Abstract
Zoonoses are diseases or infections naturally transmissible from vertebrate animals to humans, and can be bacterial, viral or parasitic. The growth of urbanization, industrialization and the advance of agriculture and livestock facilitate the spread of infectious and parasitic agents from wild animals to the human population and to their domestic animals. Among the various reservoirs of zoonotic agents, we find that didelphid species, due to their high capacity for adaptation in urban environments, as an important study case. We reviewed the literature data on the pathogens, including with zoonotic potential of marsupial species occurring in Brazil, accounted for infections by agents that we categorized into Bacteria, Viruses, Protozoa, and Helminths. Aiming identifies possible knowledge gaps, we also surveyed the origin of studied samples and the institutions leading the researches on host didelphids. Among the hosts, the genus Didelphis in the cycles of these agents stands out. Moreover, we found that the majority of reported cases are in the Southeastern Brazil, mean the data from other Brazilian localities and didelphid species could be underestimated. Most studies took place in graduate programs of public research institutions, emphasizing the importance of the funding public research for the Brazilian scientific development.
1 Introduction
Currently, three orders of marsupials are recognized in South America: Microbiotheria, Paucituberculata and Didelphimorphia (Gardner 2008), the last being the most diverse and consisting of a single family, Didelphidae, containing four subfamilies: Glironiinae, Caluromyinae, Didelphinae, and Hyladelphinae (Voss and Jansa 2009). In Brazil, there are 15 genera and 66 species, distributed across all biomes (Abreu et al. 2021; Gardner 2008; Quintela et al. 2020). However, this number is expected to increase with new taxonomic reviews and surveys in areas that have not been sampled or isolated, such as the northern and western Amazon and in southern Pampas biomes (Melo and Sponchiado 2012; Pavan 2019). These species are mostly nocturnal and have omnivorous diet, which may include fruits, nectar, invertebrates and even small vertebrates (Conceição and Bocchiglieri 2017; Dos Santos 2012). Reproductive strategies can vary according to the type of habitat and the availability of resources (Begon et al. 2006), in addition, didelphid marsupials can occupy a large number of habitats, including environments modified by man, and are commonly found inhabiting peridomiciliary areas, which denotes the importance of this taxon as a potential natural reservoir of zoonotic agents (Cantor et al. 2010; Diniz et al. 2008).
Reservoir is a complex ecological system that can be formed by one or more species, responsible for maintaining a parasite in nature (Brasil 2020), while zoonoses are diseases or infections naturally transmissible from vertebrate animals to humans, and can be bacterial, viral or parasitic (WHO 2021). There are more than 200 zoonoses types, some are preventable by vaccination, such as rabies, or another method (WHO 2021). Currently, the infectious diseases most dangerous to humans were originated from birds and mammals, such as rabies, Ebola, yellow fever, AIDS and Covid-19 (Weiss 2001; WHO 2021). The urban expansion and the advance of the agribusiness facilitate the spilling over of infectious and parasitic agents from wild animals to the human population and its domesticated animals (Corrêa and Passos 2001). It occurs mainly due resources competition, favoring the adaptability of wild animals in anthropic environments and increasing the probability of contact among the different species, including the zoophagy (Cantor et al. 2010; Da Silva et al. 2018). In Brazil, this is particularly worrisome, since the country suffered severe environmental degradation in the last years, with deforestations and burnt areas (Silva et al. 2020) and weakening environmental laws (Bragagnolo et al. 2017).
Multiple approaches in zoonotic studies meet the One Health concept of integrating human health, animal health and the environmental, aim gather data for prediction and control of zoonotic diseases (WHO 2017). Given the background, we aim investigate the zoonotic potential of didelphid species occurring in Brazil. We survey the didelphid as hosts of pathogens potentially infectious to humans, and discussed these data in the context of geographical prevalence.
2 Materials and methods
We carried out a survey in the scientific “Periódicos da CAPES” (http://www.periodicos.capes.gov.br/), “Biblioteca Virtual em Saúde” (https://www.bvsdip.icict.fiocruz.br/), “PubMed” (http://www.ncbi.nlm.nih.gov/pubmed/), “BioOne” (https://bioone.org/), “Scopus” (https://www.scopus.com), and “Google Scholar” (https://scholar.google.com.br/), using the combinations of the keywords Didelphimorphia, Brazil and marsupial, combined with the words: bacterium, virus, protozoa, helminth, nematode, platyhelminth, diseases and zoonoses (in both Portuguese and English languages). We only consider studies that provide geographic coordinates or inform the municipalities/cities of the collection site. For studies that have neighboring locations, we use a midpoint between their coordinates. We discard publications that do not identify the hosts at least at the genus level, while the identification at the species level of the marsupials were hypothesized taking into account their geographic distribution, based on Melo and Sponchiado (2012).
We organized the studies in relation to species of marsupial, locality, etiological agent and methodology used, in the latter we categorize into serological tests, culture, PCR (Polymerase Chain Reaction), and fresh microscopy/research. The marsupial nomenclature follows Abreu et al. (2021) in cases where the exact host identification might be unable to update, we have kept the original identification of the original studies. For the counting of institutions linked to projects on pathogenic agents in marsupials, we used only the institution of the first author of each study found. For the map preparation we divided the pathogens into four groups: bacteria, viruses, protozoa and helminths; and the hosts were divided by genus level. Maps were made using the QGIS program version 2.18.9 ‘Las Palmas’ (QGIS 2017).
3 Results
We found 119 studies showing didelphids infected with pathogenic agents, including with zoonotic potential (Supplementary material). Of the genera of marsupials that occur in Brazil, nine were mentioned in the surveyed studies (Figures 1 –3). The Southeastern region accounts most cases. The Supplementary material lists the positive cases of marsupials for pathogenic agents, methods, in addition to the locations, with the most specific coordinates possible, and the research institutions where the studies were carried out. Table 1 summarizes the characteristics of the main zoonoses found in marsupials in the Brazil, based on the number of records.
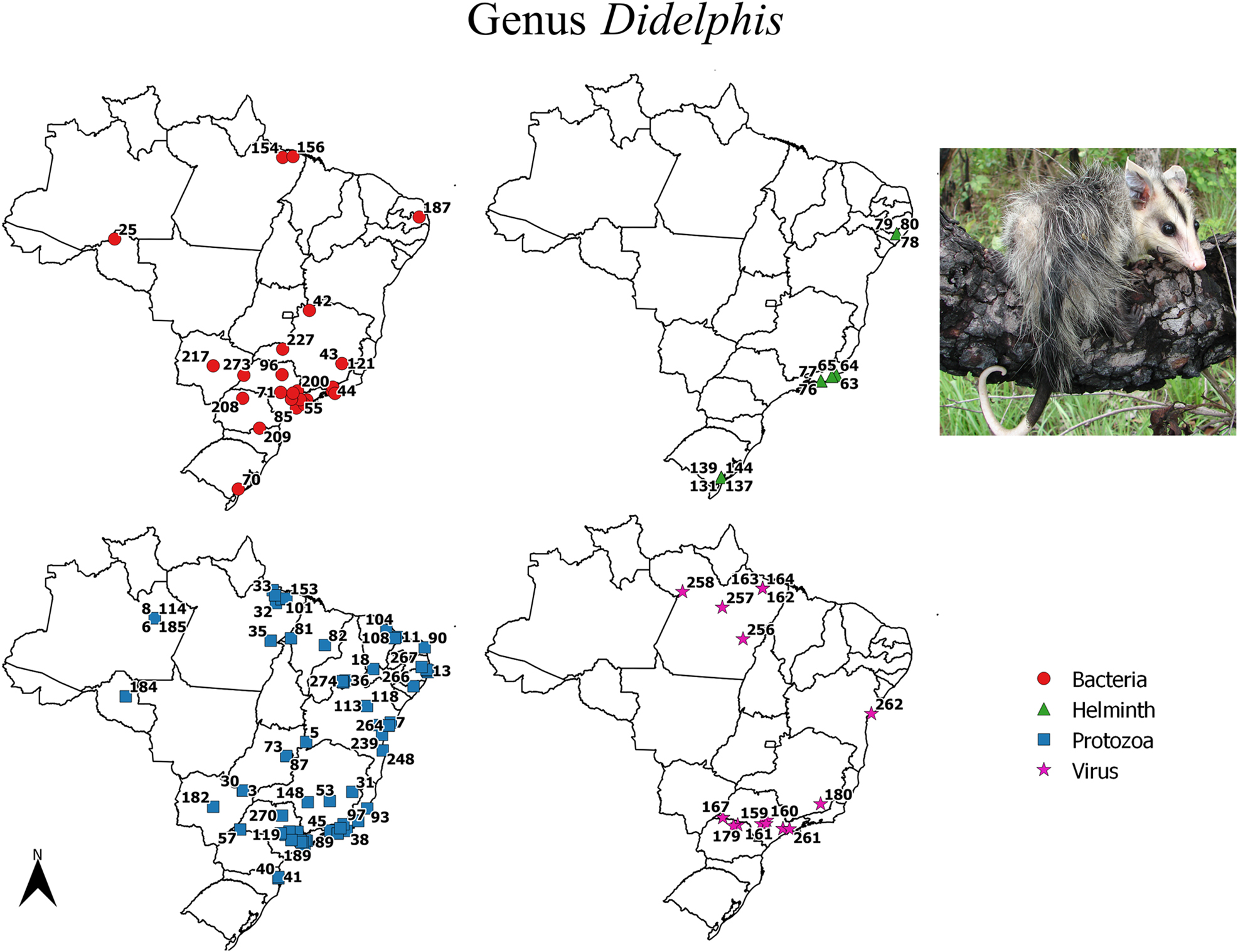
Maps with positive locations for pathogenic agents found in species of the genus Didelphis. The referenced points are listed in the complementary data. Photo credit: Alexandra M.R. Bezerra.
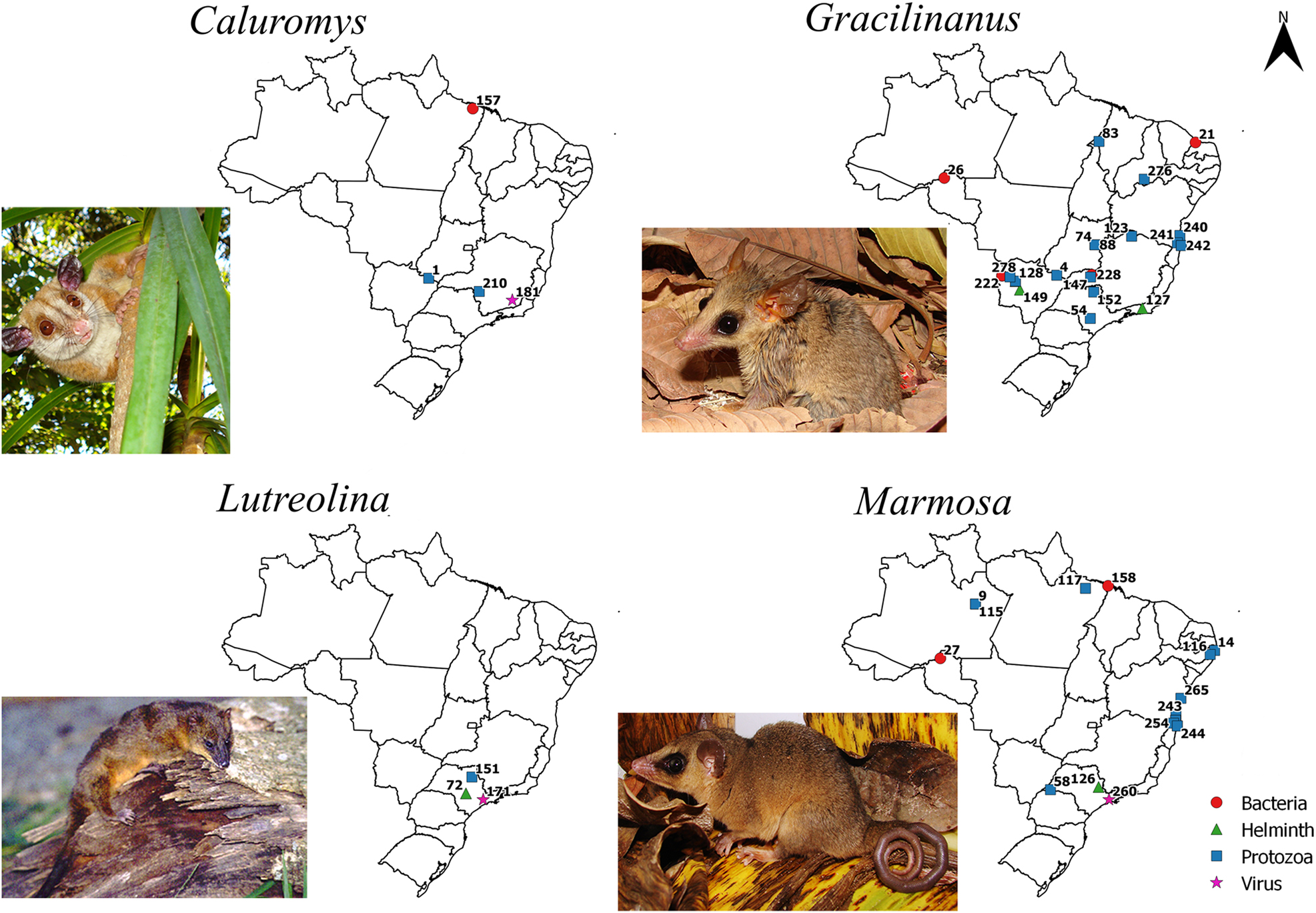
Maps with positive locations for pathogenic agents found in species of the genera Caluromys, Gracilinanus, Lutreolina, and Marmosa, respectively. The referenced points are listed in the complementary data. Photo credits: Caluromys lanatus by André F. Mendonça, Gracilinanus agilis and Marmosa (Micoureus) constantiae by Alexandra M. R. Bezerra, and Lutreolina crassicaudata by Flávio H. G. Rodrigues.
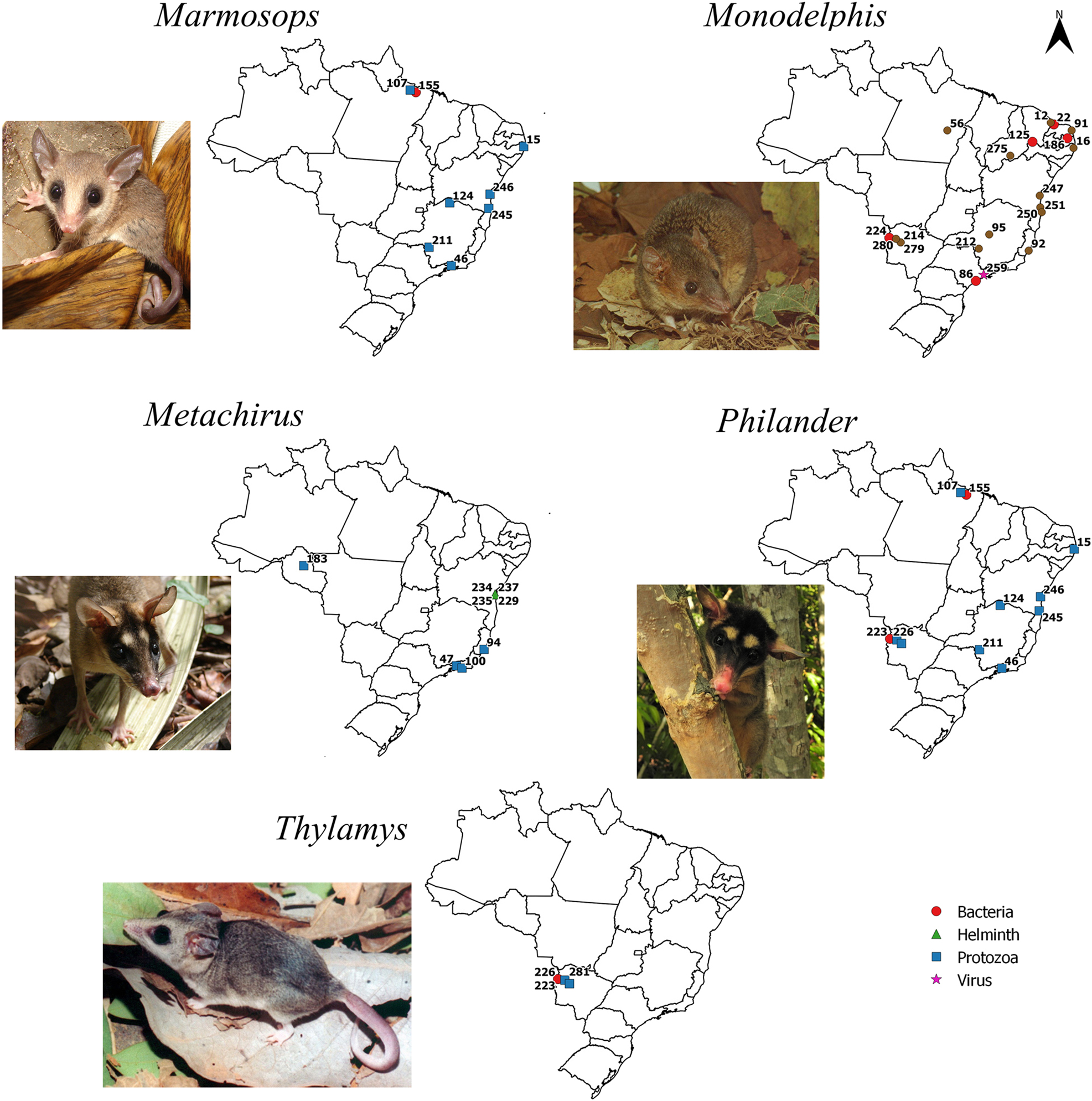
Maps with positive locations for pathogenic agents found in species of the genera Marmosops, Metachirus, Monodelphis, Philander, and Thylamys, respectively. The referenced points are listed in the complementary data. Photo credits: Marmosops ocellatus, Monodelphis domestica, Thylamys karimii by Alexandra M. R. Bezerra, and Metachirus nudicaudatus and Philander opossum by Paola da Mata.
Summary information on the main zoonoses found in marsupials in Brazil.
| Disease | Etiological agent | Symptoms | Transmission | Main hosts | References |
|---|---|---|---|---|---|
| Leptospirosis | Leptospira | Anorexia, fever, muscle pain, nausea, vomiting, constipation or diarrhea, arthralgia, conjunctival hyperemia or hemorrhage, photophobia and eye pain | Contact with urine from infected animals | Rodentia, Artiodactyla, Didelphimorphia, Carnivora, Chiroptera and Primates | Costa et al. (2001), Brasil (2020), Martins and Spink (2020) |
| Salmonellosis | Salmonella | Abdominal pain, diarrhea, low hyperthermia and emesis rarely progressing to lethal clinical events | Consumption of contaminated food and through contact with infected animals. | Mammals, birds and reptiles | Sant’ana et al. (2008), Shinohara et al. (2008) |
| Lyme-borreliosis | Borrelia | Erythema migrans, chronic atrophic acrodermatitis, Lyme arthritis, borrelian lymphocytoma, neuroborreliosis and Lyme carditis, in addition to asymptomatic infection | Through the tick bite | Rodentia and Didelphimorphia | Berglund et al. (1995), Stanek and Strle (2009), Stanek et al. (2012) |
| Anaplasmosis | Anaplasma | Fever, compromised general status, headache, myalgia, arthralgia and, to a lesser extent, maculopapular eruption | Through the tick bite | Rodentia, Cervidae, Bovidae and Canidae | Dumler et al. (2001), Abarca et al. (2008) |
| Ehrlichiosis | Ehrlichia | Fever, compromised general status, headache, myalgia, arthralgia and, to a lesser extent, maculopapular eruption | Through the tick bite | Rodentia, Cervidae, Bovidae and Canidae | McQuiston et al. (1999), Abarca et al. (2008) |
| Rocky mountain spotted fever | Rickettsia | Fever, headache, intense myalgia, nausea and vomiting, later appearing the maculopapular rash | Through the tick bite | Rodentia, Canidae and Equidae | Abramson and Givner (1999), Moraes-Filho et al. (2009) |
| Brucellosis | Brucella | Chills, headaches, constipation, generalized pain and, in the case of chronic infections, decreased fertility | Direct contact with infected animals and consumption of unpasteurized milk products | Rodentia, Carnivora, Cetacea, Bovidae, Suidae and Equidae | Ariza et al. (1995), Galinska and Zagórski (2013) |
| Bartonellosis, trench fever, cat scratch disease | Bartonella | Fever, malaise, headache, and anorexia may accompany lymphadenopathy | Through bites and scratches from infected animals and by the bite of fleas and lice | Felidae, Rodentia and Bovidae | Abbott et al. (1997), Spach and Koehler (1998) |
| Mycoplasmosis | Mycoplasma | Sore throat, cough, headache, malaise, chills, fever, rash and pneumonia | Through droplets | Rodentia, Carnivora and birds | Waites and Talkington (2004), Prezotto et al. (2015), De Sousa et al. (2017) |
| Bubonic plague | Yersinia | Headache, fever, generalized pain, myalgia, anorexia, nausea, vomiting, mental confusion, conjunctival congestion, rapid and irregular pulse, tachycardia, hypotension and prostration | Through the bite of infected fleas or by ingesting contaminated water and food, depending on the species | Rodentia, Didelphimorphia, Lagomorpha and Artiodactyla | Salyers and Whitt (2002), Dias et al. (2021) |
| Dengue, yellow fever | Flavivirus | Headache, myalgia, prostration, arthralgia, anorexia, asthenia, retroorbital pain, nausea, vomiting, exanthema, itchy skin, which may worsen to hemorrhagic manifestations and circulatory collapse | Through mosquito bites | Primates | Ribeiro and Antunes (2009), Brasil (2020) |
| Rabies | Lyssavirus | Slight increase in temperature, anorexia, headache, nausea, sore throat, numbness, irritability, restlessness and feeling of anguish. Hyperesthesia and paresthesia may occur in the path of peripheral nerves | Through the saliva of an infected animal | Chiroptera, Carnivora and Primates | De Lima and Gagliani (2014), Brasil (2020) |
| Hepatitis | Hepatovirus | Jaundice and dark urine | Ingestion of food and water contaminated with excreta and secretions | Mammals | Pereira and Gonçalves (2003), Smith and Simmonds (2018) |
| Hantaviruses | Hantavirus | Fever, hypotension, oliguria, polyuria and convalescence | Inhalation of aerosols contaminated with excreta and secretions from infected animals | Rodentia | Schönrich et al. (2008), Mattar et al. (2015) |
| Rotavirus gastroenteritis | Rotavirus | Severe watery diarrhea, fever, loss of appetite, stomach pain and vomiting | Ingestion of food and water contaminated with excreta and secretions | Mammals and birds | Cardoso et al. (1989), Dhama et al. (2015) |
| Measles | Morbillivirus | Fever, dry cough, runny nose, non-purulent conjunctivitis and Koplik’s spots | Contact with infected secretions | Mammals | Quadros et al. (2020), Takeda et al. (2020) |
| Other viroses | Ex: Alphavirus, Bunyavirus, Orthobunyavírus, Orthopoxvirus, Sigmatorquevirus, Alphatorquevirus | Febre, tosse, dor de cabeça e na garganta, coriza e perda de olfato e/ou paladar. Fever, cough, headache and throat, runny nose and loss of smell and/or taste | Contact with infected secretions and excreta | Vertebrates | Vasconcelos et al. (1991), Silva and Angerami (2008) |
| Leishmaniasis | Leishmania | Skin lesions, which may present with long-lasting fever, weight loss, asthenia, adynamia and anemia | Through the bite of several species of sandflies | Rodentia, Didelphimrphia, Carnivora and Pilosa | Carvalho et al. (2002), Brasil (2020) |
| Toxoplasmosis | Toxoplasma | Asymptomatic, but infection during pregnancy can lead to miscarriage, motor and mental retardation, or loss of vision | Ingestion of raw or undercooked meat or consumption of contaminated water and food | Felidae | Lopes and Berto (2012), Brasil (2020) |
| Chagas disease | Trypanosoma | Fever, malaise, headache, asthenia, hyporexia, edema, lymph node hypertrophy | Through triatomine feces | Mammals | Brasil (2020), Rodrigues et al. (2020) |
| Neosporosis | Neospora | – | Ingestion of raw or undercooked meat or consumption of contaminated water and food | Canidae, Mustelidae, Artiodactyla, Rodentia, Equidae and birds | Collery (1996), Tranas (1999), Dubey (2003) |
| Sarcocystosis | Sarcocystis | Fever, anorexia, prostration, pale mucous membranes, runny nose and eyes, dyspnea, salivation, which can cause death. These symptoms are usually more severe in immunocompromised patients | Ingestion of raw or undercooked meat | Mammals | Poulsen and Stensvold (2014), Dubey (2015) |
| Cryptosporidiosis | Cryptosporidium | Watery diarrhea, weight loss, cramps, nausea, vomiting, headaches and low-grade fever | Consumption of contaminated food and water | Mammals | Fayer et al. (2000), Xiao and Feng (2008) |
| Worms diseases | Platyhelminthes, Nematoda, Acanthocephala | Abdominal pain, nausea, vomiting, diarrhea, poor appetite, weight loss and anemia | Consumption of contaminated food and water, and direct contact with larvae in the soil | Vertebrates | Carneiro and Antunes 2010 |
We account 40 research institutions conducting studies on pathogenic agents in marsupials, with the Instituto Oswaldo Cruz (IOC), Rio de Janeiro state, being the institution with the largest number of studies, followed by the Universidade de São Paulo (USP) and the Universidade Estadual de São Paulo (UNESP), São Paulo state, all in the Southeastern region of Brazil. The other institutions presented a maximum of three studies on the topic (Figure 4). The most used methodology was serology, except for the helminths, which the fresh microscopy prevails (Table 2). Recently (last decade mainly), occurred the increased use of PCR in some studies, including it as complementary to the former methods (Carneiro 2018; Carreira et al. 2012; Fornazari et al. 2018; Laforente et al. 2021; Rocha et al. 2013).
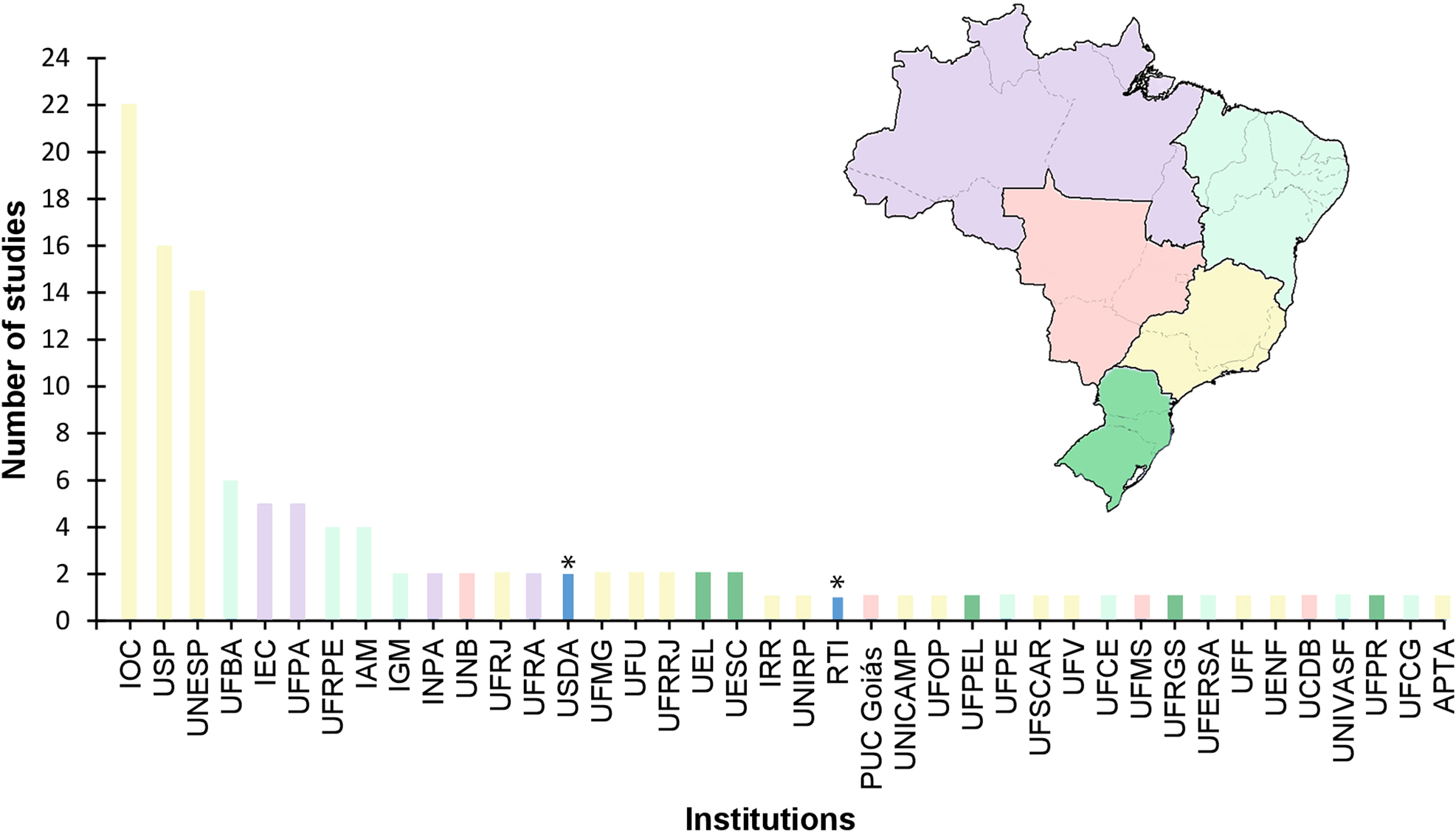
Number of studies carried out on pathogenic infections and zoonotic diseases in marsupials by institutions and Brazilian regions (Northern in lilac, Northeastern in light blue, Central in coral, Southeastern in yellow, and Southern in green). Acronyms: APTA (Agência Paulista de Tecnologia dos Agronegócios), IAM (Instituto Aggeu Magalhães), IEC (Instituto Evandro Chagas), IGM (Instituto Gonçalo Moniz), INPA (Instituto Nacional de Pesquisa da Amazônia, IOC (Instituto Oswaldo Cruz), IRR (Instituto René Rachou), PUC Goiás (Pontifícia Universidade Católica de Goiás), UCDB (Universidade Católica Dom Bosco), UEL (Universidade Estadual de Londrina), UENF (Universidade Estadual do Norte Fluminense), UESC (Universidade Estadual De Santa Cruz), UFBA (Universidade Federal da Bahia), UFCE (Universidade Federal do Ceará), UFCG (Universidade Federal de Campina Grande), UFERSA (Universidade Federal Rural do Semi-Árido), UFF (Universidade Federal Fluminense), UFMG (Universidade Federal de Minas Gerais), UFMS (Universidade Federal do Mato Grosso do Sul), UFOP (Universidade Federal de Ouro Preto), UFPA (Universidade Federal do Pará), UFPE (Universidade Federal de Pernambuco), UFPEL (Universidade Federal de Pelotas), UFPR (Universidade Federal do Paraná), UFRA (Universidade Federal Rural da Amazônia), UFRGS (Universidade Federal do Rio Grande do Sul), UFRJ (Universidade Federal do Rio de Janeiro), UFRPE (Universidade Federal Rural de Pernambuco), UFRRJ (Universidade Federal Rural do Rio de Janeiro), UFSCAR (Universidade Federal de São Carlos), UFU (Universidade Federal de Uberlândia), UFV (Universidade Federal de Viçosa), UNB (Universidade de Brasília), UNESP (Universidade Estadual Paulista), UNICAMP (Universidade Estadual de Campinas), UNIRP (Centro Universitário de Rio Preto), UNIVASF (Universidade Federal do Vale do São Francisco), and USP (Universidade de São Paulo). *RTI (Royal Tropical Institute) and USDA (United States Department of Agriculture) in dark blue, they are Netherland and American institutions, respectively.
Methods that were positive in studies of zoonoses in marsupials in Brazil (see Supplementary material for details).
| Etiological agents | Culture | Serology | PCR | Microscopy |
|---|---|---|---|---|
| Anaplasma | – | – | 2 | – |
| Bartonella | – | – | 1 | – |
| Borrelia | – | 1 | – | 1 |
| Brucella | – | 1 | – | – |
| Ehrlichia | – | – | 4 | – |
| Leptospira | 2 | 15 | 5 | – |
| Mycoplasma | – | – | 3 | – |
| Rickettsia | – | 12 | – | – |
| Salmonella | 1 | – | – | – |
| Yersinia | 3 | 2 | – | – |
| Helminths | – | – | 1 | 54 |
| Cryptosporidium | – | – | – | 1 |
| Eimeria | – | – | – | 9 |
| Leishmania | 3 | 8 | 21 | – |
| Neospora | – | 2 | 1 | – |
| Sarcocystis | 3 | – | 4 | – |
| Toxoplasma | – | 33 | 2 | – |
| Trypanosoma | 45 | 42 | 16 | 4 |
| Alphavirus | – | 1 | – | – |
| Bunyavirus | – | 1 | – | – |
| Flavivirus | – | 3 | – | – |
| Hantavirus | – | – | 1 | – |
| Hepatovirus | – | 3 | 1 | – |
| Lyssavirus | – | 7 | – | – |
| Morbillivirus | 1 | 1 | 1 | – |
| Orthobunyavírus | – | 1 | – | – |
| Orthopoxvirus | – | 1 | 5 | – |
| Rotavirus | – | 1 | – | – |
| Sigmatorquevirus Alphatorquevirus | – | – | 1 | – |
3.1 Infections caused by bacteria
Infections with Leptospira Noguchi, 1917, and Salmonella Lignieres, 1900 were found in the genera Caluromys Allen, 1900, Didelphis Linnaeus, 1758, Marmosa Gray, 1821, and Marmosops Matschie, 1916 (Casagrande et al. 2011; Da Silva et al. 2013; Fernandes et al. 2020; Fornazari et al. 2018; Horta et al. 2016; Jorge 2009; Mesquita et al. 2018; Rocha et al. 2020). As for bacteria of the genus Borrelia Swellengrebel, 1907, cases were confirmed in D. aurita Wied-Neuwied, 1826 in the Southeastern region (Abel et al. 2000; Montandon et al. 2014). Eight species of marsupials, including three species of the genus Didelphis, were found infected by Rickettsia spp. from states of the Northern and Southeastern Brazil (Barbieri 2016; Milagres et al. 2010; Silveira et al. 2015; Szabó et al. 2013; Terassini 2010; Ueno et al. 2020). The other Rickettsia spp infected marsupials were: two species of the genus Gracilinanus Gardner & Creighton, 1989, from Northern, Northeastern, and Southeastern (Coelho et al. 2016; Paiva et al. 2017; Terassini 2010), two species of the genus Monodelphis Burnett, 1830 from Northeastern and Southeastern (Paiva et al. 2017; Szabó et al. 2013), and an unidentified species of the genus Marmosa from Northern region of Brazil (Terassini 2010).
Brucella Meyer and Shaw, 1920 and Bartonella Strong et al., 1915 infections were found in D. albiventris Lund, 1840 from São Paulo state (Antunes et al. 2010), while Bartonella only was found in D. albiventris and D. aurita from Rio de Janeiro state (Alcantara et al. 2020), both Southeastern Brazil. Individuals of Monodelphis domestica and D. albiventris were found infected with Yersinia sp. in Northeastern Brazil (Almeida et al. 1987, 1989). The bacteria Mycoplasma Nowak, 1929 was found in individuals of D. albiventris from Southern and Central Brazil (Gonçalves et al. 2020; Massini et al. 2019; Pontarolo et al. 2021). Two species of marsupials, Gracilinanus agilis (Burmeister, 1854) and Thylamys macrurus (Olfers, 1818), were positive for infection by Anaplasma Theiler, 1910, both from Central Brazil (De Sousa et al. 2017b). Ehrlichia Moshkovski, 1945, was found in four species, being D. aurita from Southeastern region, and G. agilis, M. domestica (Wagner, 1842), and T. macrurus from Central Brazil (De Sousa et al. 2017b; Guimarães et al. 2018).
3.2 Infections caused by virus
The genus Flavivirus, which includes species that can cause various diseases including the dengue, was found in individuals of D. albiventris from Southeastern region (Ázara 2013), and of D. marsupialis Linnaeus, 1758 and P. opossum (Linnaeus, 1758) from Northern Brazil (Bernal et al. 2021). The genus Lyssavirus, which can cause rabies, were confirmed for D. aurita, D. albiventris, and Lutreolina crassicaudata (Desmarest, 1804), all in Southeastern Brazil (Almeida et al. 2001; Araujo et al. 2014; Bacchiega 2014).
In the Didelphis the following viruses were also found: Alphavirus, Orthobunyavirus, Morbillivirus, Sigmatorquevirus, Alphatorquevirus, Parvovirus, Hepatovirus, Rotavirus, and Orthopoxvirus, the latter being also found in Caluromys philander (Linnaeus, 1758) (Almeida et al. 2001; Bernal et al. 2021; Carneiro 2018; De Souza et al. 2018a,b; Lavorente et al. 2021; Linhares et al. 1986; Miranda et al. 2017; Peres et al. 2016, 2018; Soares et al. 1987). Individuals of P. opossum were positive for Bunyavirus infection in Northern Brazil (Bernal et al. 2021). Hantavirus was registered in D. aurita, Marmosa (Micoureus) paraguayana (Tate, 1931), and Monodelphis iheringi (Thomas, 1888), all records from Southeastern Brazil (De Araujo et al. 2012).
3.3 Infections caused by helminths
Helminths parasitizing didelphid marsupials in isolated points of all regions of Brazil, with large areas without studies. The following genres were registered: Ancylostoma Dubini, 1843, Aspidodera Railliet and Henry, 1912, Brachylaema Dujardin, 1843, Capillaria Zeder, 1800, Centrorhynchus Lühe, 1911, Cruzia Travassos, 1917, Didelphodiplostomum Dubois, 1944, Didelphonema Wolfgang, 1953, Didelphostrongylus Prestwood, 1976, Dirofilaria Railliet and Henry, 1911, Echinostoma Rudolphi, 1809, Gnathostoma Owen, 1837, Gongylonemoides Freitas and Lent, 1937, Gracilioxyuris Feijó, Tores, Maldonado Jr. and Lanfredi, 2008, Hamanniella Travassos, 1915, Mathevotaenia Akhumyan, 1946, Oligacanthorhynchus Travassos, 1915, Physaloptera mirandai Lent and Freitas, 1937, Plagiorchis Lühe, 1899, Pterygodermatites Quentin, 1969, Rhopalias Stiles and Hassall, 1898, Spirura Blanchard, 1849, Strongyloides Grassi, 1879, Travassostrongylus Orloff, 1933, Trichuris Roederer, 1761, Toxocara Stiles, 1905, Turgida Schulz, 1927, and Viannaia Travassos, 1914, occurring in D. albiventris, D. aurita, Gracilinanus microtarsus (Wagner, 1842), G. agilis, L. crassicaudata, M. m. paraguayana, Metachirus myosuros (Temminck, 1824), Philander frenatus (Olfers, 1818), and P. opossum (Antunes 2005; Araujo 2011; Bernal et al. 2015; Boullosa et al. 2017; Cardia et al. 2016; Cirino et al. 2020; Feijó et al. 2008; Gomes et al. 2003; Santos-Rondon et al. 2002; Silva et al. 2017; Torres et al. 2007). Although not all helminths presented here have been found to infect humans, we consider them to have zoonotic potentials (Antunes 2005).
3.4 Infections caused by protozoa
The genus Leishmania Ross, 1903, responsible for leishmaniasis, was confirmed in the genera Didelphis, Gracilinanus, Marmosa, Marmosops, and Monodelphis, with most occurrences in the species D. albiventris (Arias et al. 1981; Brandão-Filho et al. 2003; Cardoso et al. 2015; Carreira et al. 2012; De Alcântara 2006; Lainson and Shaw 1969; Lima et al. 2013; Monteiro 2010; Nascimento 2017; Neto 2006; Quaresma et al. 2011; Quintal 2010; Richini-Pereira et al. 2014; Schallig et al. 2007; Sherlock 1996; Sherlock et al. 1984; Silva et al. 2016; Trueb et al. 2018; Yoshida et al. 1985). The protozoan Trypanosoma Gruby, 1843 was found in Caluromys, Didelphis, Gracilinanus, Lutreolina, Marmosa, Marmosops, Metachirus Burmeister, 1854, Monodelphis, Philander Brisson, 1762, and Thylamys Gray, 1843, being confirmed in all regions of Brazil (Alencar et al. 1977; Barreto and Siqueira 1962; Barros et al. 2020; Bernal et al. 2015; Cominetti 2010; Corrêa and Barreto 1964; Da Costa et al. 2015; Dario et al. 2017; De Oliveira 2008; Fernandes et al. 1989; Ferreira 2015; Guimarães and Jansen 1943; Herrera et al. 2005, 2007, 2011; Lainson et al. 2008; Liaño 2013; Lima et al. 2012; Maldonado 2014; Mello 1982; Miles et al. 1983; Nantes et al. 2019; Pereira et al. 2021; Pinho et al. 2000; Portugal 2009; Rocha et al. 2013; Rodrigues and Melo 1942; Roque et al. 2008; Trueb et al. 2018).
For the species Toxoplasma gondii (Nicolle and Manceaux, 1908), the following genera were reported as hosts: Didelphis, Gracilinanus, Marmosa, Marmosops, and Monodelphis, with the most occurrences in the Northeastern Brazil (Brito Junior et al. 2020; De Oliveira 2011; De Siqueira 2010; Ferraroni and Marzochi 1980; Fornazari et al. 2011; Fournier 2013; Horta et al. 2016; Vitaliano et al. 2014; Yai et al. 2003). The genera Cryptosporidium Tyzzer, 1907 and Sarcocystis Lankester, 1882, and the species Neospora caninum Dubey, Carpenter, Speer, Topper and Uggla, 1988 were found only in Didelphis spp. from Southeastern and Northeastern Brazil (Cesar 2011; Da Silva 2016; Dubey et al. 2001a,b; Gallo et al. 2018; Gondim et al. 2017, 2019; Horta et al. 2016; Yai et al. 2003). The protozoan Eimeria Schneider, 1875 was reported infecting Gracilinanus agilis (Strona 2016) in Southeastern, and the genera Didelphis, Gracilinanus, Marmosa, and Monodelphis in Northeastern region (Fehlberg et al. 2018).
4 Discussion
The growth in environmental degradation caused mainly by human occupation and lack of basic sanitation, could be responsible for a higher incidence of zoonoses, due to the increase contact among people and wild animals (Neto et al. 2007); particularly worry is in relation to the mammalian species, as they share several pathogens with the humans (Mills 2006). Although several studies on zoonotic agents have been carried out with different mammalian taxonomic orders in South America (Capellão et al. 2015; Corrêa et al. 2013; Moratelli and Calisher 2015; Povill et al. 2018), the data are still qualitative and geographically underestimated. As well as the present study, previous authors (Capellão et al. 2015; Corrêa et al. 2013; Moratelli and Calisher 2015) found large geographic gaps regarding the analyzed specimens, especially in the Northern, Central and Southern of Brazil.
Of the 66 species of Didelphidae occurring in Brazil, 24 of them showed positive results for the infection of zoonotic agents, with great emphasis on the species of the genus Didelphis, mainly D. albiventris and D. aurita, known for being very well adapted to urban environments (Da Cruz and Margarido 2003; Graipel and Santos-Filho 2006). The low amount of infections found in other species could be due to the scarcity of studies to detect potential pathogens by using these species as model, but also the fact that most studies was carried out by research institutions located in the Southeastern Brazil (e.g., Instituto Oswaldo Cruz/Fiocruz, Universidade de São Paulo, and Universidade Estadual Paulista), and studying species occurring in the same region. Another highlight is the great representativeness of dissertations and theses among the surveyed studies. It corroborates the role of graduate programs in building a more accurate portrait of the Brazilian reality, by contributing to better knowledge on the studied subject and, consequently, in the improvement both mitigation measures and prevention strategies (Severino 2006).
Regarding infections by bacteria and helminths, most were concentrated in the Southeastern Brazil, with few isolated points in the Northern and Northeastern due mainly to the incidence of outbreaks, such as the bubonic plague in Paraíba state during 1980s (De Almeida 1989). Marsupials showed a greater diversity of pathogenic bacteria compared to other groups of mammals, such as bats and armadillos, highlighting the incidence of bacteria of the genus Leptospira (Capellão et al. 2015; Corrêa et al. 2013). The genus Didelphis contains most positive cases for viruses in Brazil, probably due they are larger species compared to the other didelphids and have wide distribution (Gardner 2008), being easily found in urban and rural areas (i.e., mainly the species D. aurita – Boullosa et al. 2017, and D. albiventris – Almeida et al. 2008, pers. obs.), which could facilitate the collection samplings for histopathological, serological, and PCR analysis. As the agents can express different infectious load or pathology, different techniques have dissimilar detection capabilities (Lainson and Shaw 2010). Almost all tested Didelphis individuals were positives for the presence of Lyssavirus, which is transmitted through the bite of infected animals (Murphy and Bauer 1974). The incidence of these viruses is usually higher in bats, especially in Southeastern Brazil, where also most studies are concentrated (Moratelli and Calisher 2015).
Most infections in marsupials have been caused by protozoa, with a large number of cases for the Northern and Northeastern regions. This may be facilitated due to the characteristic of transmissions, which occur mainly through arthropods, having been favored with the historical deforestation observed in these regions (Norris 2004). In Brazil, several areas are endemic for some protozoa diseases (Lopes and Chapadeiro 1983; Queiroz et al. 2004; Silveira et al. 1996). The South American marsupials are considered the oldest hosts of the protozoan Trypanosoma cruzi (Stevens et al. 1998) which lodges in the anal glands of the hosts, being asymptomatic (Urdaneta-Morales and Nironi 1996). Similar factors are verified in relation to Leishmania, with a high incidence of infection in marsupials in Brazil and other American countries, mainly in areas with anthropic impact (Carreira et al. 2012). The ancient history of the relationship of didelphid marsupials with these protozoa may be the reason for the high number of positive results, when compared with the other groups of etiological agents. In any case, it is evident the need to monitoring both hosts and the quality of their habitats. Natural environments have been systematically devastated in the last two centuries, leading to a drastic increase in the contact of wild animals with domesticated animals and humans (Burkett-Cadena and Vittor 2018; Wolfe et al. 2005), which could greatly contribute to the spilling over of several zoonoses, such as the one caused by the Coronavirus SARS-CoV 2 (Rothan and Byrareddy 2020).
5 Concluding remarks
Preliminary studies on the impact of zoonotic agents may prevent outbreaks and new pandemics from appearing (Baharoon and Memish 2019; WHO 2021). The present study shows the need of monitor hosts and vectors, mainly in endemic areas for zoonoses. Long-term studies in parasitology and population/community ecology of hosts, using different techniques for diagnosis of infections and the host species identification, including the voucher preservation by depositing it in scientific collections, should be considered priorities (Thompson et al. 2021). We highlighted the importance of marsupials as being one of the main reservoirs of zoonotic agents, with infections by bacteria, viruses, protozoa, and helminths. Public research institutions, especially those linked to graduate programs, are of unequal importance in providing, through their researches, measures for the eradication of the diseases, as well as to guarantee quality responses for other biodiversity science areas (Overbeck et al. 2018). The maintenance of these institutions is extremely important for Brazilian science, requiring a continuous increase in science funds (Bolânos-Villegas et al. 2020). Paradoxically, Brazilian science has suffered in the last years severe cut off budgets, with a consequent loss of ongoing research and graduate student dropout (Martelli-Júnior et al. 2019; Oliveira et al. 2020), and threats to the biodiversity heritage (Fernandes et al. 2017).
Funding source: Conselho Nacional de Desenvolvimento Científico e Tecnológicoico
Award Identifier / Grant number: DCR 300461/2016-0
Award Identifier / Grant number: graduate scholarship 130627/2020-8
Funding source: Fundação Amazônia de Amparo a Estudos e Pesquisas
Award Identifier / Grant number: ICAAF 018/2016
Funding source: Programa de Capacitação Institucional (2018-2023), MPEG/CNPq
Award Identifier / Grant number: 302015/2021-3
Acknowledgments
Thanks are due to José de Sousa e Silva Júnior “Cazuza” for providing the space and use of equipment in the Mammalogy section of the Museu Paraense Emílio Goeldi; Comments of Gleomar Maschio and two anonymous reviewers improved the manuscript. The authors also thank André F. Mendonça, Flávio H. G. Rodrigues, and Paola da Mata for allowing them to use their pictures.
-
Author contributions: MMB and AMRB designed the study, performed the analysis and interpretation of these data: MMB wrote the manuscript; AMRB critically reviewed the manuscript for intellectual content; MMB and AMRB are guarantors of the article. All authors read and approved the final manuscript.
-
Research funding: This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (DCR 300461/2016-0 for AMRB), Fundação Amazônia de Amparo a Estudos e Pesquisas (ICAAF 018/2016 for ARMB), and by the Programa de Capacitação Institucional (2018-2023), MPEG/CNPq (302015/2021-3).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Abarca, K., López Del, J., González, P., Dabanch, J., Torres, M., Solari, V., and Perret, C. (2008). Evidencia seroepidemiológica de exposición humana a Anaplasma sp en Santiago, Chile. Rev. Chil. Infectol. 25: 358–361.10.4067/S0716-10182008000500008Search in Google Scholar
Abbott, R.C., Chomel, B.B., Kasten, R.W., Floyd-Hawkins, K.A., Kikuchi, Y., Koehler, J.E., and Pedersen, N.C. (1997). Experimental and natural infection with Bartonella henselae in domestic cats. Comp. Immunol. Microbiol. Infect. Dis. 20: 41–51.10.1016/S0147-9571(96)00025-2Search in Google Scholar
Abel, I.D.S., Almeida-Junior, D.E.D., Fonseca, A.H.D., Soares, C.O., and Ishikawa, M.M. (2000). Borrelia sp. in naturally infected Didelphis aurita (Wied, 1826).(Marsupialia: Didelphidae). Braz. Arch. Biol. Technol. 43: 307–312.10.1590/S1516-89132000000300010Search in Google Scholar
Abramson, J.S. and Givner, L.B. (1999). Rocky Mountain spotted fever. Pediatr. Infect. Dis. J. 18: 539–540.10.1097/00006454-199906000-00012Search in Google Scholar PubMed
Abreu, E.F., Casali, D.M., Garbino, G.S.T., Loretto, D., Loss, A.C., Marmontel, M., Nascimento, M.C., Oliveira, M.L., Pavan, S.E., and Tirelli, F.P. (2021). Lista de Mamíferos do Brasil. Comitê de Taxonomia da Sociedade Brasileira de Mastozoologia (CT-SBMz), Available at: www.sbmz.org/mamiferos-do-brasil (Accessed 06 December 2021).Search in Google Scholar
Alcantara, A., Thoma, H., Campos, S., Teixeira, R., Lima, H., Pires, J., Moraes, R., and Souza, A. (2020). Molecular evidence of Bartonella spp. in free-living opossums (Didelphimorphia: Didelphidae) from peri-urban Atlantic Forest fragments of Brazil. Authorea Preprints 2020: 1–6.10.22541/au.160029787.76756825Search in Google Scholar
Alencar, J.E., Almeida, Y.M., Freitas, L.M., and Santos, A.R. (1977). Estudos sobre a epidemiologia da doença de Chagas do Ceará – Brasil. VI – Estudos de uma micro-região de Russas. Rev. Soc. Bras. Med. Trop. 11: 5–23.10.1590/S0037-86821977000100002Search in Google Scholar
Almeida, M.F., Massad, E., Aguiar, E.A, Martorelli, L.F., and Joppert, A.M. (2001). Neutralizing antirabies antibodies in urban terrestrial wildlife in Brazil. J. Wild. Dis. 37: 394–398.10.7589/0090-3558-37.2.394Search in Google Scholar PubMed
Almeida, A.J., Torquetti, C.G., and Talamoni, S.A. (2008). Use of space by Neotropical marsupial Didelphis albiventris (Didelphimorphia: Didelphidae) in an urban forest fragment. Rev. Bras. Zool. 25: 214–219.10.1590/S0101-81752008000200008Search in Google Scholar
Almeida, A.M.P., Brasil, D.P., Carvalho, F.G., and Almeida, C.R. (1987). Detection of Yersinia pestis in rodents and other small mammals in the northeast of Brazil during the period from 1966 to 1982. Rev. Saude Publica 21: 265–267.10.1590/S0034-89101987000300012Search in Google Scholar PubMed
Almeida, A.M.P., Brasil, D.P., Leal, N.C., Melo, M.E.B.D., Rêgo, R.V.B.D., and Almeida, C.R.D. (1989). Bacteriological and serological studies of a plague outbreak in the Paraíba state, Brazil. Mem. Inst. Oswaldo Cruz 84: 249–256.10.1590/S0074-02761989000200015Search in Google Scholar PubMed
Antunes, G.M. (2005). Diversidade e potencial zoonótico de parasitos de Didelphis albiventris Lund, 1841 (Marsupialia: Didelphidae), PhD thesis. Porto Alegre, Brazil: Universidade Federal do Rio Grande do Sul.Search in Google Scholar
Antunes, J.M.A.P., Machado, G.P., Costa, L.F., Fornazari, F., Cipriano, J.R.B., Appolinário, C.M., Allendorf, S.D., Bagagli, E., Teixeira, C.R., and Megid, J. (2010). Comparison of infection by Brucella spp. in free-ranging and captive wild animals from São Paulo State, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 16: 654–658.10.1590/S1678-91992010000400017Search in Google Scholar
Araujo, L.R.F. (2011). Descrição taxonômica de Cruzia sp. nov. e Aspipodera sp. nov. (Nematoda: Ascaridida), parasitas de intestino grosso de Philander opossum Linnaeus, 1758, marsupial de Carajás-Pará, Brasil, MSc dissertation. Belém, Brazil: Universidade Federal do Pará.Search in Google Scholar
Araujo, D.B., Martorelli, L.A., Kataoka, A.P.G., Campos, A.C., Rodrigues, C.S., Sanfilippo, L.F., Cunha, E.S., Durigon, E.L., and Favoretto, S.R. (2014). Antibodies to rabies virus in terrestrial wild mammals in native rainforest on the north coast of São Paulo State, Brazil. J. Wildl. Dis. 50: 469–477.10.7589/2013-04-099Search in Google Scholar
Arias, J.R., Naiff, R.D., Miles, M.A., and De Souza, A.A. (1981). The opossum, Didelphis marsupialis (Marsupialia: Didelphidae), as a reservoir host of Leishmania braziliensis guyanensis in the Amazon Basin of Brazil. Trans. R. Soc. Trop. Med. Hyg. 75: 537–541.10.1016/0035-9203(81)90194-2Search in Google Scholar
Ariza, J., Corredoira, J., Pallares, R., Viladrich, P.F., Rufi, G., Pujol, M., and Gudiol, F. (1995). Characteristics of and risk factors for relapse of brucellosis in humans. Clin. Infect. Dis. 20: 1241–1249.10.1093/clinids/20.5.1241Search in Google Scholar PubMed
Ázara, T.M.F. (2013). Detecção do dengue vírus em Aedes albopictus Skuse e pequenos mamíferos de fragmento florestal urbano, PhD thesis. Belo Horizonte, Brazil: Universidade Federal de Minas Gerais.Search in Google Scholar
Bacchiega, T.S. (2014). Circulação do vírus rábico em gambás (Didelphis albiventris e Didelphis aurita) nos municípios de Torre de Pedra, bofete e Anhembi – São Paulo, MSc dissertation. Botucatu, Brazil: Universidade Estadual Paulista.Search in Google Scholar
Baharoon, S. and Memish, Z.A. (2019). MERS-CoV as an emerging respiratory illness: a review of prevention methods. Trav. Med. Infect. Dis. 32: 101520.10.1016/j.tmaid.2019.101520Search in Google Scholar PubMed PubMed Central
Barbieri, A.R.M. (2016). Ecologia de carrapatos e riquétsias transmitidas por carrapatos em uma reserva natural do cerrado brasileiro, PhD thesis. São Paulo, Brazil: Universidade de São Paulo.Search in Google Scholar
Barretto, M.P. and De Siqueira, A.F. (1962). Natural Infection of Lutreolina crassicaudata crassicaudata by Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 4: 358–365.Search in Google Scholar
Barros, F.D.N.L., Júnior, F.D.S., Costa, S.M., de Farias, D.M., Moura, M.A.O., Júnior, P.S.B., Góes-Calvacante, G., and Scofield, A. (2020). First report of natural infection by Trypanosoma cruzi in secretions of the scent glands and myocardium of Philander opossum (Marsupialia: Didelphidae): Parasitological and clinicopathological findings. Vet. Parasitol. Reg. Stud. 22: 100463.10.1016/j.vprsr.2020.100463Search in Google Scholar PubMed
Begon, M., Townsend, C.R., and Harper, J.L. (2006). Ecology: from individuals to ecosystems, 4nd ed. Oxford: Blackwell Publishing.Search in Google Scholar
Berglund, J., Eitrem, R., Ornstein, K., Lindberg, A., Ringnér, Å., Elmrud, H., Carlsson, M., Runehagen, A., Svanborg, A., and Norrby, R. (1995). An epidemiologic study of Lyme disease in southern Sweden. N. Engl. J. Med. 333: 1319–1324.10.1056/NEJM199511163332004Search in Google Scholar PubMed
Bernal, M.K.M., Da Silva, S.K.S.M., Moraes, L.M., De Aguirra, L.R.V.M., Andrade, S.L.S., and Pereira, W.L.A. (2015). Ocorrência de Dirophilaria immitis e Trypanosoma cruzy em Philander opossum (Marsupialia, Didelphidae), capturados em Belém, Pará. Curitiba, Brazil: Anais do 42º Congresso Brasileiro de Medicina Veterinária e 1º Congresso Sul Brasileiro da ANCLIVEPA.Search in Google Scholar
Bernal, M.K.M., Chiang, J.O., Mendes, F.F., Andrade, S.L.D.S., Silva, S.K.S.M.D., and Pereira, W.L.A. (2021). Study of Arboviruses in Philander opossum, Didelphis marsupialis and Nectomys rattus captured from forest fragments in the municipality of Belém, Pará, Brazil. Ciência Rural. 51: 1–8.10.1590/0103-8478cr20200515Search in Google Scholar
Bolaños-Villegas, P., Cabrerizo, F.M., Brown, F.D., Zancan, P., Barrera, J.F., González-Muñoz, P.A., Grecco, H., Kalergis, A., Paula-Lima, A., Vargas-Balda, R., et al.. (2020). Latin America: Reduced SandT investment puts sustainable development at risk. Sci. Open 2642: 1–16. Preprints.10.14293/S2199-1006.1.SOR-.PPBPKUJ.v2Search in Google Scholar
Boullosa, R.G.M.C., Costa, S.F.C., Maldonado Junior, A., and Gentile, R. (2017). Ecological aspects of nematode parasites of Didelphis aurita (Didelphimorphia, Didelphidae) in urban sylvatic habitats in Rio de Janeiro, Brazil. Oecologia Australis 21: 54–61.10.4257/oeco.2017.2101.06Search in Google Scholar
Bragagnolo, C., Lemos, C.C., Ladle, R.J., and Pellin, A. (2017). Streamlining or sidestepping? Political pressure to revise environmental licensing and EIR in Brazil. Environ. Impact Assess. Rev. 65: 86–90.10.1016/j.eiar.2017.04.010Search in Google Scholar
Brandão-Filho, S.P., Brito, M.E., Carvalho, F.G., Ishikaw, E.A., Cupolillo, E., Floeter-Winter, L., and Shaw, J.J. (2003). Wild and synanthropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 97: 291–296.10.1016/S0035-9203(03)90146-5Search in Google Scholar
Brasil, Ministério da Saúde (2020). Vigilância em Saúde: zoonoses. Cadernos de atenção básica, n. 22 (Série A. Normas e manuais Técnicos), Available at: http://bvsms.saude.gov.br/bvs/publicacoes/vigilancia_saude_zoonoses_p1.pdf (Accessed 15 November 2020).Search in Google Scholar
Brito Junior, P.D.A., Rocha, J.M., Silva, C.A.D., Oliveira, P.M.V., Correia, J.E., Cruz, L.A.D., Sevá, A.P., Oliveira, T.V., Silva, A.V., Alvarez, M.R.V., et al.. (2020). Survey of anti-Toxoplasma gondii antibodies in wild mammals captured from Atlantic Forest fragments in Bahia, northeastern Brazil. Rev. Bras. Parasitol. Vet. 29: 1–13.10.1590/s1984-29612020083Search in Google Scholar
Burkett-Cadena, N.D. and Vittor, A.Y. (2018). Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 26: 101–110.10.1016/j.baae.2017.09.012Search in Google Scholar
Cantor, M., Ferreira, L.A., Silva, W.R., and Setz, E.Z.F. (2010). Potential seed dispersal by Didelphis albiventris (Marsupialia, Didelphidae) in highly disturbed environment. Biota Neotrop. 10: 45–51.10.1590/S1676-06032010000200004Search in Google Scholar
Capellão, R.T., Lazar, A., and Bonvicino, C.R. (2015). Infecção natural por agentes zoonóticos em tatus (Mammalia: Cingulata) na América do Sul. Bol. Soc. Bras. Mastozool. 73: 23–36.Search in Google Scholar
Cardia, D.F.F., Camossi, L.G., Fornazari, F., Babboni, S.D., Teixeira, C.R., and Bresciani, K.D.S. (2016). Primeiro relato de Strongyloides sp. (Nematoda, Strongyloididae) em Lutreolina crassicaudata (Didelphimorphia: Didelphidae). Braz. J. Biol. 76: 884–887.10.1590/1519-6984.03315Search in Google Scholar
Cardoso, D.D.D.P., De Brito, W.M.E., Martins, R.M.B., Kitajima, E.W., Souza, M.P., Barbosa, A.J., Oliveira, S.A., and Rascopi, S.B. (1989). Ocorrência de Rotavirus e Adenovirus em amostras fecais de crianças com gastrenterite, na cidade de Goiânia. Rev. Soc. Bras. Med. Trop. 22: 67–71.10.1590/S0037-86821989000200001Search in Google Scholar
Cardoso, R.M., De Ara, N.N.S.L., and Romero, G.A.S. (2015). Expanding the knowledge about Leishmania species in wild mammals and dogs in the Brazilian savannah. Parasites Vectors 8: 1–8.10.1186/s13071-015-0780-ySearch in Google Scholar
Carneiro, I.O. (2018). Infecções Virais em Marsupiais no Estado da Bahia, PhD thesis. Salvador, Brazil: Universidade Federal da Bahia.Search in Google Scholar
Carneiro, M. and Antunes, C.M.F. (2000). Epidemiologia: introdução e conceito. In: Neves, D.P., Melo, A.L., Genaro, O., and Linardi, P.M. (Eds.), Parasitologia humana, 11th ed. São Paulo: Atheneu, pp. 10–25.Search in Google Scholar
Carreira, J.C., Da Silva, A.V., Pereira, D., and Brazil, R.P. (2012). Natural infection of Didelphis aurita (Mammalia: Marsupialia) with Leishmania infantum in Brazil. Parasites Vectors 5: 1–5.10.1186/1756-3305-5-111Search in Google Scholar
Carvalho, F.A., Charest, H., Tavares, C.A.P., Matlashewski, G., Valente, E.P., Rabello, A., Gazzinelli, R.T., and Fernandes, A.P. (2002). Diagnosis of American visceral leishmaniasis in humans and dogs using the recombinant Leishmania donovani A2 antigen. Diagn. Microbiol. Infect. Dis. 43: 289–295.10.1016/S0732-8893(02)00410-8Search in Google Scholar
Casagrande, R.A., Lopes, L.F.L., Reis, E.M.D., Rodrigues, D.D.P., and Matushima, E.R. (2011). Isolation of Salmonella enterica in opossum (Didelphis aurita and Didelphis albiventris) of the São Paulo. Ciência Rural. 41: 492–496.10.1590/S0103-84782011005000016Search in Google Scholar
Cesar, M.O. (2011). Sarcocystis sp. eliminados por Didelphis aurita e Didelphis albiventris (gambás) de vida livre no estado de São Paulo: Infecção experimental em periquitos australianos (Melopsittacus undulatus) e camundongos Balb/c nude, MSc dissertation. São Paulo, Brazil: Universidade de São Paulo.Search in Google Scholar
Cirino, B.S., Costa Neto, S.F.D., Maldonado Júnior, A., and Gentile, R. (2020). First study on the helminth community structure of the neotropical marsupial Metachirus myosuros (Didelphimorphia, Didelphidae). Rev. Bras. Parasitol. Vet. 29: 1–13.10.1590/s1984-29612020064Search in Google Scholar PubMed
Coelho, M.G., Ramos, V.N., Limongi, J.E., De Lemos, E.R.S., Guterres, A., Da Costa Neto, S.F., Rozental, T., Bonvicino, C.B., D’Andrea, P.S., Moraes-Filho, J., et al.. (2016). Serologic evidence of the exposure of small mammals to spotted-fever Rickettsia and Rickettsia bellii in Minas Gerais, Brazil. J. Infect. Dev. Ctries. 10: 275–282.10.3855/jidc.7084Search in Google Scholar PubMed
Collery, P. (1996). Neosporosis in domestic animals. Ir. Vet. J. 49: 152–156.Search in Google Scholar
Cominetti, M.C. (2010). Infecção natural por Trypanosoma sp. em Triatoma sordida, Didelphis albivetris e Sus scrofa em comunidade rural do Mato Grosso do Sul, Brasil, MSc dissertation. Campo Grande, Brazil: Universidade Federal do Mato Grosso do sul.Search in Google Scholar
Conceição, A.M. and Bocchiglieri, A. (2017). Seleção de invertebrados na dieta de marsupiais (Mammalia: Didelphimorphia) em fragmento de Mata Atlântica no nordeste do Brasil. Bol. do Mus. Biol. Mello Leitao 39: 117–126.Search in Google Scholar
Corrêa, F.M.A. and Barreto, M.P. (1964). Estudos sobre reservatórios e vetores silvestres do Trypanosoma cruzi. III. Infecção natural do marsupial Marmosa agilis agilis por tripanossomo semelhante a T. cruzi. Rev. Inst. Med. Trop. Sao Paulo 6: 157–166.Search in Google Scholar
Corrêa, S.H.R. and Passos, E.C. (2001). Wild animals and public health. In: Fowler, M.E., and Cubas, Z.S. (Eds.), Biology, medicine, and surgery of South American wild animals. Lowa: Lowa State University Press/Ames, pp. 493–499.10.1002/9780470376980.ch42Search in Google Scholar
Corrêa, M.M.D.O., Lazar, A., Dias, D., and Bonvicino, C.R. (2013). Quirópteros hospedeiros de zoonoses no Brasil. Bol. Soc. Bras. Mastozool. 67: 23–38.Search in Google Scholar
Costa, E., Costa, Y.A., Lopes, A.A., Sacramento, E., and Bina, J.C. (2001). Formas graves de leptospirose: aspectos clínicos, demográficos e ambientais. Rev. Soc. Bras. Med. Trop. 34: 261–267.10.1590/S0037-86822001000300006Search in Google Scholar PubMed
Da Costa, A.P., Costa, F.B., Soares, H.S., Ramirez, D.G., Mesquita, E.T.K.C., Gennari, S.M., and Marcili, A. (2015). Trypanosoma cruzi and Leishmania infantum chagasi infection in wild mammals from Maranhão State, Brazil. Vector Borne Zoonotic Dis. 15: 656–666.10.1089/vbz.2015.1771Search in Google Scholar PubMed
Da Cruz, A.C.C. and Margarido, T.C.C. (2003). Características reprodutivas de Didelphis albiventris Lund, 1840 (Mammalia-Marsupialia) na região metropolitana de Curitiba, Paraná, Brasil. Arq. Ciênc. Vet. Zool. UNIPAR 6: 119–126.Search in Google Scholar
Da Silva, E.M. (2016). Detecção de Cryptosporidium spp., Leishmania spp. e identificação de ixodídeos e sifonápteros em Didelphis albiventris Lund 1841 (Marsupialia: Didelphidae), MSc dissertation. Recife, Brazil: Universidade Federal Rural de Pernambuco.Search in Google Scholar
Da Silva, F.J., Silva, T.R., Silva, G.C.P., Dos Santos, C.E.P., Júnior, J.R.F.A., and Mathias, L.A. (2013). Isolation of Leptospira borgpetersenii in synanthropic Didelphis albiventris in Jaboticabal, São Paulo, Brazil. Braz. J. Vet. Res. Anim. Sci. 50: 457–461.10.11606/issn.1678-4456.v50i6p457-461Search in Google Scholar
Da Silva, M.B., Portela, J.M., Wei, L.I., Jackson, M., Gonzalez-Juarrero, M., Hidalgo, A.S., Belisle, J.T., Bouth, R.C., Gobbo, A.R., Barreto, J.G., et al.. (2018). Evidence of zoonotic leprosy in Pará, Brazilian Amazon, and risk associated with human contact or consumption of armadillos. PLoS Neglected Trop. Dis. 12: 1–19.10.1371/journal.pntd.0006532Search in Google Scholar PubMed PubMed Central
Dario, M.A., Lisboa, C.V., Costa, L.M., Moratelli, R., Nascimento, M.P., Costa, L.P., Leite, Y.L.R., Llewellyn, M.S., Xavier, S.C.C., Roque, A.L.R., et al.. (2017). High Trypanosoma spp. diversity is maintained by bats and triatomines in Espírito Santo state, Brazil. PLoS One 12: 1–22.10.1371/journal.pone.0188412Search in Google Scholar PubMed PubMed Central
De Alcântara, A.C. (2006). ELISA indireto e mkDNA PCR-RFLP para o diagnóstico e avaliação da infecção por Leishmania sp. em reservatórios domésticos (cães) e silvestres (marsupiais) em Barra do Pojuca, Camaçari, Bahia, MSc dissertation. Salvador, Brazil: Universidade Federal da Bahia.Search in Google Scholar
De Almeida, A.M. (1989). Estudos bacteriológicos e sorológicos de um surto de peste no Estado da Paraíba, Brasil. Mem. Inst. Oswaldo Cruz 84: 249–256.10.1590/S0074-02761989000200015Search in Google Scholar PubMed
De Araujo, J., Thomazelli, L.M., Henriques, D.A., Lautenschalager, D., Ometto, T., Dutra, L.M., Aires, C.C., Favorito, S., and Durigon, E.L. (2012). Detection of hantavirus in bats from remaining rain forest in São Paulo, Brazil. BMC Res. Notes 5: 690.10.1186/1756-0500-5-690Search in Google Scholar PubMed PubMed Central
De Lima, F.G. and Gagliani, L.H. (2014). Raiva: aspectos epidemiológicos, controle e diagnóstico laboratorial. UNILUS Ensino Pesqui. 11: 45–62.Search in Google Scholar
De Oliveira, A. (2011). Interface entre pequenos mamíferos silvestres e animais domésticos em área fragmentada do Alto Paranaparema, estado de São Paulo, Brasil, MSc dissertation. São Paulo, Brazil: Universidade de São Paulo.Search in Google Scholar
De Oliveira, F.C.G. (2008). Avaliação preliminar de impacto ambiental sobre a fauna de pequenos mamíferos e suas taxas de infecção por Trypanosoma cruzi e hantavírus na área de influência da usina hidrelétrica Espora, Aporé – GO, MSc dissertation. Goiânia, Brazil: Universidade Católica de Goiás.Search in Google Scholar
De Siqueira, D.B. (2010). Detecção de anticorpos anti-Toxoplasma gondii em marsupiais e roedores silvestres da Mata Atlântica do estado de Pernambuco, Nordeste do Brasil, MSc dissertation. Recife, Brazil: Universidade Federal Rural de Pernambuco.Search in Google Scholar
De Sousa, K.C.M., Herrera, H.M., Secato, C.T., Do Vale Oliveira, A., Santos, F.M., Rocha, F.L., Barreto, W.T.G., Macedo, G.C., Pinto, P.C.E.A., Machado, R.Z., et al.. (2017a). Occurrence and molecular characterization of hemoplasmas in domestic dogs and wild mammals in a Brazilian wetland. Acta Trop. 171: 172–181.10.1016/j.actatropica.2017.03.030Search in Google Scholar
De Sousa, K.C.M., Calchi, A.C., Herrera, H.M., Dumler, J.S., Barros-Battesti, D.M., Machado, R.Z., and André, M.R. (2017b). Anaplasmataceae agents among wild mammals and ectoparasites in Brazil. Epidemiol. Infect. 145: 3424–3437.10.1017/S095026881700245XSearch in Google Scholar
De Souza, W.M., Dennis, T., Fumagalli, M.J., Araujo, J., Sabino-Santos, G., Maia, F.G.M., Acrani, G.O., Carrasco, A.O.T., Romeiro, M.F., Modha, S., et al.. (2018a). Novel parvoviruses from wild and domestic animals in Brazil provide new insights into parvovirus distribution and diversity. Viruses 10: 1–10.10.3390/v10040143Search in Google Scholar
De Souza, W.M., Fumagalli, M.J., De Araujo, J., Sabino-Santos, G.Jr, Maia, F.G.M., Romeiro, M.F., Modha, S., Nardi, M.S., Queiroz, L.H., Durigon, E.L., et al.. (2018b). Discovery of novel anelloviruses in small mammals expands the host range and diversity of the Anelloviridae. Virology 514: 9–17.10.1016/j.virol.2017.11.001Search in Google Scholar
Dhama, K., Saminathan, M., Karthik, K., Tiwari, R., Shabbir, M.Z., Kumar, N., and Singh, R.K. (2015). Enterite por rotavírus aviário – uma revisão atualizada. Vet. Q. 35: 142–158.10.1080/01652176.2015.1046014Search in Google Scholar
Dias, Y.H.F., Moreira, M.V., Blassioli, V.C., Rezende, E.A., Garcia, L.R., Felizardo, F.M., Rezende, M.C.L., Almeida, A.B., Langner, D., and Tavares, R.L. (2021). Yersinia Pestis e a Peste: uma visão holística no século 21. Braz. J. Health Rev. 4: 8641–8656.10.34119/bjhrv4n2-379Search in Google Scholar
Diniz, S.A., Silva, F.L., Neta, A.C.C., Bueno, R., Guerra, R.M., Abreu-Silva, A.L., and Santos, R.L. (2008). Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J. Infect. Dev. Ctries. 2: 24–33.10.3855/jidc.318Search in Google Scholar
Dos Santos, E.M. (2012). Predação do roedor Calomys sp. (Cricetidae) pelo marsupial Monodelphis domestica (Didelphidae) em Buíque-PE, Brasil. Biotemas 25: 317–320.10.5007/2175-7925.2012v25n3p317Search in Google Scholar
Dubey, J.P. (2003). Review of Neospora caninum and neosporosis in animals. Kor. J. Parasitol. 41: 1–16.10.3347/kjp.2003.41.1.1Search in Google Scholar
Dubey, J.P. (2015). Foodborne and waterborne zoonotic sarcocystosis. Food Waterborne Parasitol. 1: 2–11.10.1016/j.fawpar.2015.09.001Search in Google Scholar
Dubey, J.P., Lindsay, D.S., Kerber, C.E., Kasai, N., Pena, H.F.J., Gennari, S.M., Kwok, O.C.H., Shen, S.K., and Rosenthal, B.M. (2001a). First isolation of Sarcocystis neurona from the South American opossum, Didelphis albiventris, from Brazil. Vet. Parasitol. 95: 295–304.10.1016/S0304-4017(00)00395-2Search in Google Scholar
Dubey, J.P., Lindsay, D.S., Rosenthal, B.M., Kerber, C.E., Kasai, N., Pena, H.F.J., Kwok, O.C.H., Shen, S.K., and Gennari, S.M. (2001b). Isolates of Sarcocystis falcatula–like organisms from South American opossums Didelphis Marsupialis and Didelphis Albiventris from São Paulo, Brazil. J. Parasitol. 87: 1449–1453.10.1645/0022-3395(2001)087[1449:IOSFLO]2.0.CO;2Search in Google Scholar
Dumler, J.S., Barbet, A.F., Bekker, C.P., Dash, G.A., Palmer, G.H., Ray, S.C., Rikihisa, Y., and Rurangirwa, F.R. (2001). Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales. Int. J. Syst. Evol. Microbiol. 51: 2145–2165.10.1099/00207713-51-6-2145Search in Google Scholar
Fayer, R., Morgan, U., and Upton, S.J. (2000). Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30: 1305–1322.10.1016/S0020-7519(00)00135-1Search in Google Scholar
Fehlberg, H.F., Brito Junior, P.D.A., Alvarez, M.R.D.V., Berto, B.P., and Albuquerque, G.R. (2018). Eimeria spp. (Apicomplexa: Eimeriidae) of marsupials (Mammalia: Didelphimorphia) in southern Bahia, Brazil. Rev. Bras. Parasitol. Vet. 27: 604–608.10.1590/s1984-296120180062Search in Google Scholar
Feijó, I.A., Torres, E.J.L., Maldonado, A., and Lanfreid, R.M. (2008). A new oxyurid genus and species from Gracilinanus agilis (Marsupialia: Didelphidae) in Brazil. J. Parasitol. 94: 847–851.10.1645/GE-1428.1Search in Google Scholar
Fernandes, A.J., Diotaiuti, L., Dias, J.C.P., Romanha, A.J., and Chiari, E. (1989). Infecção natural das glândulas anais de gambás (Didelphis albiventris) pelo Trypanosoma cruzi no município de Bambuí-MG. Mem. Inst. Oswaldo Cruz 84: 87–93.10.1590/S0074-02761989000100016Search in Google Scholar
Fernandes, G.W., Vale, M.M., Overbeck, G.E., Bustamante, M.M.C., Grelle, C.E.V., Bergallo, H.G., Magnusson, W.E., Akama, A., Alves, S.S., Amorim, A., et al.. (2017). Dismantling Brazil’s science threatens global biodiversity heritage. Perspect. Ecol. Conserv. 15: 239–243.10.1016/j.pecon.2017.07.004Search in Google Scholar
Fernandes, J.J., Peixoto, A.L., de Farias, A.S.S., Pinheiro, T.J., da Costa, D.F., Silva, M.L.C.R., Júnior, J.P.A., Malossi, C.D., Ullmann, L.S., Sergio Santos de Azevedo, S.S., et al.. (2020). Didelphis albiventris as a carrier of Leptospira sp. in the central nervous tissue in the semiarid region of Northeast, Brazil. Comp. Immunol. Microbiol. Infect. Dis. 101560.10.1016/j.cimid.2020.101560Search in Google Scholar
Ferraroni, J.J. and Marzochi, M.C.A. (1980). Prevalência da infecção pelo Toxoplasma gondii em animais domésticos, silvestres e grupamentos humanos da Amazônia. Mem. Inst. Oswaldo Cruz 75: 99–109.10.1590/S0074-02761980000100010Search in Google Scholar
Ferreira, J.I.G.S. (2015). Diversidade, isolamento e filogenia de parasitas do gênero Trypanosoma em vertebrados silvestres da ilha pluvial e Estação Ecológica de Piapitinga, Minas Gerais, MSc dissertation. São Paulo, Brazil: Universidade de São Paulo.Search in Google Scholar
Fornazari, F., Teixeira, C.R., Da Silva, R.C., Leiva, M., De Almeida, S.C., and Langoni, H. (2011). Prevalence of antibodies against Toxoplasma gondii among Brazilian white-eared opossums (Didelphis albiventris). Vet. Parasitol. 179: 238–241.10.1016/j.vetpar.2011.02.005Search in Google Scholar
Fornazari, F., Langoni, H., Marson, P.M., Nóbrega, D.B., and Teixeira, C.R. (2018). Leptospira reservoirs among wildlife in Brazil: beyond rodents. Acta Trop. 178: 205–212.10.1016/j.actatropica.2017.11.019Search in Google Scholar PubMed
Fournier, G.F.S.R. (2013). Estudo epidemiológico de Toxoplasma gondii em animais silvestres e gatos domésticos de duas unidades de conservação na cidade de Natal, RN, MSc dissertation. São Paulo, Brazil: Universidade de São Paulo.10.11606/D.42.2013.tde-03062014-101711Search in Google Scholar
Galinska, E.M. and Zagórski, J. (2013). Brucellosis in humans-etiology, diagnostics, clinical forms. Ann. Agric. Environ. Med. 20: 233–238.Search in Google Scholar
Gallo, S.S.M., Lindsay, D.S., Ederli, N.B., Matteoli, F.P., Venancio, T.M., and De Oliveira, F.C.R. (2018). Identification of opossums Didelphis aurita (Wied-Neuweid, 1826) as a definitive host of Sarcocystis falcatula-like sporocysts. Parasitol. Res. 117: 213–223.10.1007/s00436-017-5695-4Search in Google Scholar PubMed
Gardner, A.L. (2008). Mammals of South America. In: Marsupials, xenarthrans, shrews, and bats, Vol. 1. Chicago: Chicago University Press.10.1007/s10914-007-9053-7Search in Google Scholar
Gomes, D.C., Da Cruz, R.P., Vicente, J.J., and Pinto, R.M. (2003). Nematode parasites of marsupials and small rodents from the Brazilian Atlantic rain forest in the State of Rio de Janeiro, Brazil. Rev. Bras. Zool. 20: 699–707.10.1590/S0101-81752003000400024Search in Google Scholar
Gonçaalves, L.R., Herrera, H.M., Nantes, W.A.G., Santos, F.M., Porfirio, G.E.O., Barreto, W.T.G., Macedo, G.C., Assis, W.O., Campos, J.B.V., Silva, T.M.V., et al.. (2020). Genetic diversity and lack of molecular evidence for hemoplasma cross-species transmission between wild and synanthropic mammals from Central-Western Brazil. Acta Trop. 203: 105303.10.1016/j.actatropica.2019.105303Search in Google Scholar PubMed
Gondim, L.S., Jesus, R.F., Ribeiro-Andrade, M., Silva, J.C., Siqueira, D.B., Marvulo, M.F., Aléssio, F.M., Mauffrey, J.F., Julião, F.S., Savani, E.S.M.M., et al.. (2017). Sarcocystis neurona and Neospora caninum in Brazilian opossums (Didelphis spp.): Molecular investigation and in vitro isolation of Sarcocystis spp. Vet. Parasitol. 243: 192–198.10.1016/j.vetpar.2017.07.002Search in Google Scholar PubMed
Gondim, L.F., Soares, R.M., Tavares, A.S., Borges-Silva, W., de Jesus, R.F., Llano, H.A., and Gondim, L.Q. (2019). Sarcocystis falcatula-like derived from opossum in Northeastern Brazil: in vitro propagation in avian cells, molecular characterization and bioassay in birds. Int. J. Parasitol. 10: 132–137.10.1016/j.ijppaw.2019.08.008Search in Google Scholar PubMed PubMed Central
Graipel, M.E. and Santos Filho, M. (2006). Reprodução e dinâmica populacional de Didelphis aurita Wied-Neuwied (Mammalia: Didelphimorphia) em ambiente periurbano na Ilha de Santa Catarina, Sul do Brasil. Biotemas 19: 65–73.Search in Google Scholar
Guimarães, A., Raimundo, J.M., Silva, A.T.D., Carpintero, F.M., Pires, J.R., Benevenute, J.L., Machado, R.Z., André, M.R., and Baldani, C.D. (2018). Detection of a putative novel genotype of Ehrlichia sp. from opossums (Didelphis aurita) from Brazil. Rev. Bras. Parasitol. Vet. 28: 140–144.10.1590/s1984-296120180068Search in Google Scholar
Guimarães, F.N. and Jansen, G. (1943). Um foco potencial de Tripanosomíase Americana na cidade do Rio de Janeiro. Mem. Inst. Oswaldo Cruz 39: 405–416.10.1590/S0074-02761943000600014Search in Google Scholar
Herrera, L., D’Andrea, P.S., Xavier, S.C.C., Mangia, R.H., Fernandes, O., and Jansen, A.M. (2005). Trypanosoma cruzi infection in wild mammals of the National Park ‘Serra da Capivara’and its surroundings (Piaui, Brazil), an area endemic for Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 99: 379–388.10.1016/j.trstmh.2004.07.006Search in Google Scholar
Herrera, H.M., Rademaker, V., Abreu, U.G.P., D‘Andrea, P.S., and Jansen, A.M. (2007). Variables that modulate the spatial distribution of Trypanosoma cruzi and Trypanosoma evansi in the Brazilian Pantanal. Acta Trop. 102: 55–62.10.1016/j.actatropica.2007.03.001Search in Google Scholar
Herrera, H.M., Rocha, F.L., Lisboa, C.V., Rademaker, V., Mourão, G.M., and Jansen, A.M. (2011). Food web connections and the transmission cycles of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida, Trypanosomatidae) in the Pantanal Region, Brazil. Trans. R. Soc. Trop. Med. Hyg. 105: 380–387.10.1016/j.trstmh.2011.04.008Search in Google Scholar
Horta, M.C., Ragozo, A.M.A., Casagrande, R.A., Reiko, E., and Gennari, S.M. (2016). Occurrence of anti-Toxoplasma gondii, Neospora caninum and Leptospira spp. antibodies in opossums (Didelphis spp.) in São. Braz. Braz. J. Vet. Res. Anim. Sci. 53: 1–9.10.11606/issn.1678-4456.bjvras.2016.110381Search in Google Scholar
Jorge, S. (2009). Identificação molecular e perfil sorológico de Leptospira spp. Isolada de gambás-de-orelha-branca (Didelphis albiventris) no sul do Brasil, MSc dissertation. Pelotas, Brazil: Universidade Federal de Pelotas.Search in Google Scholar
Lainson, R. and Shaw, J.J. (1969). Leishmaniasis in Brazil. III. Cutaneous leishmaniasis in an opossum, Marmosa murina (Marsupialia, Didelphidae) from the lower amazon region. Trans. R. Soc. Trop. Med. Hyg. 63: 738–740.10.1016/0035-9203(69)90117-5Search in Google Scholar
Lainson, R. and Shaw, J.J. (2010). New World leishmaniasis. In: Collier, L., Balows, A., and Sussman, M. (Eds.), Topley and Wilson’s microbiology and microbial infections. New York: John Wiley and Sons, pp. 313–349.10.1002/9780470688618.taw0182Search in Google Scholar
Lainson, R., Da Silva, F.M.M., and Franco, C.M. (2008). Parasite of Monodelphis emiliae (Marsupialia: Didelphidae) from Amazonian Brazil. Parasite 15: 99–103.10.1051/parasite/2008152099Search in Google Scholar PubMed
Lavorente, F.L.P., De Matos, A.M.R.N., Lorenzetti, E., Oliveira, M.V., Pinto‐Ferreira, F., Michelazzo, M.D.M.Z., Viana, N.E., Lunardi, M., Headley, S.A., Alfieri, A.A., et al.. (2021). First detection of Feline morbillivirus infection in white‐eared opossums (Didelphis albiventris, Lund, 1840), a non‐feline host. Transbound Emerg. Dis. 00: 1–12.10.1111/tbed.14109Search in Google Scholar PubMed
Liaño, G.A. (2013). Ecologia de pequenos mamíferos silvestres reservatórios de zoonoses na Represa de Ribeirão das Lajes, Piraí, RJ, MSc dissertation. Rio de Janeiro, Brazil: Universidade Federal do Rio de Janeiro.Search in Google Scholar
Lima, B.S., Dantas-Torres, F., De Carvalho, M.R., Marinho-Junior, J.F., De Almeida, E.L., Brito, M.E., Gomes, F., and Brandão-Filho, S.P. (2013). Small mammals as hosts of Leishmania spp. in a highly endemic area for zoonotic leishmaniasis in north-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 107: 592–597.10.1093/trstmh/trt062Search in Google Scholar PubMed
Lima, M.M., Sarquis, O., and Oliveira, T.G. (2012). Investigation of Chagas disease in four periurban areas in northeastern Brazil: epidemiologic survey in man, vectors, non- human hosts and reservoirs. Trans. R. Soc. Trop. Med. Hyg. 106: 143–149.10.1016/j.trstmh.2011.10.013Search in Google Scholar PubMed
Linhares, A.C., Pereira, J.D.M., Nakauth, C.M., and Gabbay, Y.B. (1986). Rotavirus infection in wild marsupials (Didelphis marsupialis) of the Amazon region. Trans. R. Soc. Trop. Med. Hyg. 80: 20–24.10.1016/0035-9203(86)90186-0Search in Google Scholar
Lopes, C.C.H. and Berto, B.P. (2012). Aspectos associados à toxoplasmose: Uma referência aos principais surtos no Brasil. Saúde & Ambiente em Revista 7: 1–7.Search in Google Scholar
Lopes, E.R. and Chapadeiro, E. (1983). Morte súbita em área endêmica da doença de Chagas. Rev. Soc. Bras. Med. Trop. 16: 79–84.10.1590/S0037-86821983000200003Search in Google Scholar
Maldonado, I.F.R. (2014). Estudo da diversidade genética das subpopulações de Trypanosoma cruzi I isoladas do gênero Didelphis no Brasil baseado no Multilocus Sequênce Typing (MLST), MSc dissertation. Rio de Janeiro, Brazil: Instituto Oswaldo Cruz.Search in Google Scholar
Martelli-Júnior, H., Martelli, D.R., Silva, A.C.S., Oliveira, M.C.L., and Oliveira, E.A. (2019). Brazil’s endangered postgraduate system. Science 363: 240.10.1126/science.aav9015Search in Google Scholar PubMed
Martins, M.H.D.M. and Spink, M.J.P. (2020). A leptospirose humana como doença duplamente negligenciada no Brasil. Ciênc. Saúde Colet. 25: 919–928.10.1590/1413-81232020253.16442018Search in Google Scholar PubMed
Massini, P.F., Drozino, R.N., Otomura, F.H., Mongruel, A.C.B., Valente, J.D.M., Toledo, M.J.D.O., Martins, T.F., Vidotto, O., Vieira, T.S.W.J., and Vieira, R.F.D.C. (2019). Detection of Hemotropic Mycoplasma sp. in white-eared opossums (Didelphis albiventris) from Southern Brazil. Rev. Bras. Parasitol. Vet. 28: 797–801.10.1590/s1984-29612019058Search in Google Scholar
Mattar, S., Guzman, C., and Figueiredo, L.T. (2015). Diagnosis of Hantavirus infection in humans. Expert Rev. Anti-infect. Ther. 13: 939–946.10.1586/14787210.2015.1047825Search in Google Scholar PubMed
McQuiston, J.H., Paddock, C.D., Holman, R.C., and Childs, J.E. (1999). The human ehrlichiosis in the United States. Emerg. Infect. Dis. 5: 635–642.10.3201/eid0505.990504Search in Google Scholar PubMed PubMed Central
Mello, D.A. (1982). Roedores, marsupiais e triatomíneos silvestres capturados no município de Mambaí-Goiás. Infecção natural pelo Trypanosoma cruzi. Rev. Saude Publica 16: 282–291.10.1590/S0034-89101982000500003Search in Google Scholar PubMed
Melo, G. and Sponchiado, J. (2012). Distribuição geográfica dos marsupials no Brasil. In: Cáceres, N.C. (Ed.), Os marsupiais do Brasil: biologia, ecologia e conservação. Campo Grande: Editora Universidade Federal do Mato Grosso do Sul, pp. 95–112.Search in Google Scholar
Mesquita, G.S.D.S., Rocha, K.D.S., Monteiro, T.R.M., Rosário, M.K.S.D., Baia, I.W.M., Pereira, H.D.S., Cerqueira, V.D., and Moraes, C.C.G.D. (2018). Detection of antibodies against Leptospira spp. in free-living marsupials caught in the eastern Amazon. Rev. Soc. Bras. Med. Trop. 51: 368–371.10.1590/0037-8682-0236-2017Search in Google Scholar PubMed
Milagres, B.S., Padilha, A.F., Barcelos, R.M., Gomes, G.G., Montandon, C.E., Pena, D.C.H., Nieri-Bastos, F.A., Silveira, I., Pacheco, R., Labruna, M.B., et al.. (2010). Rickettsia in synanthropic and domestic animals and their hosts from two areas of low endemicity for Brazilian spotted fever in the eastern region of Minas Gerais, Brazil. Am. J. Trop. Med. Hyg. 83: 1305–1307.10.4269/ajtmh.2010.10-0239Search in Google Scholar PubMed PubMed Central
Miles, M.A., Arias, J.R., Valente, S.A.D.S., Naiff, R.D., De Souza, A.A., Póvoa, M.M., Lima, J.A.N., and Cedillos, R.A. (1983). Vertebrate hosts and vectors of Trypanosoma rangeli in the Amazon Basin of Brazil. Am. J. Trop. Med. Hyg. 32: 1251–1259.10.4269/ajtmh.1983.32.1251Search in Google Scholar PubMed
Mills, J.N. (2006). Biodiversity loss and emerging infectious disease: an example from the rodent-borne hemorrhagic fevers. Biodiversity 7: 9–17.10.1080/14888386.2006.9712789Search in Google Scholar
Miranda, J.B., Borges, I.A., Campos, S.P., Vieira, F.N., De Ázara, T.M., Marques, F.A., Costa, G.B., Luis, A.P.M.F., Oliveira, J.S., Ferreira, P.C.P., et al.. (2017). Serologic and molecular evidence of vaccinia virus circulation among small mammals from different biomes, Brazil. Emerg. Infect. Dis. 23: 931–938.10.3201/eid2306.161643Search in Google Scholar PubMed PubMed Central
Montandon, C.E., Yoshinari, N.H., Milagres, B.S., Mazioli, R., Gomes, G.G., Moreira, H.N., Padilha, A.F., Wanderley, G.G., Mantovani, E., Galvão, M.A.M., et al.. (2014). Evidence of Borrelia in wild and domestic mammals from the state of Minas Gerais, Brazil. Rev. Bras. Parasitol. Vet. 23: 287–290.10.1590/S1984-29612014040Search in Google Scholar PubMed
Monteiro, S.R.D. (2010). Participação dos gambás na epidemiologia da leishmaniose na Mata Atlântica do estado de Pernambuco, Brasil, MSc dissertation. Recife, Brazil: Universidade Federal Rural de Pernambuco.Search in Google Scholar
Moraes-Filho, J., Pinter, A., Pacheco, R.C., Gutmann, T.B., Barbosa, S.O., Gonzáles, M.A.R., Muraro, M.A., Cecilio, S.R.M., and Labruna, M.B. (2009). New epidemiological data on Brazilian spotted fever in an endemic area of the state of São Paulo, Brazil. Vector Borne Zoonotic Dis. 9: 73–78.10.1089/vbz.2007.0227Search in Google Scholar PubMed
Moratelli, R. and Calisher, C.H. (2015). Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem. Inst. Oswaldo Cruz 110: 1–22.10.1590/0074-02760150048Search in Google Scholar PubMed PubMed Central
Murphy, F.A. and Bauer, S.P. (1974). Early street rabies virus infection in striated muscle and later progression to the central nervous system. Intervirology 3: 256–268.10.1159/000149762Search in Google Scholar PubMed
Nantes, W.A.G., Barreto, W.T.G., Santos, F.M., de Macedo, G.C., Rucco, A.C., Assis, W.O., Porfírio, G.E.O., Andrade, G.B., Jansen, A.M., and Herrera, H.M. (2019). The influence of parasitism by Trypanosoma cruzi in the hematological parameters of the white ear opossum (Didelphis albiventris) from Campo Grande, Mato Grosso do Sul, Brazil. Int. J. Parasitol. 9: 16–20.10.1016/j.ijppaw.2019.03.015Search in Google Scholar
Nascimento, A.M.R. (2017). Ocorrência e infecção natural de flebotomíneos e pequenos mamíferos por Leishmania em matas de galeria do Distrito Federal, Brasil, PhD thesis. Brasília, Brazil: Universidade de Brasília.Search in Google Scholar
Neto, C.M.B.G. (2006). Pesquisa sobre o envolvimento do marsupial Didelphis albiventris lund, 1840 (Didelphimorphia, Didelphidae) e de cães domiciliados no ciclo de transmissão da leishmaniose visceral no município de Camaçari, localidade de Barra do Pojuca, Bahia, MSc dissertation. Salvador, Brazil: Universidade Federal da Bahia.Search in Google Scholar
Neto, G.D.A., De Angelis, B.L.D., and Soares, P.F. (2007). Áreas urbanas degradadas: relações com a gestão dos resíduos sólidos. Rev. Desenvolv. Econ. 8: 86–92.Search in Google Scholar
Norris, D.E. (2004). Mosquito-borne diseases as a consequence of land use change. EcoHealth 1: 19–24.10.1007/s10393-004-0008-7Search in Google Scholar
Oliveira, E.A., Martelli-Júnior, H., Silva, A.C.S.E., Martelli, D.R.B., and Oliveira, M.C.L. (2020). Science funding crisis in Brazil and COVID-19: deleterious impact on scientific output. An Acad. Bras Ciências 92: 1–3.10.1590/0001-3765202020200700Search in Google Scholar PubMed
Overbeck, G.E., Bergallo, H.G., Grelle, C.E.V., Akama, A., Bravo, F., Colli, G.R., Magnusson, W.E., Tomas, W.M., and Fernandes, G.W. (2018). Global biodiversity threatened by science budget cuts in Brazil. Bioscience 68: 11–12.10.1093/biosci/bix130Search in Google Scholar PubMed PubMed Central
Paiva, K.A., Pereira, J.S., Fonseca, Z.A., Coelho, W.A., Teixeira, G.M., Oliveira, M.F.D., and Ahid, S.M. (2017). Rickettsia amblyommii associado a roedores e marsupiais nativos da Estação Experimental Rafael Fernandes da UFERSA, Rio Grande do Norte. Pesqui. Vet. Bras. 37: 621–626.10.1590/s0100-736x2017000600015Search in Google Scholar
Pavan, S.E. (2019). A revision of the Monodelphis glirina group (Didelphidae: Marmosini), with a description of a new species from Roraima. J. Mammal. 100: 103–117.10.1093/jmammal/gyy165Search in Google Scholar
Pereira, F.E. and Gonçalves, C.S. (2003). Hepatite A. Rev. Soc. Bras. Med. Trop. 36: 387–400.10.1590/S0037-86822003000300012Search in Google Scholar
Pereira, H.S., Scofield, A., Júnior, P.S.B., Lirados, D.S., De Sousa, J.S., Chaves, J.F., De Jesus, R.C., Dos Anjos, A.H.L., Sarmento, N.M.F.P., Júnior, F.D., et al.. (2021). Chagas disease in urban and peri-urban environment in the Amazon: sentinel hosts, vectors, and the environment. Acta Trop. 1: 105858–105843.10.1016/j.actatropica.2021.105858Search in Google Scholar
Peres, M.G., Barros, C.B., Appolinário, C.M., Antunes, J.M., Mioni, M.S., Bacchiega, T.S., Allendorf, S.D., Vicente, A.F., Fonseca, C.R., and Megid, J. (2016). Dogs and opossums positive for vaccinia virus during outbreak affecting cattle and humans, São Paulo State, Brazil. Emerg. Infect. Dis. 22: 271–273.10.3201/eid2202.140747Search in Google Scholar
Peres, M.G., Bacchiega, T.S., Appolinário, C.M., Vicente, A.F., Mioni, M.D.S.R., Ribeiro, B.L., Fonseca, C.R.S., Pelícia, V.C., Ferreira, F., Oliveira, G.P., et al.. (2018). Vaccinia virus in blood samples of humans, domestic and wild mammals in Brazil. Viruses 10: 1–12.10.3390/v10010042Search in Google Scholar
Pinho, A.P., Cupolillo, E., Mangia, R.H., Fernandes, O., and Jansen, A.M. (2000). Trypanosoma cruzi in the sylvatic environment: distinct transmission cycles involving two sympatric marsupials. Trans. R. Soc. Trop. Med. Hyg. 94: 509–514.10.1016/S0035-9203(00)90069-5Search in Google Scholar
Pontarolo, G.H., Kühl, L.F., Pedrassani, D., Campos, M., Figueiredo, F.B., Valente, J.D.M., Gonçalves, L.R., André, M.R., Vieira, T.S.W.J.J., Vieira, R.F.C., et al.. (2021). ‘Candidatus Mycoplasma haemoalbiventris’, a novel hemoplasma species in white‐eared opossums (Didelphis albiventris) from Brazil. Transbound Emerg. Dis. 68: 565–572.10.1111/tbed.13716Search in Google Scholar PubMed
Portugal, L.G. (2009). Ecologia populacional de pequenos mamíferos e o parasitismo por Trypanosoma cruzii em uma área rural do Rio de Janeiro, MSc dissertation. Rio de Janeiro, Brazil: Instituto Oswaldo Cruz.Search in Google Scholar
Poulsen, C.S. and Stensvold, C.R. (2014). Current status of epidemiology and diagnosis of human sarcocystosis. J. Clin. Microbiol. 52: 3524–3530.10.1128/JCM.00955-14Search in Google Scholar PubMed PubMed Central
Povill, C., Lazar, A., and Bonvicino, C.R. (2018). Levantamento de agentes zoonóticos encontrados em pequenos marsupiais da América do Sul. Heringeriana 11: 13–32.10.17648/heringeriana.v11i2.917765Search in Google Scholar
Prezotto, C.F., Araújo, T.S., Gomez, S.Y.M., and Sousa, N.R.D.S.M. (2015). Anemia infecciosa das galinhas e micoplasmose aviária: Uma breve revisão e abordagem de coinfecções. Rev. Eletrôn. Pesquisa Anim. 3: 127–144.Search in Google Scholar
QGIS (2017). QGIS 2.18.9 ‘Las Palmas’. Boston, MA: Free Software Foundation, Inc. Available at: http://www.qgis.org/en/site/forusers/download.html (Accessed 14 January 2020).Search in Google Scholar
Quadros, G.T.S., Da Silva, J.D.L.S., Da Silva, J.C., Santos, L.M., Neves, O.L.S., and Pereira, E.B. (2020). Análise epidemiológica situacional do sarampo no município de Bragança-Pará em 2019 e 2020. Braz. J. Dev. 6: 99392–99398.10.34117/bjdv6n12-435Search in Google Scholar
Quaresma, P.F., Rêgo, F.D., Botelho, H.A., Da Silva, S.S., Moura, A.J., Teixeira-Neto, R.G., Madeira, F.M., Carvalho, M.B., Paglia, A.P., Melo, M.N., et al.. (2011). Wild, synanthropic and domestic hosts of Leishmania in an endemic area of cutaneous leishmaniasis in Minas Gerais State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 105: 579–585.10.1016/j.trstmh.2011.07.005Search in Google Scholar PubMed
Queiroz, M.J., Alves, J.G., and Correia, J.B. (2004). Leishmaniose visceral: características clínico-epidemiológicas em crianças de área endêmica. J. Pediatr. 80: 141–146.10.1590/S0021-75572004000200012Search in Google Scholar
Quintal, A.P.N. (2010). Leishmania spp. em Didelphis albiventris e Micoureus paraguayanus (Mammalia: Didelphimorphia), MSc dissertation. Araçatuba, Brazil: Universidade Estadual Paulista.Search in Google Scholar
Quintela, F., Da Rosa, C.A., and Feijó, A. (2020). Updated and annotated checklist of recent mammals from Brazil. An Acad. Bras Ciências 92: 1–57.10.1590/0001-3765202020191004Search in Google Scholar PubMed
Ribeiro, M. and Antunes, C.M.D.F. (2009). Febre amarela: estudo de um surto. Rev. Soc. Bras. Med. Trop. 42: 523–531.10.1590/S0037-86822009000500009Search in Google Scholar
Richini-Pereira, V.B., Marson, P.M., Hayasaka, E.Y., Victoria, C., Silva, R.C.D., and Langoni, H. (2014). Molecular detection of Leishmania spp. in road-killed wild mammals in the Central Western area of the State of São Paulo, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 20: 1–7.10.1186/1678-9199-20-27Search in Google Scholar PubMed PubMed Central
Rocha, F.L., Roque, A.L., Arrais, R.C., Santos, J.P., Lima, V.D.S., Xavier, S.C.D.C., Cordeir-Estrela, P., D’Andrea, P.S., and Jansen, A.M. (2013). Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology 140: 160–170.10.1017/S0031182012001539Search in Google Scholar PubMed
Rocha, K.S., Schupp, G.S.M., Silva, M.F.F., Leite, F.N.B., Macedo, R.C.S.L., Araújo, E.S., Mendes-Oliveira, A.C., Duarte, V.C., Scofield, A., Goes, G.C., et al.. (2020). New records of Leptospira spp. in wild marsupials and a rodent in the eastern Brazilian Amazon through PCR detection. Acta Amaz. 50: 305–308.10.1590/1809-4392201903683Search in Google Scholar
Rodrigues, B.A. and Melo, G.B. (1942). Contribuição ao estudo da tripanossomíase americana. Mem. Inst. Oswaldo Cruz 37: 77–90.10.1590/S0074-02761942000100006Search in Google Scholar
Rodrigues, G.M., Queiroz, E.P., Alexandre, K.V., and Rabelo, L.M. (2020). Agravos causados pela Doença de Chagas no ser humano: Revisão sobre as características do Trypanosoma cruzi. Rev. Liberum accessum 1: 1–14.Search in Google Scholar
Roque, A.L.R., Xavier, S.C.C., Rocha, M.R., Duarte, A.C.M., D’Andrea, P.S., and Jansen, A.M. (2008). Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 79: 742–749.10.4269/ajtmh.2008.79.742Search in Google Scholar
Rothan, H.A. and Byrareddy, S.N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109: 102433.10.1016/j.jaut.2020.102433Search in Google Scholar PubMed PubMed Central
Salyers, A.A. and Whitt, D.D. (2002). Yersinia pestis, the cause of plague, and its relatives. In: Salyers, A.A., and Whitt, D.D. (Eds.), Bacterial pathogenesis: a molecular approach. Washington, DC: American Society for Microbiology, pp. 202–215.Search in Google Scholar
Sant’ana, T.M., Monteiro, M.E.Z., Bignarde, J.M.P., Ueda, F.D.S., Penha, G.D.A., and Pereira, R.E.P. (2008). Salmonelose em animais silvestres e exóticos. Rev. Cient. Eletrôn. Med. Vet. 11: 1–7.Search in Google Scholar
Santos-Rondon, M.V., Pires, M.M., Dos Reis, S.F., and Ueta, M.T. (2002). Marmosa paraguayana (Marsupialia: Didelphidae) as a new host for Gracilioxyrus agilisis (Nematoda: Oxyuridae) in Brazil. J. Parasitol. 98: 170–174.10.1645/GE-2902.1Search in Google Scholar PubMed
Schallig, H.D., Da Silva, E.S., Van Dermeide, W.F., Schoone, G.J., and Gontijo, C.M. (2007). Didelphis marsupialis (common opossum): a potential reservoir host for zoonotic leishmaniasis in the metropolitan region of Belo Horizonte (Minas Gerais, Brazil). Vector Borne Zoonotic Dis. 7: 387–393.10.1089/vbz.2006.0651Search in Google Scholar PubMed
Schönrich, G., Rang, A., Lütteke, N., Raftery, M.J., Charbonnel, N., and Ulrich, R.G. (2008). Hantavirus‐induced immunity in rodent reservoirs and humans. Immunol. Rev. 225: 163–189.10.1111/j.1600-065X.2008.00694.xSearch in Google Scholar PubMed
Severino, A.J. (2006). A avaliação do PNPG 2005-2010 e a política de pós-graduação no Brasil. In: Ferreira, N.S.C. (Ed.), Políticas públicas e gestão da educação: polêmicas, fundamentos e análises. Brasília: Líber Livro, pp. 51–74.Search in Google Scholar
Sherlock, I.A. (1996). Ecological interactions of visceral leishmaniasis in the State of Bahia. Mem. Inst. Oswaldo Cruz 91: 671–683.10.1590/S0074-02761996000600003Search in Google Scholar PubMed
Sherlock, I.A., Miranda, J.C., Sadigursky, M., and Grimaldi Junior, G. (1984). Natural infection of the opossum Didelphis albiventris (Marsupialia, Didelphidae) with Leishmania donovani, in Brazil. Mem. Inst. Oswaldo Cruz 79: 511.10.1590/S0074-02761984000400020Search in Google Scholar PubMed
Shinohara, N.K.S., Barros, V.B.D., Jimenez, S.M.C., Machado, E.D.C.L., Dutra, R.A.F., and Lima Filho, J.L.D. (2008). Salmonella spp., important pathogenic agent transmitted through foodstuffs. Ciência Saúde Coletiva 13: 1675–1683.10.1590/S1413-81232008000500031Search in Google Scholar PubMed
Silva, E.M., Alves, L.C., Guerra, N.R., Farias, M.P.O., Oliveira, E.L.R., De Souza, R.C., Da Cunha, C., Ramos, R.A.N., and Porto, W.J.N. (2016). Leishmania spp. in Didelphis spp. from northeastern Brazil. J. Zoo Wildl. Med. 47: 942–944.10.1638/2015-0148.1Search in Google Scholar PubMed
Silva, E.M., Lima, V.S.S., Borges, J.C.G., and Porto, W.J.N. (2017). Ocorrência de parasitas gastrointestinais zoonóticos em uma população de Didelphis albiventris (Lund, 1841) de uma área urbana no nordeste do Brasil. Rev. Electrón. Vet. 18: 1–11.Search in Google Scholar
Silva, L.J.D. and Angerami, R.N. (2008). Viroses emergentes no Brasil. Rio de Janeiro: Editora Fiocruz.10.7476/9788575413814Search in Google Scholar
Silva, C.A., Santilli, G., Sano, E.E., and Rodrigues, S.W.P. (2020). Análise qualitativa do desmatamento na Floresta Amazônica a partir de sensores SAR, óptico e termal. An. Insti. Geoci. 42: 18–29.10.11137/2019_4_18_29Search in Google Scholar
Silveira, I., Martins, T.F., Olegário, M.M., Peterka, C., Guedes, E., Ferreira, F., and Labruna, M.B. (2015). Rickettsial infection in animals, humans and ticks in Paulicéia, Brazil. Zoonoses Public Health 62: 525–533.10.1111/zph.12180Search in Google Scholar
Silveira, T.G.V., Teodoro, U., Lonardoni, M.V.C., Guilherme, A.L.F., De Toledo, M.J.O., Ramos, M., Arraes, S.M.A.A., Bertolini, D.A., Spinoza, R.P., and Barbosa, O.C. (1996). Aspectos epidemiológicos da leishmaniose tegumentar em área endêmica do Estado do Paraná, Brasil. Cad. Saúde Pública 12: 141–147.10.1590/S0102-311X1996000200003Search in Google Scholar
Smith, D.B. and Simmonds, P. (2018). Classification and genomic diversity of enterically transmitted hepatitis viruses. Cold Spring Harb. Perspect. Med. 8: a031880.10.1101/cshperspect.a031880Search in Google Scholar
Spach, D.H. and Koehler, J.E. (1998). Bartonella-associated infections. Emerg. Infect. Dis. 12: 137–155.10.1016/S0891-5520(05)70414-1Search in Google Scholar
Soares, M.D.C.P., Bensabath, G., and Rosa, A. (1987). The presence of antibodies for hepatitis a virus in amazonia Didelphis marsupialis (Vertebrata, Marsupialia). Rev. Instit. Med. Trop. São Paulo 29: 110–111.10.1590/S0036-46651987000200008Search in Google Scholar
Stanek, G. and Strle, F. (2009). Lyme borreliosis: a European perspective on diagnosis and clinical management. Curr. Opin. Infect. Dis. 22: 450–454.10.1097/QCO.0b013e32832ee880Search in Google Scholar
Stanek, G., Wormser, G.P., Gray, J., and Strle, F. (2012). Lyme borreliosis. Lancet 379: 461–473.10.1016/S0140-6736(11)60103-7Search in Google Scholar
Stevens, J., Noyes, H., and Gibson, W. (1998). The evolution of trypanosomes infecting humans and primates. Mem. Inst. Oswaldo Cruz 93: 669–676.10.1590/S0074-02761998000500019Search in Google Scholar PubMed
Strona, A.L.S. (2016). Infestação diferencial de Eimeria spp. (Apicomplexa: Eimeriidae) em indivíduos de Gracilinanus agilis (Didelphimorphia: Didelphidae) na Estação Ecológica do Panga, Uberlândia-MG: Padrões e processos, MSc dissertation. Uberlândia, Brazil: Universidade Federal de Uberlândia.10.14393/ufu.di.2018.96Search in Google Scholar
Szabó, M.P.J., Nieri-Bastos, F.A., Spolidorio, M.G., Martins, T.F., Barbieri, A.M., and Labruna, M.B. (2013). In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology 140: 719–728.10.1017/S0031182012002065Search in Google Scholar PubMed
Takeda, M., Seki, F., Yamamoto, Y., Nao, N., and Tokiwa, H. (2020). Animal morbilliviruses and their cross-species transmission potential. Curr. Opin. Virol. 41: 38–45.10.1016/j.coviro.2020.03.005Search in Google Scholar PubMed
Terassini, F.A. (2010). Levantamento de carrapatos, seus hospedeiros e agentes infecciosos associados, na Estação Ecológica Samuel, Rondônia, Brasil, MSc dissertation. São Paulo, Brazil: Universidade de São Paulo.Search in Google Scholar
Thompson, C.W., Phelps, K.L., Allard, M.W., Cook, J.A., Dunnum, J.L., Ferguson, A.W., Gelang, M., Khan, F.A.A., Paul, D.L., Reederet, D.M., et al.. (2021). Preserve a voucher specimen! The critical need for integrating natural history collections in infectious disease studies. mBio 12: e02698–20.10.1128/mBio.02698-20Search in Google Scholar PubMed PubMed Central
Torres, E.L., Maldonado-Junior, A., and Lanfreid, R.M. (2007). Pterygodermatites (Paucipectines) ja gerskio ldi (Nematoda: Rictulariidae) from Gracilinanus agilis and G. microtarsus (Marsupialia: Didelphidae) in brazilian pantanal and atlantic forest by light and scanning electron microscopy. J. Parasitol. 93: 274–279.10.1645/GE-986R2.1Search in Google Scholar PubMed
Trueb, I., Portela, R.D., Franke, C.R., Carneiro, I.O., Ribeiro, G.J.Jr, Soares, R.P., and Barrouin-Melo, S.M. (2018). Trypanosoma cruzi and Leishmania sp. infection in wildlife from urban rainforest fragments in Northeast Brazil. J. Wildl. Dis. 54: 76–84.10.7589/2017-01-017Search in Google Scholar PubMed
Ueno, T.E.H., Cutolo, A.A., Martins, T.F., Moraes-Filho, J., Azevedo, S.S.D., and Labruna, M.B. (2020). Rickettsial infection in equids, opossums and ticks in the municipality of Monte Mor, state of São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 29: 1–9.10.1590/s1984-29612020073Search in Google Scholar PubMed
Urdaneta-Morales, S. and Nironi, I. (1996). Trypanosoma cruzi in the anal glands of urban opossums. I – Isolation and experimental infections. Mem. Inst. Oswaldo Cruz 91: 399–403.10.1590/S0074-02761996000400002Search in Google Scholar PubMed
Vasconcelos, P.F.D.C., Travassos da Rosa, J.F.S., Travassos da Rosa, A.P.D.A., Dégallier, N., and Pinheiro, F.D.P. (1991). Epidemiologia das encefalites por arbovírus na Amazônia brasileira. Rev. Inst. Med. Trop. Sao Paulo 33: 465–476.10.1590/S0036-46651991000600007Search in Google Scholar
Vitaliano, S.N., Soares, H.S., Pena, H.F.J., Dubey, J.P., and Gennari, S.M. (2014). Serologic evidence of Toxoplasma gondii infection in wild birds and mammals from southeast Brazil. J. Zoo Wildl. Med. 45: 197–199.10.1638/2013-0179R.1Search in Google Scholar PubMed
Voss, R.S. and Jansa, S.A. (2009). Phylogenetic relationships and classification of didelphid marsupials, an extant radiation of new world metatherian mammals. Bull. Am. Mus. Nat. Hist. 322: 1–177.10.1206/322.1Search in Google Scholar
Waites, K.B. and Talkington, D.F. (2004). Mycoplasma pneumoniae e seu papel como patógeno humano. Clin. Microbiol. Rev. 17: 697–728.10.1128/CMR.17.4.697-728.2004Search in Google Scholar PubMed PubMed Central
Weiss, R.A. (2001). Animal origins of human infectious disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356: 957–977.10.1098/rstb.2001.0838Search in Google Scholar PubMed PubMed Central
Wolfe, N.D., Daszak, P., Kilpatrick, A.M., and Burke, D.S. (2005). Bushmeat hunting, deforestation, and prediction of zoonotic disease. Emerg. Infect. Dis. 11: 1822–1827.10.3201/eid1112.040789Search in Google Scholar PubMed PubMed Central
World Health Organization – WHO (2017). One health, Available at: https://www.who.int/news-room/q-a-detail/one-health (Accessed 11 April 2021).Search in Google Scholar
World Health Organization – WHO (2021). Zoonoses, Available at: https://www.who.int/news-room/fact-sheets/detail/zoonoses (Accessed 23 February 2021).Search in Google Scholar
Xiao, L. and Feng, Y. (2008). Zoonotic cryptosporidiosis. FEMS Immunol. Med. Microbiol. 52: 309–323.10.1111/j.1574-695X.2008.00377.xSearch in Google Scholar PubMed
Yai, L.E.O., Cañón-Franco, W.A., Geraldi, V.C., Summa, M.E.L., Camargo, M.C.G., Dubey, J.P., and Gennari, S.M. (2003). Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in the South American opossum (Didelphis marsupialis) from the city of São Paulo, Brazil. J. Parasitol. 89: 870–871.10.1645/GE-83RSearch in Google Scholar PubMed
Yoshida, E.L.A., Corrêa, F.M.A., Pacheco, R.S., Momen, H., and Grimaldi-Junior, G. (1985). Leishmania mexicana in Didelphis marsupialis aurita in Sao Paulo State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 27: 172.10.1590/S0036-46651985000400002Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/mammalia-2021-0134).
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Ecology
- Infection agents of Didelphidae (Didelphimorphia) of Brazil: an underestimated matter in zoonoses research
- Habitat type impacts small mammal diversity in the Ukaguru Mountains, Tanzania
- Roosting ecology of insectivorous bats in a tropical agricultural landscape
- Bats in wetlands: composition and structure of assemblage in Reserva Natural Don Luis, Esteros del Iberá, Argentina
- Bats can reach 3626 m a.s.l. in Papua New Guinea: altitudinal range extensions for six rainforest bat species
- Original record of deposited skins of hedgehogs (Erinaceidae) at red fox den
- Conservation
- Distribution update, male genitalia, natural history, and conservation of the stump-tailed porcupine Coendou rufescens in South America
- Growth, weights, and measurements of female wild sheep from Iran
- Ethology
- How fishing cats Prionailurus viverrinus Bennett, 1833 fish: describing a felid’s strategy to hunt aquatic prey
- Biogeography
- New altitudinal records of Panthera onca (Carnivora: Felidae) in the Andean region of Ecuador
- Taxonomy/Phylogeny
- Taxonomic status and phylogenetic position of Oxymycterus juliacae Allen 1900 (Rodentia: Cricetidae)
Articles in the same Issue
- Frontmatter
- Ecology
- Infection agents of Didelphidae (Didelphimorphia) of Brazil: an underestimated matter in zoonoses research
- Habitat type impacts small mammal diversity in the Ukaguru Mountains, Tanzania
- Roosting ecology of insectivorous bats in a tropical agricultural landscape
- Bats in wetlands: composition and structure of assemblage in Reserva Natural Don Luis, Esteros del Iberá, Argentina
- Bats can reach 3626 m a.s.l. in Papua New Guinea: altitudinal range extensions for six rainforest bat species
- Original record of deposited skins of hedgehogs (Erinaceidae) at red fox den
- Conservation
- Distribution update, male genitalia, natural history, and conservation of the stump-tailed porcupine Coendou rufescens in South America
- Growth, weights, and measurements of female wild sheep from Iran
- Ethology
- How fishing cats Prionailurus viverrinus Bennett, 1833 fish: describing a felid’s strategy to hunt aquatic prey
- Biogeography
- New altitudinal records of Panthera onca (Carnivora: Felidae) in the Andean region of Ecuador
- Taxonomy/Phylogeny
- Taxonomic status and phylogenetic position of Oxymycterus juliacae Allen 1900 (Rodentia: Cricetidae)

