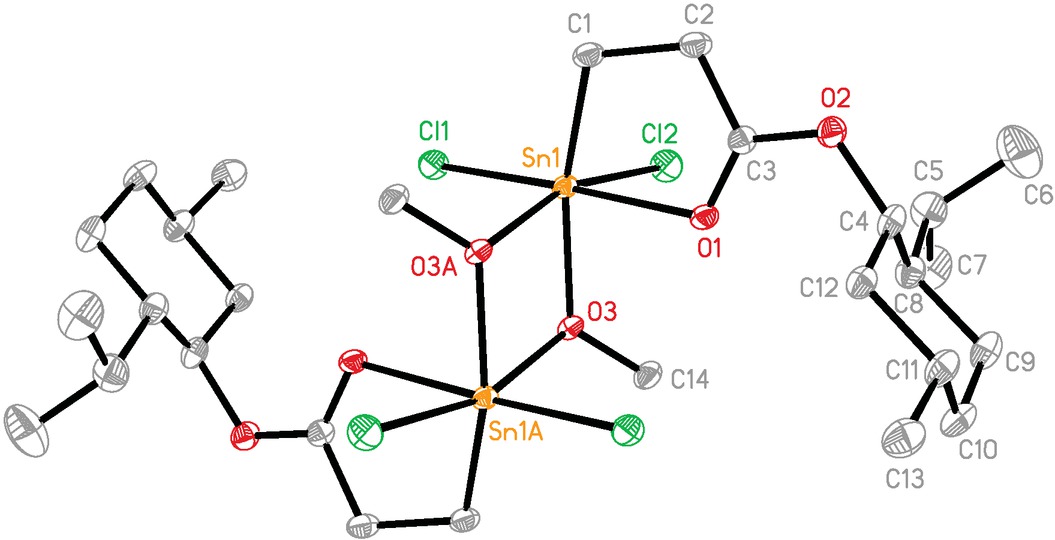
The molecular structure of 4. Ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity.
A = -x, -y+1, -z+1.
3 Conclusion
Four new complexes of 2-(menthoxycarbonyl) ethyltin chloride, MenOCOCH2CH2SnCl3⋅L (1-3) and [MenOCOCH2CH2SnCl2(OCH3)]2 (4), have been synthesized by the reaction of 2-(menthoxycarbonyl)ethyltin trichloride with pbSO, bpy, phen or py in methanol solution and characterized by NMR spectra and single crystal X-ray diffraction analysis. The tin atoms of the complexes are hexa-coordinated and adopt a distorted octrahedron geometry with [CSnCl3O2], [CSnCl3N2], and [CSnCl2O3], respectively. Compounds 1-3 are molecular addition complexes, and 4 is a centrosymmetric dimer with a four-membered Sn2O2 ring. The intramolecular C=O→Sn coordination still exists in 1 and 4, and has been replaced by the chelating bpy and phen ligands, respectively, leaving the carbonyl moieties non-coordinating in 2 and 3.
Experimental
Materials and physical measurements
2-(Menthoxycarbonyl)ethyltin trichloride was prepared according to the reported method (Tian et al., 2005). The other chemicals (Sinopharm Chemical Reagent Company Limited, Shanghai, China) were of reagent grade and were used without further purification. Carbon, hydrogen and nitrogen analyses were obtained using a Perkin Elmer 2400 Series II elemental analyzer (Perkin Elmer, Waltham, Massachusetts, USA). IR spectra were recorded on a Nicolet 470 FT-IR spectrophotometer using KBr discs in the range 4000-400 cm-1 (Thermo Nicolet Corporation, Madison, Wisconsin, USA). 1H and 13C NMR spectral data were collected using a Bruker Avance HD 500 NMR spectrometer (Bruker BioSpin, Switzerland) with CDCl3 as solvent and TMS as internal standard. 119Sn NMR spectra were recorded in CDCl3 on a Varian Mercury Vx300 spectrometer using Me4Sn external reference (Varian Corporation, USA).
Synthesis of the complexes
A solution of 2-(menthoxycarbonyl)ethyltin trichloride (0.872 g, 2 mmol) in methanol (30 mL) was added to the solutions of the ligand (2 mmol) (for bpSO, 0.432 g; for bpy, 0.312 g; for phen, 0.360 g; for py, 0.158 g) in methanol (30 mL) under stirring. The mixture was heated at reflux for 1 h, and then the solution was concentrated under reduced pressure by a rotary evaporator. The residual solution was cooled to room temperature and filtered. The obtained solid was washed with cold methanol and dried in a vacuum dryer for 12 h. The characterization data of the products are shown below (see Scheme 2):
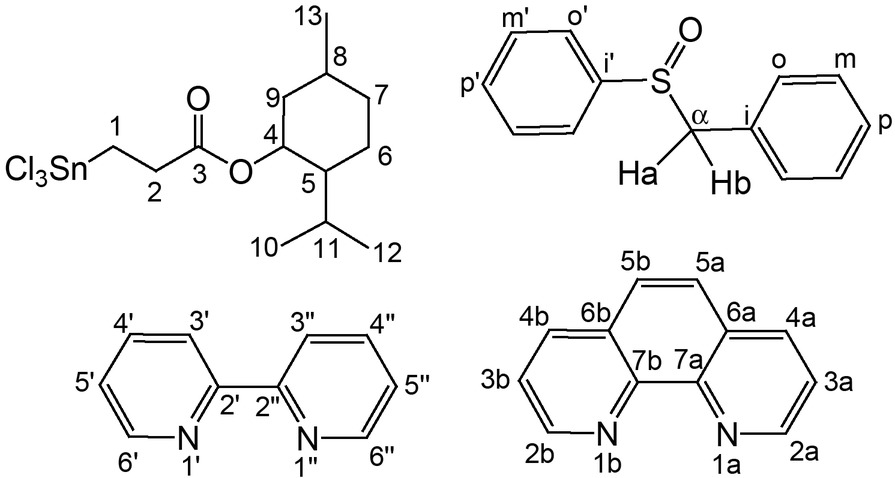
The numbering scheme in 1-4 for the NMR assignment.
MenOCOCH2CH2SnCl3⋅bpSO (1)
Yield 1.069 g (82%), m.p. 106-108°C. Anal. Found: C, 47.23; H, 5.19. Calc. for C26H35Cl3O3SSn: C, 47.84; H, 5.41%. IR (KBr,
ν, cm-1): 1645 (C=O), 955 (S=O). 1H NMR (CDCl3, d, ppm): 0.71 (d, J = 7.0 Hz, 3H, H-13), 0.84 (d, J = 7.0 Hz, 3H, H-12), 0.862.07 (m, 14H, H-1+H-5∼H-11), 2.86 (t, J = 7.5 Hz, J(119/117Sn-1H) = 208/198 Hz, 2H, H-2), 4.15 (AB system, d, J = 12.5 Hz, 1H, H-a), 4.31 (AB system, d, J = 12.5 Hz, 1H, H-b), 4.97 (dt, J = 4.5, 11.0 Hz, 1H, H-4), 6.94 (d, J = 7.0 Hz, 2H, o-H), 7.23 (t, J = 7.5 Hz, 2H, m-H), 7.28-7.33 (m, 3H, p-H + o′-H), 7.43 (t, J = 7.5 Hz, 2H, m′-H), 7.50 (t, J = 7.5 Hz, 1H, p′-H). 13C NMR (CDCl3, d, ppm): 30.11 (C-1), 28.73 (J(119/117Sn-13C) = 78 Hz, C-2), 181.66 (C-3), 80.25 (C-4), 46.99 (C-5), 23.24 (C-6), 33.86 (C-7), 31.29 (C-8), 40.41 (C-9), 20.60 (C-10), 26.41 (C-11), 21.89 (C-12), 16.28 (C-13); 130.80 (i-C), 128.89 (o-C), 128.55 (m-C), 127.18 (p-C), 137.72 (i′-C), 125.39 (o′-C), 129.23 (m′-C), 132.32 (p′-C), 61.57 (α-C). 119Sn NMR (CDCl3, d, ppm): -332.6.
MenOCOCH2CH2SnCl3⋅bpy (2)
Yield 1.055 g (89%), m.p. 194-1195°C. Anal. Found: C, 46.65; H, 5.17, N, 4.69. Calc. for C23H31Cl3N2O2Sn: C, 46.62; H, 5.27; N, 4.73%. IR (KBr, ν, cm-1): 1719 (C=O), 1601, 1446 (C=N+C=C). 1H NMR (CDCl3, d, ppm): 0.74 (d, J = 7.0 Hz, 3H, H-13), 0.83-2.02 (m, 15H, H-5∼H-12), 2.43 (t, J = 7.5 Hz, J (119/117Sn-1H) = 104/99 Hz, 2H, H-1), 3.02 (t, J = 7.5 Hz, J (119/117Sn-1H) = 194/186 Hz, 2H, H-2), 4.70 (dt, J = 4.5, 11.0 Hz, 1H, H-4), 7.80 (t, J = 6.5 Hz, 1H, H-5′), 7.88 (t, J = 6.5 Hz, 1H, H-5′′), 8.23 (t, J = 7.5 Hz, 1H, H-4′), 8.30 (t, J = 7.5 Hz, 1H, H-4′′), 8.42 (d, J = 8.0 Hz, 1H, H-3′), 8.46 (d, J = 8.0 Hz, 1H, H-3′′), 9.24 (d, J = 5.0 Hz, 1H, H-6′), 9.90 (d, J = 5.0 Hz, 1H, H-6′′). 13C NMR (CDCl3, d, ppm): 31.31 (C-1), 30.63 (J(119/117Sn-13C) = 46 Hz, C-2), 174.03 (C-3), 74.48 (C-4), 47.01 (C-5), 23.38 (C-6), 34.25 (C-7), 31.43 (C-8), 40.90 (C-9), 20.84 (C-10), 26.06 (C-11), 22.06 (C-12), 16.36 (C-13); 145.34 (C-2′), 145. 98 (C-2′′), 127.92 (C-3′, C-3′′), 142.43 (C-4′), 142.50 (C-4′′), 123.02 (C-5′), 123.17 (C-5′′), 142.76 (C-6′), 143.25 (C-6′′). 119Sn NMR (CDCl3, d, ppm): -278.3.
MenOCOCH2CH2SnCl3⋅phen (3)
Yield 1.048 g (85%), m.p. 189-190°C. Anal. Found: C, 48.79; H, 4.96, N, 4.49. Calc. for C23H31Cl3N2O2Sn: C, 48.70; H, 5.07; N, 4.54%. IR (KBr, ν, cm-1): 1730 (C=O), 1523, 1429 (C=N+C=C). 1H NMR (CDCl3, d, ppm): 0.73 (d, J = 7.0 Hz, 3H, H-13), 0.83-2.04 (m, 15H, H-5∼H-12), 2.54 (t, J = 7.5 Hz, J(119/117Sn-1H) = 108/102 Hz, 2H, H-1), 3.09 (t, J = 7.5 Hz, J(119/117Sn-1H) = 196/185 Hz, 2H, H-2), 4.72 (dt, J = 4.5, 11.0 Hz, 1H, H-4), 8.10 (dd, J = 5.0, 8.0 Hz, 1H, H-3a), 8.15 (dd, J = 5.0, 8.0 Hz, 1H, H-3b), 8.16 (s, 2H, H-5a+H-5b), 8.73 (d, J = 8.0 Hz, 1H, H-4a), 8.75 (d, J = 8.0 Hz, 1H, H-4b), 9.61(d, J = 5.0 Hz, 1H, H-2a), 10.22 (d, J = 5.0 Hz, 1H, H-2b). 13C NMR (CDCl3, d, ppm): 31.17 (C-1), 30.60 (J(119Sn-13C) = 46 Hz, C-2), 174.12 (C-3), 74.46 (C-4), 47.02 (C-5), 23.37 (C-6), 34.26 (C-7), 31.43 (C-8), 40.90 (C-9), 20.83 (C-10), 26.05 (C-11), 22.05 (C-12), 16.35 (C-13); 145.83 (C-2a), 146.30 (C-2b), 126.04 (C-3a, C-3b), 134.18 (C-4a), 135.32 (C-4b), 127.56 (C-5a), 127.79 (C-5b), 129.81 (C-6a, C-6b), 140.92 (C-7a), 141.14 (C-7b). 119Sn NMR (CDCl3, d, ppm): -293.7.
[(CH3O)Cl2SnCH2CH2COOMen]2 (4)
Yield 0.518 g (60%), m.p. 98-99°C. Anal. Found: C, 38.74; H, 5.89. Calc. for C14H26Cl2O3Sn: C, 38.93; H, 6.07%. IR (KBr,
ν, cm-1): IR (KBr): 1648 (C=O), 1222 (C-O). 1H NMR (CDCl3, d, ppm): 0.77-2.04 (m, 40H, H-1+H-5∼H-13), 2.83 (t, J = 7.5 Hz, J(119/117Sn-1H) = 194/188 Hz, 4H, H-2), 3.79 (s, J(119/117Sn-1H) = 38 Hz, 6H, OCH3), 5.07 (dt, J = 4.5, 11.0 Hz, 2H, H-4). 13C NMR (CDCl3, d, ppm): 26.43 (C-1), 28.17 (C-2), 182.14 (C-3), 81.71 (C-4), 46.90 (C-5), 23.34 (C-6), 33.97 (C-7), 31.41 (C-8), 40.41 (C-9), 20.72 (C-10), 26.40 (C-11), 21.92 (C-12), 16.42 (C-13), 52.35 (CH3O). 119Sn NMR (CDCl3, d, ppm): -364.5.
Crystal structure determination
The colorless single crystals of the complexes were obtained from chloroform or methanol by slow evaporation at room temperature. The intensity data were measured at 295(2) K on a Bruker Smart Apex area-detector fitted with graphite monochromatized Mo-Kα radiation (0.71073 Å) using the ϕ and ω scan technique. The structure was solved by direct method and refined by a full-matrix least squares procedure based on F2 using the SHELXL-97 (Sheldrick, 2008). The non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed at calculated positions. In the complex 4, the menthyl (C(4)-C(13)) is disordered over two conformations. The site occupancies were refined to 0.517(4):0.483(4). In refinements, the C-C bonds and 1,3-distances of the disorderly menthyl were restrained to 1.52(1) and 2.50(2) Å, respectively. The crystallographic parameters and refinements are summarized in Table 2. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 1856984-1856986.
Crystallographic and refinement data of 1,3 and 4.
| Compound | 1 | 3⋅CHCl 3 | 4 |
|---|---|---|---|
| Empirical formula | C26H35Cl3O3SSn | C26H32Cl6N2O2Sn | C28H52Cl4O6Sn2 |
| Formula weight | 652.64 | 735.93 | 863.88 |
| Crystal system | Orthorhombic | monoclinic | triclinic |
| Space group | P212121 | P21 | P-1 |
| a /Å | 8.0424(6) | 8.2101(13) | 8.8045(10) |
| b /Å | 10.3818(7) | 42.742(7) | 10.3430(12) |
| c /Å | 35.337(3) | 9.4003(15) | 11.6693(14) |
| α /(°) | 90 | 90 | 66.019(2) |
| β /(°) | 90 | 105.545(2) | 83.732(2) |
| γ /(°) | 90 | 90 | 72.472(2) |
| Volume /Å3 | 2950.4(4) | 3178.0(9) | 925.75(19) |
| Z | 4 | 4 | 1 |
| Dc / (g⋅cm-3) | 1.469 | 1.538 | 1.550 |
| μ / mm-1 | 1.233 | 1.334 | 1.672 |
| F(000) | 1328 | 1480 | 436 |
| Crystal size / mm | 0.32 × 0.20 × 0.02 | 0.35 × 0.30 × 0.25 | 0.38 × 0.22 × 0.04 |
| θ range /(°) | 2.04 to 26.00 | 1.91 to 26.00 | 2.25 to 26.00 |
| Tot. reflections | 19018 | 24106 | 5848 |
| Uniq. Reflections, Rint | 5800, 0.0728 | 12371, 0.0245 | 3596, 0.0308 |
| Reflections with I>2σ(I) | 4420 | 11499 | 3338 |
| GOF on F2 | 0.981 | 1.099 | 1.122 |
| Flack parameter | -0.03(3) | 0.06(2) | - |
| R indices [I>2σ(I)] | R=0.0479, wR=0.0994 | R=0.0530, wR=0.1309 | R=0.0346, wR=0.0934 |
| R indices (all data) | R=0.0678, wR=0.1075 | R=0.0569, wR=0. 1331 | R=0.0371, wR=0. 0949 |
| Δρmin, Δρmax/(e·Å-3) | -0.323, 0.568 | -1.757, 0.875 | -0.558, 0.783 |
Acknowledgments
This work was supported by the Experimental Teaching Reform Project of Qufu Normal University (sj201401), the Undergraduate Innovation Project in Qufu Normal University (201710446040), and Shandong Provincial Natural Science Foundation, China (ZR2013BM007).
References
Airapetyan D.V., Petrosyan V.S., Gruener S.V., Korlyukov A.A., Arkhipov D E., Bowden A.A., et al., Structure of hypercoordinated monoorganodihalostannanes in solutions and in the solid state: the halogen effect. Inorg. Chim. Acta, 2015, 432, 142-148.10.1016/j.ica.2015.04.006Search in Google Scholar
Balasubramanian R., Chohan Z.H., Doidge-Harrison S.M.S.V., Howie R.A., Wardell J.L., Some further studies of estertin compounds: crystal structures of [NEt4[MeO2CCH2CH2Sn(dmio)2 (dmio = 1,3-dithiole-2-one-4,5-dithiolato) and (MeO2CCH2CH22SnX2 [X2 = I2 (NCS)2 or Cl, Br]. Polyhedron, 1997, 16, 4283-4295.10.1016/S0277-5387(97)00226-XSearch in Google Scholar
Buchanan H., Howie R.A., Khan A., Spencer G.M., Wardell J.L., Preparations and crystal structures of Sn(CH2CH2CO2Me)2(C3S5 and [Q][Sn(CH2CH2CO2Me)(C3S52 (Q = NEt4 or 1,4-dimethylpyridinium, C3S5= 4,5-disulfanyl-1,3-dithiole-2-thionate). J. Chem. Soc., Dalton Trans, 1996, 541-548.10.1039/dt9960000541Search in Google Scholar
Chandrasekar S., Balachandran V., Evans H.-S., Latha A., Synthesis, crystal structures HOMO-LUMO analysis and DFT calculation of new complexes of p-substituted dibenzyltin chlorides and 1,10-phenanthroline. Spectrochim. Acta A, 2015, 143, 136-146.10.1016/j.saa.2015.01.112Search in Google Scholar
Davies A.G., Organotin Chemistry (2nd ed.). Wiley-VCH, Weinheim, 2004, P174. DOI:10.1002/978047075809010.1002/9780470758090Search in Google Scholar
de Morais B.P., Donnici C.L., Rodrigues B.L., de Lima G.M., Wardell J.L., Bitzer R.S., Transesterification reactions of dimethoxycarbonylethyltin pyrrolidinodithiocarbamate, [Sn(PDTC)2(CH2CH2COOCH32 spectroscopic and X-ray structural characterization of new estertin dithiocarbamate complexes. J. Organometal. Chem., 2017, 832, 57-65.10.1016/j.jorganchem.2016.12.034Search in Google Scholar
Fuller A.L., Aitken R.A., Ryan B.M., Slawin A.M.Z., Woollins J.D., The X-Ray Structures of Sulfoxides. J. Chem. Crystallogr., 2009, 39, 407-415.10.1007/s10870-008-9493-9Search in Google Scholar
Garad M.V., Gopinathan M.S., Gopinathan C., Molecular addition complexes of β-carbalkoxyethyltin chlorides. Indian J. Chem., 1981, 20A, 412-414.Search in Google Scholar
Glowka M.L., Martynowski D., Kozaowska K., Stacking of six-membered aromatic rings in crystals. J. Mol. Struct., 1999, 474, 81-89.10.1016/S0022-2860(98)00562-6Search in Google Scholar
Guo M., Lin Y., Yao Y., Tian L., The crystal structures of two 2-methoxycarbonylethyltin trichloride adducts. Main Group Met. Chem., 2017, 40, 101-104.10.1515/mgmc-2017-0024Search in Google Scholar
Hall V.J., Tiekink E.R.T., Benzyltriehloro(1,10-phenanthroline-N,N’) tin(IV) benzene solvate. Acta Crystallogr. C, 1996, 52, 2141-2143.10.1107/S0108270195009334Search in Google Scholar
Harrison P.G., King T.J., Healy M.A., Structural studies in main group chemistry: Estertin derivatives, structural and spectroscopic studies. J. Organometal. Chem., 1979, 182, 17-36.10.1016/S0022-328X(00)85873-8Search in Google Scholar
Holecek J., Nadvornik M., Handlir K., Lycka A., 13C and 119Sn NMR spectra of di-n-butyltin(IV) compounds. J. Organomet. Chem., 1986, 315, 299-308.10.1016/0022-328X(86)80450-8Search in Google Scholar
Howie R.A., Wardell S.M.S.V., (2-Carbomethoxyethyl)triiodotin at 120 K. Acta Crystallogr. E, 2002, 58, m220-m222.10.1107/S1600536802007080Search in Google Scholar
Howie R.A., de Lima G.M., Wardell J.L., Wardell S.M.S.V., Harrison W.T.A., Solid state study of sulfoxide adducts of (2-amidoethyl-C,O)trihalostannanes: supramolecular networks constructed from hydrogen-bonds involving the amido units. J. Organometal. Chem., 2012, 716, 62-69.10.1016/j.jorganchem.2012.05.050Search in Google Scholar
Howie R.A., Harrison W.T.A., Lima G.M.D., Wardell J.L., Wardell S.M.S.V., Four distannoxane derivatives containing the bidentate 2-amidoethyl ligand. Z Kristallogr., 2011, 226,739-747.10.1524/zkri.2011.1408Search in Google Scholar
Hutton R.E., Burley J.W., Oakes V., β-substituted alkyltin halides: I. Monoalkyltin trihalides: Synthetic, mechanistic and spectroscopic aspects. J. Organometal. Chem., 1978, 156, 369-382.10.1016/S0022-328X(00)93543-5Search in Google Scholar
Lima G.M.D., Milne B.F., Pereira R.P., Rocco A.M., Skakle J.M.S., Travis A.J., et al., Experimental and ab initio structural study of estertin compounds, X3SnCH2CH2CO2Me: Crystal structures of Cl3SnCH2CH2CO2Me at 120 K and Br3SnCH2CH2CO2Me at 120 and 291 K. J. Mol. Struct., 2009, 921, 244-250.10.1016/j.molstruc.2008.12.064Search in Google Scholar
Liu Q., Shi X., Zhang C., Tian L., Synthesis and structure of [2-((L)-menthoxycarbonyl)ethyl]diphenyltin halides. Main Group Met. Chem., 2015, 38, 93-97.10.1515/mgmc-2015-0020Search in Google Scholar
Lu N., Chang W.-H., Wei R.-J., Fang Y.-C., Han T.-W., Wang G.-Q., et al., Pyridinium saccharinate salts as efficient recyclable acylation catalyst: a new bridge between heterogeneous and homogeneous catalysis. Tetrahedron, 2016, 72, 3468-3476.10.1016/j.tet.2016.04.073Search in Google Scholar
Maughan D., Wardell J.L., Burley J.W., Lewis acidity of carboxyethyltin chlorides, Cl3SnCH2CH2CO2R and Cl2Sn(CH2CH2CO2R)2 J. Organomet. Chem., 1981, 212, 59-70.10.1016/S0022-328X(00)85526-6Search in Google Scholar
Sheldrick G.M., A short history of SHELX. Acta Crystallogr. A, 2008, 64, 112-122.10.1107/S0108767307043930Search in Google Scholar PubMed
Sousa G.F.D., Sabino J.R., Vencato I., Filgueiras C.A.L., Ardisson J.D., Synthesis, structural and spectral studies of five- and six-coordinate adducts of organotin(IV) halides containing dibenzylsulfoxide (dbso) as ligand. J. Braz. Chem. Soc., 2009, 20, 1425-1433.10.1590/S0103-50532009000800006Search in Google Scholar
Tian L.-J., Sun Y.-X., Liu X.-J., Yang G.-M., Shang Z.-C., Synthesis and structural characterization of 2-(-)-menthoxycarbonylethyltin compounds. Polyhredron, 2005, 24, 2027-2034.10.1016/j.poly.2005.05.025Search in Google Scholar
Tian L., Zhang S., Yao Y., Shi X., Synthesis and crystal structure of [2-(menthoxycarbonyl)ethyl]tris(8-quinolinato)tin. Main Group Met. Chem., 2016, 39, 209-212.10.1515/mgmc-2016-0030Search in Google Scholar
Tian L., Yao Y., Wang Y., Liu J., Synthesis, structure and property of diorganotin complexes with chiral N-(5-chlorosalicylidene) valinate ligand. J. Mol. Struct., 2018, 1156, 441-449.10.1016/j.molstruc.2017.11.132Search in Google Scholar
Van Koten G., Noltes J.G., Novel chiral triorganotin halides. Stabilization of optically active tin centers by intramolecular coordination. J .Am. Chem. Soc., 1976, 98, 5393-5395.10.1021/ja00433a058Search in Google Scholar
Varga R.A., Silvestru C., Deleanu C., Synthesis, solution behaviour and X-ray structures of [2-(Me2NCH2C6H4SnCl3 and [2-(Me2NCH2 C6H4SnCl3⋅DMSO. Appl. Organometal. Chem., 2005, 19, 153-160.10.1002/aoc.817Search in Google Scholar
Wang L., Chen M., Zhang P., Li W., Zhang J., Palladium/PC-Phos– catalyzed enantioselective arylation of general sulfenate anions: scope and synthetic applications. J. Am. Chem. Soc., 2018, 140, 3467-3473.10.1021/jacs.8b00178Search in Google Scholar PubMed
Wang P., Shu H., Zhu G., Zhou Z., Two dimensional nuclear magneitc resonance analysis of bis(2,2′-bipyriidne)(2,2′-bipyriidne-4,4-dicarboxylic acid) rurthenium(II). Chinese J. Anal. Chem., 1999, 27, 1043-1046.Search in Google Scholar
© 2019 Lin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Article
- Optimization of pH, temperature and carbon source for bioleaching of heavy metals by Aspergillus flavus isolated from contaminated soil
- A new Pb(II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: crystal structure and anti-lung cancer activity
- A new Pb(II)-based coordination polymer constructed by 5-((4-carboxyphenoxy)methyl)benzene-1,3-dioic acid and N-donor co-ligand: crystal structure and anti-glioma activity
- Calcium(II)-mediated resolution of methyl o-chloromandelate with chiral O,O′-dibenzoyltartaric acid in preparative scale
- Sensor application of doped C60 fullerenes in detection of 1-(3-trifluoromethylphenyl)piperazine as an alternative to ecstasy
- Synthesis, spectroscopic characterization and computational studies of Schiff base complexes of tin(IV) chloride
- Synthesis and structural characterization of the complexes of 2-(menthoxycarbonyl)ethyltin chloride
- Bismuth⋅⋅⋅π arene interaction in [Bi{OC6H4(CH2C6H5)-2}3]2
- Synthesis, characterization and electroluminescence properties of a new mixed-ligand diorganotin(IV) complex
- Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb(II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands
- The interaction of morin and morin-5’-sulfonic acid with lead(II): Study of the 1:1 complex formation process in aqueous solution
- Synthesis and biological evaluation of Piroxicam derivative as a lead chelator
- The stabilities and geometries of Re-encapsulated Sin(n=16, 20, 24, 28, 32, 36, and 40) clusters: A computational investigation
- Synthesis, structural characterization, cytotoxicity and encapsulation studies of N,Nʹ-(1, 2-dicyano-1,2-vinylene)-bis(4-hydroxysalicylideneaminato) di(p-chlorobenzyl)tin as potential anticancer drug
- Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD
- Transition metals doped ZnO nanocluster for ethylene oxide detection: A DFT study
- Thermal rearrangement of a 1,2-bis(silylene): Synthesis and crystal structure of a silyl-silylene
- Toxicity of arsenic on isolated human lymphocytes: The key role of cytokines and intracellular calcium enhancement in arsenic-induced cell death
- Theoretical insight of alpha amino acid phenylalanine adsorption on pristine and decorated fullerenes
- DFT/QTAIM analysis of favipiravir adsorption on pristine and silicon doped C20 fullerenes
- New insights into the oxidation of phenoxatellurine with sulfuric acid
Articles in the same Issue
- Research Article
- Optimization of pH, temperature and carbon source for bioleaching of heavy metals by Aspergillus flavus isolated from contaminated soil
- A new Pb(II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: crystal structure and anti-lung cancer activity
- A new Pb(II)-based coordination polymer constructed by 5-((4-carboxyphenoxy)methyl)benzene-1,3-dioic acid and N-donor co-ligand: crystal structure and anti-glioma activity
- Calcium(II)-mediated resolution of methyl o-chloromandelate with chiral O,O′-dibenzoyltartaric acid in preparative scale
- Sensor application of doped C60 fullerenes in detection of 1-(3-trifluoromethylphenyl)piperazine as an alternative to ecstasy
- Synthesis, spectroscopic characterization and computational studies of Schiff base complexes of tin(IV) chloride
- Synthesis and structural characterization of the complexes of 2-(menthoxycarbonyl)ethyltin chloride
- Bismuth⋅⋅⋅π arene interaction in [Bi{OC6H4(CH2C6H5)-2}3]2
- Synthesis, characterization and electroluminescence properties of a new mixed-ligand diorganotin(IV) complex
- Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb(II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands
- The interaction of morin and morin-5’-sulfonic acid with lead(II): Study of the 1:1 complex formation process in aqueous solution
- Synthesis and biological evaluation of Piroxicam derivative as a lead chelator
- The stabilities and geometries of Re-encapsulated Sin(n=16, 20, 24, 28, 32, 36, and 40) clusters: A computational investigation
- Synthesis, structural characterization, cytotoxicity and encapsulation studies of N,Nʹ-(1, 2-dicyano-1,2-vinylene)-bis(4-hydroxysalicylideneaminato) di(p-chlorobenzyl)tin as potential anticancer drug
- Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD
- Transition metals doped ZnO nanocluster for ethylene oxide detection: A DFT study
- Thermal rearrangement of a 1,2-bis(silylene): Synthesis and crystal structure of a silyl-silylene
- Toxicity of arsenic on isolated human lymphocytes: The key role of cytokines and intracellular calcium enhancement in arsenic-induced cell death
- Theoretical insight of alpha amino acid phenylalanine adsorption on pristine and decorated fullerenes
- DFT/QTAIM analysis of favipiravir adsorption on pristine and silicon doped C20 fullerenes
- New insights into the oxidation of phenoxatellurine with sulfuric acid

