Synthesis and characterization of a new tin(IV) complex with anthracene-9-carboxylic acid as a precursor in the preparation of an organic light-emitting diode
Abstract
To prepare a new tin(IV) complex (1), [(SnMe2)2(C15H9O2)(OCH2CH3)(O)]2, with strong optical absorption, anthracene-9-carboxylic acid (HL) with a large conjugated π-system and rich optical properties has been used as a ligand. The characterization of title complex was performed by various spectroscopic methods, including 1H NMR, 13C NMR, 119Sn NMR, UV, IR, and elemental analyses (CHN). The crystal structure of complex 1 was determined by single-crystal X-ray diffraction. The results of spectroscopic data along with structural analyses showed that complex 1 is a tetranuclear, centrosymmetric dimeric and contains two Sn atoms, one in a five-coordinate environment and the other in a six-coordinate environment. Thermogravimetric and differential thermal analyses (TGA and DTA) have been used to study the thermal behavior of 1. The investigation of photoluminescence properties and thermal behavior of complex 1 showed that this compound has the necessary conditions for its use as a precursor in the manufacture of optical devices. This compound was used as an emitting layer for the preparation of an organic light-emitting diode. The effect of the complex concentration on the electrical properties of the diode was investigated.
Introduction
During the past two decades, the synthesis and design of compounds with strong optical absorption is one of the most important concerns of chemists and scientists (Koh and Vittal, 2008; Zhou et al., 2016; Hayati et al., 2017a,b). These are due to the increasing demands of human society to light and optical devices and widespread use of these compounds in a variety of fields such as transistors, lasers, telecommunication technology and fluorescent sensors for highly specific probes and bioanalysis (Sreejith et al., 2016; Lee et al., 2016; Hanifehpour et al., 2017). One of the most important factors in the preparation of optical materials with high usability is the selection of proper ligand and metal. Until now, substantial attempts have been dedicated to the combination of appropriate organic ligands and metal ions for the preparation of potential coordination compounds with relevant properties (Ngoune et al., 2016; Im and Lee, 2016; Tatikonda et al., 2016). Lewis acid property of tin compounds provides the necessary conditions for their use in the preparation of organotin coordination polymers with carboxylic acid ligands (Carcelli et al., 2001; Boshra et al., 2010; Miller et al., 2016; Deng et al., 2017). The anthracene-9-carboxylate anion (L−) represents a ligand having a fused ring whose conjugated π-system influences the coordinating behavior of the resulting metal carboxylate. More importantly, the conjugated π-system is a fluorophore that can be exploited for use in fluorescence signaling, a theme of interest in the development of fluorescent materials for applications in electroluminescence (Herrmann et al., 1984; Byrnes et al., 2004; Liu et al., 2007; Wang et al., 2008). Therefore, the anthracene-9-carboxylic acid (HL) can be a proper ligand in the preparation of coordination compounds with different metal ions with strong optical absorption.
In this work, following our interest in the preparation of luminescence coordination compounds, a new dimethyltin(IV) complex with HL ligand was prepared. Investigating the optical properties of the prepared compound showed that this compound has the necessary optical capability to be used in the manufacture of optical devices. In order to prove this point, this complex was used as an emitting layer in the construction of an organic light-emitting diode (OLED), and its electroluminescence property was investigated.
Results and discussion
Synthesis
A new tin(IV) complex (Scheme 1) has been successfully synthesized with 9-anthracenecarboxylic acid ligand. All spectroscopic data of prepared tin(IV) complex showed that this complex has the formula of [(SnMe2)2(C15H9O2)(OCH2CH3)(O)]2.
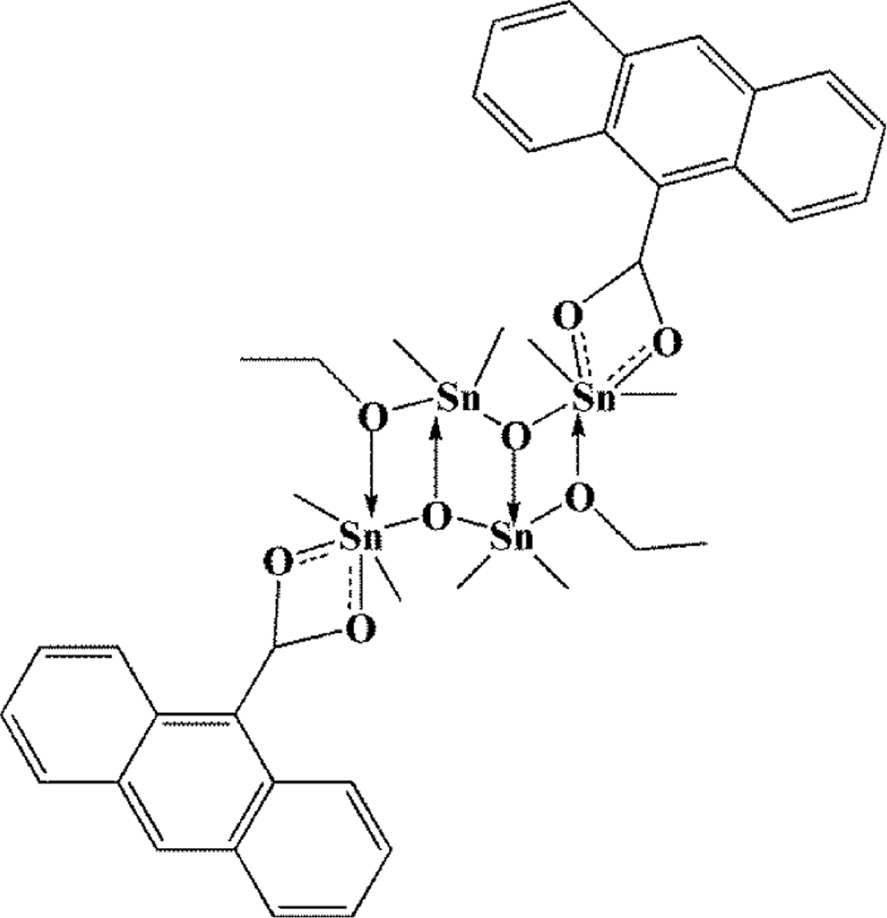
Line drawing structure of the prepared tin(IV) compound.
Description of crystal structure of tin complex
Crystal structure analysis of the prepared compound displays the attendance of a tetranuclear complex that crystallizes in the triclinic space group P1̅. Crystal data and refinement parameters are provided in Table 1. Selected angles and bond lengths are listed in Table 2. The crystal structure of the complex can be considered as a tetramer that was made of a building block of Sn2O2, as a dimeric unit with a center of inversion and a three-rung-staircase Sn4O4 core. The four tin atoms and four oxygen atoms are basically coplanar. The prepared complex belongs to the class of dimeric tetraorganodistannoxanes with a ‘ladder structure’. In spite of these compounds being well known and well described in literature with different carboxylate ligands, a survey of the literature shows that there is not any report of tetraorganodistannoxanes with anthracene-9-carboxylate ligand. However, until now, several anthracene-9-carboxylate metal complexes of zinc, cadmium, and nickel with interesting structural and optical features have been reported (Herrmann et al., 1984; Byrnes et al., 2004; Liu et al., 2007; Wang et al., 2008). As can be seen in Figure 1, the asymmetric unit of the compound, [(SnMe2)2(C15H9O2)(OCH3)(O)]2, comprises two independent Sn(IV) atoms, one 9-anthracenecarboxylic acid ligand, one ethoxy anion, and one oxo oxygen atom. The distannoxane features two Sn atoms, one in a five-coordinate environment and the other in a six-coordinate environment. The six-coordinate Sn atom (Sn2) as a terminal tin exists in a distorted octahedral geometry for which the O atoms comprise the rhombus-shaped plane; one of the Sn-O bonds is somewhat long (2.847(3) Å) as the carboxyl unit engages in anisobidentate chelation. The two methyl C atoms are located over the longest side of the plane (Figure 1). Geometry around the five-coordinate central tin atom is a distorted trigonal bipyramid. The axial positions of the tin atom, Sn(1), were occupied by one oxygen atom from bridging ethoxy and one oxo oxygen atom with bond angle of 147.08°, which in comparison to the ideal value indicates a significant deviation. Two carbon atoms, C1 and C2, from the methyl groups attached to the tin atom and an oxo oxygen atom occupied equatorial positions. Ethoxy groups through the oxygen atom are bridges between exo-cyclic and endo-cyclic tin atoms, Sn1-O4 (2.141(3) Å) and Sn2-O4 (2.207(3) Å). Each bridging oxygen atom in the Sn2O2 ring is attached to three Me2Sn units, and as a result these oxygen atoms are three coordinated. The carboxylate ion is disordered over two positions such that the anthracene portion is twisted along the axis linking the carbon atoms at the 1- and 9-positions in a 1:1 ratio and connected to the exo-cyclic tin atoms by using two carboxylate O atom in bidentate coordination mode. The ethoxide ion is also disordered but in a slightly different ratio. Restraints were imposed. As can be seen in Figure 1, the dimeric units are additionally connected to a center of inversion to produce a ladder-like structure. All bond lengths in the tetranuclear distannoxane are in the same range found for other diorganotin ladder structures reported in the literature (Table 2) (Zhang et al., 2008; Wang et al., 2013). As shown in Figure 2, the interaction between the oxygen atom of the carboxylate ligand with the tin atom of a neighboring molecule forms a chain running along the b axis of the triclinic unit cell. This structural feature of 1 is quite similar to that of {(Me2Sn)2(O)[(COO)(CH2C5H3NCl)]2}n, a one-dimensional infinite chain in which the carboxylic oxygen atoms link the dimeric tetraorganodistannoxane units (Zhang et al., 2011). The junctions between the one-dimensional infinite chains by the hydrogen bonding interactions (Table 3) lead to their growth into a 3D supramolecular network.
Crystal data and refinement details for tin(IV) complex.
| Chemical formula | C42H52O8Sn4 |
| Mr | 1159.60 |
| Crystal system, space group | Triclinic, P1̅ |
| Temperature (K) | 100 |
| a, b, c (Å) | 7.4956 (4), 11.3698 (6), 14.3530 (9) |
| α, β, γ (°) | 68.342 (5), 79.658 (5), 74.256 (4) |
| V (Å3) | 1089.89 (11) |
| Z | 1 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.31 |
| Crystal size (mm) | 0.40×0.20×0.05 |
| No. of measured, independent and observed [I>2σ(I)] reflections | 17828, 5051, 3981 |
| Rint | 0.056 |
| (sin θ/λ)max (Å−1) | 0.651 |
| Refinement | |
| R[F2 >2σ(F2)], wR(F2), S | 0.044, 0.124, 1.06 |
| No. of reflections | 5051 |
| No. of parameters | 241 |
| No. of restraints | 112 |
| Δρmax, Δρmin (e Å−3) | 1.90, −1.09 |
Selected bond lengths (Å) and bond angles (°) for complex 1.
| Bond lengths (Å) | |||
| Sn(1)-O(3) | 2.030(4) | Sn(1)-O(4) | 2.139(5) |
| Sn(2)-O(1) | 2.190(4) | Sn(2)-O(4) | 2.208(4) |
| Sn(2)-O(3) | 2.030(4) | Sn(2)-C(4) | 2.109(6) |
| Sn(1)-C(1) | 2.117(7) | Sn(1)-C(2) | 2.119(7) |
| Sn(2)-C(3) | 2.102(6) | ||
| Bond angles (°) | |||
| O(3)-Sn(1)-O(4) | 73.39(15) | O(3)-Sn(1)-C(1) | 113.3(2) |
| O(3)-Sn(1)-C(2) | 120.5(2) | O(4)-Sn(1)-C(1) | 98.1(2) |
| O(4)-Sn(1)-C(2) | 97.4(2) | C(1)-Sn(1)-C(2) | 126.2(3) |
| O(1)-Sn(2)-O(3) | 81.20(17) | O(1)-Sn(2)-O(4) | 153.14(17) |
| O(1)-Sn(2)-C(3) | 95.8(2) | O(1)-Sn(2)-C(4) | 94.2(2) |
| O(3)-Sn(2)-O(4) | 71.95(16) | O(3)-Sn(2)-C(3) | 105.5(2) |
| O(3)-Sn(2)-C(4) | 107.0(2) | O(4)-Sn(2)-C(3) | 91.8(2) |
| O(4)-Sn(2)-C(4) | 93.3(2) | C(3)-Sn(2)-C(4) | 147.1(3) |
| Sn(2)-O(1)-C(5) | 108.0(3) | ||
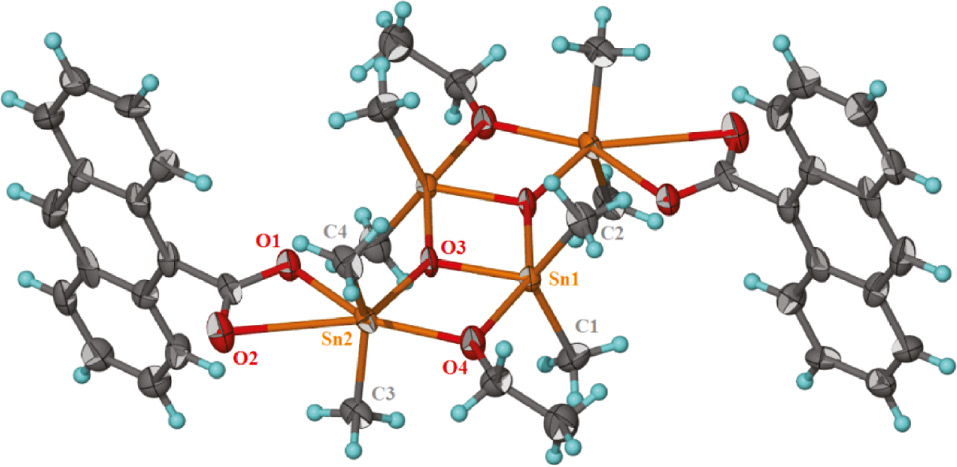
Molecular structure of complex 1.
Thermal ellipsoids scaled up to the 40% probability level.
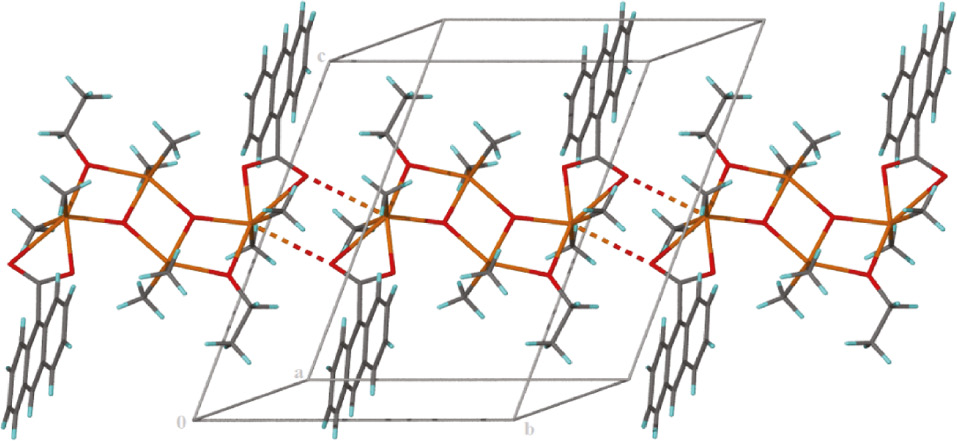
A perspective view of the interaction between the oxygen atom of the carboxylate ligand with the tin atom of a neighboring molecule to form a chain running along the b axis.
Significant intermolecular interactions (interatomic) distance (Å) and bond angles (°) in complex 1.
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| C(3)-H3B)…O(2)a | 0.98 | 2.58 | 3.207(7) | 122 |
Symmetry transformations used to generate equivalent atoms: a1−x, −y, 1−z.
Spectroscopic data of prepared complex
The results of CHN analysis are in good agreement with the results of the X-ray analyses. The distinct difference between the IR spectrum of the title complex and free ligand and also the presence of the stretching vibration of Sn-O at 461 cm−1 indicate unambiguously the coordination of 9-anthracenecarboxylic acid ligand to Sn(IV) ion. The Sn-O absorption band appeared at frequencies similar to those discerned in other Sn(IV) complexes. A Sn-O-Sn bridged structure is suggested by the presence of a strong stretching vibration at about 631 cm−1 that is relevant to the ν(Sn-O-Sn) (Azadmeher et al., 2008). The IR spectra can provide detailed information about the carboxylate group. The values of the asymmetric stretching vibration (νas), the symmetric stretching vibration (νs), and ∆ν(νas(CO2)–νs(CO2)) of compound 1 are 1668, 1480, and 188 cm−1, respectively. The value of ∆ν is less than the free acid and can be ascribed to the bidentate coordination mode of the carboxylate groups (Scheme 1) (Dudev and Lim, 2007; Karthikeyan et al., 2016). The tin carboxylate formation can be confirmed by the red shift of νas and νs of complex 1 in comparison with those of free 9-anthracenecarboxylic acid. There is a good agreement between the results obtained from IR spectra and X-ray crystal structural analyses.
The provision of valuable information about metal-ligand binding and determination of additional details of structural properties of complex 1 in the solution, the 1H, 13C, and 119Sn NMR spectra of the prepared organotin(IV) complex were recorded.
The 1H NMR spectrum of the complex exhibits the anticipated aliphatic and aromatic peaks with the exact integration which somewhat shifted to the low field relative to those of free ligand. The reason of this shift can be related to the electron density transfer from the ligands to the tin atom as an acceptor. The presence of two different types of tin atoms in the 1H NMR spectrum of the complex can be confirmed by the attendance of two singlet signals at 0.61 and 0.95 ppm which are assigned to the methyl groups attached to a tin atom (CH3Sn). As previously reported, 87 and 98 Hz values for 2J117/119Sn-H are associated with the presence of five coordinated tin atoms in the structure of the compound (Li et al., 2016). The results of 13C NMR spectrum of the complex confirmed 1H NMR data. The 119Sn NMR data of the complex demonstrate two signals for two nonequivalent tin atoms at −142.8 and −149.1 ppm. These chemical shifts indicate the presence of two five-coordinated tin atoms (Ma et al., 2003; Coza et al., 2015).
Thermal inspection of prepared complex
In order to prove that the prepared compound has the necessary thermal stability to be used in the manufacture of optical devices, thermal analysis of the title complex was carried out under air atmosphere from room temperature up to 800°C (Figure 3). As can be seen, the prepared tin complex is stable to approximately 200°C. The first endothermic peak in the DTA curve at 210°C without a mass loss in the TGA curve is associated with complex melting. The decomposition of the framework and the loss of organic components of the complex show an extensive exothermic peak in the DTA curve together with a significant weight loss in the TGA curve in the temperature range of 450–650°C. Finally, the remaining residue can be attributed to tin(IV) oxide particles. The results of this investigation showed that the prepared coordination polymer has proper thermal stability for the preparation of optical devices such as OLEDs.
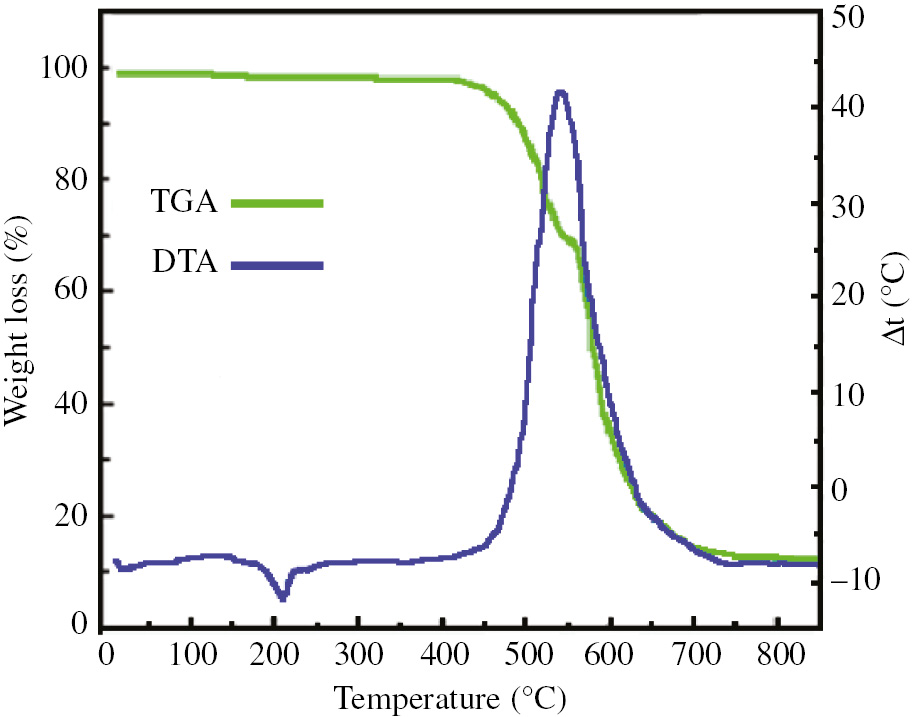
TGA and DTA diagrams of complex 1.
Photophysical properties
The UV-Vis absorption spectra of complex 1 and free HL ligand were recorded in the solid state at room temperature. As can be seen in Figure 4, K-band and B-band of 9-anthracenecarboxylic acid at 258 and 368 nm, respectively, are present in its spectrum. The origin of these bands can be attributed to the n→π* and π→π* transitions (Kubota et al., 2015). Absorption spectrum of the prepared compound with the 9-anthracenecarboxylic acid ligand showed similar bands with a slight shift which indicate the origin of their transitions is also relevant to n→π* and π→π* transitions of ligand. A barely blue-shift in the maximum of absorption bands of complex 1 relative to those of ligand probably is due to the formation of a strict conjugated system of ligands after coordination to tin(IV) atom. The room temperature solid state photoluminescence of 9-anthracenecarboxylic acid ligand and complex 1 show peaks at 492 and 520 nm, respectively; the source of these peaks can be related to the spin allowed π-π* transitions of the ligand (Figure 5). The nature of these transitions cannot be attributed to d-d transitions or metal-to-ligand charge transfer, inasmuch as Sn(IV) is hard to reduce or to oxidize because of the full electronic configuration of Sn(IV). However, metallic elements can sensitize the emission intensity of their coordinated organic ligands.
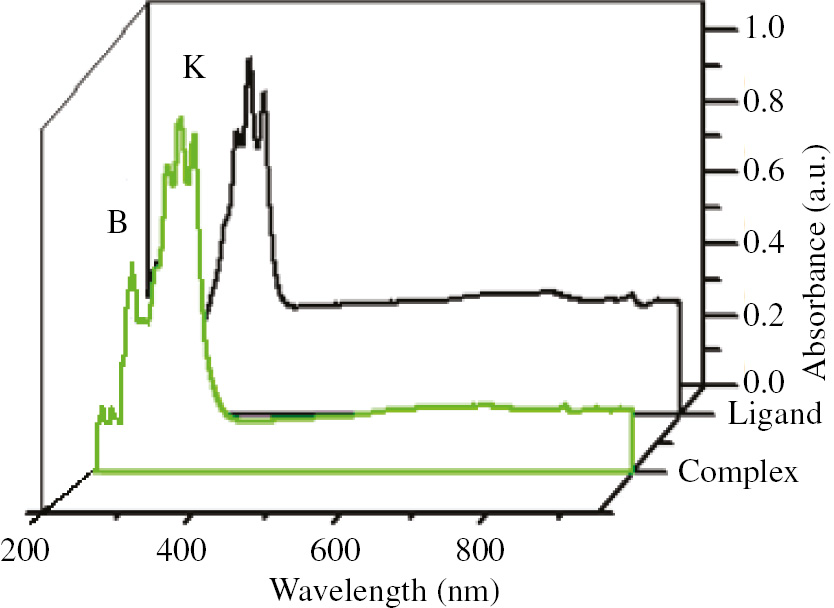
The absorption spectra of complex 1 and free HL ligand.
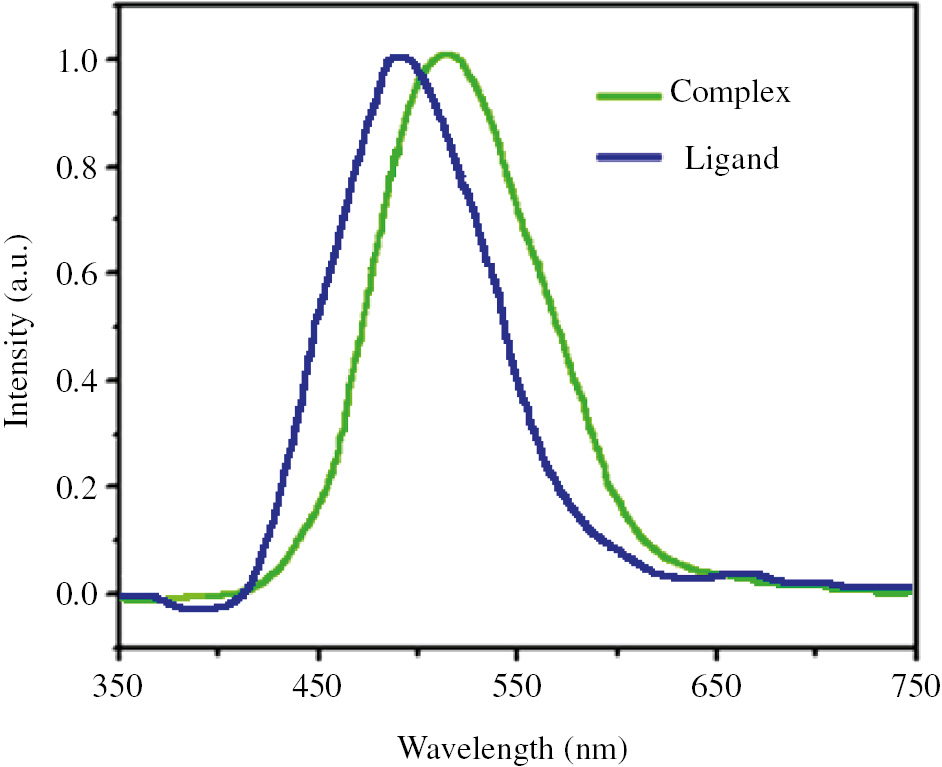
The photoluminescence spectra of complex 1 and free HL ligand.
The absolute quantum yield for free HL ligand and complex 1 in solid state is acquired, 0.55 and 0.69, as described by Moreno (2012). The higher quantum yield of complex 1 relative to free HL ligand could be due to a decline in the nonradiative decay of intraligand excited states. The investigation of the optical properties of the complex shows that this complex can be a suitable fluorescent material in the preparation of luminescence devices like light-emitting diodes.
Electroluminescence characteristics
In order to prove that the prepared complex has the necessary potential that can be used in the manufacture of optical devices and to investigate the effect of the complex concentration as the emitting layer on the electroluminescence properties of an OLED, complex 1 was used with two different concentrations of 10% and 20% for the preparation of two OLEDs with a general configuration of ITO/PEDOT:PSS(90 nm)/PVK:PBD:tin-complex (80 nm)/Al (200 nm) (Figure 6). By applying the driving voltage to the electrodes, the injection of electrons from Al and holes from ITO into the bulk begins. A large number of electrons and holes combine with the application of this electric force and cause the formation of excitons and the emission of light. Of course, there is the possibility of excimers formation in PVK molecules as hole transport layer due to the overlapping of its absorption and emission spectra; this reduces the electroluminescence properties. Increasing the PBD as electron transport layer (ETL) into the PVK is a good solution to reduce the formation of excimer. Mixing simultaneously the holes and electron-transporting functions and forming a single bipolar host material can be the best solution for the proper balance of electrons and holes. It should be noted that the high use of the PBD relative to the PVK leads to a reduction in the stability of the device. Experience has shown that the ratio of PVK:PBD=100:40 is the most effective ratio. This ratio reduces the risk of excimer formation and increases the stability of the device (Janghouri et al., 2013). The PEDOT:PSS interlayer is used as an auxiliary layer for better injection of holes into the emissive layers. Using this layer can increase the electrical current inside the device and increase the probability of exciton formation. In order to show that complex 1 acts as an emission layer in the structure of prepared diode, a diode was prepared without the use of complex 1 in its structure as PVK:PBD-based device. The spectrum of this diode showed a relatively broad peak in the blue wavelength region. However, according to Figure 7, the present peaks in the electroluminescence (EL) spectra of prepared tin(IV) complex-based device show a long red shift rather than PVK:PBD-based device. This displacement indicates that complex 1 acts as the emission layer in the structure of diodes.
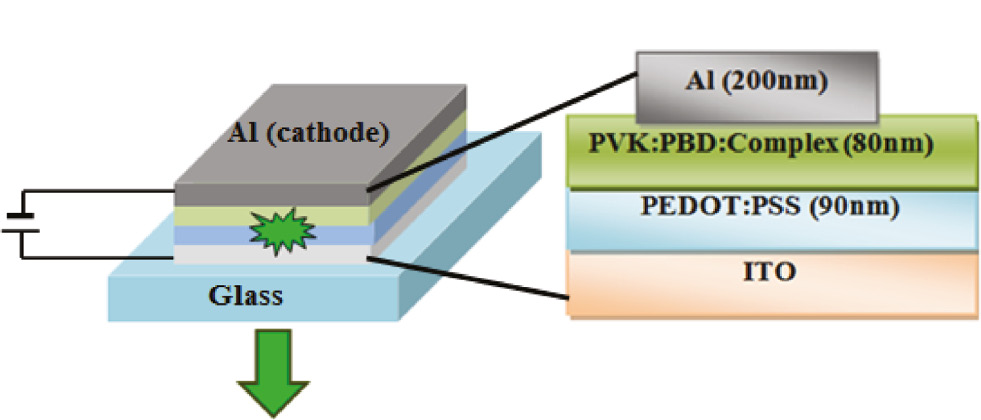
Layer arrangement and layer thickness of the OLED devices.
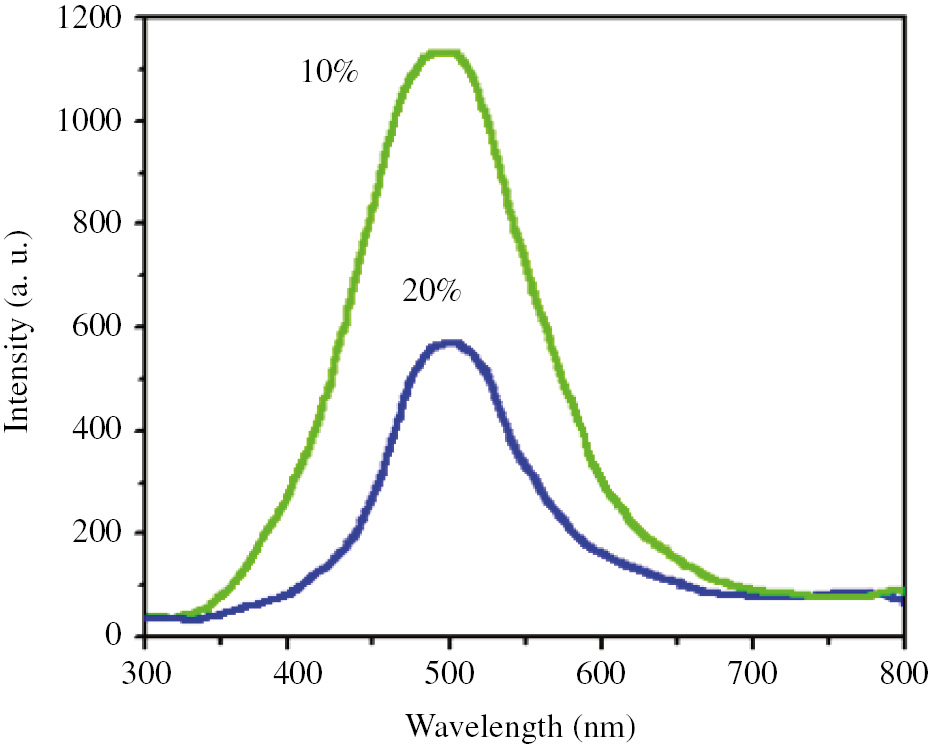
Electroluminescence spectra of prepared OLED devices with 10 and 20% (w/w) of complex 1 as the dopant.
The electroluminescence spectra of prepared devices with 10% and 20% (w/w) of tin(IV) complex as a dopant revealed a green emission at 505 nm.
The effect of the concentration of doped complex in the structure of diodes on their electroluminescence properties can be determined by a simple look at the electroluminescence spectra of prepared diodes. As shown in Figure 7, high concentration of the complex leads to a decrease in the electroluminescence intensity of the diodes. The reason for this can be attributed to a trap effect in the current voltage, which leads to the self-quenching of emission and a decrease in the efficiency of the device.
According to atomic force microscope (AFM) images of the premix of PVK:PBD:tin(IV) complex with a scanning area of 1×1 μm, it is possible to understand the decrease in the electroluminescence intensity of prepared diode with high concentration of the complex (Figure 8). The average root-mean-square surface morphology of the films for concentrations of 10 and 20% was about 11.8 and 38.2 nm, respectively. These results show that the surface roughness of a layer with high concentration of the complex is relatively higher than that of a layer with low concentration of the prepared complex. Reducing surface roughness is associated with increase in contact area between the ETL and the cathode and more effective injection of electrons and holes in the system.
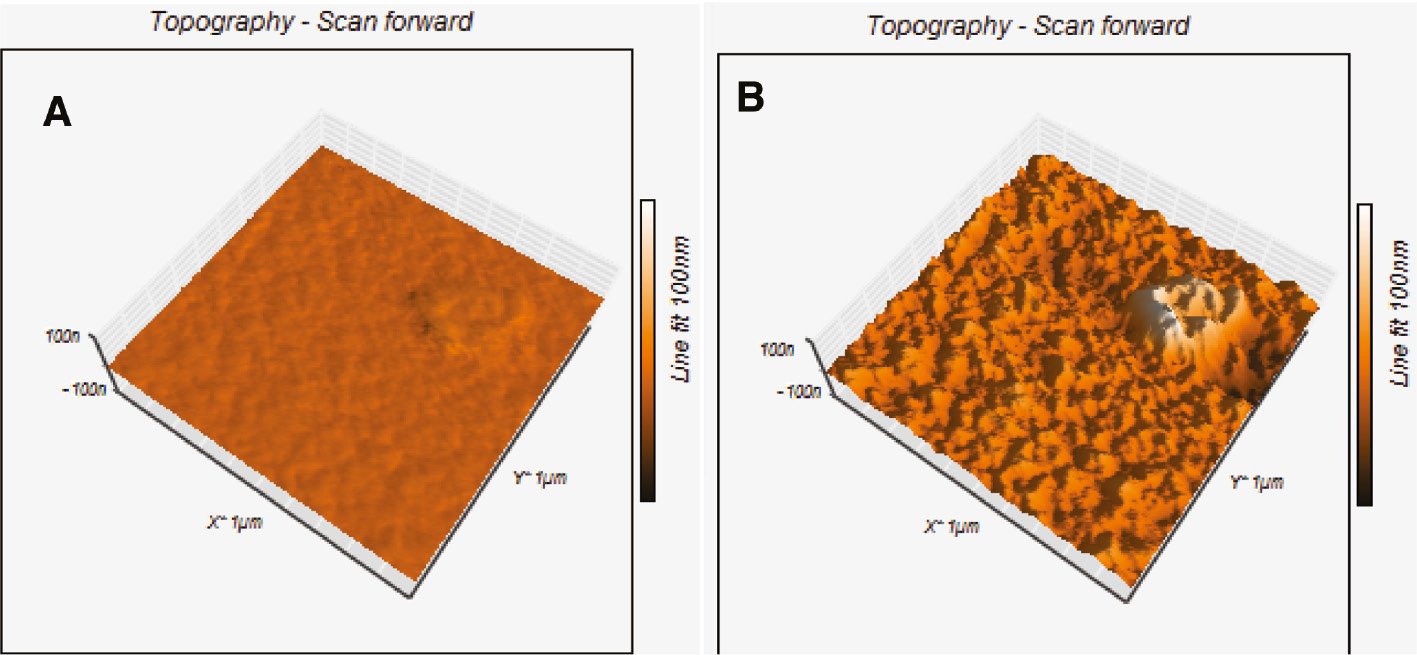
AFM images of premixed PVK:PBD:complex thin films with concentration ratios of 100:40:10 (A) and 100:40:20 (B).
The voltage versus the current density of prepared diodes shows that there is an inverse relationship between changes in the current density and the complex concentration at a specific voltage (Figure 9). In general, by injecting an electron hole into the layers by applying voltage, current density within the system increases. As can be seen, a diode with lower concentration of complex has lower operation voltage. This voltage reduction improves the efficiency of the diode. The result is that the device with high concentration of complex requires more driving voltage than the other device. The reason of higher driving voltage of this device can be related to higher electron injection barrier in it. High electron injection barrier prevents further injection of electrons from the electrodes to bulk. The high operating voltage of device with high concentration of complex will shorten the lifetime of the device because of impurity diffusion under a high electric field and thermal aging.
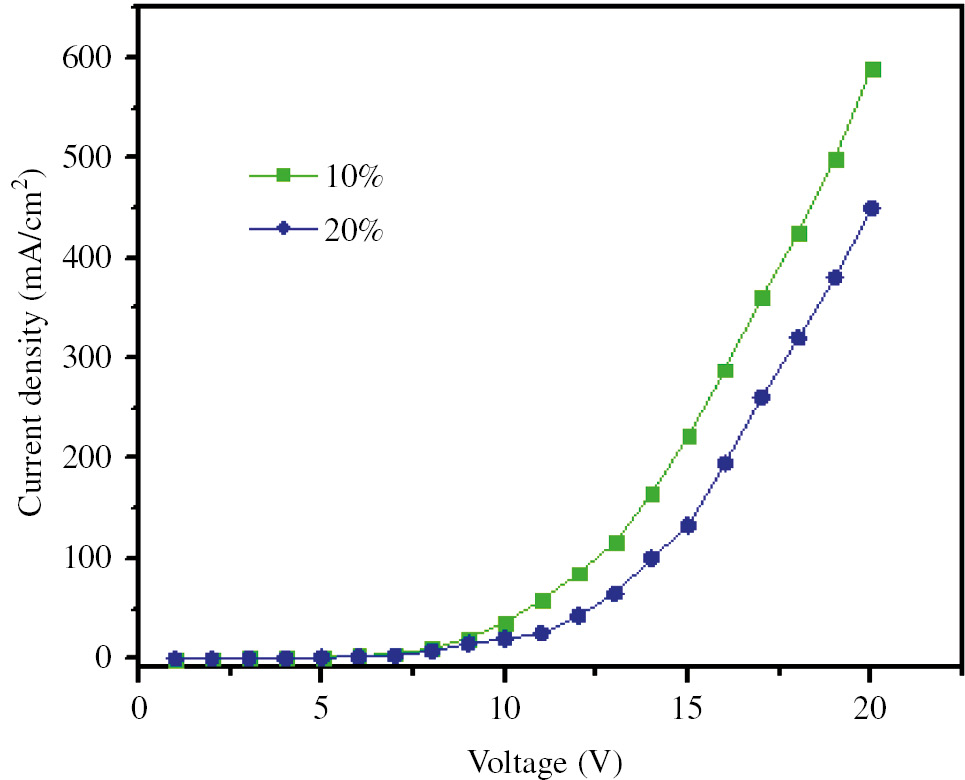
Current density (J)-voltage (V) characteristics of prepared OLED devices.
The luminescence-voltage characteristics of prepared devices are visible in Figure 10. The concentration of the complex is effective on the turn-on voltage which is defined as the bias voltage at L=1 cd/m2. The maximum brightness of devices is at 130 and 910 cd/m2 for concentrations of 10 and 20%, respectively. As can be seen, the device with low concentration of the complex has a higher brightness. High surveys show that the prepared tin compounds can be used as suitable precursors in the manufacture of light-emitting diodes, and adjusting the concentration of the complex used in the preparation of the diode can be a good factor in improving the quality of its light emission intensity.
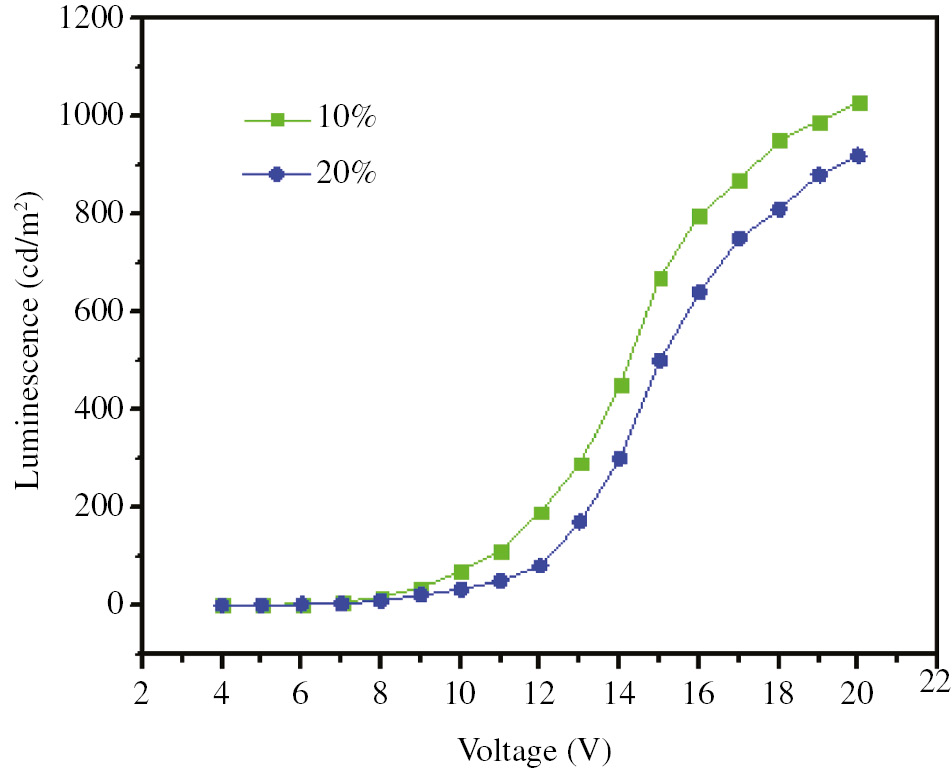
Luminescence-voltage characteristics of the prepared OLED devices.
Conclusion
A novel tin(IV) complex was synthesized and fully characterized by spectroscopic methods. In addition, its crystalline structure was determined by X-ray diffraction. The results of X-ray diffraction showed that complex 1 belongs to the class of dimeric tetraorganodistannoxanes with a ‘ladder structure’. Due to the fact that this complex showed good fluorescence emission at room temperature in solid state and acceptable thermal stability according to thermogravimetric and differential thermal analysis (TGA-DTA), it was used as a precursor in the construction of an OLED. The study of the effect of prepared complex concentration on the electrical properties of prepared diode showed that there is an inverse relationship between the complex concentration and the driving voltage of the diodes. The final result is that this complex can be used as a light emission layer in the production of OLEDs with green light emission.
Experimental
All reagents were acquired from Merck. It should be noted that all purchased compounds were used without additional purification. All solvents before applying were dried according to standard procedures (Perrin et al., 1980). The OLEDs were prepared according to the method used in our previous works (Janghouri et al., 2014). C, H, and N microanalyses of compounds have been evaluated by Thermo Finnigan Flash-1112EA microanalyzer. For recording of infrared spectra, the Bomem MB-series FT-IR instrument has been utilized. NMR spectra were inscribed on a Bruker AVANCE 300 operating at 300.3 MHz, respectively. The thickness of the samples was measured by using a DekTak 8000 profilometer. EL and photoluminescence measurements of the fabricated OLEDs were performed by USB2000 and HR4000 Ocean Optic. Current-voltage-luminance characteristics and AFM measurements were checked by a Keithley source meter (model 2400), optical meter (Mastech-MS6612), and Easyscan 2, respectively. The absorption spectra of compounds were obtained on Shimadzu 2100 spectrometers. The thermal analysis (TG-DTA) was carried out on a Bahr STA-503 instrument under air atmosphere. The method reported by Moreno was used to measure absolute quantum yields of compounds (Moreno, 2012). Crystallographic measurements were prepared utilizing a Bruker SMART APEX diffractometer supplied with a graphite monochromated Mo-Ka radiation at 100 K. Accurate unit cell parameters and orientation matrices were achieved from least-squares refinements. The structure solved by direct method was refined by full-matrix least-squares procedure on F2. The SHELXL97 crystallographic software package was used for all refinements.
Preparation of tin(IV) complex (1)
For the preparation of complex 1, 9-anthracenecarboxylic acid (1 mmol) was dissolved in 15 mL ethanol, and then triethylamine (1 mmol) was added to the reaction mixture under stirring at room temperature. After 10 min, a solution of dimethyltin(IV) dichloride (2 mmol) in 4 mL ethanol was added to the mixture and refluxed for 3 h. The resulting precipitate was collected and loaded into the longer arm of a convection tube, and then the tube was carefully filled with dry ethanol and sealed. The longer arm of the tube was submerged in an oil bath at 60°C. After a week, the yellow crystals were gathered in the cold and shorter arm of tube (yield 82%, m.p: 210–212°C). Anal. Calc. (%) for C42H52O8Sn4: C, 43.50; H, 4.52. Found (%): C, 43.52; H, 4.50. IR (KBr, cm−1): υ(w, PhH), 3032; υ(m, CH), 2938; υas(COO), 1668; υs(COO), 1420; υ(Sn-C), 548, 583; υ(Sn-O), 461; υ(Sn-O-Sn), 631. 1H NMR (CDCl3), δ (ppm): 0.61 (s, 12H, 2J117/119Sn-H=87 Hz, SnCH3), 0.95 (s, 12H, 2J117/119Sn-H=98 Hz, SnCH3), 1.25 (t, 6H, -OCH2CH3), 3.42 (q, 4H, -OCH2CH3), 7.32–8.34 (m, 18H, Ar-H). 13C NMR (CDCl3), δ (ppm): 168.8 (COO), 142.8, 130.2, 129.2, 128.3, 126.7, 126.8, 126.9, 125.3 (Ar-C), 19.3, 56.3 (OCH2CH3), 5.1, 5.8 (SnCH3). 119Sn NMR (CDCl3), δ (ppm): −142.8 and −149.1.
Supplementary material
CCDC 1531453 includes the crystallographic data for the complex reported in this article. Copies of the data can be obtained from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Acknowledgments
We thank the Graduate Study Councils of Shahid Beheshti University and Payame Noor University for supporting this study.
References
Azadmeher, A.; Amini, M. M.; Hadipour, N.; Khavasi, H. R.; Fun, H.; Chen, C. Synthesis and structural characterization of diorganotin(IV) complexes with 2,6-pyridinedicarboxylic acid. Appl. Organometal. Chem.2008, 22, 19–24.10.1002/aoc.1343Search in Google Scholar
Boshra, R.; Doshi, A.; Venkatasubbaiah, K.; Jäkle, F. Binding of a 2-pyridyl moiety to a B/Sn bidentate Lewis acid. Inorg. Chim. Acta2010, 364, 162–166.10.1016/j.ica.2010.09.010Search in Google Scholar
Byrnes, M. J.; Chisholm, M. H.; Dye, D. F.; Hadad, C. M.; Pate, B. D.; Wilson, P. J.; Zaleski, J. M. 9,10-Anthracene dicarboxylate bridged complexes with M2 quadruply bonded dimetal units: [{M2(O2CtBu)3}2(μ-9,10-An(CO2)2)], where M=Mo or W. Dalton Trans.2004, 523–529.10.1039/B312613GSearch in Google Scholar PubMed
Carcelli, M.; Fochi, A.; Pelagatti, P.; Pelizzi, G.; Russo, U. The behaviour of polydentate hydrazonic ligands derived from 2-acetylpyridine towards organotin compounds: Part II. The monoorganotin(IV) complexes. J. Organomet. Chem.2001, 626, 161–167.10.1016/S0022-328X(01)00677-5Search in Google Scholar
Coza, C.; Stegarescu, A.; Şuteu, R.; Silvestru, A. Organotin(IV) hypervalent pseudohalides. Synthesis and structural characterization. J. Organomet. Chem.2015, 777, 71–80.10.1016/j.jorganchem.2014.11.026Search in Google Scholar
Deng, H.; Wei, Z.; Wang, X. Enhanced adsorption of active brilliant red X-3B dye on chitosan molecularly imprinted polymer functionalized with Ti(IV) as Lewis acid. Carbohydr. Polym.2017, 157, 1190–119710.1016/j.carbpol.2016.10.087Search in Google Scholar PubMed
Dudev, T.; Lim, C. Effect of carboxylate-binding mode on metal binding/selectivity and function in proteins. Acc. Chem. Res.2007, 40, 85–93.10.1021/ar068181iSearch in Google Scholar PubMed
Hanifehpour, Y.; Morsali, A.; Soltani, B.; Mirtamizdoust, B.; Joo, S. W. Ultrasound-assisted fabrication of a novel nickel(II)- bis-pyrazolyl borate two-nuclear discrete nano-structured coordination compound. Ultrason. Sonochem.2017, 34, 519–524.10.1016/j.ultsonch.2016.06.032Search in Google Scholar PubMed
Hayati, P.; Rezvani, A. R.; Morsali, A.; Retailleau, P.; Granda, S. G. Influences of temperature, power ultrasound and reaction time on the morphological properties of two new mercury(II) coordination supramolecular compounds. Ultrason. Sonochem.2017a, 34, 968–977.10.1016/j.ultsonch.2016.07.019Search in Google Scholar PubMed
Hayati, P.; Rezvani, A. R.; Morsali, A.; Retailleau, P. Ultrasound irradiation effect on morphology and size of two new potassium coordination supramolecule compounds. Ultrason. Sonochem.2017b, 34, 195–205.10.1016/j.ultsonch.2016.05.031Search in Google Scholar PubMed
Herrmann, U.; Tiimmler, B.; Maass, G.; Mew, P. K. T.; Vogtle, F. Anthracenoyl crown ethers and cryptands as fluorescent probes for solid-phase transitions of phosphatidylcholines: syntheses and phospholipid membrane studies. Biochemistry1984, 23, 4059–4067.10.1021/bi00313a008Search in Google Scholar PubMed
Im, H. J.; Lee, S. W. Two-dimensional 3d–4f coordination polymers based on compartment compounds: [NiLn(L)(NO3)2(4-pca)(H2O)] (Ln=Nd, Eu, Tb; H2L=1,3-bis((3-methoxysalicylidene)amino)propane); 4-Hpca=pyridine-4-carboxylic acid). Polyhedron2016, 110, 24–30.10.1016/j.poly.2016.02.023Search in Google Scholar
Janghouri, M.; Mohajerani, E.; Amini, M. M.; Najafi, E.; Hosseini, H. Yellow–orange electroluminescence of novel tin complexes. J. Electron. Mater.2013, 42, 2915–2925.10.1007/s11664-013-2694-9Search in Google Scholar
Janghouri, M.; Mohajerani, E.; Amini, M. M.; Najafi, E. Green–white electroluminescence and green photoluminescence of zinc complexes. J. Lumin.2014, 154, 465–474.10.1016/j.jlumin.2014.05.022Search in Google Scholar
Karthikeyan, A.; Muthiah, P. T.; Perdih, F. Supramolecular architectures in CoII and CuII complexes with thiophene-2-carboxylate and 2-amino-4,6-dimethoxypyrimidine ligands. Act. Cryst. C. Struct. Chem.2016, 72, 442–450.10.1107/S2053229616006148Search in Google Scholar PubMed
Koh, L.; Vittal, J. J. Photodimerization of a 1D hydrogen-bonded zwitter-ionic lead(II) complex and its isomerization in solution. Chem. Commun.2008, 441–443.10.1039/B714355ASearch in Google Scholar PubMed
Kubota, Y.; Niwa, T.; Jin, J.; Funabiki, K.; Matsui, M. Synthesis, absorption, and electrochemical properties of quinoid-type bisboron complexes with highly symmetrical structures. Org. Lett.2015, 17, 3174–3177.10.1021/acs.orglett.5b01547Search in Google Scholar PubMed
Lee, J. H.; Im, S. Y.; Lee, S. W. 1D and 2D Zn–Ln coordination polymers based on compartment compounds: [ZnLn(L)(NO3)2(4-ppa)(EtOH)] and [ZnLn(L)(NO3)2(4-pca)(H2O)] (Ln=Eu, Tb; H2L=1,3-bis((3-methoxysalicylidene)amino)propane; 4-Hppa=4-pyridinepropionic acid; 4-Hpca=4-pyridinecarboxylic acid). Polyhedron2016, 118, 125–132.10.1016/j.poly.2016.08.014Search in Google Scholar
Liu, C. S.; Wang, J. J.; Yan, L. F.; Chang, Z.; Bu, X. H.; Udo, E. C. S.; Ribas, J. Copper(II), cobalt(II), and nickel(II) complexes with a bulky anthracene-based carboxylic ligand: syntheses, crystal structures, and magnetic properties. Inorg. Chem.2007, 46, 6299–6310.10.1021/ic070086ySearch in Google Scholar PubMed
Li, Q.; Liu, X.; Cheng, S.; Zhang, R.; Shi, Y.; Ma, C. Novel organotin complexes derived from 2,2′-selenodiacetic acid: synthesis and biological evaluation. RSC Adv.2016, 6, 32484–32492.10.1039/C6RA01948JSearch in Google Scholar
Ma, C.; Jiang, Q.; Zhang, R. Synthesis and characterization of a linked, π-π stacked organotin compound of 2-mercapto-6-nitrobenzothiazolyl diphenyltin chloride with 2-ethoxyl-6- nitrobenzothiazole. Can. J. Chem.2003, 81, 825–831.10.1139/v03-087Search in Google Scholar
Miller, C. J.; Chadha, U.; Ulibarri-Sanchez, J. R.; Dickie, D. A.; Kemp, R. A. Structure and Lewis-base reactivity of bicyclic low-valent germanium and tin complexes bridged by bis(diisopropylphosphino)amine. Polyhedron2016, 114, 351–359.10.1016/j.poly.2016.01.028Search in Google Scholar
Moreno, L. A. Absolute quantum yield measurement of powder samples. J. Vis. Exp.2012, 12, e3066.10.3791/3066Search in Google Scholar PubMed PubMed Central
Ngoune, J.; Anguile, J. J.; Nenwa, J.; Djimassinga, G.; Pettinari, C.; Álvarez, E.; Pandolfo, L. Synthesis, characterization and molecular structure of a zinc(II) formate-2,2′-bipyridine mono-dimensional coordination polymer. Comparison with other 2,2-bipyridine coordination compounds. Inorg. Chim. Acta2016, 453, 263–267.10.1016/j.ica.2016.08.005Search in Google Scholar
Perrin, D. D.; Armaredo, W. L.; Perrin, D. R. Purification of Laboratory Chemicals, 2nd Ed. Pergamon Press: Oxford, 1980.Search in Google Scholar
Sreejith, S. S.; Mohan, N.; Aiswarya, N.; Kurup, M. R. P. Inclusion, pseudo-inclusion compounds and coordination polymer of Pd(II), Zn(II) and Cd(II) from salen-type Schiff base ligand with a 1,3-diimino spacer group: crystal structures, spectroscopic and thermal studies. Polyhedron2016, 115, 180–192.10.1016/j.poly.2016.05.007Search in Google Scholar
Tatikonda, R.; Kalenius, E.; Haukka, M. Synthesis and characterization of Zwitterionic Zn(II) and Cu(II) coordination compounds with ring-substituted 2,2′-biimidazole derivatives. Inorg. Chim. Acta2016, 453, 298–304.10.1016/j.ica.2016.08.015Search in Google Scholar
Wang, J. J.; Liu, C. S.; Hu, T. L.; Chang, Z.; Li, C. Y.; Yan, L. F.; Chen, P. Q.; Bu, X. H.; Wu, Q.; Zhao, L. J.; et al. Zinc(II) coordination architectures with two bulky anthracene-based carboxylic ligands: crystal structures and luminescent properties. Cryst. Eng. Comm. 2008, 10, 681–692.10.1039/b710209gSearch in Google Scholar
Wang, Q. F.; Mab, C. L.; He, G. F.; Li, Z. Synthesis and characterization of new tin derivatives derived from 3,5,6-trichlorosalicylic acid: cage, chain and ladder X-ray crystal structures. Polyhedron2013, 49, 177–182.10.1016/j.poly.2012.09.057Search in Google Scholar
Zhang, R. F.; Wang, Q. F.; Yang, M. Q.; Wang, Y. R.; Ma, C. L. Five new dicarboxylate organotin complexes under hydrothermal condition: syntheses, characterization and crystal structures of 1D, 2D and 3D polymers constructed from dimeric tetraorganodistannoxane units. Polyhedron2008, 27, 3123–3131.10.1016/j.poly.2008.06.039Search in Google Scholar
Zhang, J. H.; Zhang, R. F.; Maa, C. L.; Wang, D. Q.; Wang, H. Z. New organotin carboxylates derived from 6-chloro-3-pyridineacetic acid exhibiting discrete molecular, drum-like, linear polymeric and ladder structures constructed from dimeric tetraorganodistannoxane units. Polyhedron2011, 30, 624–631.10.1016/j.poly.2010.11.035Search in Google Scholar
Zhou, Q.; Qian, J.; Zhang, C. Three interesting coordination compounds based on metalloligand and alkaline-earth ions: syntheses, structures, thermal behaviors and magnetic property. J. Mol. Struct.2016, 1119, 340–345.10.1016/j.molstruc.2016.04.034Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Lead acetate toxicity on human lymphocytes at non-cytotoxic concentrations detected in human blood
- Synthesis, characterization and biological activity of tin(IV) macrocyclic complexes derived from 1,2-diphenylethane-1,2-dione dihydrazone
- Synthesis and characterization of a new tin(IV) complex with anthracene-9-carboxylic acid as a precursor in the preparation of an organic light-emitting diode
- Synthesis of propargylamines catalyzed by nano-colloidal silica-tethered polyhedral oligomeric silsesquioxanes with eight branches of 3-aminopropyltriethoxysilane as an efficient catalyst
- Short Communication
- Synthesis, characterization, and thermal properties of N,N,N′,N′-tetramethyl guanidinium tribromidocadmate(II) exhibiting an unusual coordination geometry
Articles in the same Issue
- Frontmatter
- Research Articles
- Lead acetate toxicity on human lymphocytes at non-cytotoxic concentrations detected in human blood
- Synthesis, characterization and biological activity of tin(IV) macrocyclic complexes derived from 1,2-diphenylethane-1,2-dione dihydrazone
- Synthesis and characterization of a new tin(IV) complex with anthracene-9-carboxylic acid as a precursor in the preparation of an organic light-emitting diode
- Synthesis of propargylamines catalyzed by nano-colloidal silica-tethered polyhedral oligomeric silsesquioxanes with eight branches of 3-aminopropyltriethoxysilane as an efficient catalyst
- Short Communication
- Synthesis, characterization, and thermal properties of N,N,N′,N′-tetramethyl guanidinium tribromidocadmate(II) exhibiting an unusual coordination geometry


