Abstract
Two species of warthog are currently widely recognised, the poorly known desert warthog Phacochoerus aethiopicus and the widely distributed common warthog Phacochoerus africanus. Spatial data for both species were collected during field surveys and from the literature, museums, colleagues, naturalists, local experts, and online resources to assess their biogeography in the Horn of Africa (HoA). Their distributions were overlaid with ArcGIS datasets for altitude, rainfall, temperature, and ecoregions. Phacochoerus aethiopicus appears to be restricted to Ethiopia, Kenya, and Somalia, with no records west of the Eastern Rift Valley (ERV). The estimated current geographic distribution of P. aethiopicus is 1,109,000 km2. Phacochoerus africanus occurs in all five countries of the HoA and has an estimated current geographic distribution in the HoA of 1,213,000 km2. Phacochoerus africanus appears to be the more adaptable species although P. aethiopicus is able to live where mean annual rainfall is more variable. Although both species are allopatric over vast regions, they are sympatric in central east Ethiopia, north Somalia, central Kenya, north coast of Kenya, and southeast Kenya. Both suids remain locally common, their populations are, however, in decline due to the negative impacts on the environment by the rapidly growing human populations in all five countries.
1 Introduction
Two species of warthog Phacochoerus F. Cuvier, 1826 (Artiodactyla, Suidae), are currently widely recognised, desert warthog Phacochoerus aethiopicus (Pallas, 1766) and common warthog Phacochoerus africanus (Gmelin, 1788). These two suids are morphologically distinct (Figure 1; d’Huart and Grubb 2005; Ewer 1957; Grubb 1993, 2005, 2013; Grubb and d’Huart 2010; Roosevelt and Heller 1914) and genetically distinct (Garcia-Erill et al. 2022; Gongora et al. 2011; Randi et al. 2002). Both species occur in the Horn of Africa, here taken as Eritrea, Ethiopia, Djibouti, Somalia, and Kenya (hereafter, referred to as ‘HoA’). Overviews of the geography, climate, and habitats of the HoA can be found in Ash and Miskell (1998), Fjeldså and de Klerk (2001), Friis et al. (2005), Happold and Lock (2013), Jenner (2020), Kingdon (2015), Livingstone and Kingdon (2013), Morley and Kingdon (2013), Redman et al. (2009), and Yalden et al. (1996).

Main traits for distinguishing desert warthog Phacochoerus aethiopicus from common warthog Phacochoerus africanus in the field. Photographs by ©Yvonne de Jong and Tom Butynski.
Two subspecies of P. aethiopicus are currently recognised; the extinct Cape warthog P. a. aethiopicus (Pallas, 1766) from South Africa and perhaps Namibia, and the extant Somali warthog P. a. delamerei Lönnberg, 1909, in the HoA (De Jong and Butynski 2018; De Jong et al. 2016a; d’Huart and Grubb 2001; Grubb 1993, 2005, 2013; Grubb and d’Huart 2010; Meijaard et al. 2011). Photographs of both species, posted on an interactive distribution map, can be viewed at: https://www.wildsolutions.nl/photomaps/phacochoerus.
The taxonomy of P. africanus is uncertain and in need of revision (Butynski and De Jong 2018; Groves and Grubb 2011; Grubb and d’Huart 2013). We here follow the classification of Grubb (1993, 2005 who acknowledges four subspecies of P. africanus based on skull size and proportions: northern warthog P. a. africanus (Gmelin, 1788), Eritrean warthog P. a. aeliani (Cretzschmar, 1828), Central African warthog P. a. massaicus Lönnberg, 1909, and southern warthog P. a. sundevallii Lönnberg, 1909 (Figure 2). This taxonomy gains some support from the molecular study of Muwanika et al. (2003), but not from Garcia-Erill et al. (2022). The HoA supports three of the four putative subspecies: P. a. africanus (Ethiopia and probably extreme northwest Kenya), P. a. aeliani (coastal hinterland of Eritrea, Djibouti and, perhaps, extreme northwest Somalia), and P. a. massaicus (Kenya) (Butynski and De Jong 2018; De Jong et al. 2016a).
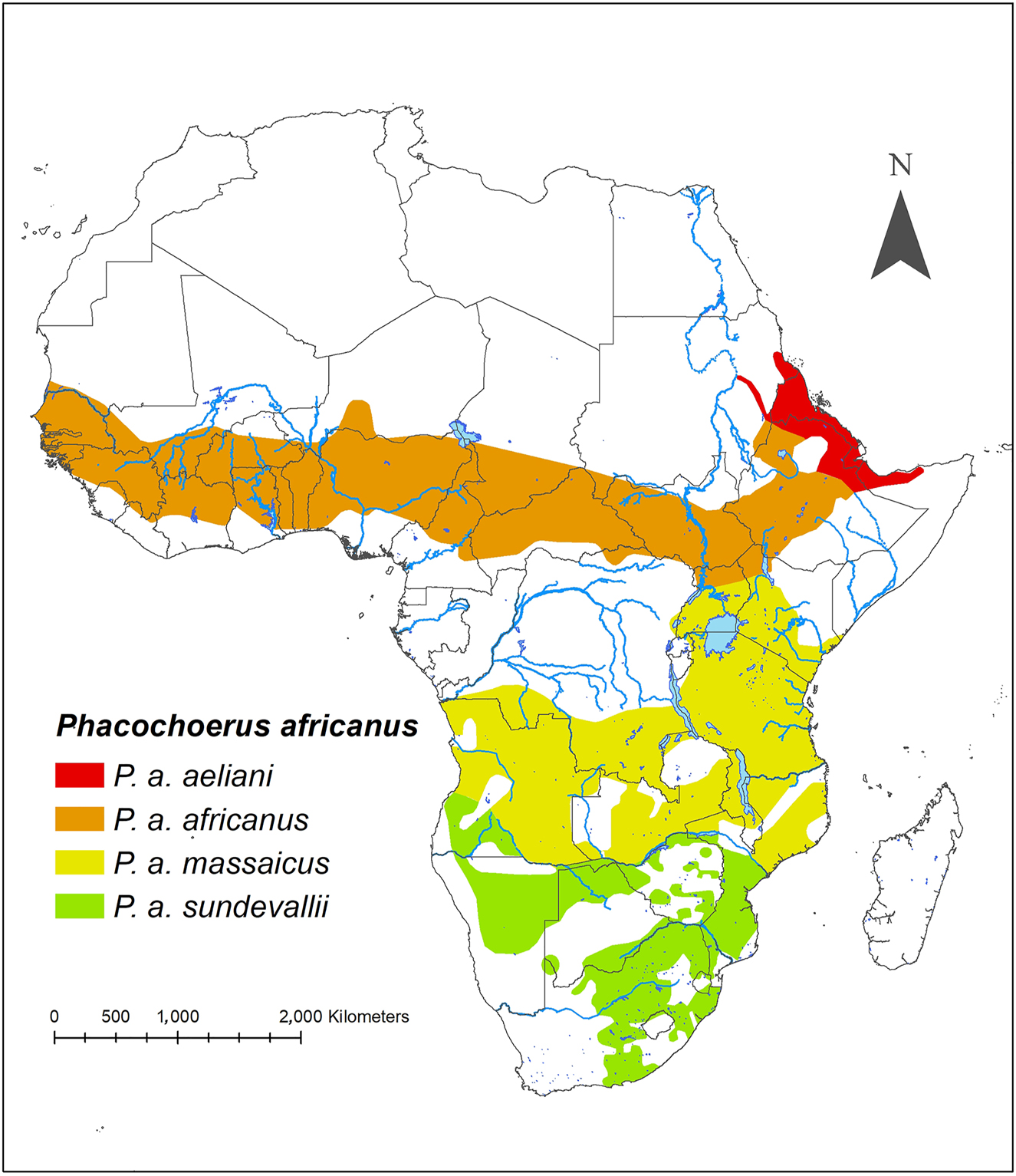
Geographic distribution of the four putative subspecies of common warthog Phacochoerus africanus. The geographic limits are poorly understood and, therefore, are approximate. Based on Butynski and De Jong (2018), De Jong et al. (2016b), Muwanika et al. (2003), Vercammen and Mason (1993), and this study.
The geographic distribution, abundance, ecology, behaviour, and conservation status of P. aethiopicus remain poorly known (De Jong and Butynski 2018; De Jong et al. 2016a; Grubb 1993). In fact, P. aethiopicus is probably Africa’s least studied non-forest large mammal. In contrast, P. africanus is distributed over much of sub-Saharan Africa and is relatively well-studied (Butynski and De Jong 2018; Cumming 1975, 2013; De Jong et al. 2016b).
A better understanding of the natural history of P. aethiopicus is of considerable scientific interest and important to the development of effective conservation and management plans for this species. The IUCN/SSC Pigs, Peccaries, and Hippos Status Survey and Conservation Action Plan emphasises the large gaps in our knowledge of P. aethiopicus (Oliver 1993). One of the ‘priority projects’ listed in this plan includes studying the biogeography and comparative ecology of P. aethiopicus and P. africanus to determine whether they are allopatric, sympatric, or parapatric, and how they differ ecologically (d’Huart and Oliver 1993; Grubb 1993; Vercammen and Mason 1993). To this end, d’Huart and Grubb (2001) provided the first map of the geographic distribution of P. a. delamerei. Here we follow-up on their work, presenting what we currently know about the geographic distribution of P. a. delamerei (hereafter, referred to as ‘P. aethiopicus’) and P. africanus in the HoA after a further 20 years of compiling locality records. As in d’Huart and Grubb (2001), this study relates the geographic distributions of these two species to altitude, mean annual rainfall, mean annual temperature, ecoregion, and geological and ecological barriers. We present estimates of the current geographic distributions of these two species in the HoA, discuss their conservation status, and put forth priorities for future research.
2 Materials and methods
This study covers the land area of the HoA, a total of 2,463,130 km2 (Eritrea: 117,600 km2; Ethiopia: 1,104,300 km2; Djibouti: 23,200 km2; Somalia: 580,370 km2; Kenya: 637,660 km2; World Bank 2021).
Spatial data for P. aethiopicus and P. africanus were collected during surveys in Kenya by YDJ and TMB (e.g., De Jong and Butynski 2009, 2014), from the literature, from 15 museum collections [including data taken from d’Huart and Grubb (2001) for 12 museums], colleagues, naturalists, local experts, digital repositories [i.e., Global Biodiversity Information Facility (GBIF; https://www.gbif.org/), iNaturalist (https://www.inaturalist.org/), VertNet (http://vertnet.org/)], additional online platforms (e.g., Facebook.com, Africaimagelibrary.com, national park websites), and naturalist blogs (e.g., SafariTalk.net). Species identification of all records was done by the authors with the help of skulls and photographs.
The above sources of data derive from the period 1828–2021, but mostly for the period 1950–2021. Spatial data were imported into a Microsoft Access database (hereafter, referred to as ‘WarthogBase’). WarthogBase is a ‘living’ database as localities for each species continue to be added as they become available. As of July 2022, WarthogBase contained 549 records, 219 records for P. aethiopicus (Ethiopia 8; Kenya 161; Somalia 50), 265 records for P. africanus (Djibouti 7; Eritrea 10; Ethiopia 88; Kenya 153; Somalia 7), and 65 unknown. Due to the large number of records from popular tourist destinations in Kenya [e.g., Maasai Mara National Reserve (NR), Nairobi National Park (NP), Samburu NR] and Ethiopia (e.g., Bale Mountains NP), we limited the number of records in WarthogBase from such areas.
WarthogBase forms the basis of the current geographic distribution maps for P. aethiopicus and P. africanus presented in the latest IUCN Red List of Threatened Species (De Jong et al. 2016a,b), as well as in Butynski and De Jong (2018) and in De Jong and Butynski (2018).
Maps of the historic geographic distribution of Phacochoerus in the HoA are provided by Stewart and Stewart (1963) for Kenya (based on presence or absence in 10-min squares), Kingdon (1979) for Kenya, Funaioli and Simonetta (1966) for Somalia, and Yalden et al. (1984) for Djibouti, Eritrea, Ethiopia, and extreme northwest Somalia. Yalden et al. (1984) provide 124 localities. These maps are based on field observations of the authors, and their detailed reviews of the literature and unpublished reports, examination of museum specimens, and correspondence with colleagues and others. None of these maps, however, distinguishes between the two species of Phacochoerus. All four of these historic maps were georeferenced in ArcMAP (ArcGIS Desktop 10.7.1). Where these maps show presence of an unidentified species of Phacochoerus for a locality for which we did not already have a record for P. aethiopicus and/or P. africanus, that locality was added to our map and referred to as ‘Phacochoerus sp.?’. The result is an ArcMAP shapefile (hereafter, referred to as the ‘Phacochoerus Shapefile’) that shows what is known about the combined historic and current geographic distributions for the two species of Phacochoerus, as well as for unidentified Phacochoerus, in the HoA. The Phacochoerus sp.? Shapefile is depicted in light grey in Figure 3 where it contrasts against the shapefiles of the two species.
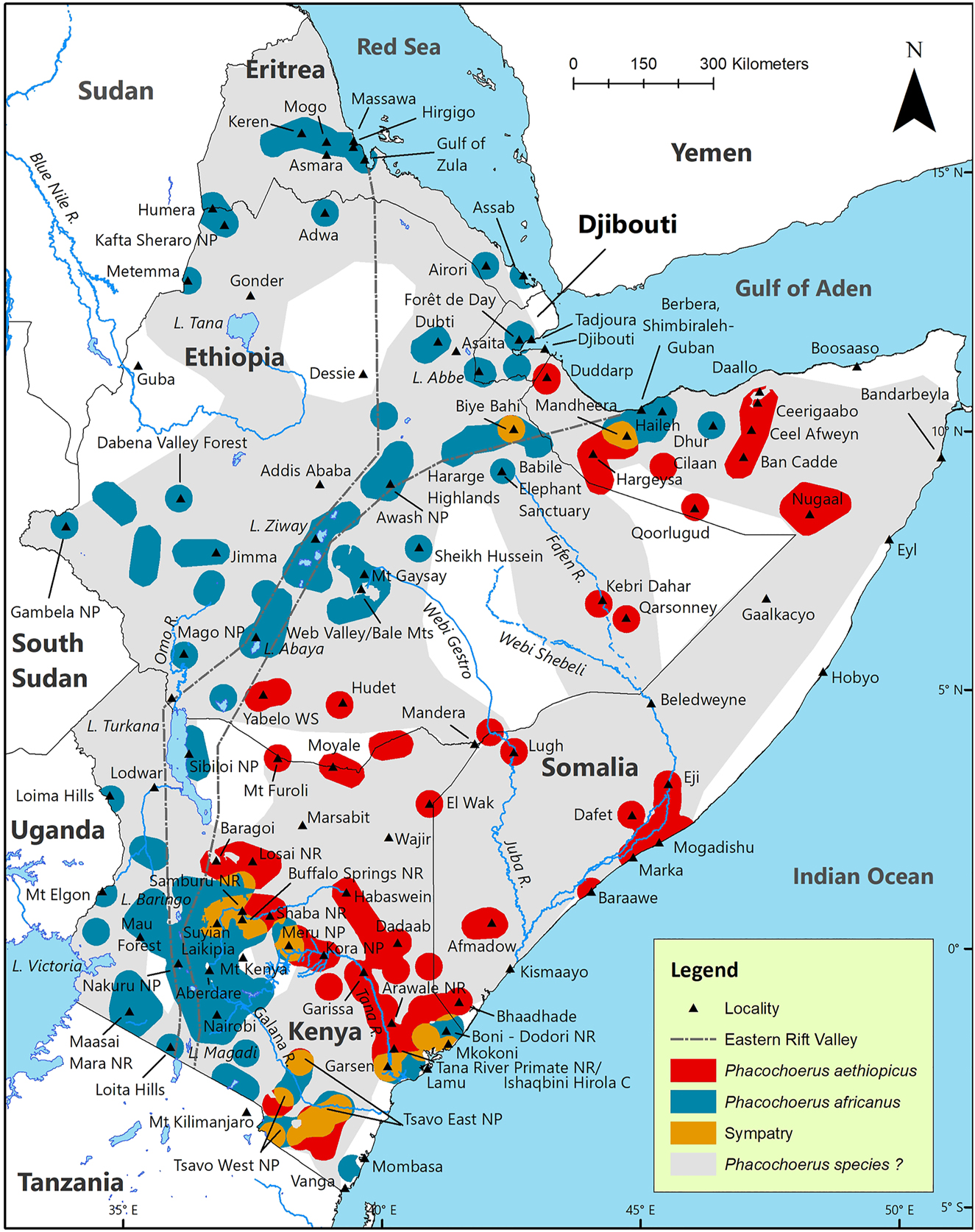
Estimated historic geographic distribution of desert warthog Phacochoerus aethiopicus, common warthog Phacochoerus africanus, and Phacochoerus sp.? In the Horn of Africa. Coordinates of the records used to compile this map are presented in WarthogBase at: www.wildsolutions.nl/warthogbase. ‘Phacochoerus species?’ is based on maps presented in Stewart and Stewart (1963) and Kingdon (1979) for Kenya, Funaioli and Simonetta (1966) for Somalia, and Yalden et al. (1984) for Eritrea, Ethiopia, and extreme northwest Somalia.
‘Presence only’ spatial data, from records identified to species level in WarthogBase, were projected in ArcMAP. A buffer zone with a radius of 30 km was applied to each locality to simulate the geographic distribution of each species. A radius of 30 km was selected as this seems reasonably conservative given the large scale of the maps, the paucity of sites in the HoA where Phacochoerus has been reported to occur, and that P. africanus is known to move at least 16 km in less than 2 months (Cumming 1975). Touching or overlaying buffer zones were placed into polygons. All polygons were combined in an ArcMap shapefile that depicts the minimum historic geographic distributions of P. aethiopicus and P. africanus.
The shapefiles for the two species were used as proxies for historic and current geographic distributions. To gain insights into altitudinal and climatological variables, each species shapefile was overlaid with datasets for altitude (DEM of Africa, resolution 30 arc-second), mean annual rainfall (WorldClim 2.1 Bioclimatic variable 12, resolution 30 arc-second; Fick and Hijmans 2017; WorldClim 2016), mean annual temperature (WorldClim 2.1 Bioclimatic variable 1, resolution 30 arc-second; Fick and Hijmans 2017; WorldClim 2016), and ecoregions (Burgess et al. 2004; Olson et al. 2001) in ArcMap. These shapefiles were then modified by removing those altitudes and ecoregions considered unsuitable for each species. The highest altitude record is 1895 m above sea level (m asl) for P. aethiopicus and 3500 m asl for P. africanus. Areas >1895 m asl were, therefore, removed from the P. aethiopicus shapefile and those >3500 m asl were removed from the P. africanus shapefile. ‘East Africa Mangrove Ecoregion’ was removed from the shapefile for both species, and ‘Eastern Arc Forest Ecoregion’ and ‘East African Montane Forest Ecoregion were removed from the P. aethiopicus shapefile as these species are not known to occur within these ecoregions.
The spelling of locality names used in this study is mainly adopted from the following sources: Polhill (1988) for Kenya, United States Board on Geographic Names (1982), Ash and Miskell (1998) for Somalia, and GeoNames Geographical Database (https://www.geonames.org). For the sake of clarity, some alternative names are provided in the text and in WarthogBase.
3 Results
3.1 Historic distribution
Phacochoerus aethiopicus appears to be endemic to Ethiopia, Somalia, and Kenya. We found no P. aethiopicus records for Djibouti. This species is, however, recorded for Duddarp, northwest Somalia, about 18 km from Djibouti. Phacochoerus aethiopicus occurs in Kenya less than 12 km from Tanzania. As far as we are aware, there is no record of this species for Tanzania. Locality records are almost all from east of the Eastern (Gregory) Rift Valley (ERV), the exceptions being north Ethiopia and northwest Somalia where P. aethiopicus occurs on the graben (= floor) of the ERV (Figure 3; Table 1).
Geographic limits of desert warthog Phacochoerus aethiopicus and altitude of the locality. See Figure 3.
| Geographic limit | Locality | Coordinates | Altitude (m asl) | Source |
|---|---|---|---|---|
| North | Duddarp, northwest Somalia near Djibouti | N11.05000; E43.18333 | 200 | d’Huart and Grubb (2001) |
| East | Nugaal, northeast Somalia | N8.22840; E48.82148 | 445 | K. Lorenz pers. comm.; P. Moehlman pers. comm. |
| South | Tsavo West NP, southeast Kenya near Tanzania | S3.61318; E37.87187 | 998 | J. Culverwell pers. comm. |
| West | Suyian Ranch, Laikipia, central Kenya | N0.58438; E36.69335 | 1895 | Butynski and De Jong (2021); Powys (2020) |
3.2 Sympatry
There are five known, widely-spaced, areas of sympatry between P. aethiopicus and P. africanus (Figure 3); central east Ethiopia (3300 km2), north Somalia (3300 km2), central Kenya (four sites; 13,400 km2), north coast of Kenya (two sites; 8000 km2), and southeast Kenya (five sites; 14,100 km2).
At Suyian Ranch, on the Laikipia Plateau, central Kenya, one mixed-species sounder was encountered in 2021 (Figure 4; Butynski and De Jong 2021). In addition, the authors observed individuals here that they judged to be atypical for either P. aethiopicus or P. africanus.

Adult male desert warthog Phacochoerus aethiopicus (left) and adult male common warthog Phacochoerus africanus on Suyian Ranch, Laikipia, central Kenya. To the best of our knowledge, this is the first photograph of these two species together. Photograph by ©Yvonne de Jong and Tom Butynski.
3.3 Altitude
The known altitudinal range of P. aethiopicus is from sea level to 1895 m asl (Suyian Ranch, Laikipia, central Kenya), with most of the geographic distribution <1000 m asl (Figure 5; Table 2). Phacochoerus africanus occupies a much broader altitudinal range in the HoA, occurring from sea level to 3500 m asl (Bale Mountains, central south Ethiopia), with most of the geographic distribution between 1000 m asl and 2000 m asl. Although these two species overlap in altitude from sea level to 1895 m asl, P. aethiopicus is primarily a lowland species while P. africanus is primarily a mid-altitude and highland species (Butynski and De Jong 2018, 2021; De Jong and Butynski 2014, 2018; d’Huart and Grubb 2001).
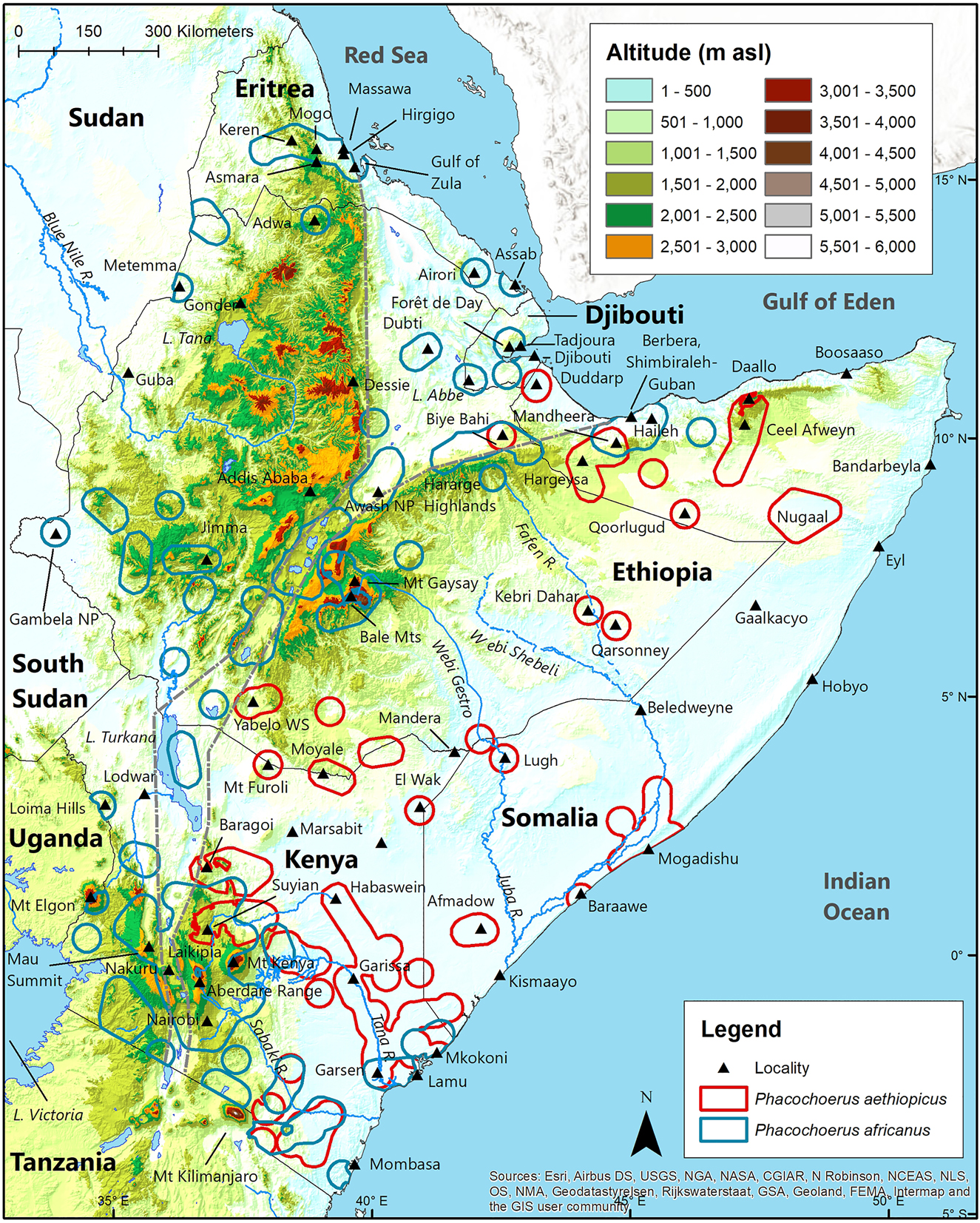
Geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa relative to altitude.
Lowest and highest known altitudes, by country, of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa.
| Country | Altitude range (m asl) | |||
|---|---|---|---|---|
| Phacochoerus aethiopicus | Phacochoerus africanus | |||
| Lowest | Highest | Lowest | Highest | |
| Eritrea | – | – | 0, Zula and Arkiko (d’Huart and Grubb 2001) | 1730, Mogo (Abraha 2016) |
| Ethiopia | 545, Qarsonney, Ogaden (X. Y. Kaariye pers. comm. via J. O. Heckel pers. comm.) | 1400, Yabelo Wildlife Sanctuary (H. Pohlstrand pers. comm.) | 350, Dubti, Afar (d’Huart and Grubb 2001; P. Moehlman pers. comm.) | 3500, Mount Gaysay, Bale Mountains (Deribe et al. 2008; C. Roche pers. comm. 2014) |
| Djibouti | – | – | 240, Lake Abbe (Künzel et al. 2004; A. Laurent pers. comm. 2015) | 1440, Forêt de Day (Künzel et al. 2004) |
| Somalia | 0, Baraawe, Marka (d’Huart and Grubb 2001) | 1690, Ceel Afweyn (D. Mallon pers. comm. 2014; N. Redman pers. comm. 2012) | 130, Shimbiraleh Guban, Berbera (d’Huart and Grubb 2001) | 960, Mandheera (Elliot 1897) |
| Kenya | 0, Mkokoni, Kiunga Marine Reserve (d’Huart and Grubb 2001) | 1895, Suyian Ranch (Butynski and De Jong 2021; Powys 2020) | 0, Lamu (d’Huart and Grubb 2001); Mkokoni (Amin et al. 2017) | 2385, Mau Summit (Hollister 1924) |
As mentioned above, the ‘Phacochoerus Shapefile’ does not distinguish between the two species of Phacochoerus and is, therefore, not included in Table 2. Nonetheless, in some cases, the ‘Phacochoerus Shapefile’ strongly suggests altitudinal limits that are greater than those presented in Table 2. Here is a summary of where we suspect these maps extend the altitudinal limits beyond those given in Table 2.
Eritrea: Yalden et al. (1984) show Phacochoerus near Asmara (2300 m asl). The P. africanus buffer zone for Mogo (1730 m asl; Abraha 2016) also reaches Asmara. There is no record for P. aethiopicus in Eritrea, the nearest record to Asmara being 665 km to the southeast at Duddarp, northwest Somalia (Figure 5). It is, therefore, highly probable that the altitudinal range for P. africanus in Eritrea is 0–2300 m asl.
Ethiopia: Künzel et al. (2004) found P. africanus at Lake Abbe, Djibouti, near Ethiopia at 240 m asl (Figure 5). The buffer zone for P. africanus at Lake Abbe runs into Ethiopia at the same altitude. It is probable that the altitudinal range for P. africanus in Ethiopia is about 240–3500 m asl.
Djibouti: Yalden et al. (1984) show Phacochoerus at sea level west of Tadjoura. The buffer zone for the Forêt de Day record for P. africanus (Table 2) also reaches the coast of Djibouti. It is, therefore, likely that the altitudinal range for P. africanus in Djibouti is 0–1440 m asl. There is no record for P. aethiopicus in Djibouti, the nearest record to Tadjoura being about 90 km to the southeast at Duddarp, Somalia (Figure 5).
Somalia: Phacochoerus africanus occurs on the north coast of Somalia where the lowest altitudinal record is 30 m asl (Table 2). This species occurs at sea level just south of Somalia on the north coast of Kenya (Figure 5). Mandheera (960 m asl) represents the highest confirmed altitudinal record for P. africanus in Somalia. D. Mallon (pers. comm. 2021), however, observed Phacochoerus at Daallo at 2130 m asl in Buxus-Juniperus habitat. It is probable that the altitudinal range for P. africanus in Somalia is 0–2130 m asl.
Kenya: Given that P. africanus occurs to 3500 m asl on the Bale Mountains, 7° north of the equator in Ethiopia (Table 2), its known highest altitude in Kenya (2385 m asl on the equator) is surprisingly low. This is especially unexpected given the similarities in the montane and alpine plant community, and that many of the other species of large mammal on the volcanic Bale Mountains (e.g., Yalden et al. 1984) achieve greater altitude on Kenya’s highest volcanic mountains (Coe and Foster 1972; Moreau 1944a,b; Young and Evans 1993). Nonetheless, the distribution maps in Stewart and Stewart (1963) and Kingdon (1979) do not show records above the foothills of Kenya’s three highest mountains; Mount Elgon, Aberdare Range, and Mount Kenya. This also corresponds to the other literature (e.g., Butynski 1999; Butynski and De Jong 2016; Young and Evans 1993). Similarly, on Mount Kilimanjaro, in north Tanzania on Kenya’s south border, there is no record for P. africanus above the foothills (Child 1965; Grimshaw et al. 1995; Guest and Leedal 1954; Moreau 1944a; True 1892).
Lowest and highest mean annual rainfall, by country, over the geographic distribution of desert Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa.
| Country | Mean annual rainfall (mm) | |||
|---|---|---|---|---|
| Phacochoerus aethiopicus | Phacochoerus africanus | |||
| Lowest | Highest | Lowest | Highest | |
| Eritrea | – | – | 50 | 850 |
| Ethiopia | 200 | 700 | 100 | 1950 |
| Djibouti | – | – | 50 | 450 |
| Somalia | 100 | 650 | 50 | 550 |
| Kenya | 250 | 1200 | 150 | 1950 |
3.4 Rainfall
Mean annual rainfall over the geographic distribution of P. aethiopicus ranges from 100 mm (northeast coast of Somalia) to 1200 mm (central Kenya; Figure 6; Table 3). Most records are from regions where mean annual rainfall is between 200 mm and 500 mm. Mean annual rainfall over the geographic distribution of P. africanus ranges from about 50 mm (southeast Eritrea and north coast of Somalia) to 1950 mm (central Kenya and west Ethiopia), with most records from areas where mean annual rainfall is 200–1200 mm.
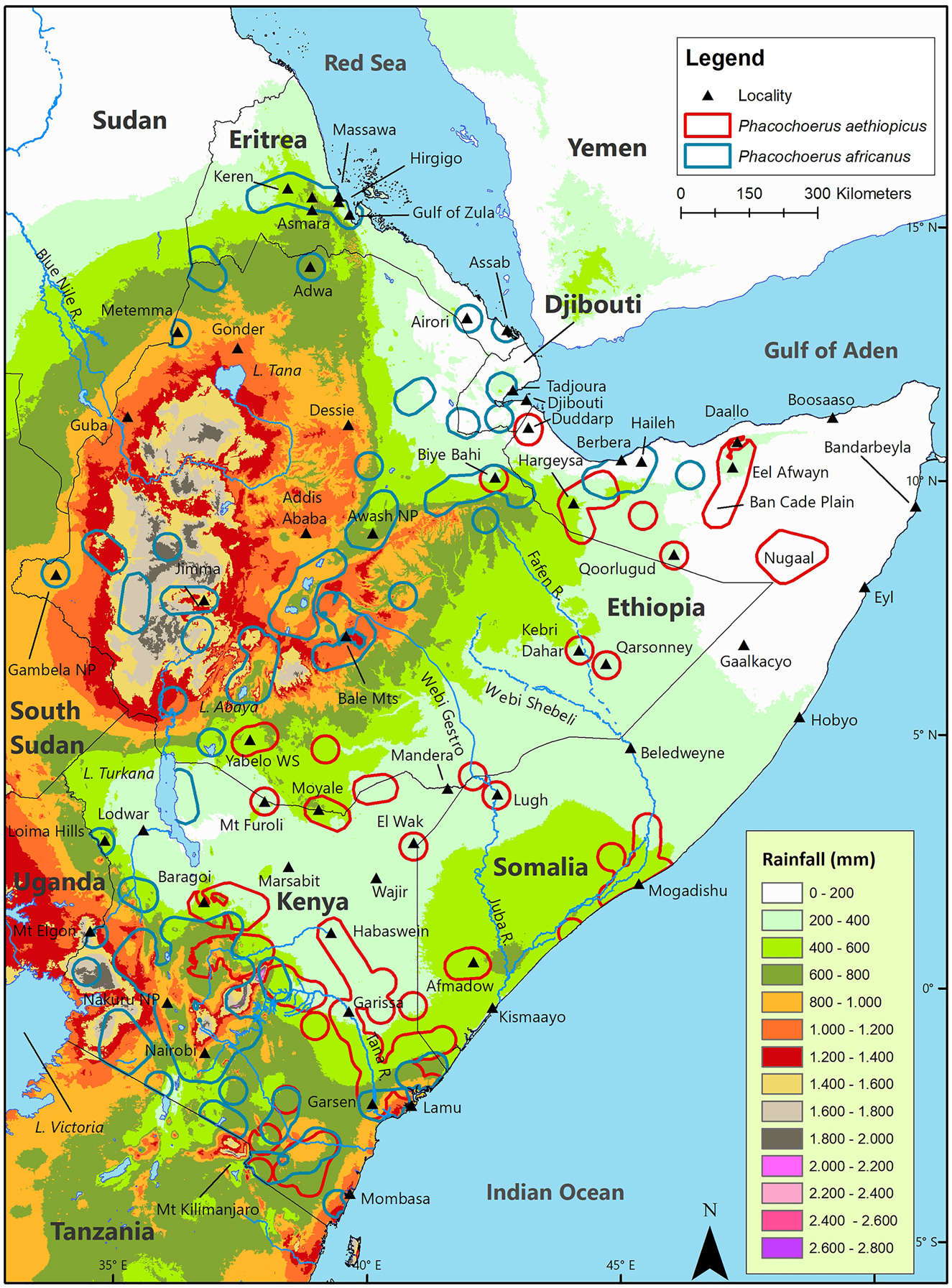
Geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa relative to mean annual rainfall.
Rainfall is low and temperatures are high (see below) along the south coast of the Red Sea, south coast of the Gulf of Aden, and west coast of the Indian Ocean, but these extreme conditions are often ameliorated by the high humidity that results from the proximity to these large bodies of warm water and the mist carried inland by the Northeast Trades (Kingdon 1990). On the north coast of Somalia, where some sites support both species, mean annual rainfall is as low as 50 mm (Berbera) but mean relative humidity is 67% (mean monthly range = 44–81%). On the coast of Eritrea, P. africanus occurs where mean annual rainfall is 219 mm (Massawa) but mean relative humidity is 66% (mean monthly range = 54–76%). These levels of humidity help support a higher level of plant growth and greater plant diversity than would otherwise be possible in these low rainfall regions. Regardless of the high humidity, neither P. aethiopicus nor P. africanus occurs where mean annual rainfall is <50 mm (e.g., in the vicinity of Boosaaso and Cape Guardafui near the east tip of the HoA; Figure 6).
3.5 Temperature
Mean annual temperature over the geographic distribution of P. aethiopicus ranges from as low as 17 °C (central north coast of Somalia and Laikipia, central Kenya) to as high as 31 °C (Lugh, south Somalia; Figure 7; Table 4), while mean annual temperature across the geographic distribution is typically between 20 °C and 27 °C. The extremes are about 5.5 °C (Ceerigaabo, north Somalia) and 49 °C (Berbera, north Somalia; weatherspark.com).
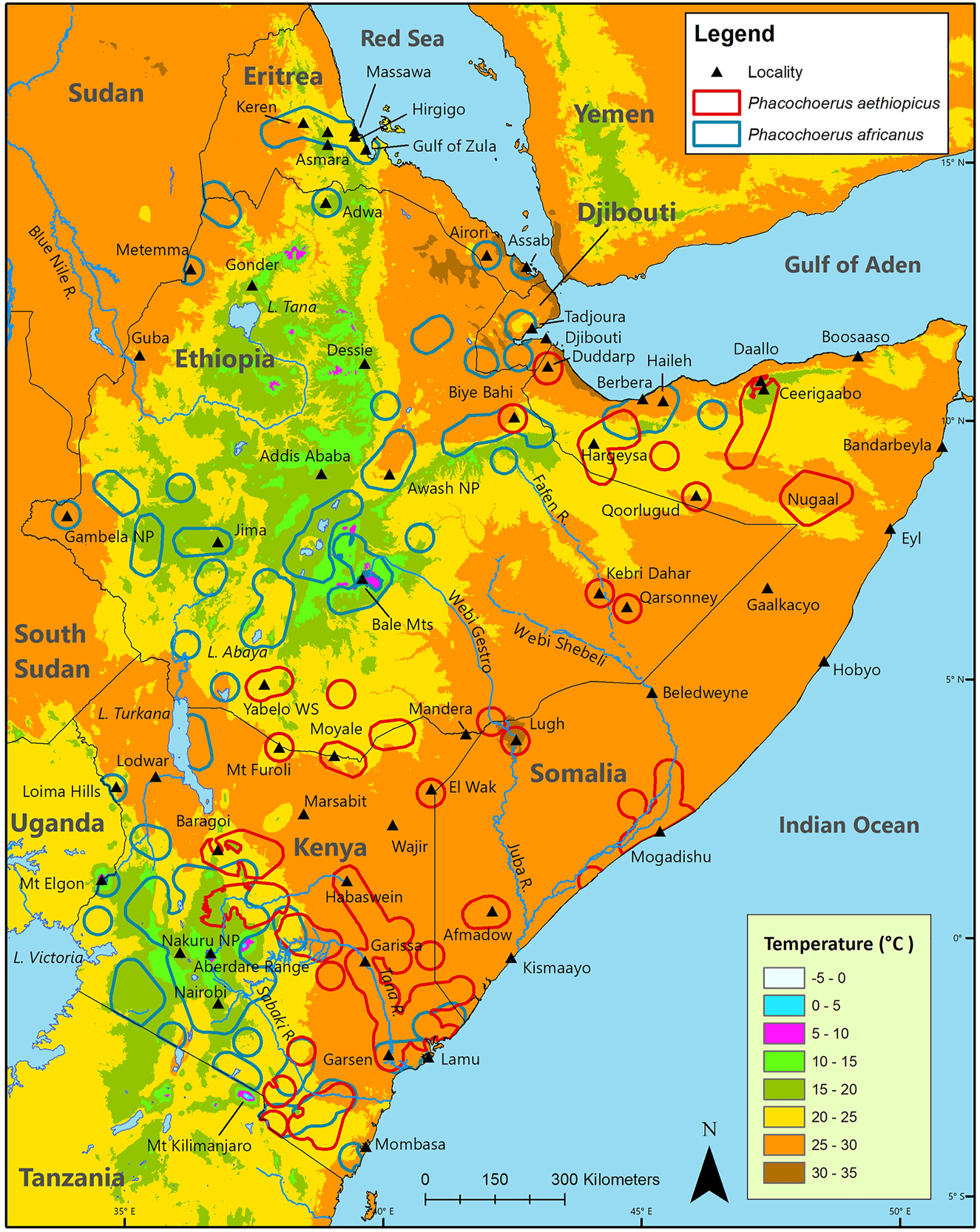
Geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa relative to mean annual temperature.
Lowest and highest mean annual temperatures, by country, over the geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa.
| Country | Mean annual temperature (°C) | |||
|---|---|---|---|---|
| Phacochoerus aethiopicus | Phacochoerus africanus | |||
| Lowest | Highest | Lowest | Highest | |
| Eritrea | – | – | 16 | 32 |
| Ethiopia | 20 | 30 | 8 | 30 |
| Djibouti | – | – | 21 | 32 |
| Somalia | 17 | 31 | 17 | 30 |
| Kenya | 17 | 29 | 8 | 30 |
Mean annual temperature over the geographic distribution of P. africanus ranges from as low as 8 °C (Aberdare Range, central Kenya, and Bale Mountains, central south Ethiopia), to as high as 32 °C (central Djibouti and Assab, southeast Eritrea), while mean annual temperature across the geographic distribution is typically between 15 °C and 25 °C. Temperature can drop to about −7 °C in the Web Valley, Bale Mountains (C. Gordon pers. comm. 2018), and reach 45 °C at Tadjoura, Djibouti.
3.6 Biomes, biotic zones, and ecoregions
In the HoA, P. aethiopicus and P. africanus both occur mainly in the ‘Tropical and Subtropical Grasslands, Savannas, Shrublands, and Woodlands Biome’, but also in the ‘Deserts and Xeric Shrublands Biome’, and the ‘Tropical and Subtropical Moist Broadleaf Forest Biome. In addition, P. africanus occurs in the ‘Montane Grasslands and Shrublands Biome’.
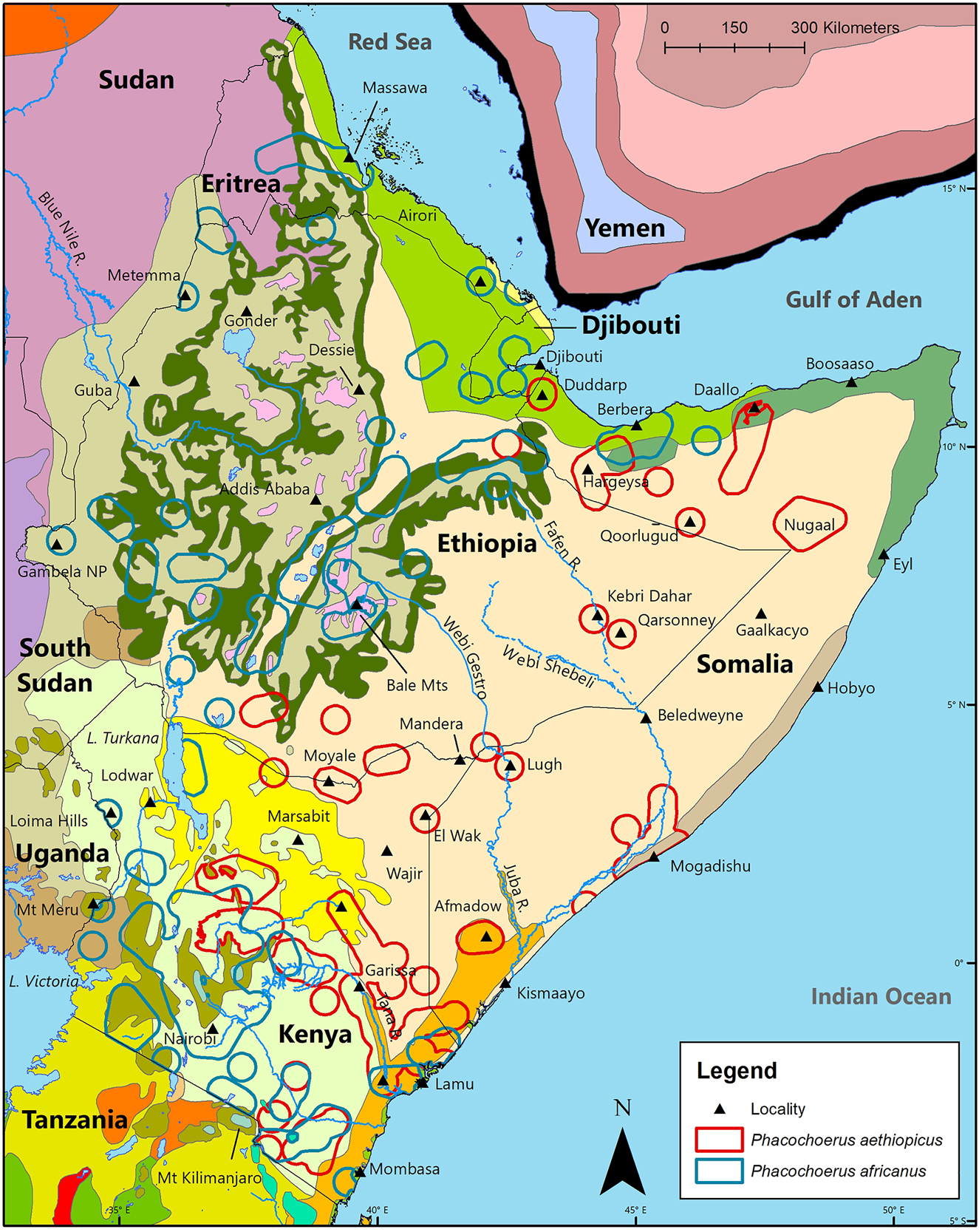
Geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the ecoregions of the Horn of Africa (Burgess et al. 2004; Olson et al. 2001).
The 16 ecoregions (Burgess et al. 2004; Olson et al. 2001), by country, in which desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus occur in the Horn of Africa.
| Ecoregion | Eritrea | Ethiopia | Djibouti | Somalia | Kenya |
|---|---|---|---|---|---|
| East African Montane Forests | africanus | ||||
| East Sudanian Savanna | africanus | africanus | |||
| Eastern Arc Forest | africanus | ||||
| Eritrean Coastal Desert | africanus | ||||
| Ethiopian Montane Forests | africanus | africanus | |||
| Ethiopian Montane Grasslands and Woodlands | africanus | africanus | |||
| Ethiopian Montane Moorlands | africanus | africanus | |||
| Ethiopian Xeric Grasslands and Shrublands | africanus | africanus | africanus |
aethiopicus
africanus |
|
| Hobyo Grasslands and Shrublands | aethiopicus | ||||
| Masai Xeric Grasslands and Shrublands |
aethiopicus
africanus |
||||
| Northern Acacia-Commiphora Bushlands and Thickets |
aethiopicus
africanus |
||||
| Northern Zanzibar-Inhambane Coastal Forest Mosaic | aethiopicus |
aethiopicus
africanus |
|||
| Sahelian Acacia Savanna | africanus | africanus | |||
| Somali Acacia-Commiphora Bushlands and Thickets | africanus |
aethiopicus
africanus |
aethiopicus
africanus |
aethiopicus | |
| Somali Montane Xeric Woodlands |
aethiopicus
africanus |
||||
| Victoria Basin Forest-Savanna Mosaic | africanus |
Most of the geographic distribution of P. aethiopicus lies within the ‘Somalia-Masai Bushland Biotic Zone’ (Happold and Lock 2013). This is White’s (1983) ‘Somalia-Masai Regional Centre of Endemism’. This biotic zone has a long history of isolation and aridity, and is characterised by low, very unpredictable, and highly variable rainfall.
Phacochoerus aethiopicus occurs in at least seven ecoregions (Figure 8; Table 5). See Figure 2.2 in Burgess et al. (2004) and Figure 2 in Olson et al. (2001). Most of the geographic distribution of this species lies in the ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’, while there is also substantial presence in the ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ and ‘Northern Zanzibar-Inhambane Coastal Forest Mosaic Ecoregion’
Phacochoerus africanus is present in at least 15 ecoregions in the HoA, but particularly in the ‘Northern Acacia-Commiphora Bushlands and Thickets Ecoregion’, ‘Somali Acacia-Commiphora Bushlands and Thickets Ecoregion’, ‘Ethiopian Xeric Grasslands and Shrublands Ecoregion’, and ‘Ethiopian Lower Montane Forests, Woodlands, and Bushlands Ecoregion’.
The only ecoregion where P. aethiopicus is present, but where P. africanus is apparently absent, is the ‘Hobyo Grasslands and Shrublands Ecoregion’ which is located along the east coast of Somalia. This highly distinct ecoregion, also known as the ‘Obbia’, is marked by dune grasslands with a sparse covering of bushes, small shrubs, and herbs adapted to the persistent sand-laden winds (Kingdon 1979, 1990; White 1983).
The five regions of sympatry between P. aethiopicus and P. africanus occur in five ecoregions (Figures 3 and 8). This indicates considerable similarities in the physiology, ecology, and behaviour of these two species, and that the major differences are not likely to be determined without detailed studies in areas with extreme climatic conditions.
3.7 Habitats
Phacochoerus aethiopicus appears to prefer relatively flat and sandy ground with open areas of short, perennial grass in Acacia spp. woodland and Acacia spp./Commiphora spp. bushland. Edges of water courses, swamps, lakes, drainage lines, vegetation mosaics, and bases of rocky outcrops offer the habitat characteristics that seem favoured by P. aethiopicus. On Suyian Ranch, P. aethiopicus and P. africanus both appear to prefer abandoned cattle bomas (livestock enclosures or kraals) where nutritious grasses are dominant, particularly Cynodon spp. (T. Butynski and Y. de Jong pers. obs.). Burrows are probably a prerequisite. Phacochoerus aethiopicus apparently occurs where perennial sources of drinking water are unavailable. Elliot (1897, p. 110) states, “They appeared to be somewhat independent of water, for we met them in the middle of the Haud where certainly the nearest water-hole must have been 50 miles away, too far one might suppose for them to seek daily.”
Of the two species, P. africanus occupies a wider range of habitats, but is typically a species of open country; grasslands, open bushlands, open woodlands, savanna, pans, floodplains, glades, and vleis. This species is most abundant on alluvial soils in lightly wooded vegetation mosaics where water is always present (Butynski and De Jong 2018; Grubb et al. 1998; Skinner and Chimimba 2005), but also occurs, at least at low density, where perennial sources of water are not available (Bigourdan 1948; Butynski and De Jong 2018; Dorst and Dandelot 1970; Smithers 1971). Phacochoerus africanus is also present in some forests where it is probably reliant on large open areas of short grass (Deribe et al. 2008; Künzel et al. 2004; Yalden et al. 1996).
Phacochoerus africanus requires burrows for thermal-regulation, water conservation, sleeping, giving birth, rearing young, and predator-avoidance, yet does not dig its own burrows (Bradley 1971; Cumming 1975; Geigy 1955). These are typically modified aardvark Orycteropus afer burrows, but sometimes those of Cape crested porcupineHystrix africaeaustralis or North African crested porcupine Hystrix cristata are used, as well as rock caves and underground erosion gullies (Butynski and De Jong 2018; Cumming 1975; Guiraud 1948; Shortridge 1934; White and Cameron 2009). Orycteropus afer is very broadly, if not completely, sympatric both with P. aethiopicus and P. africanus, while Hystrix spp. occurs over most of the geographic distributions of these two species, with the notable exceptions of Djibouti and Somalia.
4 Discussion
4.1 Ecology
Consistent with the findings of d’Huart and Grubb (2001), P. aethiopicus is endemic to Ethiopia, Somalia, and Kenya. This species is not known to occur at altitudes >1895 m asl, nor where mean annual rainfall is <100 mm or >1200 mm or where the mean annual temperature is <17 °C or >31 °C (Table 6). It appears that the geographic distribution of P. aethiopicus is more limited by altitude, rainfall, and temperature than is P. africanus. The altitudinal range of P. africanus overlaps that of P. aethiopicus from sea level to 1895 m asl, but extents to 3500 m asl (Table 6). Examination of major environmental gradients, shown in Table 6, indicate that P. africanus is able to live in more extreme environments than P. aethiopicus.
Altitudinal, mean annual rainfall, and mean annual temperature limits of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in the Horn of Africa.
| Environmental gradients | Phacochoerus aethiopicus | Phacochoerus africanus |
|---|---|---|
| Altitude (m asl) | 0–1895 | 0–3500 |
| Mean annual rainfall (mm) | 100–1200 | 50–1950 |
| Mean annual temperature (°C) | 17–31 | 8–32 |
The geographic distribution of P. aethiopicus is restricted to low altitude, semi-arid, landscapes east of the ERV, only occupying the graben of the ERV in north Ethiopia and northwest Somalia (Figure 3). Here the graben is wide (>300 km) and the escarpment is relatively gradual and low. In contrast, the geographic distribution of P. africanus is not limited by the ERV. The ERV appears to be an ecological barrier for P. aethiopicus south of N 9.6°. It is likely that the relatively high annual rainfall (>1250 mm) and lower mean annual temperatures (<17 °C) associated with the higher altitudes of the east escarpment of the ERV (>1895 m asl) cannot be tolerated by P. aethiopicus, especially the young.
The skull of P. aethiopicus is slightly smaller than that of P. africanus (Grubb 1993). Although body measurements for P. aethiopicus are lacking, it appears (in the field) to be the marginally smaller species, as well as more gracile with less muscle mass (at least over the hindquarters) and relatively longer legs. These characters give P. aethiopicus a relatively greater body surface area than P. africanus. In addition, P. aethiopicus often appears lean with wrinkled (‘desiccated’) skin, whereas P. africanus typically appears fat with tight skin. It may be that P. aethiopicus has less capacity to accumulate sub-dermal fat than does P. africanus. Both species, but particularly P. aethiopicus, have a sparse pelage that affords little protection from the cold. While these traits probably provide an advantage to P. aethiopicus in extreme heat, they are likely disadvantages under conditions of cold and drought.
At the northeast extreme of the ERV, P. aethiopicus occurs on the graben of the ERV at Biye Bahi (920 m asl) and Duddarp (185 m asl; Figure 5). In three regions south of N9.6°, the east escarpment of the ERV is <1895 m asl (lower Omo River Basin, southwest Ethiopia; Lake Turkana Basin, northwest Kenya; Lake Magadi Basin, central south Kenya). In two of these relatively low regions, P. aethiopicus reaches the east escarpment of the ERV; Yabelo Wildlife Sanctuary (>1430 m asl) east of the Omo River and south of Baragoi (1425 m asl).
Phacochoerus aethiopicus occurs mainly in the ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ and ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ (Figure 8). Both of these ecoregions are low in altitude, relatively flat, and arid to semi-arid, with a mean annual rainfall that can fluctuate greatly year to year (<100 mm/year to 600 mm/year; Burgess et al. 2004), and temperatures that can reach >38 °C. A small part of the geographic distribution of P. aethiopicus lies in the ‘Northern Zanzibar-Inhambane Coastal Forest Mosaic Ecoregion’. This ecoregion is also low in altitude, mostly flat, and relatively wet with a mean annual rainfall of <1000–2000 mm (Burgess et al. 2004). Located along the north coast of Kenya and along the Tana River, this ecoregion represents a transition zone between the ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ to the north and the ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ to the west. Another small part of the geographic distribution of P. aethiopicus lies in the hot and dry ‘Hobyo Grasslands and Shrublands Ecoregion’ of east Somalia (see above). Maximum temperature over most of this ecoregion is between 30 °C and 33 °C, while mean annual rainfall ranges from 200 mm to 430 mm. The highest temperatures occur over the south part of this ecoregion (38 °C at Mogadishu) where mean annual rainfall is 429 mm.
The south extreme of the geographic distribution of P. aethiopicus is a region of sympatry with P. africanus. This is where the ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ meets the ‘Southern Acacia-Commiphora Bushland and Thickets Ecoregion’. There is no evidence for P. aethiopicus in the ‘Southern Acacia-Commiphora Bushland and Thickets Ecoregion’, the north-most extent of which reaches the Kenya-Tanzania border.
A broken chain of highlands and mountains runs northeastwards along the southeast escarpment of the ERV from central Ethiopia almost to the tip of the HoA at Cape Guardafui (Figure 5; Happold and Lock 2013). Along this chain, in central east Ethiopia, lie the Hararge Highlands, while farther to the northeast, across a lowland gap (where Hargeysa is located), are the Ogo Highlands (= Galgodon Highlands). The Ogo Highlands include the range of mountains that runs along the north coast of Somalia from Mandheera eastwards to near Cape Guardafui. It appears that the population of P. africanus on the foothills of the Hararge Highlands is now isolated from the population on the foothills of the Ogo Highlands. The presence of these two currently disconnected populations suggests that the Hararge Highlands and Ogo Highlands were once part of a more homogeneous ecosystem that has since been fragmented by geologic and climatic changes.
4.2 Estimated geographic distribution
Our best estimates of the current geographic distributions in the HoA for P. aethiopicus and P. africanus (Figure 9) are based on the historic map for Phacochoerus (Figure 3), their altitudinal and climatic limits (Table 6), as well as ecoregions, threats, and conservation status.
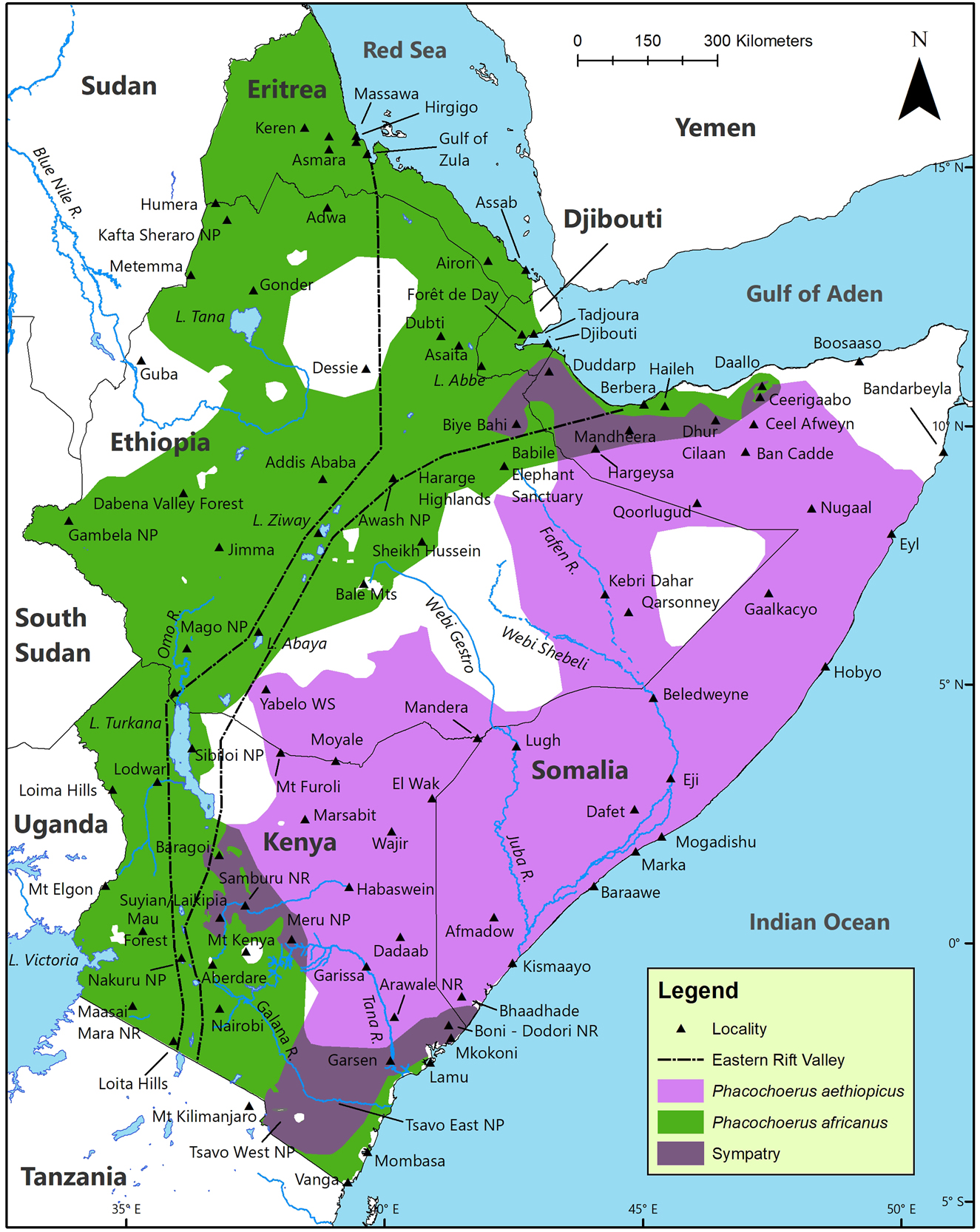
Estimated current geographic distribution of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus, and the areas of sympatry, in the Horn of Africa.
The geographic distribution of P. aethiopicus extends from southeast Kenya north to the Red Sea and Gulf of Aden, and from the east flank of the eastern escarpment (below 1895 m asl) of the ERV eastwards to the Indian Ocean (Figures 3 and 9). The estimated current geographic distribution is 1,109,000 km2 (45% of the land surface of the HoA; Figure 9). This includes most of central and east Kenya, all but the driest parts of southeast Ethiopia (mean annual rainfall <200 mm), and all but the driest parts of Somalia (mean annual rainfall <100 mm). The only records for P. africanus over the vast region comprised of east Ethiopia and Somalia come from Berbera and Dhur Cilaan in extreme north Somalia. We expect that P. africanus also occurs in Daallo (extreme north Somalia) which lies in the ‘Somali Montane Xeric Woodlands Ecoregion’.
The estimated current distribution of P. africanus in the HoA is shown in Figure 9. Since P. africanus is present on the north coast of Kenya, where it is sympatric with P. aethiopicus, we expect it to also occur in the ‘Northern Zanzibar-Inhambane Coastal Forest Mosaic Ecoregion’ in extreme south Somalia. Phacochoerus africanus occurs within and west of the ERV throughout the HoA. The estimated current geographic distribution of P. africanus in the HoA is 1,213,000 km2 (49% of the land surface of the HoA; Figure 9).
4.3 Threats and conservation
Phacochoerus aethiopicus and P. africanus are both listed as ‘Least Concern’ on the IUCN Red List of Threatened Species (De Jong et al. 2016a,b). Both species do relatively well in regions unsuitable for growing crops. Outside the HoA, P. africanus is widespread, locally abundant and, in some areas, expanding its geographic distribution (e.g., South Africa; Swanepoel et al. 2016). Nonetheless, overall, the abundance and geographic distribution of both species continues to decline. The ultimate cause of this decline in the HoA is humans, the populations of which continue to grow rapidly in all five countries (see www.wildsolutions.nl/warthogbase). Details on the threats to the long-term survival of P. aethiopicus and P. africanus are presented in Butynski and De Jong (2018), De Jong and Butynski (2018), and De Jong et al. (2016a,b).
The ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ (1,053,900 km2) and, to a lesser extent, the ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ (326,000 km2), are the strongholds for P. aethiopicus. The ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ is the second richest ecoregion in the HoA in terms of the number of endemic vertebrate species (49). As such, it is regarded as ‘Globally Outstanding’ in WWF’s Biological Distinctiveness Index (Burgess et al. 2004). The ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ is the richest ecoregion in the HoA in terms of the number of vertebrate species (1012; Burgess et al. 2004). The ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ is among Africa’s top 10 ecoregions with the highest extinction risk scores (Burgess et al. 2004). To the northwest, the ecoregions of the Ethiopian Highlands on both sides of the ERV, where P. africanus is present, also have an outstanding biodiversity. They are, however, listed as “Critically Threatened” as they are among Africa’s ecoregions with the highest percentage of habitat loss (Burgess et al. 2004).
Phacochoerus aethiopicus occurs at low densities over most of its geographic distribution and there are large areas within the distribution polygon where it is apparently absent (De Jong and Butynski 2018; De Jong et al. 2016a). In Arawale NR, central east Kenya, Njoroge et al. (2009) report a mean density of 0.8 individuals/km, with highest densities in thickets (1.3 individuals/km). Low abundance or absence are likely a result of scarcity of food and perennial water, given the low annual rainfall throughout much of the geographic distribution and the great variability and extremes of rainfall. For example, mean annual rainfall at Mogadishu is 429 mm but varies more than 16-fold, from 60 mm to 1000 mm (Ashford 1998).
Rainfall is strongly correlated with plant productivity and, thus, food for herbivores. The lower the annual rainfall, the higher the annual rainfall variability and, therefore, the greater the variability of plant foods for herbivores. Jones and Thornton (2013) and Thornton (2014) used the coefficient of variation of mean annual rainfall as a measure of rainfall variability. Phacochoerus aethiopicus typically lives under conditions of considerably higher annual rainfall variability and, therefore, probably food variability, than does P. africanus. Over the northeast 20% or so of the HoA, where the mean annual rainfall is <250 mm, annual rainfall variability is >45%. Over most of the remainder of the geographic distribution of P. aethiopicus, where mean annual rainfall is 200–500 mm, annual rainfall variability is >30%. Much of the geographic distribution of P. africanus in the HoA receives a mean annual rainfall of 200–1200 mm. Here rainfall variability ranges from 15% to 40%. It may be that, while P. africanus is able to survive under a greater range of altitude, rainfall, and temperature than P. aethiopicus, the latter is able to cope with greater variability in the availability of water and food.
Where water and food are reliably available, P. aethiopicus can be common. Aggregations of up to 30 individuals occur in bushland around permanent wells and close to towns in Ogaden, southeast Ethiopia (Wilhelmi et al. 2004). Phacochoerus aethiopicus occurs at high density during the dry season on patches of green grass along the Ewaso N’yiro River in Samburu NR, central Kenya, and is sometimes common and confiding near small, remote, villages and lodges where hunting is absent (A. Caron pers. comm. 2004; De Jong and Butynski 2018; De Jong et al. 2016a; Grubb and d’Huart 2010).
Hybridization is important from a species conservation perspective as it is a potential threat. Currently, there is no solid evidence for hybridization between P. aethiopicus and P. africanus. Recent analyses of the genetic structure and phylogeography of Phacochoerus, based on the whole genome sequencing of four P. aethiopicus and 35 P. africanus, did not identify recent hybrids (Garcia-Erill et al. 2022). This result is, however, not surprising given the small sample size (n = 39). This study did, however, find one P. africanus from an area of sympatry in Kenya (Tsavo West NP) that showed signs of enhanced P. aethiopicus ancestry. As mentioned above, in an area of sympatry on the Laikipia Plateau, several individuals judged to be atypical for either P. aethiopicus or P. africanus were observed (Butynski and De Jong 2021). In short, on-going hybridization between P. aethiopicus and P. africanus cannot be excluded.
4.4 Protected areas
Phacochoerus aethiopicus occurs in at least 12 protected areas in Kenya [Arawale NR (533 km2), Boni NR (1339 km2), Buffalo Springs NR (131 km2), Dodori NR (877 km2), Kora NP (1787 km2), Losai NR (1806 km2), Meru NP (870 km2), Samburu NR (165 km2), Shaba NR (239 km2), Tsavo East NP (13,747 km2), Tsavo West NP (7065 km2), and Ishaqbini Hirola Conservancy (72 km2)], and Ethiopia (Yabelo Wildlife Sanctuary; 2496 km2) (Culverwell et al. 2008; De Jong and Butynski 2014, 2018; De Jong et al. 2016a; d’Huart and Grubb 2001; Grubb and d’Huart 2010, 2013). This species is expected to be present in the Tana River Primate NR, Rahole NR, Bisanadi NR, Kenya, in Babile Elephant Sanctuary, Ethiopia, and in Daallo Mountain NP, Lag Badana-Bushbush NP, and Kismayo NP, Somalia. Phacochoerus africanus is present in many protected areas in the HoA.
Unfortunately, protected areas in the HoA typically face many challenges, particularly high levels of illegal activities (e.g., poaching, taking of fuelwood, timber, and charcoal, livestock grazing and browsing, and agricultural encroachment). Government funding is often insufficient for training, equipping, and supporting the required numbers of management, security, education, and research personnel. Corruption and mismanagement are other problems (Butynski and De Jong 2018; De Jong and Butynski 2014, 2018; De Jong et al. 2016a). In addition, parks and reserves are increasingly fragmented and isolated, lacking connectivity with other protected areas. This connectivity is essential for gene-flow and species range shifts, particularly in this time of rapid climate change (Saura et al. 2018).
4.5 Future research
As stated above, the natural history of P. aethiopicus remains poorly known. While this study addressed questions concerning the biogeography of Phacochoerus in the HoA, it also generated important questions, including:
Does P. aethiopicus occur in Djibouti, Eritrea, or Tanzania? Was this species historically present in these countries?
Does P. africanus occur in south Somalia?
Does P. africanus occur in Samburu NR, Buffalo Springs NR, or Shaba NR, central Kenya, and in Daallo Forest Reserve, north Somalia?
Which species is present in those areas depicted as ‘Phacochoerus sp.?’ in Figure 3?
Where else are P. aethiopicus and P. africanus sympatric?
Do P. aethiopicus and P. africanus hybridize?
What does the fossil record tell us about the historic distribution of P. aethiopicus and P. africanus?
Which subspecies of P. africanus are valid and what are their geographic distributions?
What is the diet of P. aethiopicus? How do the diets of P. aethiopicus and P. africanus differ?
Do P. aethiopicus and P. africanus differ in their dependence on sources of perennial water for drinking?
Do P. aethiopicus and P. africanus differ in the level of nocturnality?
5 Conclusions
Phacochoerus aethiopicus and P. africanus are widespread in the HoA, with P. africanus having the greater geographic distribution. This difference is probably related, in large part, to the fact that P. africanus is able to live in more extreme environments than P. aethiopicus as indicated by gradients of altitude, rainfall, and temperature. The ERV appears to be a geographic barrier for P. aethiopicus, while the semi-arid bushlands and sub-deserts of much of the east half of the HoA appear to be barriers for P. africanus. The two main ecoregions in which P. aethiopicus occurs are the ‘Somali Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’ and the ‘Northern Acacia-Commiphora Deciduous Bushlands and Thickets Ecoregion’. Large portions of each of these two ecoregions occur in Somalia. Relative to many other species of large mammals in the HoA, these two suids remain widespread and locally common. They are, nonetheless, in decline as a result of a rapidly growing human population in all five countries. These findings support the current IUCN Red List assessments of ‘Least Concern’ for these two species (De Jong et al. 2016a,b).
Funding source: Zoo Atlanta; Zoo New England; Margot Marsh Biodiversity Foundation; Primate Action Fund (Re:wild); Critical Ecosystem Partnership Fund; Conservation International; Zoological Society for the Conservation of Species and Populations; Northern Rangeland Trust; Primate Conservation Inc.; National Geographic Society
Acknowledgments
This paper is dedicated to zoologist Dr. Peter Grubb, who passed away on 23 December 2006 (Oates et al. 2008). Peter’s main research focus was the taxonomy and distribution of Africa’s mammals, particular ungulates and primates. He led the ‘re-discovery’ of P. aethiopicus and published much of the early information on the taxonomy, biogeography, and conservation of this species. Together with Jean-Pierre d’Huart, Peter compiled the first distribution map for Phacochoerus in the Horn of Africa in 2001, paving the way for us, 20 years later, to present what we currently know about one of Africa’s least known large mammals. We acknowledge and thank the following people for their unpublished information and/or assistance: Yilma Abebe, Paolo Agnelli, Tony Allport, Raj Amin, Nicki Austin, Fausto Barbagli, Sheila Bell-Cross, Joan Bennun, Max Bennun, Shivani Bhalla, Jean-Paul Boerekamps, Luca Borgesio, Nik Borrow, Greg Brownlee, Alexandre Caron, Andre Coetzee, Tim Collins, Evan Craig, Ian Craig, Johnnie Cross, James Culverwell, David Cumming, Peter Cunningham, Zeke Davidson, Matthias de Beenhouwer, Arnoud de Jong, Tom de Maar, Emmanuel de Merode, Jean-Pierre Dekker, Lois Dekker, Noah Dekker, Yilma Dellelegn, Jacqueline d’Huart, John Doherty, Grégoire Dubois, Helen Dufreshne, Scott Dyson, Richard Estes, Marc Faucher, Peggy Faucher, Jim Feely, Craig Feibel, Brian Finch, Gérard Galat, Anh Galat-Luong, Spartaco Gippoliti, Kathy Goins, Georgia Gold, Chris Gordon, Olivier Hamerlynck, Meridith Happold, Curtis Hart, Jens Ove Heckel, Forest Jarvis, Henning Jensen, Julia Kalinkina, Xassan Yussuf Kaariye, George Kashouh, Fanuel Kebede, Julian Kerbis, Juliet King, Jonathan Kingdon, Alisa Klamm, Richard Kock, Michael Kohn, Hassan Kossar, Eric Lafforgue, Alain Laurent, Louise Leakey, Artemy Lebedev, Markus Lilje, Dick Looij, Klaus Lorenze, Belinda Low Mackey, Patrick Malonza, Trish Luke, Quentin Luke, David Mallon, Brian McMorrow, Rafael Medina, Dominique Mignard, Patricia Moehlman, Simon Musila, Béla Nagy, Vincent Obanda, Bernard Ogwoka, Wesley Overman, Martin Pickford, Håkan Pohlstrand, Anne Powys, Gilfrid Powes, John Rawnsley, Nigel Redman, Johannes Refisch, Chris Roche, Jason Roussos, Avery Sean, Sidney Shema, Ludwig Siege, Etotépé Sogbohossou, Malte Sommerlatte, Antoine Souron, Steve Spawls, Siva Sundaresan, Gloria Svampa, Tatiana Thomas, Simon Tonge, Vlada Trailin, Laura Tranter, Lulu Vallings, Janco van Gelderen, Dick van Hoffen, Ariadne Van Zandbergen, Vincenzo Vomero, Archie Voorspuy, Tim Wacher, Washington Wachira, John Weir, and Stuart Williams. We especially thank Lorna Depew and Carly Butynski for reviewing the draft manuscript. We extend our appreciation to the National Council for Science and Technology, Government of Kenya. We thank the Institute of Primate Research, National Museums of Kenya, and Kenya Wildlife Service for serving as our research affiliates in Kenya.
-
Author contributions: Conceptualization, study design, methodology, data collection, analyses, and preparation of the text by YdJ, JPdH, and TMB. Kenya fieldwork by TMB and YdJ. Data curation and mapping by YdJ.
-
Research funding: Fieldwork was funded by Zoo Atlanta, Zoo New England, Margot Marsh Biodiversity Foundation, Primate Action Fund (re:wild), Critical Ecosystem Partnership Fund, Conservation International, Zoological Society for the Conservation of Species and Populations, Northern Rangeland Trust, Primate Conservation Inc., and National Geographic Society.
-
Conflict of interest statement: The authors declare that they have no conflicts of interest regarding this article.
-
Data availability: Locality data used in this study for P. aethiopicus and P. africanus in the HoA are available in ‘WarthogBase’ at: www.wildsolutions.nl/warthogbase. More than 400 geotagged photographs of Phacochoerus spp. throughout their geographic distributions are available on ‘Warthog Photographic Map’ at: https://wildsolutions.nl/photomaps/phacochoerus/. Supporting information can be found at: www.wildsolutions.nl/warthogbase.
-
Research ethics: Fieldwork in Kenya conducted with permission from the National Commission for Science, Technology & Innovation (NACOSTI permits: NCST/RRI/12/1/MAS/61; NCST/RRI/12/1/MAS/47; MOEST 13/001/33C 283/3; MOEST 13/001/33C 283; NACOSTI/P/15/2864/4867; NACOSTI/P/18/2864/21939).
References
Abraha, E.T. (2016). First camera trap record of leopards in Eritrea. Cat News 64: 17–18.Search in Google Scholar
Amin, R., Wacher, T., and Butynski, T.M. (2017). Sympatry among three suid species (Family Suidae) on the north coast of Kenya. J. East Afr. Nat. Hist. 106: 67–78, https://doi.org/10.2982/028.106.0202.Search in Google Scholar
Ash, J.S. and Miskell, J.E. (1998). Birds of Somalia. Pica Press, Sussex, UK.Search in Google Scholar
Ashford, O.M. (1998). The climate of Somalia. In: Ash, J.S. and Miskell, J.E. (Eds.), Birds of Somalia. Pica Press, Sussex, UK, pp. 66–68.Search in Google Scholar
Bigourdan, J. (1948). Le phacochère et les suidés dans l’Ouest Africain. Bull. IFAN 10: 285–360.Search in Google Scholar
Bradley, R.M. (1971). Warthog (Phacochoerus aethiopicus Pallas) burrows in Nairobi National Park. Afr. J. Ecol. 9: 149–152, https://doi.org/10.1111/j.1365-2028.1971.tb00227.x.Search in Google Scholar
Burgess, N.D., Hales, J., Underwood, E., Dinerstein, E., Olson, D., Itoua, I., Schipper, J., Rickketts, T., and Newman, K. (2004). Terrestrial ecoregions of Africa and Madagascar: a conservation assessment. Island Press, Washington, DC.Search in Google Scholar
Butynski, T.M. (1999). Aberdares National Park and Aberdares Forest Reserves wildlife fence placement study and recommendations. In: Unpublished report for the Kenya Wildlife Service and the Kenya Forest Department. Nairobi, Kenya. Available at: https://www.wildsolutions.nl/wp-content/uploads/Butynski-1999-Aberdare_fence_placement_Part1.pdf.Search in Google Scholar
Butynski, T.M. and De Jong, Y.A. (2016). South Western Mau Forest Reserve; game-proof barrier feasibility study. In: Unpublished report for ISLA/IDH by Rhino Ark Charitable Trust. Nairobi, Kenya. Available at: https://www.wildsolutions.nl/wp-content/uploads/Butynski-De-Jong-SWMauReport20Oct16-mk-1.pdf.Search in Google Scholar
Butynski, T.M. and De Jong, Y.A. (2018). Common warthog Phacochoerus africanus (Gmelin, 1788). In: Melletti, M., and Meijaard, E. (Eds.), Ecology, conservation and management of wild pigs and peccaries. Cambridge University Press, Cambridge, UK, pp. 85–100.10.1017/9781316941232.011Search in Google Scholar
Butynski, T.M. and De Jong, Y.A. (2021). Sympatry between desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus in Kenya, with particular reference to Laikipia County. Suiform Sound. 20: 33–44.Search in Google Scholar
Child, G.S. (1965). Some notes on the mammals of Kilimanjaro. Tanganyika Notes and Records 64: 77–89.Search in Google Scholar
Coe, M.J. and Foster, J.B. (1972). The mammals of the northern slopes of Mount Kenya. J. East Afr. Nat. Soc. Natl. Mus. 131: 1–18.Search in Google Scholar
Culverwell, J., Feely, J., Bell-Cross, S., De Jong, Y.A., and Butynski, T.M. (2008). A new pig for Tsavo. Swara 31: 50–52.Search in Google Scholar
Cumming, D.H.M. (1975). A field study of the ecology and behaviour of warthog. Museum Memoir no. 7. Nat. Mus. Monu. Rhod. Salisbury, Zimbabwe.Search in Google Scholar
Cumming, D.H.M. (2013). Phacochoerus africanus common warthog. In: Kingdon, J., and Hoffmann, M. (Eds.), Mammals of Africa. Pigs, hippopotamuses, chevrotain, giraffes, deer and bovids, Vol. 6. Bloomsbury, London, pp. 54–60.Search in Google Scholar
De Jong, Y.A. and Butynski, T.M. (2009). Primate biogeography, diversity, taxonomy and conservation of the coastal forests of Kenya. In: Unpublished report, Eastern Africa Primate Diversity and Conservation Program, Nanyuki, Kenya. Available at: https://www.wildsolutions.nl/wp-content/uploads/De-JongButynski2009PrimatesCoastalForestKenya.pdf.Search in Google Scholar
De Jong, Y.A. and Butynski, T.M. (2014). Distribution, abundance, ecology, and conservation status of the desert warthog (Phacochoerus aethiopicus) in northern Kenya. In: Unpublished report to the National Geographic Society. Washington, DC. Available at: https://www.wildsolutions.nl/wp-content/uploads/DeJongButynski-WarthogSurveyReport-May-143.pdf.Search in Google Scholar
De Jong, Y.A. and Butynski, T.M. (2018). Desert warthog Phacochoerus aethiopicus (Pallas, 1766). In: Melletti, M. and Meijaard, E. (Eds.), Ecology, evolution and management of wild pigs and peccaries. Cambridge University Press, Cambridge, UK, pp. 101–113.10.1017/9781316941232.012Search in Google Scholar
De Jong, Y.A., Butynski, T.M., and d’Huart, J.P. (2016a). Phacochoerus aethiopicus. IUCN Red List of Threatened Species 2016: e.T41767A44140316. IUCN/SSC. Available at: https://www.iucnredlist.org/details/41767/0.Search in Google Scholar
De Jong, Y.A., Cumming, D.H.M., d’Huart, J.P., and Butynski, T.M. (2016b). Phacochoerus africanus, The IUCN Red List of Threatened Species 2016: e.T41768A109669842. IUCN/SSC. Available at: https://www.iucnredlist.org/species/41768/109669842.Search in Google Scholar
Deribe, E., Bekele, A., and Balakrishnan, M. (2008). Population status and diurnal activity patterns of the common warthog (Phacochoerus africanus) in the Bale Mountains national park, Ethiopia. Int. J. Ecol. Environ. Sci. 34: 91–97.Search in Google Scholar
d’Huart, J.P. and Grubb, P. (2001). Distribution of the common warthog (Phacochoerus africanus) and the desert warthog (Phacochoerus aethiopicus) in the Horn of Africa. Afr. J. Ecol. 39: 156–169, https://doi.org/10.1046/j.0141-6707.2000.00298.x.Search in Google Scholar
d’Huart, J.P. and Grubb, P. (2005). A photographic guide to the differences between the common warthog (Phacochoerus africanus) and the desert warthog (Ph. aethiopicus). Suiform Sound. 5: 4–8.Search in Google Scholar
d’Huart, J.P. and Oliver, W.L.R. (1993). Review of priorities of conservation action and future research on Afrotropical suids. In: Oliver, W.L.R. (Ed.), Pigs, peccaries and hippos. Status survey and conservation action plan. IUCN/SSC Pigs and Peccaries Specialist Group and IUCN/SSC Hippo Specialist Group. IUCN, Gland, Switzerland, pp. 101–106.Search in Google Scholar
Dorst, J. and Dandelot, P. (1970). A field guide to the large mammals of Africa. Collins, London.Search in Google Scholar
Elliot, D.G. (1897). List of mammals obtained by the Field Columbian Museum’s East African Expedition to Somali-land in 1896. Field Columbian Mus. Publ. Zool. Ser. 19 1: 109–155.Search in Google Scholar
Ewer, R.F. (1957). A collection of Phacochoerus aethiopicus teeth from the Kalkbank Middle Stone Age site, central Transvaal. Palaeontol. Afr. 5: 5–20.Search in Google Scholar
Fick, S.E. and Hijmans, R.J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37: 4302–4315, https://doi.org/10.1002/joc.5086.Search in Google Scholar
Fjeldså, J. and de Klerk, H. (2001). Avian endemism in northeastern tropical Africa. Biol. Skr. 54: 259–271.Search in Google Scholar
Friis, I., Thulin, M., Adsersen, H., and Bürger, A.M. (2005). Patterns of plant diversity and endemism in the Horn of Africa. Biol. Skr. 55: 289–314.Search in Google Scholar
Funaioli, U. and Simonetta, A.M. (1966). The mammalian fauna of the Somali Republic: status and conservation problems. Monit. Zool. Ital. 74: 285–347, https://doi.org/10.1080/03749444.1966.10736746.Search in Google Scholar
Garcia-Erill, G., Jørgensen, C.H.F., Muwanika, V.B., Wang, X., Rasmussen, M.S., De Jong, Y.A., Gaubert, P., Olayemi, A., Salmona, J., Butynski, T.M., et al.. (2022). Warthog genomes resolve a biogeographical conundrum and reveal interspecies introgression of disease resistance genes. Mol. Biol. Evol. 39: msac134, https://doi.org/10.1093/molbev/msac134.Search in Google Scholar PubMed PubMed Central
Geigy, R. (1955). Observations sur les phacochères du Tanganyika. Rev. Suisse Zool. 62: 139–162.Search in Google Scholar
Gongora, J., Cuddahee, R.E., Ferreira do Nascimento, F., Palgrave, C.J., Lowden, S., Ho, S.Y.W., Simond, D., Damayanti, C.S., White, D.J., Tay, W.T., et al.. (2011). Rethinking the evolution of extant sub-Saharan African suids (Suidae, Artiodactyla). Zool. Scripta 40: 327–335, https://doi.org/10.1111/j.1463-6409.2011.00480.x.Search in Google Scholar
Grimshaw, J.M., Cordeiro, N.J., and Foley, C.A.H. (1995). The mammals of Kilimanjaro. J. East Afr. Nat. Hist. 84: 105–139, https://doi.org/10.2982/0012-8317(1995)84[105:tmok]2.0.co;2.10.2982/0012-8317(1995)84[105:TMOK]2.0.CO;2Search in Google Scholar
Groves, C. and Grubb, P. (2011). Ungulate taxonomy. The Johns Hopkins University Press, Baltimore, Maryland, USA.10.56021/9781421400938Search in Google Scholar
Grubb, P. (1993). The afrotropical suids Phacochoerus, Hylochoerus and Potamochoerus. 4.1 taxonomy and description. In: Oliver, W.L.R. (Ed.), Pigs, peccaries and hippos. Status survey and conservation action plan. IUCN/SSC Pigs and Peccaries Specialist Group and IUCN/SSC Hippo Specialist Group. IUCN, Gland, Switzerland, pp. 66–73.Search in Google Scholar
Grubb, P. (2005). Order Artiodactyla. In: Wilson, D.E. and Reeder, D.M. (Eds.), Mammal species of the world: a taxonomic and geographic reference, 3rd ed 1. The Johns Hopkins University Press, Baltimore, Maryland, USA, pp. 637–722.Search in Google Scholar
Grubb, P. (2013). Phacochoerus warthogs. In: Kingdon, J., and Hoffmann, M. (Eds.), Mammals of Africa. Pigs, hippopotamuses, chevrotain, giraffes, deer and bovids, Vol. 6. Bloomsbury, London, p. 50.Search in Google Scholar
Grubb, P. and d’Huart, J.P. (2010). Rediscovery of the cape warthog Phacochoerus aethiopicus: a review. J. East Afr. Nat. Hist. 99: 77–102, https://doi.org/10.2982/028.099.0204.Search in Google Scholar
Grubb, P. and d’Huart, J.P. (2013). Phacochoerus aethiopicus desert warthog. In: Kingdon, J., and Hoffmann, M. (Eds.), Mammals of Africa. Pigs, hippopotamuses, chevrotain, giraffes, deer and bovids, Vol. 6. Bloomsbury, London, pp. 51–53.Search in Google Scholar
Grubb, P., Jones, T.S., Davies, A.G., Edberg, E., Starin, E.D., and Hill, J.E. (1998). Mammals of Ghana, Sierra Leone and the Gambia. The Trendrine Press, Cornwall, UK.Search in Google Scholar
Guest, H.J. and Leedal, G.P. (1954). Notes on the fauna of Kilimanjaro. Tanganyika Notes and Records 36: 43–49.Search in Google Scholar
Guiraud, M. (1948). Contribution à l’étude du Phacochoerus aethiopicus Pallas. Mammalia 12: 55–66, https://doi.org/10.1515/mamm.1948.12.2.54.Search in Google Scholar
Happold, D. and Lock, M. (2013). The biotic zones of Africa. In: Kingdon, J., Happold, D., Hoffmann, M., Butynski, T.M., Happold, M., and Kalina, J. (Eds.), Mammals of Africa, Vol. 1. Introductory chapters and Afrotheria. Bloomsbury, London, 57–74.Search in Google Scholar
Hollister, N. (1924). East African mammals in the United States National Museum. Part III. Primates, Artiodactyla, Proboscidea, and Hyracoidea. Bull. U.S. Natl. Mus. 99: 1–164.10.5479/si.03629236.99.3Search in Google Scholar
Jenner, T. (2020). Mammals of Ethiopia, Eritrea, Djibouti and Somalia. Meru Publishing, Buckinghamshire, UK.Search in Google Scholar
Jones, P.G. and Thornton, P.K. (2013). Generating downscaled weather data from a suite of climate models for agricultural modelling applications. Agric. Syst. 114: 1–5, https://doi.org/10.1016/j.agsy.2012.08.002.Search in Google Scholar
Kingdon, J. (1979). East African mammals: an atlas of evolution in Africa, part B: large mammals. Vol. 3. Academic Press, London.Search in Google Scholar
Kingdon, J. (1990). Island Africa: the evolution of Africa’s rare animals and plants. Collins, London.Search in Google Scholar
Kingdon, J. (2015). The Kingdon field guide to African mammals, 2nd ed. Bloomsbury, London.Search in Google Scholar
Künzel, T., Rayaleh, H.A., and Heckel, J.-O. (2004). Warthogs of Djibouti. Suiform Sound. 4: 55–57.Search in Google Scholar
Livingstone, D. and Kingdon, J. (2013). The evolution of a continent: geography and geology. In: Kingdon, J., Happold, D., Hoffmann, M., Butynski, T.M., Happold, M., and Kalina, J. (Eds.), Mammals of Africa. Introductory chapters and Afrotheria, Vol. 1. Bloomsbury, London, 27–42.Search in Google Scholar
Meijaard, E., d’Huart, J.P., and Oliver, W.L.R. (2011). Family Suidae (pigs). In: Wilson, D.E. and Mittermeier, R.A. (Eds.), Handbook of the mammals of the world. Hoofed mammals, 2. Lynx Edicions, Barcelona, Spain, pp. 248–291.Search in Google Scholar
Moreau, R.E. (1944a). Kilimanjaro and Mount Kenya: some comparisons, with special reference to the mammals and birds and with a note on Mount Meru. Tanganyika Notes and Records. 18: 28–68.Search in Google Scholar
Moreau, R.E. (1944b). Mount Kenya: a contribution to the biology and bibliography. J. East Afr. Nat. Hist. 18: 61–92.Search in Google Scholar
Morley, R.J. and Kingdon, J. (2013). Africa’s environmental and climatic past. In: Kingdon, J., Happold, D., Hoffmann, M., Butynski, T.M., Happold, M., and Kalina, J. (Eds.), Mammals of Africa. Introductory chapters and Afrotheria, Vol. 1. Bloomsbury, London, pp. 43–56.Search in Google Scholar
Muwanika, V.B., Nyakaana, S., Siegismund, H.R., and Arctander, P. (2003). Phylogeography and population structure of the common warthog (Phacochoerus africanus) inferred from variation in mitochondrial DNA sequences and microsatellite loci. Heredity 91: 361–372, https://doi.org/10.1038/sj.hdy.6800341.Search in Google Scholar PubMed
Njoroge, P., Yeggo, R., Muchane, M., Githiru, M., Njeri, T., and Giani, A. (2009). A survey of the large and medium sized mammals of Arawale National Reserve, Kenya. J. East Afr. Nat. Hist. 98: 119–128, https://doi.org/10.2982/028.098.0108.Search in Google Scholar
Oates, J.F., Groves, C.P., Brandon-Jones, D., and Hughes, B. (2008). Peter Grubb (1942–2006). Primate Conserv. 23: 143–145.10.1896/052.023.0118Search in Google Scholar
Oliver, W.L.R. (Ed.), (1993). Pigs, peccaries and hippos. Status survey and conservation action plan. IUCN/SSC Pigs and Peccaries Specialist Group and IUCN/SSC Hippo Specialist Group, Gland, Switzerland.Search in Google Scholar
Olson, D.M., Dinerstein, E., Wikramanayake, E.D., Burgess, N.D., Powell, G.V.N., Underwood, E.C., D’amico, J.A., Itoua, L., Strand, H.E., Morrison, J.C., et al.. (2001). Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51: 933–938, https://doi.org/10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2.10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2Search in Google Scholar
Polhill, D. (1988). Flora of tropical East Africa: index of collecting localities. Royal Botanic Gardens, Kew, UK.Search in Google Scholar
Powys, A. (2020). New large mammal for the Laikipia Plateau. Forum Focus. Available at: https://laikipia.org/2020/04/02/new-large-mammal-for-the-laikipia-plateau/.Search in Google Scholar
Randi, E., d’Huart, J.P., Lucchini, V., and Aman, R. (2002). Evidence of two genetically deeply divergent species of warthog Phacochoerus africanus and P. aethiopicus (Artiodactyla: Suiformes) in East Africa. Mamm. Biol. 67: 91–96, https://doi.org/10.1078/1616-5047-00013.Search in Google Scholar
Redman, N., Stevenson, T., and Fanshawe, J. (2009). Birds of the Horn of Africa. Princeton University Press, Princeton, New Jersey.Search in Google Scholar
Roosevelt, T. and Heller, E. (1914). Life-histories of African game animals, Vol. 1. Paladium Press, Birmingham, Alabama.Search in Google Scholar
Saura, S., Bertzky, B., Bastin, L., Battistella, L., Mandrici, A., and Dubois, G. (2018). Protected area connectivity: shortfalls in global targets and country-level priorities. Biol. Conserv. 219: 53–67, https://doi.org/10.1016/j.biocon.2017.12.020.Search in Google Scholar
Shortridge, G.C. (1934). The mammals of South West Africa. William Heinemann Ltd., London.Search in Google Scholar
Skinner, J.D. and Chimimba, C.T. (2005). The mammals of the southern Africa Subregion, 3rd ed. Cambridge University Press, Cape Town, South Africa.10.1017/CBO9781107340992Search in Google Scholar
Smithers, R.H.N. (1971). The mammals of Botswana. The National Museums of Rhodesia, Salisbury, Museum Memoir no. 4.Search in Google Scholar
Stewart, D.R.M. and Stewart, J. (1963). The distribution of some large mammals in Kenya. J. East Afr. Nat. Hist. Soc. Natl. Mus. 24: 1–152.Search in Google Scholar
Swanepoel, M., Schulze, E., and Cumming, D.H.M. (2016). A conservation assessment of Phacochoerus africanus. In: Child, M.D., Raimondo, D., Do Linh San, E., Roxburgh, L., and Davies-Mostert, H. (Eds.), The Red List of Mammals of South Africa, Swaziland and Lesotho 2016. South African National Biodiversity Institute and Endangered Wildlife Trust, Pretoria, South Africa.Search in Google Scholar
Thornton, P. (2014). Rainfall and rainfall variability. In: Sebastian, E. (Ed.), Atlas of African agriculture research and development: revealing agriculture’s place in Africa. International Food Policy Research Institute, Washington, DC, pp. 38–39.Search in Google Scholar
True, F.W. (1892). An annotated catalogue of the mammals collected by Dr. W.L. Abbott in the Kilimanjaro region, East Africa. Proc. U. S. Natl. Mus. 15: 445–480, https://doi.org/10.5479/si.00963801.15-915.445.Search in Google Scholar
United States Board on Geographic Names (1982). Gazetteer of Ethiopia: names approved by the United States Board on Geographic Names. Defense Mapping Agency, Washington DC. USA.Search in Google Scholar
Vercammen, P. and Mason, D.R. (1993). The warthogs (Phacochoerus africanus and P. aethiopicus). In: Oliver, W.L.R. (Ed.), Pigs, peccaries, and hippos. Status survey and conservation action plan. IUCN/SSC Pigs and Peccaries Specialist Group and IUCN/SSC Hippo Specialist Group, Gland, Switzerland, pp. 75–84.Search in Google Scholar
White, F. (1983). The vegetation of Africa: a descriptive memoir to accompany the UNESCO/AETFAT/UNSC vegetation map of Africa. UNESCO, Paris, France.Search in Google Scholar
White, A.M. and Cameron, E.Z. (2009). Communal nesting is unrelated to burrow availability in the common warthog. Anim. Behav. 77: 87–94, https://doi.org/10.1016/j.anbehav.2008.08.030.Search in Google Scholar
Wilhelmi, F., Kaariye, X.Y., and Heckel, J.-O. (2004). The desert warthog in the Ogaden, Ethiopia. Suiform Sound. 4: 52–54.Search in Google Scholar
World Bank (2021). World Bank open data, https://data.worldbank.org/Worldclim (2016). Global Climate Data. Version 2. Available at: http://www.worldclim.com.Search in Google Scholar
Yalden, D.W., Largen, M.J., and Kock, D. (1984). Catalogue of the mammals of Ethiopia, 5: Artiodactyla. Monit. Zool. Ital. 19: 67–221, https://doi.org/10.1080/00269786.1984.11758579.Search in Google Scholar
Yalden, D.W., Largen, M.J., Kock, D., and Hillman, J.C. (1996). Catalogue of the mammals of Ethiopia and Eritrea, 7. Revised checklist, zoogeography and conservation. Trop. Zool. 9: 73–164, https://doi.org/10.1080/03946975.1996.10539304.Search in Google Scholar
Young, T.P. and Evans, M.R. (1993). Alpine vertebrates of Mount Kenya, with particular notes on the rock hyrax. J. East Afr. Nat. Hist. Soc. Natl. Mus. 82: 55–79.Search in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Biogeography
- Biogeography and conservation of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus (Artiodactyla: Suidae) in the Horn of Africa
- How far westward? Revisiting the distribution of Arctonyx badgers in the westernmost global range
- Solitary, in the night, it goes: summary of records and range expansion of the common genet in Italy
- Conservation
- Rarity and conservation status of the Colombian Speckled Tree Rat, Pattonomys semivillosus (I. Geoffroy, 1838)
- Ecology
- Trophic structure and foraging strategies in a bat community in northern Pantanal, Brazil
- “Brown hare never goes underground”: the exception that proves the rule
- New records of the rare bushy-tailed opossum, Glironia venusta Thomas, 1912 (Didelphimorphia), in Brazil, with notes on the species’ diet
- First records of albinism and leucism in Ctenodactylus gundi (Rodentia: Ctenodactylidae)
- Paint it black: first record of melanism in Canada lynx (Lynx canadensis)
- Taxonomy/Phylogeny
- Morphological redescription, phylogenetic position, and distribution of the near threatened cavy Microcavia shiptoni (Thomas, 1925), with a key for the living species of Microcavia
- Taxonomic reassessment of the chaco mice of the genus Andalgalomys Williams and Mares, 1978 (Rodentia, cricetidae) with a redefinition of Andalgalomys olrogi Williams and Mares, 1978
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Mammalia volume 86 (2022)
Articles in the same Issue
- Frontmatter
- Biogeography
- Biogeography and conservation of desert warthog Phacochoerus aethiopicus and common warthog Phacochoerus africanus (Artiodactyla: Suidae) in the Horn of Africa
- How far westward? Revisiting the distribution of Arctonyx badgers in the westernmost global range
- Solitary, in the night, it goes: summary of records and range expansion of the common genet in Italy
- Conservation
- Rarity and conservation status of the Colombian Speckled Tree Rat, Pattonomys semivillosus (I. Geoffroy, 1838)
- Ecology
- Trophic structure and foraging strategies in a bat community in northern Pantanal, Brazil
- “Brown hare never goes underground”: the exception that proves the rule
- New records of the rare bushy-tailed opossum, Glironia venusta Thomas, 1912 (Didelphimorphia), in Brazil, with notes on the species’ diet
- First records of albinism and leucism in Ctenodactylus gundi (Rodentia: Ctenodactylidae)
- Paint it black: first record of melanism in Canada lynx (Lynx canadensis)
- Taxonomy/Phylogeny
- Morphological redescription, phylogenetic position, and distribution of the near threatened cavy Microcavia shiptoni (Thomas, 1925), with a key for the living species of Microcavia
- Taxonomic reassessment of the chaco mice of the genus Andalgalomys Williams and Mares, 1978 (Rodentia, cricetidae) with a redefinition of Andalgalomys olrogi Williams and Mares, 1978
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Mammalia volume 86 (2022)

