Abstract
Faunas of old oceanic islands often have extremely high levels of endemism and are considered highly susceptible to anthropogenic disruption. We surveyed the richly endemic small mammal fauna on Mt. Amuyao in the Central Cordillera of northern Luzon Island, Philippines. We tested hypotheses regarding elevational patterns of species richness and community composition, community response to habitat disturbance, and interactions of native and non-native mammals. Our study revealed greater species richness and faunal heterogeneity within the Central Cordillera than previously suspected. We documented 15 native species (14 rodents and 1 insectivore), and two species of non-native rodents. All of the native species are endemic to the Philippines, eight being restricted to the Cordillera. Twelve of the 14 native rodents belong to two ancient endemic clades, indicating that most of the regional diversity is the product of in situ speciation. Native mammal assemblages are ecologically diverse, and include species with varied trophic habits, activity patterns, and climbing ability. Some native species are restricted to relatively pristine habitat, whereas others are highly tolerant of disturbance. Non-native species are restricted to highly disturbed habitats and apparently are displaced by natives where habitat has regenerated from past disturbance.
Introduction
Oceanic islands are well known for their richly endemic biotas, but mammals on such islands have been surprisingly poorly documented. At 103,000 km2 (United Nations Environmental Programme 2010), Luzon, in the northern Philippine archipelago, is the largest island that is entirely oceanic. Recent studies on Luzon have documented at least 47 native non-flying mammals, of which 42 (89%) are endemic (Heaney et al. 2013a, 2014a,b, 2016, Balete et al. 2015), and it seems certain that other endemic species are yet to be discovered in poorly explored areas.
Luzon has a long and complex geological history (Hall 2012) which is closely associated with faunal diversification, much of which resulted from autochthonous speciation (Jansa et al. 2006, Heaney et al. 2011, 2013b, 2016, Justiniano et al. 2014). Although progress has been made in documenting the mammal faunas in many portions of Luzon, little has been published on mammals of the largest and most species-rich area, the Central Cordillera, and this has limited our perspective on the evolution, ecology, and conservation of this richly endemic fauna.
The Cordilleran mammal fauna was introduced to science in the late 19th century through specimens obtained from Mt. Data (2310 m) and elsewhere by British naturalist John Whitehead (Figure 1, Thomas 1898). During the 20th century, Mt. Data remained the principal focus of collecting efforts on Luzon (Sanborn 1952, Rabor 1955). Our 2000–2003 study in Balbalasang-Balbalan National Park (Rickart et al. 2011b) was the first comprehensive survey of mammals in the Cordillera region, involving standardized sampling along a local elevational habitat gradient to facilitate quantitative comparisons with gradient surveys conducted in other regions of the Philippines (Heaney et al. 1989, 2006, Rickart et al. 1991). However, that study was conducted on Mt. Bali-it, a peak that reached only 2238 m, well short of the maximum elevation in the Cordillera (2930 m).
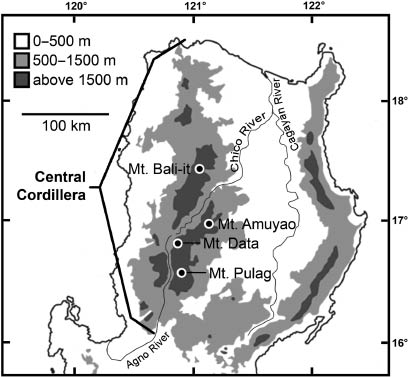
Map of northern Luzon Island showing areas of highland habitat and location of Mt. Amuyao in relation to other peaks in the Central Cordillera.
Here, we report results of surveys undertaken in 2007 and 2011 on Mt. Amuyao (2702 m), the fourth highest mountain on Luzon. To determine the nature and extent of possible faunal variation within the Central Cordillera, we sought to survey a new site geographically distinct from Balbalasang. We were particularly interested in accessing areas at elevations higher than those available at Balbalasang. In order to gain greater insight into the effects of anthropogenic disturbance on native and non-native mammals, we surveyed habitats ranging from agricultural lands and early second growth to relatively undisturbed mature forest.
Some of the data from the Mt. Amuyao survey have appeared in previous publications describing newly discovered taxa (Balete et al. 2012, Heaney et al. 2014a), new distributional records of bats (Heaney et al. 2012), and the impact of habitat disturbance on small mammals (Rickart et al. 2011a). Here, we present the comprehensive results from our elevational transect survey of the non-flying small mammals, placing them within the broader context of our earlier gradient studies. Much of the data presented here constitutes the first information on several species endemic to the Central Cordillera.
Materials and methods
Study area
Mt. Amuyao is located in the east-central portion of the Central Cordillera, approximately 60 km south of Mt. Bali-it from which is it separated by intervening land below 1500 m elevation, and approximately 30 km northeast of Mt Data and 50 km north of Mt. Pulag (2922 m), both of which are located within the same contiguous area of highland habitat above 1500 m (Figure 1). The Central Cordillera, extending nearly 300 km from the northern tip of Luzon south to the Central Valley, is an uplifted magmatic arc associated with subduction in both the East Luzon Trench and the Manila Trench. The core of the Cordillera consists of Eocene–Oligocene plutonic rocks with associated marine sedimentary rocks. Uplift and volcanic activity occurred during the Late Oligocene–Middle Miocene (33–12 Ma) in conjunction with westward subduction of the Philippine Sea Plate along the Manila Trench, followed by Pliocene–Quaternary (5–0 Ma) uplift and extensive magmatism (Ringenbach et al. 1990, Hall 2002, 2012, Hollings et al. 2011). Much of the Cordillera west of Mt. Amuyao has exposed extrusive and intrusive igneous rocks (andesite and granite), whereas to the east underlying sedimentary rocks include sandy shale and sandstone, of which the latter predominates on the surface of Mt. Amuyao (Smith 1915: 192).
We surveyed mammals from 17 February to 26 March 2007 and from 5 to 27 April 2011 at 15 localities along an elevational gradient on the northern aspect of Mt. Amuyao from the nearby town of Barlig (ca. 1500 m) to the mountain summit (2702 m). These localities encompassed the range of natural forest habitats and included areas with varied levels of natural and anthropogenic disturbance from agricultural land to nearly pristine forest (Figure 2). Detailed information on the plants of Mt. Amuyao, including an assessment of plant diversity across elevation, can be found elsewhere (Salcedo 2001). The following descriptions of our survey localities are based on our field notes. Where our localities and those of Salcedo (2001) coincided, we list the dominant plants from her study.
Locality 1. 1.0 km S, 0.6 km E Barlig Municipal Hall, 1510 m elev., 17.04030°N, 121.10583°E. 21–22 February 2007. At this locality southeast of Barlig we trapped in early second-growth habitat on abandoned rice terraces overgrown with saplings, tree ferns (Cyathea), shrubs, and cane grass (Saccharum).
Locality 2. 1.1 km S, 0.6 km E Barlig Municipal Hall, 1530 m elev., 17.03797°N, 121.10424°E. 18–20 February 2007. At this locality we trapped in areas of active agriculture that included terraced vegetable gardens and along grassy margins of rice fields (Figure 2A).
Locality 3. 0.5 km S, 0.2 km E Barlig Municipal Hall, 1535 m elev., 17.04109°N, 121.10291°E. 18–20 February 2007. This locality was on a steep slope with grasses, shrubs, scattered pines (Pinus kesiya), cultivated banana (Musa), and sweet potato (Ipomoea).
Locality 4. 2.15 km N, 1.25 km W Mt. Amuyao summit, 1650 m elev., 17.03270°N, 121.11604°E. 19–27 April 2011. This locality was in lightly disturbed lower montane forest southeast of Barlig (Figure 2C). The terrain was relatively steep (ca. 40° average slope). Height of forest canopy was 15–20 m, with emergent trees 25–30 m. Canopy and emergent trees had diameters at breast height (dbh) of 20–60 cm. Dominant trees included oaks (Lithocarpus), myrtles (Syzygium), and laurels in areas with deep soils, with pines and figs (Ficus) predominant in rocky areas. Canopy ephiphytes included mosses, ferns, and orchids. Canopy vines included Schefflera and Tetrastigma. Understory plants included tree ferns, palms (Pinanga), wild banana (Musa) and various flowering trees and shrubs (Astronia, Calophyllum, Ficus, Helicia, Psychotria). Ground cover plants included ferns, orchids, taro (Alocasia) and gingers (Zingiber). There was 3–5 cm of leaf litter over a humus layer of 5–7 cm.
Locality 5. 0.2 km N, 0.3 km E Barlig Municipal Hall, 1675 m elev., 17.04444°N, 121.10260°E. 21–22 February 2007. Habitat at this locality was secondary pine forest with undergrowth of cane grass, forbs, and bracken fern (Pteridium). Undergrowth was periodically burned to promote herbaceous vegetation for livestock forage.
Locality 6. 0.3 km N Barlig Municipal Hall, 1760 m elev., 17.04694°N, 121.09801°E. 18–23 February 2007. This locality (Figure 2B) was along a ridge in open pine forest with trees between 12 and 20 meters in height, the largest of which had dbh of ca. 60 cm. The understory, which was regularly burned, included cane grass and bracken fern with scattered shrubs (Rubus, Melastoma, and Vaccinium). Ground cover included pine needles and dead grass over moderately rocky volcanic soil.
Locality 7. 0.6 km N Barlig Municipal Hall, 1800 m elev., 17.04820°N, 121.09977°E. 21–25 February 2007. This locality was in remnant montane forest near secondary pine forest (locality 6). Height of the forest canopy was 10–15 m, and emergent trees reaching 20 m. Canopy and emergent trees had dbh of 30–60 cm. Dominant trees included oaks and figs that supported epiphytic mosses, ferns, and orchids. In addition to figs, fruiting plants included vines (Piper) and small trees and shrubs (Melastoma, Helicia, and Vaccinium). Other understory plants included herbaceous ferns, tree ferns, and palms. Ground cover included moss, ferns, small palms, and cane grass. There was a thin cover of leaf litter over a 3–10 cm layer of humus.
Locality 8. 1.75 km N, 0.4 km W Mt. Amuyao summit, 1885 m elev., 17.02929°N, 121.12466°E. 23–26 March 2007. This locality was situated on the northern aspect of Mt. Amuyao, on steep terrain in mature montane forest. Dominant woody plants noted by Salcedo (2001) near this locality and locality 11 included Decaspermum fruticosum (Myrtaceae), Horsfieldia megacarpa (Myristicaceae), Lithocarpus jordanae and L. solerianus (Fagaceae), Phyllocladus hypophyllus (Podocarpaceae), Piper sp. (Piperaceae), Schefflera sp. (Araliaceae), and Vernonia benguetensis (Asteraceae).
Locality 9. 1.75 km N, 1.5 km W Mt. Amuyao summit, 1950 m elev., 17.02595°N, 121.11301°E. 24–26 March 2007. This locality was on a steep slope in mature montane forest. Dominant trees included oaks, laurels, and myrtles up to 20 m high with dbh of 25–50 cm, with ephiphytic mosses, ferns, and orchids.
Locality 10. 1.25 km N, 0.5 km W Mt. Amuyao summit, 1990 m elev., 17.02602°N, 121.12276°E. 22 March 2007. This locality was on steep terrain in mature montane forest.
Locality 11. 1.0 km N, 1.0 km W Mt. Amuyao summit, 2100 m elev., 17.02213°N, 121.11791°E. 16–26 March 2007; 5–10 April 2011. 3040 trap-nights. This locality was in mature montane forest along a ridge northwest of Mt. Amuyao summit. Average slope was ca. 25°, ranging between 10° and 60°. The forest canopy was at 15–20 m with about 80% cover. Emergent trees were 25–30 m in height. Dominant trees included oaks, laurels, myrtles, and eleaocarps, with some scattered pines along ridgelines. Abundant canopy epiphytes included mosses, ferns, orchids, and pitcher plants (Nepenthes). Vines included climbing bamboo (Schizostachyum), pandans (Freycinetia), Piper, Tetrastigma, and other woody species. Understory plants included tree ferns, saplings, and shrubs, with a ground cover of ferns, herbaceous plants, and club mosses. Grass was present under pines. Leaf litter was 3–10 cm thick and continuous over a layer of humus 10–30 cm thick. Moss-covered logs were abundant. Disturbance included numerous trails and pit-traps for wild pigs.
Locality 12. 0.75 km W Mt. Amuyao summit, 2300 m elev., 17.01467°N, 121.12196°E. 9–14 March 2007. This locality was in a ravine with a shallow stream. Habitat was mossy forest with oaks, myrtles, laurels, and podocarps 15 m or less in height and dbh of 20–40 cm. The terrain was steep and rocky with shallow soil and humus. Dominant woody plants recorded by Salcedo (2001) near this locality included Cyrtandra sp. (Gesneriaceae), Dacrycarpus steupii (Podocarpaceae), Diplycosia luzonica (Ericaceae), Eurya nitida (Theaceae), Perrottetia alpestris (Celastraceae), Psychotria sp. (Rubiaceae), Rubus pectinellus (Rosaceae), Vernonia benguetensis (Asteraceae), and Viburnum luzonicum (Caprifoliaceae).
Locality 13. 0.4 km N, 0.4 km W Mt. Amuyao summit, 2480 m elev., 17.01727°N, 121.12393°E. 27 February–13 March 2007. This locality was in a moist ravine within mossy forest similar to that at locality 12.
Locality 14. 0.5 km N, 0.5 km W Mt. Amuyao summit, 2530 m elev., 17.01717°N, 121.12188°E. 27 February–14 March 2007; 12–17 April 2011. This locality was in mossy forest along a ridge northwest of Mt Amuyao summit (Figure 2D). Slope varied from nearly level on the ridge top to ca. 80° on the sides of the ridge. Tree canopy varied from 5 m along the ridge top to 20 m on the sides, and was discontinuous due to tree falls and landslides. Dominant trees included podocarps, myrtles, laurels, and oaks, with dbh of 50–80 cm. Epiphytic mosses, ferns, and orchids were abundant. Understory plants included saplings, herbaceous ferns, a dwarf, grass-like bamboo, tree ferns, Melastoma, Rhododendron, Vaccinium, and laurels. Leaf litter was 4–10 cm thick and continuous over humus 10–100 cm deep. Between 26 February and 12 March, mean daily maximum temperature was 14.6°C (13.5–16°) and mean daily minimum was 9.2°C (7.5–11°).
Locality 15. Mt. Amuyao summit, 2690 m elev., 17.01330°N, 121.12807°E. 2–11 March 2007. Habitat at the summit of Mt. Amuyao was dwarfed mossy forest. Height of woody vegetation on the summit was 2 m or less and up to 5 m on slopes immediately below the summit. Vegetation was dense and continuous except for disturbed areas along trails and in clearings. Dominant plants forming the “canopy” included myrtles, laurels, oaks, and Rhododendron, with understory vegetation including ginger, ferns, orchids, and dwarf bamboo. Moss covered the ground and most understory surfaces. Small fallen branches were common, and there was sparse leaf litter over a thin (<5 cm) layer of humus covering rocky soil. Dominant woody plants listed by Salcedo (2001) included Lithocarpus jordanae and Lithocarpus luzoniensis (Fagaceae), Eurya spp. (Theaceae), Schefflera simplicifolia (Araliaceae), Rhododendron subsessile (Ericaceae), Tasmania piperita (Winteraceae), Rubus pectinellus (Rosaceae), Discocalyx montana (Myrsinaceae), and Melastoma sp. (Melastomataceae).
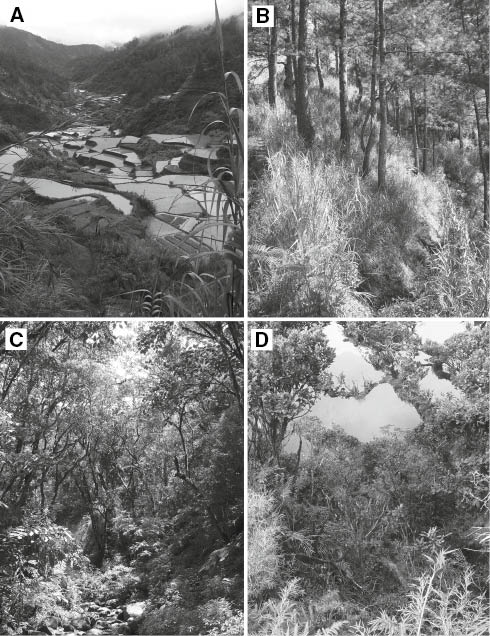
Habitats represented along the Mt. Amuyao survey gradient. (A) Rice terraces near Barlig, ca. 1550 m elevation, illustrating the disturbed agricultural and early second growth habitats at localities 1–3. (B) Secondary pine (Pinus keysia) forest and understory grasses at locality 6, ca. 1760 m elevation. (C) Lower montane forest near locality 4, ca. 1650 m elevation. (D) Mossy forest near locality 14, ca. 2550 m elevation.
Survey procedures
Surveys involved trapping of small (<500 g body mass) mammals including shrews (Soricidae) and rodents (Muridae). Methods followed those used in previous surveys conducted elsewhere on Luzon (Heaney et al. 1999, 2013a,c, Rickart et al. 1991, 2011b, 2013, Balete et al. 2009, 2011, 2013a,b, Alviola et al. 2011, Duya et al. 2011). Survey effort was expressed as trap-nights (i.e. 1 trap set for 24 h). Nearly two-thirds of the total trapping effort consisted of ground trapping, principally under root tangles, at burrow entrances, along runways, and beside or on top of fallen logs. At localities in mature forest, some traps were set 1–5 m above the ground surface on inclined tree trunks, horizontal branches, within tree cavities, and at intersecting vines (hereafter, “arboreal trapping”). Traps were baited either with pieces of sliced coconut lightly fried in cooking oil and coated with peanut butter (“coconut bait”), or with live annelid earthworms obtained locally. Earthworm-baited traps were set exclusively on the ground and constituted nearly one-third of the total trapping effort. Although our trapping methods did not target larger species (>500 g), we gathered information on their occurrence from local residents and obtained a few specimens from hunters. Voucher specimens for all documented species were deposited at the Field Museum of Natural History, of which half will be returned to the National Museum of the Philippines.
Data analysis
In assessing the distribution of native species across the elevational gradient, we used the “range-through” assumption of occurrence (Rowe 2009), where the number of species considered present at a given sampling locality included those directly recorded plus those assumed to be present because of their occurrence at both lower and higher elevations. Secondary localities with limited sampling were excluded from our assessment of elevational patterns. However, data from secondary localities were included in assessments of overall capture frequencies of species based on bait type, diel period, and trap position. To measure the adequacy of sampling, we plotted species accumulation curves for each of the principal sampling localities as well as a cumulative curve for all localities combined, beginning with the lowest elevation locality and proceeding upward.
Relative abundance of species was measured through trapping success expressed as capture rate (i.e. the number of captures per 100 trap-nights). The number of relevant trap-nights varied for different species based on their climbing habits (i.e. strictly ground-dwelling, scansorial, or strictly arboreal) and body size (i.e. <100 g vs. >100 g), because these characteristics determined likelihood of capture in traps set above ground and in traps of different sizes.
We used χ2-tests to assess patterns of bait attractiveness, ground vs. arboreal capture frequencies, and diel activity where total samples included 20 or more individuals and there were at least five individuals per category. For smaller samples we used a binomial test. Expected frequencies for bait type and trap position were based on the number of trap-nights at localities where species were either documented or inferred to occur. For diel activity, expected frequencies for diurnal and nocturnal/crepuscular captures were 0.42 and 0.58 representing the approximate length of the two periods (10 h and 14 h, respectively). The relationship of capture parameters with elevation was assessed using Spearman’s rank correlation (rs ).
Results
Small mammals
Trapping at 15 localities for a total of 13,019 trap-nights, we captured 665 small mammals representing 15 species including 1 native shrew, 12 native murid rodents, and two non-native murid rodents (Table 1). Three of the native rodents (Archboldomys maximus Balete et al., 2012, Musseromys inopinatus Heaney et al., 2014a, and Soricomys montanus Balete et al., 2012) were newly discovered during our surveys of Mt. Amuyao. Subsequently, we discovered that S. montanus also occurs on Mt. Data and Mt. Pulag (Rickart et al. 2011a); at present, the other two species are known only from Mt. Amuyao. The remaining native species are Philippine endemics with geographic distributions ranging from the greater Cordillera region to the oceanic Philippines, and the two non-native species occur throughout the Philippines and beyond (Table 1, Heaney et al. 2010).
Distribution of small mammal species along the elevational gradient of Mt. Amuyao.
| Species | Distributiona | Adult mass X±SD (n) | Locality elevation (m) | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1510 | 1530 | 1535 | 1650 | 1675 | 1760 | 1800 | 1885 | 1950 | 1990 | 2100 | 2300 | 2480 | 2530 | 2690 | ||||
| Crocidura grayi | P | 9.9±0.5 (30) | 0 | 0 | 0 | 4 | 0 | 1 | 1 | 5 | 0 | 0 | 4 | 2 | 8 | 15 | 17 | 57 |
| Apomys abrae | C | 62.1±6.1 (15) | 0 | 0 | 0 | 16 | 0 | 5 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| Apomys datae | C | 77.2±6.5 (30) | 0 | 0 | 0 | 28 | 0 | 0 | 3 | 24 | 5 | 4 | 72 | 32 | 19 | 45 | 62 | 294 |
| Apomys musculus | P | 21.2±1.8 (8) | 0 | 0 | 0 | 12 | 0 | 0 | 0c | 0 | 1 | 0 | 12 | 1 | 1 | 4 | 1 | 32 |
| Archboldomys maximus | C | 42.4±6.0 (13) | 0 | 0 | 0 | 1 | 0 | 0 | 0c | 2 | 0 | 0 | 2 | 3 | 3 | 3 | 1 | 15 |
| Batomys granti | L | 178.2±11.5 (5) | 0 | 0 | 0 | 2 | 0 | 0 | 0c | 2 | 0 | 1 | 7 | 0c | 1 | 1 | 0 | 14 |
| Bullimus luzonicus | L | 463.8±28.7 (4) | 0 | 0 | 1 | 5 | 0 | 0 | 0c | 4 | 0 | 0 | 3 | 2 | 0c | 0c | 1 | 16 |
| Chrotomys silaceus | C | 131.8±17.8 (17) | 0 | 0 | 0 | 3 | 0 | 0 | 0c | 1 | 0 | 1 | 13 | 10 | 13 | 2 | 3 | 46 |
| Chrotomys whiteheadi | C | 126.8±22.2 (14) | 4 | 0 | 2 | 1 | 4 | 1 | 4 | 0c | 1 | 0 | 3 | 0c | 0c | 0c | 3 | 23 |
| Musseromys inopinatus | C | 17.3±2.0 (3) | 0 | 0 | 0 | 2 | 0 | 0 | 0c | 0c | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 5 |
| Rattus everetti | P | 311.0±35.1 (9) | 0 | 0 | 4 | 6 | 0 | 2 | 2 | 2 | 0 | 0 | 3 | 0c | 1 | 6 | 0 | 26 |
| Rattus exulansb | W | 66.8±6.6 (20) | 2 | 1 | 8 | 0 | 6 | 33 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 51 |
| Rattus tanezumib | W | 183.5±14.5 (8) | 3 | 6 | 7 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 |
| Rhynchomys soricoides | C | 159.9±17.4 (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0c | 1 | 1 | 4 | 13 |
| Soricomys montanus | C | 24.7±2.7 (7) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0c | 1 | 0 | 3 | 1 | 0c | 3 | 1 | 10 |
| Total captures | 9 | 7 | 22 | 80 | 13 | 44 | 33 | 40 | 9 | 7 | 129 | 52 | 47 | 80 | 93 | 665 | ||
| Total trap nights | 50 | 75 | 247 | 2270 | 157 | 472 | 457 | 569 | 106 | 129 | 3040 | 1222 | 625 | 2470 | 1130 | 13,019 | ||
| No. native species (+ inferred) | 1 | 0 | 3 | 11 | 1 | 4 | 6 (+6) | 7 (+3) | 5 | 4 | 12 | 8 (+4) | 8 (+3) | 9 (+2) | 9 | 13 | ||
| No. non-native species | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | ||
Principal localities in mature forest are shown in italic.
aC, Endemic to Central Cordillera; L, endemic to Luzon faunal region; P, endemic to oceanic Philippines; W, widespread beyond the Philippines.
bNon-native species.
cPresence inferred from occurrence at both higher and lower elevations (principal localities only).
At three of the nine principal localities (2100 m, 2530 m, and 2690 m) the number of native species trapped reached terminal plateaus that extended for more than 500 trap-nights of sampling. However, at 2530 m we inferred the presence of two additional native species that were documented at higher and lower elevations (Table 1). At the other principal trapping localities, species accumulation curves did not achieve plateaus. The curve for the combined localities (Figure 3) shows a rapid increase to 11 species after fewer than 2000 cumulative trap nights with the sequential addition of localities up to 1650 m. Two more species were added after 4500 trap nights following the addition of localities in montane forest between 1800 and 2000 m. An additional 8000 trap-nights at the five localities above 2100 m in upper montane and mossy forest did not add any new species.
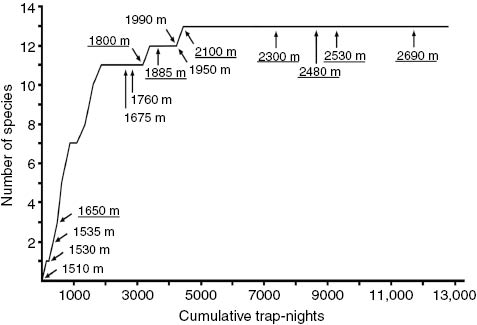
Combined species accumulation curve for native small mammals at all survey localities on the Mt. Amuyao gradient. Localities added sequentially from lowest to highest elevation, with initial sampling at each locality indicated by an arrow. Principal localities in mature forest are underlined.
Most native species were broadly distributed across the survey gradient (Table 1; Figure 4), and all but one (Musseromys inopinatus) were captured ten or more times. Seven of the 13 native species [Crocidura grayi Dobson, 1890, Apomys datae (Meyer, 1899), Apomys musculus Miller, 1911, Archboldomys maximus, Bullimus luzonicus Thomas, 1895,Chrotomys silaceus (Thomas, 1895), and Chrotomys whiteheadi Thomas, 1895] were trapped or inferred to occur at all of the principal localities in forest habitat from 1650 m to the summit of Mt. Amuyao (2690 m). Documented ranges of three other species (Batomys granti Thomas, 1895,Rattus everetti GÜnther, 1897, and Soricomys montanus) were nearly as broad, and even Musseromys inopinatus, with only five captures, was recorded at four localities between 1650 and 2300 m elevation. Rhynchomys soricoides was not recorded below ca. 2000 m, whereas Apomys abrae, which had the narrowest documented range, was not recorded above 1800 m. The two non-native species [Rattus exulans (Peale, 1848) and Rattus tanezumi (Temminck, 1845)] were recorded only at 1800 m or lower (Figure 4). Across all localities, native species richness (the number of species documented or inferred to occur at a given locality) ranged from 1 to 13 (Table 1). At the eight principal sampling localities in relatively undisturbed mature forest (1650 m to 2690 m), species richness ranged between nine and 13 and was not correlated with elevation (rs =-0.217; p>0.2).
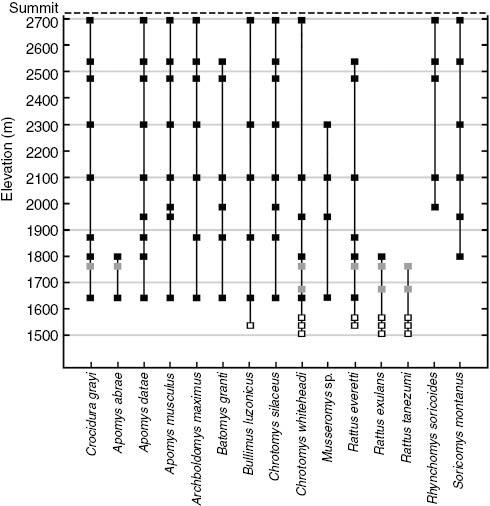
Distributions of small mammals along the Mt. Amuyao survey gradient. Black squares denote localities in mature forest, gray squares localities in secondary pine forest, and open squares localities in deforested second-growth habitat.
Habitat disturbance had a major impact on the occurrence patterns of individual species. The two non-native species (Rattus exulans and Rattus tanezumi) were common or abundant below 1600 m at localities with the most disturbed habitat (cropland and early second growth), and also at two localities in secondary pine forest (1675 and 1760 m) where undergrowth was periodically burned to maintain herbaceous vegetation. Aside from one individual of R. exulans trapped at 1800 m in remnant montane forest near secondary pine forest, the non-native species were not documented in mature forest (Table 1, Figure 4). Among the 13 native species, five were found at disturbed localities outside of forest. Chrotomys whiteheadi had the greatest association with disturbed habitat; it was documented at four of the five disturbed localities accounting for 11 of 23 total captures (48%) for this species. Rattus everetti was recorded at two disturbed localities where six out of 26 captures (23%) occurred. Apomys abrae was common in pine secondary forest, accounting for five out of 42 captures (12%). Two other native species, Bullimus bagobus and Crocidura grayi, had singleton captures in disturbed habitat. Captures for the remaining eight native species were restricted to localities in mature forest (Table 1, Figure 4).
Trap success across the sampling gradient differed for the two types of bait (Table 2). At three of the eight principal localities in mature forest (1650 m, 2480 m, and 2530 m) and for all localities combined, capture rates were significantly greater for ground traps baited with earthworms compared to those with coconut. Trap success with either bait was not significantly correlated with elevation, nor were measures of overall trap success, either weighted by bait type (percent success for all trap nights) or unweighted (mean success for the two baits; Table 2).
Captures of native small mammals along the elevation gradient on Mt. Amuyao by bait type, trap position, and diel period.
| Locality elevation (m) | Total | rs (p)a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1510 | 1530 | 1535 | 1650 | 1675 | 1760 | 1800 | 1885 | 1950 | 1990 | 2100 | 2300 | 2480 | 2530 | 2690 | |||
| Ground traps (coconut) | |||||||||||||||||
| Trap-nights | 50 | 63 | 199 | 737 | 64 | 353 | 143 | 344 | 25 | 82 | 767 | 347 | 222 | 631 | 344 | 4371 | |
| Captures | 4 | 0 | 5 | 26 | 0 | 6 | 14 | 30 | 2 | 4 | 49 | 18 | 7 | 27 | 33 | 225 | |
| Captures/100 trap-nights | 3.53 | 9.79 | 8.72 | 6.39 | 5.19 | 3.15 | 4.28 | 9.59 | 5.15 | -0.07 (>0.2) | |||||||
| Ground traps (earthworm) | |||||||||||||||||
| Trap-nights | 0 | 12 | 48 | 595 | 93 | 115 | 185 | 187 | 50 | 39 | 610 | 458 | 325 | 505 | 504 | 3726 | |
| Captures | 0 | 2 | 35 | 4 | 3 | 18 | 9 | 5 | 3 | 55 | 28 | 39 | 40 | 59 | 300 | ||
| Captures/100 trap-nights | 5.88 | 9.73 | 4.81 | 9.02 | 6.11 | 12.00 | 7.92 | 11.71 | 8.05 | 0.524 (>0.1) | |||||||
| X2 | 3.827 | 0.001 | 2.389 | 3.302 | 0.287 | 12.718 | 6.677 | 0.993 | 26.246 | ||||||||
| p | 0.050 | 0.972 | 0.122 | 0.069 | 0.592 | 0.001 | 0.010 | 0.319 | 0.001 | ||||||||
| Arboreal traps (coconut) | |||||||||||||||||
| Trap-nights | 0 | 0 | 0 | 938 | 0 | 4 | 129 | 38 | 31 | 8 | 1663 | 417 | 78 | 1334 | 282 | 4922 | |
| Captures | 19 | 0 | 0 | 1 | 2 | 0 | 25 | 5 | 1 | 13 | 1 | 67 | |||||
| Captures/100 trap-nights | 2.03 | 0 | 2.63 | 1.50 | 1.20 | 1.28 | 0.97 | 0.35 | 1.36 | -0.43 (>0.2) | |||||||
| Overall trap success | |||||||||||||||||
| Weighted | 4.58 | 9.76 | 7.34 | 7.55 | 7.71 | 8.41 | 5.90 | 10.85 | 6.48 | 0.405 (>0.2) | |||||||
| Unweighted | 4.71 | 9.76 | 6.77 | 7.70 | 5.65 | 7.58 | 6.10 | 10.65 | 6.60 | 0.286 (>0.2) | |||||||
| Diel period | |||||||||||||||||
| Diurnal | 0 | 0 | 0 | 6 | 0 | 0 | 1 | 3 | 1 | 0 | 10 | 4 | 4 | 12 | 16 | 57 | |
| Nocturnal/crepuscular | 4 | 0 | 7 | 74 | 4 | 9 | 31 | 37 | 9 | 7 | 120 | 48 | 47 | 66 | 93 | 556 | |
| Percent diurnal | 7.5 | 3.1 | 7.5 | 7.7 | 7.7 | 7.8 | 15.4 | 14.7 | 10.3 | 0.928 (<0.01) | |||||||
Principal trapping localities in mature forest are shown in italic. Capture rates calculated for principal localities only. Capture frequencies greater than expected (p<0.05) are shown in bold.
aSpearman’s coefficient, capture variables vs. elevation.
Species differed in their responses to the two types of bait (Table 3). Earthworms were significantly more effective than coconut in capturing four species (Apomys datae, Crocidura grayi, Chrotomys silaceus, and Soricomys montanus), with two others (Archboldomys maximus and Rhynchomys soricoides) approaching significance. For three species (Apomys musculus, Bullimus luzonicus, and Rattus tanezumi), coconut bait was significantly more effective, and approached significance for two others (Batomys granti and Rattus everetti). The remaining species showed no significant differences in bait preference reflecting more omnivorous food habits (e.g. Apomys abrae and Rattus exulans), frequent captures in traps placed in confined runways or at burrow openings that were unrelated to bait attractiveness (Chrotomys whiteheadi), or insufficient number of captures to detect patterns.
Captures of small mammal species on Mt Amuyao by trap position, bait type, and diel period.
| Species | Bait typea | Diel period | Trap postion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coconut | Earthworm | X2 | p-Value | Nocturnal-crepuscular | Diurnal | X2 | p-Value | Ground surface | Above ground | X2 | p-Value | |
| Crocidura grayi | 11 (0.12) | 46 (1.25) | 74.230 | <0.001 | 24 | 33 | 5.967 | 0.015 | 52 | 5 | 21.827 | <0.001 |
| Apomys abrae | 25 (1.06) | 17 (1.72) | 2.662 | 0.130 | 42 | 0 | 30.295 | <0.001 | 40 | 2 | <0.001 | |
| Apomys datae | 136 (1.51) | 158 (4.31) | 87.287 | <0.001 | 289 | 5 | 196.062 | <0.001 | 276 | 18 | <0.001 | |
| Apomys musculus | 31 (0.63) | 1 (0.03) | <0.001 | 31 | 1 | <0.001 | 1 | 31 | <0.001 | |||
| Archboldomys maximus | 6 (0.15) | 9 (0.25) | 0.124 | 4 | 11 | 0.011 | 15 | 0 | <0.001 | |||
| Batomys granti | 11 (0.13) | 3 (0.09) | 0.160 | 14 | 0 | <0.001 | 12 | 2 | 0.138 | |||
| Bullimus luzonicus | 15 (0.35) | 1 (0.03) | <0.001 | 15 | 1 | 0.002 | 16 | 0 | 0.007 | |||
| Chrotomys silaceus | 17 (0.42) | 29 (0.79) | 4.779 | 0.029 | 45 | 1 | 29.895 | <0.001 | 46 | 0 | <0.001 | |
| Chrotomys whiteheadi | 11 (0.25) | 12 (0.32) | 0.449 | 0.503 | 22 | 1 | 13.494 | <0.001 | 23 | 0 | <0.001 | |
| Musseromys inopinatus | 5 (0.15) | 0c | 4 | 1 | 0.238 | 0 | 5 | 0.008 | ||||
| Rattus everetti | 21 (0.24) | 5 (0.16) | 0.057 | 26 | 0 | <0.001 | 24 | 2 | <0.01 | |||
| Rattus exulansb | 29 (1.80) | 22 (2.10) | 0.363 | 0.547 | 49 | 2 | 30.302 | <0.001 | 51 | 0 | <0.001 | |
| Rattus tanezumib | 19 (1.30) | 2 (0.23) | 0.004 | 17 | 4 | 0.018 | 21 | 0 | <0.001 | |||
| Rhynchomys soricoides | 4 (0.17) | 9 (0.37) | 0.087 | 13 | 0 | <0.001 | 13 | 0 | 0.007 | |||
| Soricomys montanus | 4 (0.06) | 6 (0.21) | 0.032 | 0 | 10 | <0.001 | 9 | 1 | 0.036 | |||
Capture frequencies significantly greater than expected (p<0.05) are shown in bold.
aNumber of individuals (percent trap success in parentheses).
bNon-native species.
cNo arboreal traps baited with earthworms.
Eleven species (Apomys abrae, Apomys datae, Apomys musculus, Batomys granti, Bullimus luzonicus, Chrotomys silaceus, Chrotomys whiteheadi, Rattus everetti, Rattus exulans, Rattus tanezumi,and Rhynchomys soricoides) had significantly greater activity during the nocturnal/crepuscular period, whereas three species (Crocidura grayi, Archboldomys maximusand Soricomys montanus) were significantly diurnal. All but one of the five specimens of Musseromys inopinatus were captured at night, but the sample was too small to detect a significant diel pattern (Table 3). For the eight principal survey localities in mature forest, the percentage of diurnal captures for native species was positively correlated with elevation, principally due to a nearly two-fold increase at the two uppermost localities (Table 2).
Capture frequencies by trap position (ground surface vs. arboreal) revealed differences in the climbing ability and habitat use of species (Table 3). Eight species (Crocidura grayi, Apomys abrae, Apomys datae, Archboldomys maximus, Bullimus luzonicus, Chrotomys silaceus, Chrotomys whiteheadi, and Soricomys montanus) were captured on the ground more frequently than expected, indicating primary activity on or beneath the ground surface and infrequent climbing. In contrast, two species (Apomys musculus and Musseromys inopinatus) had significantly more arboreal captures, indicating that they are adept climbers that are primarily (if not exclusively) arboreal. The remaining species did not show significant differences in capture frequency; Batomys granti and Rattus everetti were captured more frequently on the ground, but arboreal captures indicated climbing abilities in both species. Rattus exulans and Rattus tanezumi were captured only on the ground, but this is inconclusive because both of these non-native species occurred at localities in disturbed habitats where trees were scarce and we did little or no arboreal trapping. Arboreal trapping effort was uneven across the eight principal localities in mature forest, but there was an overall trend of decreased arboreal trap success with elevation; for the five localities with more than 250 arboreal trap-nights (Table 2) there was a perfect inverse correlation of trap success with elevation, indicating a highly significant decrease in arboreal captures with increasing elevation (rs =-1; p<0.01).
Large mammals
In 2007 we obtained specimens of two large arboreal murid rodents from local hunters. Three specimens of the slender-tailed giant cloud rat, Phloeomys pallidus Nehring, 1890, were taken at undetermined locations on the north-facing slope of Mt. Amuyao. One was shot in mid-January in an area of montane forest with oak, pine, bamboo, and climbing pandan at ca. 2200 m. Two others were shot at night on 19 and 20 February in montane forest on the north slope of Mt. Amuyao, one from ca. 12 m up in a large tree (ca. 60 cm dbh). On 24 February, one specimen of the bushy-tailed cloud rat, Crateromys schadenbergi (Meyer, 1895), taken approximately 4 km W of Barlig, was shot at night from a tree ca. 5 m above the ground. Both species of cloud rats are endemic to Luzon. C. schadenbergi is restricted to the Central Cordillera where it occurs in pine forest and mossy forest from 2000 to 2740 m elevation; P. pallidus is more broadly distributed in central and northern Luzon with an elevational range from sea level to ca. 2300 m (Heaney et al. 2010, 2016, unpublished FMNH specimen records). Both species were reported to be relatively common on Mt. Amuyao.
According to local hunters, long-tailed macaques, Macaca fascicularis (Raffles, 1821), were uncommon in montane forest and absent from higher elevations. Philippine warty pigs, Sus philippensis Nehring, 1886, and Philippine deer, Cervus mariannus Desmarest, 1822, were said to be uncommon, generally in areas dominated by oaks. In 2011, we observed diggings made by pigs in montane forest at 1650 and 2100 m elevation. There are no museum specimen records for these three species from Mountain Province (Heaney et al. 2010). The common palm civet, Paradoxurus hermaphroditus (Pallas, 1777), was the only carnivore reported by local residents and we found signs of their presence in 2011; there is a specimen from Sagada, ca. 25 km west of Mt. Amuyao (Heaney et al. 2010).
Discussion
Mt. Amuyao and the fauna of the Central Cordillera
Species accumulation curves and the range-through assumption of continuous distribution suggest that further sampling likely would have added species at several of our sampling localities. However, the accumulation curve for the combined localities (Figure 3) indicates that sampling was effective in documenting most, if not all, native species of rodents and shrews on Mt. Amuyao. Trapping revealed a rich assemblage of 13 native small mammals including three previously unknown, two of which (Archboldomys maximus and Musseromys inopinatus) currently are known only from Mt. Amuyao. Although either or both may be locally endemic, additional surveys over sufficiently broad elevational gradients within the Cordillera are necessary to test this supposition. Nocturnal spotlighting surveys would be particularly useful in documenting the occurrence of arboreal species.
With a total of 15 species (1 shrew and 14 rodents, including the large arboreal murids, Crateromys schadenbergi and Phloeomys pallidus), Mt. Amuyao supports one of the richest native small mammal assemblages on Luzon. In comparison, we found 14 species at Balbalasang (Rickart et al. 2011b), and on Mt. Data, combined historical and recent surveys documented 17 native species (Thomas 1898, Sanborn 1952, Field Museum unpublished records). Seven native rodents that are endemic to Luzon have been recorded elsewhere within the Central Cordillera but were not documented on Mt. Amuyao: Abditomys latidens (Sanborn, 1952), Apomys microdon Hollister, 1913, Batomys dentatus Miller, 1911, Carpomys melanurus Thomas, 1895, Carpomys phaeurus Thomas, 1895, Soricomys kalinga (Balete et al., 2006), and Tryphomys adustus Miller, 1910 (Heaney et al. 2010, Balete et al. 2012). These “missing” species include some that may be restricted to other portions of the Central Cordillera (e.g. B. dentatus and S. kalinga). Others may only occur at elevations below our lowest sampling locality (e.g. A. microdon). Some are arboreal species that are particularly difficult to capture and thus may have eluded detection (Carpomys spp.). Many of these species are simply very poorly known; this is a clear indication of the need for additional intensive faunal surveys in the Cordillera.
Elevation and species richness
At 2702 m, Mt. Amuyao is one of the highest mountains on Luzon. Our previous surveys revealed a clear pattern for native small mammals in which total species richness increases significantly with peak elevation (Rickart et al. 2011b, Heaney et al. 2013a,c). Mt. Amuyao is nearly 500 m higher than Mt. Bali-it, which at 2238 m was the highest point surveyed on the Balbalasang transect. Although we would predict greater species richness on Amuyao compared to Bali-it, we recorded 13 small mammal species on both mountains. However, the lower end of the Mt. Amuyao sampling gradient was at a higher elevation, so its actual extent was ca. 100 m less than that of Balbalasang (1197 vs. 1313 m, respectively). Equally important, the Amuyao transect did not include elevations below 1500 m (and no localities in mature forest below 1650 m). It is possible that other species, including Apomys microdon (discussed above), are present at lower elevations near Mt. Amuyao; this can be ascertained through additional field work.
Many previous studies of small mammals (e.g. Heaney 2001, McCain 2005, Rowe 2009) have noted curvilinear, “hump-shaped” patterns of species richness across local elevational gradients. However, this is only the case where sampling gradients reflect the full elevational range within a region. For both Mt. Bali-it and Mt. Amuyao, the local gradients we were able to survey were incomplete; the Mt. Bali-it gradient did not include sufficient high elevation habitat, and there were no forested localities available at lower elevations on Mt. Amuyao. However, when combined (Figure 5), the two gradients describe a composite hump-shaped pattern of elevation and species richness, from a low of five native species at ca. 1050 m on Mt. Bali-it, increasing to peak values of 12 species at localities between 1800 and 2300 m on Mt. Amuyao, and declining to nine species at 2690 m elevation near the summit of Mt. Amuyao. Furthermore, eight of the 13 species that occur above 1800 m on Mt. Amuyao are endemic to the Central Cordillera, and most of these are either restricted to, or achieve highest densities in, high elevation habitat (Table 1). When compared to the species richness-elevation curves for Luzon mountains we have surveyed outside of the Cordillera (Rickart et al. 2011b, Heaney et al. 2013a, 2013c), it is clear that the higher mid-elevation peak for the composite cordilleran curve is due entirely to the presence of these high-elevation endemic species.
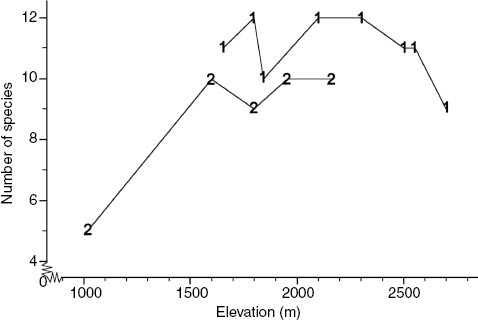
Species richness of native small mammals (insectivores and rodents) captured or inferred to occur at survey localities along elevational gradients on 1) Mt. Amuyao (this study) and 2) Mt. Bali-it (Rickart et al. 2011b).
Community structure
The mammals of Mt. Amuyao exhibit great morphological and ecological diversity. Among the murid rodents, the range in body size spans more than two orders of magnitude from Musseromys inopinatus (15–20 g) to Phloeomys pallidus (up to 2.6 kg). Species vary in trophic habits, spatial habitat use, temporal activity patterns, and adaptations to habitat disturbance. The native community is dominated by narrowly endemic species that are restricted to the Central Cordillera region, many of which are ecological specialists.
Remarkably, most of this diversity (12 of the 15 native species) is contained within just two of the “old endemic” clades of Philippine murid rodents (Musser and Heaney 1992). Four species (Batomys granti, Crateromys schadenbergi, Musseromys inopinatus, and Phloeomys pallidus) belong to the arboreal cloud rat clade that is the sister group to the rest of the subfamily Murinae (Jansa et al. 2006, Heaney et al. 2009, Schenk et al. 2013). Eight others (Apomys abrae, Apomys datae, Apomys musculus, Archboldomys maximus, Chrotomys silaceus, Chrotomys whiteheadi, Rhynchomys soricoides, and Soricomys montanus) are members of the “earthworm mouse” clade (Steppan et al. 2003, Jansa et al. 2006). The remaining two native species (Bullimus luzonicus and Rattus everetti) are derived from much more recent colonization of the Philippines and are members of a clade widespread in Indo-Australia that includes the non-native commensal species Rattus exulans and Rattus tanezumi (Jansa et al. 2006, Schenk et al. 2013). The phylogenetic structure of the Mt. Amuyao fauna reflects the infrequency of colonization from outside the oceanic Philippines, the great age of the Cordillera region, the early colonization of the region by murid rodents during the Miocene, and the overriding importance of autochthonous diversification in the formation of the Luzon fauna (Jansa et al. 2006, Heaney et al. 2011, 2014a, 2014b, 2016, Justiniano et al. 2014).
Conservation
Mt. Amuyao supports an ecologically diverse mammalian fauna that includes two species that may be restricted to the mountain, and several others that occur only in the Central Cordillera. Small mammal communities in forest habitats consist solely of native species, and natives are numerically dominant even in disturbed forest. Some native species appear to be most abundant where habitat is moderately disturbed and a few are common in highly disturbed habitats including deforested agricultural areas. In contrast, non-native species rarely occur in forest, are abundant only where habitat is severely disturbed, and appear to be displaced by native species where habitat is actively regenerating. Results demonstrate that native small mammal communities are resistant to invasion by alien species, can tolerate substantial habitat disturbance, and can recover from severe disturbance where habitat is allowed to regenerate. These findings call into question the general perception that highly endemic insular faunas are uniformly susceptible to disruption from disturbance and invasive species (Rickart et al. 2011a, 2013).
Although natural habitat on Mt. Amuyao is extensive and appear to be stable, the mountain has no protected status. Local communities have long traditions of sustainable use of forest products and wildlife, however, regional population growth and economic development have brought changes that may have negative impacts. The construction of telecommunications facilities on the mountain summit has provided greater access to the peak, which is now a popular destination for increasing numbers of trekkers and ecotourists. Without greater protection, further development may have dire consequences. Mt. Data National Park, where the expansion of commercial vegetable production has destroyed all but a tiny remnant of natural forest, represents a worst-case scenario (Heaney et al. 2006). Given the anticipated effects of climate change on the distribution of montane habitat in the tropics (Foster 2001) and uncertainty in how species may respond in a topographically complex region (Elsen and Tingley 2015), further loss of forest on Mt. Amuyao and elsewhere in the Central Cordillera would only augment the negative impacts on this unique regional fauna.
Acknowledgments
Our project was approved by the National Commission on Indigenous Peoples (NCIP), with thanks to L Carante-Gallardo, B Masweng, R Alawas, A Olsim, V Sal-ly, WK Kalangeg, and F Falinchao. Research permits were issued by the Philippine Department of Environment and Natural Resources (DENR), and we particular thank S Penafiel, TM Lim, J DeLeon, A Tagtag, C Custodio, and M Mendoza. We thank C Fiasnilon and J Away of Barlig, and local residents who served as guides, porters, and camp assistants. We thank N Antoque, T Atiwag, R Away, A Ayuga, J Barcelona, R Buenviaje, S Legasi, R Ngaya, R Plutado, P Puchana, J Riddell, A Reginaldo, and particularly J Sarmiento for assistance with fieldwork. For assistance at the Field Museum we thank T DeCoster, J Phelps, A Niedzielski, and WT Stanley. Funding was provided by the Negaunee Foundation, Grainger Foundation, and the Barbara Brown, Ellen Thorne Smith, and Marshall Field funds of the Field Museum. Research was conducted in accordance with all relevant laws and regulations of the Philippines.
References
Alviola, P.A., M.R.M. Duya, M.V. Duya, L.R. Heaney and E.A. Rickart. 2011. Mammalian diversity patterns on Mount Palali, Caraballo Mountains, Luzon. Fieldiana: Life Earth Sci. 2: 61–74.10.3158/2158-5520-2.1.61Search in Google Scholar
Balete, D.S., L.R Heaney, M.J. Veluz and E.A. Rickart. 2009. Diversity patterns of small mammals in the Zambales Mts., Luzon, Philippines. Mamm. Biol. 74: 456–466.10.1016/j.mambio.2008.05.006Search in Google Scholar
Balete, D.S., P.A. Alviola, M.R.M. Duya, M.V. Duya, L.R. Heaney and E.A. Rickart. 2011. The mammals of the Mingan Mountains, Luzon: evidence for a new center of mammalian endemism. Fieldiana: Life Earth Sci. 2: 75–87.10.3158/2158-5520-2.1.75Search in Google Scholar
Balete, D.S., E.A. Rickart, L.R. Heaney, P.A. Alviola, M.V. Duya, M.R.M. Duya, T. Sosa and S. Jansa. 2012. Archboldomys (Muridae: Murinae) reconsidered: a new genus and three new species of shrew-mice from Luzon Island, Philippines. Amer. Mus. Nov. 3754: 1–60.10.1206/3754.2Search in Google Scholar
Balete, D.S., L.R. Heaney, P.A. Alviola and E.A. Rickart. 2013a. Diversity and distribution of small mammals in the Bicol Volcanic Belt of southern Luzon Island, Philippines. Nat. Mus. Phil.: J. Nat. Hist. 1: 67–93.Search in Google Scholar
Balete, D.S., L.R. Heaney and E.A. Rickart. 2013b. The mammals of Mt. Irid, southern Sierra Madre, Luzon Island, Philippines. Nat. Mus. Phil.: J. Nat. Hist. 1: 17–31.Search in Google Scholar
Balete, D.S., E.A. Rickart, L.R. Heaney and S.A. Jansa. 2015. A new species of Batomys from southern Luzon Island, Philippines. Proc. Biol. Soc. Washington 128: 22–39.10.2988/0006-324X-128.1.22Search in Google Scholar
Duya, M.R.M., M.V. Duya, P.A. Alviola, D.S. Balete and L.R. Heaney. 2011. Diversity of small mammals in montane and mossy forests on Mount Cetaceo, Cagayan Province, Luzon. Fieldiana: Life Earth Sci. 2: 88–95.10.3158/2158-5520-2.1.88Search in Google Scholar
Elsen, P.R. and M.W. Tingley. 2015. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Change 5: 772–776.10.1038/nclimate2656Search in Google Scholar
Foster, P. 2001. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Sci. Rev. 55: 73–106.10.1016/S0012-8252(01)00056-3Search in Google Scholar
Hall, R. 2002. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. J. Asian Earth Sci. 20: 353–431.10.1016/S1367-9120(01)00069-4Search in Google Scholar
Hall, R. 2012. Late Jurassic–Cenozoic reconstructions of the Indonesian region and the Indian Ocean. Tectonophysics 570–571: 1–41.10.1016/j.tecto.2012.04.021Search in Google Scholar
Heaney, L.R. 2001. Small mammal diversity along elevational gradients in the Philippines: an assessment of patterns and hypotheses. Global Ecol. Biogeogr. 10: 15–39.10.1046/j.1466-822x.2001.00227.xSearch in Google Scholar
Heaney, L.R., P.D. Heideman, E.A. Rickart, R.B. Utzurrum and J.S.H. Klompen. 1989. Elevational zonation of mammals in the central Philippines. J. Trop. Ecol. 5: 259–280.10.1017/S0266467400003643Search in Google Scholar
Heaney, L.R., D.S. Balete, E.A. Rickart, R.C.B. Utzurrum and P.C. Gonzales. 1999. Mammalian diversity on Mt. Isarog, a threatened center of endemism on southern Luzon Island, Philippines. Fieldiana: Zool. ns 95: 1–62.Search in Google Scholar
Heaney, L.R., D.S. Balete, J. Sarmiento and P.A. Alviola. 2006. Losing diversity and courting disaster: The mammals of Mt. Data National Park. Haring Ibon 25: 15–23.Search in Google Scholar
Heaney, L.R., D.S. Balete, E.A. Rickart, M.J. Veluz and S.A. Jansa. 2009. A new genus and species of small “tree mouse” (Rodentia, Muridae) related to the Philippine giant cloud-rats. Bull. Amer. Mus. Nat. Hist. 331: 205–229.10.1206/582-7.1Search in Google Scholar
Heaney, L.R., M.L. Dolar, D.S. Balete, J.A. Esselstyn, E.A. Rickart and J.L. Sedlock. 2010. Synopsis of Philippine Mammals. Available from http://www.fieldmuseum.org/philippine_mammals/.Search in Google Scholar
Heaney, L.R, D.S. Balete, E.A. Rickart, P.A. Alviola, M.R.M. Duya, M.V. Duya, M.J. Veluz, L. VandeVrede and S. Steppan. 2011. Seven new species and a new subgenus of forest mice (Rodentia: Muridae: Apomys) from Luzon Island. Fieldiana: Life Earth Sci. 2: 1–60.10.3158/2158-5520-2.1.1Search in Google Scholar
Heaney, L.R., D.S. Balete, P.A. Alviola, E.A. Rickart and M. Ruedi. 2012. Nyctalus plancyi and Falsistrellus petersi (Chiroptera: Vespertilionidae) from northern Luzon, Philippines: ecology, phylogeny, and biogeographic implications. Acta Chiropterol. 14: 265–278.10.3161/150811012X661602Search in Google Scholar
Heaney, L.R., D.S. Balete, P.A. Alviola, M.R.M. Duya and E.A. Rickart. 2013a. The mammals of Mt. Anacuao, NE Luzon Island, Philippines: a test of predictions of Luzon small mammal biodiversity patterns. Nat. Mus. Phil.: J. Nat. Hist. 1: 3–15.Search in Google Scholar
Heaney, L.R., D.S. Balete and E.A. Rickart. 2013b. Models of oceanic island biogeography: changing perspectives on biodiversity dynamics in archipelagoes. Front. Biogeogr. 5: 249–257.10.21425/F5FBG18991Search in Google Scholar
Heaney, L.R., D.S. Balete, R.G.B. Rosell-Ambal, M.J. Veluz and E.A. Rickart. 2013c. The small mammals of Mt. Banahaw – San Cristobal National Park, Luzon, Philippines: elevational distribution and ecology of a highly endemic fauna. Nat. Mus. Phil.: J. Nat. Hist. 1: 49–64.Search in Google Scholar
Heaney, L.R., D.S. Balete, E.A. Rickart, M.J. Veluz and S.A. Jansa. 2014a. Three new species of Musseromys (Muridae, Rodentia), the endemic Philippine tree mouse from Luzon Island. Amer. Mus. Novit. 3802: 1–27.10.1206/3802.1Search in Google Scholar
Heaney, L.R., D.S. Balete, M.J. Veluz, S.J. Steppan, J.A. Esselstyn, A. Pfeiffer and E.A. Rickart. 2014b. Two new species of Philippine forest mice (Apomys, Muridae, Rodentia) from Lubang and Luzon islands, with a redescription of Apomys sacobianus Johnson, 1962. Proc. Biol. Soc. Washington 126: 395–413.10.2988/0006-324X-126.4.395Search in Google Scholar
Heaney, L.R., D.S. Balete and E.A. Rickart. 2016. The mammals of Luzon Island: biogeography and natural history of a Philippine Fauna. Johns Hopkins University Press, Baltimore.10.1353/book.44856Search in Google Scholar
Hollings, P., R. Wolfe, D.R. Cooke and P.J. Waters. 2011. Geochemistry of Tertiary igneous rocks of northern Luzon, Philippines: evidence for a back-arc setting for alkalic porphyry copper-gold deposits and a case for slab roll-back? Econ. Geol. 106: 1257–1277.10.2113/econgeo.106.8.1257Search in Google Scholar
Jansa, S., K. Barker and L.R. Heaney. 2006. The pattern and timing of diversification of Philippine endemic rodents: evidence from mitochondrial and nuclear gene sequences. Syst. Biol. 55: 73–88.10.1080/10635150500431254Search in Google Scholar PubMed
Justiniano, R., J.J. Schenk, D.S. Balete, E.A. Rickart, J.A. Esselstyn, L.R. Heaney and S.J. Steppan. 2014. Testing diversification models of endemic Philippine forest mice (Apomys) with nuclear phylogenies across elevational gradients reveals repeated colonization of isolated mountain ranges. J. Biogeogr. 42: 51–64.10.1111/jbi.12401Search in Google Scholar
McCain, C.M. 2005. Elevational gradients in diversity of small mammals. Ecology 86: 336–372.10.1890/03-3147Search in Google Scholar
Musser, G.G. and L.R. Heaney. 1992. Philippine rodents: definitions of Tarsomys and Limnomys plus a preliminary assessment of phylogenetic patterns among native Philippine murines (Murinae, Muridae). Bull. Amer. Mus. Nat. Hist. 211: 1–138.Search in Google Scholar
Rabor, D.S. 1955. Notes on the mammals and birds of the central northern Luzon highlands, Philippines. Part I: notes on mammals. Silliman J. 2: 193–218.Search in Google Scholar
Rickart, E.A., L.R. Heaney and R.C.B. Utzurrum. 1991. Distribution and ecology of small mammals along an elevational transect in southeastern Luzon, Philippines. J. Mammal. 72: 458–469.10.2307/1382128Search in Google Scholar
Rickart, E.A., D.S. Balete, R.J. Rowe and L.R. Heaney. 2011a. Mammals of the northern Philippines: tolerance for habitat disturbance and resistance to invasive species in an endemic fauna. Diversity Distrib. 17: 530–541.10.1111/j.1472-4642.2011.00758.xSearch in Google Scholar
Rickart, E.A., L.R. Heaney, D.S. Balete and B.R. Tabaranza. 2011b. Small mammal diversity along an elevational gradient in northern Luzon, Philippines. Mamm. Biol. 76: 12–21.10.1016/j.mambio.2010.01.006Search in Google Scholar
Rickart, E.A., L.R. Heaney, D.S. Balete, P.A. Alviola, M.R.M. Duya, M.V. Duya, G. Rosell-Ambal and J.L. Sedlock. 2013. Mammals of Mt. Natib, Bataan Province, Luzon, Philippines. Nat. Mus. Phil.: J. Nat. Hist. 1: 33–46.Search in Google Scholar
Ringenbach, J.C., J.F. Stephan, P. Maleterr and H. Bellon. 1990. Structure and geological history of the Lepanto-Cervantes releasing bend on the Abra River Fault, Luzon Central Cordillera, Philippines. Tectonophysics 183: 225–241.10.1016/0040-1951(90)90418-8Search in Google Scholar
Rowe, R.J. 2009. Environmental and geometric drivers of small mammal diversity along elevational gradients in Utah. Ecography 32: 411–422.10.1111/j.1600-0587.2008.05538.xSearch in Google Scholar
Salcedo, P.V.G. 2001. Floral diversity and vegetation zones of the northern slope of Mt. Amuyao, Mountain Province, Luzon, Philippines. Asia Life Sci. 10: 119–157.Search in Google Scholar
Sanborn, C.C. 1952. Philippine zoological expedition 1946–1947. Mammals. Fieldiana Zool. 33: 89–158.Search in Google Scholar
Schenk, J.J., K.C. Rowe and S.J. Steppan. 2013. Ecological opportunity and incumbency in the diversification of repeated continental colonizations by muroid rodents. Syst. Biol. 62: 837–864.10.1093/sysbio/syt050Search in Google Scholar PubMed
Smith, W.D. 1915. Notes on a geologic reconnaissance of Mountain Province, Luzon, P. I. Phil. J. Sci. 10: 177–209.Search in Google Scholar
Steppan, S.J., C. Zawadski and L.R. Heaney. 2003. Molecular phylogeny of the endemic Philippine rodent Apomys (Muridae) and the dynamics of diversification in an oceanic archipelago. Biol. J. Linn. Soc. 80: 699–715.10.1111/j.1095-8312.2003.00274.xSearch in Google Scholar
Thomas, O. 1898. On the mammals obtained by Mr. John Whitehead during his recent expedition to the Philippines. Trans. Zool. Soc. Lond. 14: 377–412.10.1111/j.1096-3642.1898.tb00062.xSearch in Google Scholar
United Nations Environmental Programme. 2010. Island directory: basic environmental and geographic information on the significant islands of the world. Available from http://islands.unep.ch/isldr.htm.Search in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Original Studies
- The mammals of Mt. Amuyao: a richly endemic fauna in the Central Cordillera of northern Luzon Island, Philippines
- Social organization and demography of reintroduced Dorcas gazelle (Gazella dorcas neglecta) in North Ferlo Fauna Reserve, Senegal
- Reproductive characteristics of the water chevrotain, Hyemoschus aquaticus
- First data on the presence and diet of common genet (Genetta genetta, Linnaeus 1758) in the Ebro Delta (NE Iberian Peninsula)
- Band size, activity pattern and occupancy of the coati Nasua narica (Carnivora, Procyonidae) in the Southeastern Mexican rainforest
- Partial support for the classical ring species hypothesis in the Chaerephon pumilus species complex (Chiroptera: Molossidae) from southeastern Africa and western Indian Ocean islands
- Recording at water bodies increases the efficiency of a survey of temperate bats with stationary, automated detectors
- Effect of wheat (Triticum aestivum L.) seed color and hardness genes on the consumption preference of the house mouse (Mus musculus L.)
- Short Notes
- New records of Isothrix (Wagner 1845) (Rodentia: Echimyidae) from Ecuador
- Notes on the distribution of the genus Andalgalomys (Rodentia, Cricetidae), with the first record of A. pearsoni (Myers 1978) from Argentina
- Reviewer acknowledgement Mammalia volume 80 (2016)
Articles in the same Issue
- Frontmatter
- Original Studies
- The mammals of Mt. Amuyao: a richly endemic fauna in the Central Cordillera of northern Luzon Island, Philippines
- Social organization and demography of reintroduced Dorcas gazelle (Gazella dorcas neglecta) in North Ferlo Fauna Reserve, Senegal
- Reproductive characteristics of the water chevrotain, Hyemoschus aquaticus
- First data on the presence and diet of common genet (Genetta genetta, Linnaeus 1758) in the Ebro Delta (NE Iberian Peninsula)
- Band size, activity pattern and occupancy of the coati Nasua narica (Carnivora, Procyonidae) in the Southeastern Mexican rainforest
- Partial support for the classical ring species hypothesis in the Chaerephon pumilus species complex (Chiroptera: Molossidae) from southeastern Africa and western Indian Ocean islands
- Recording at water bodies increases the efficiency of a survey of temperate bats with stationary, automated detectors
- Effect of wheat (Triticum aestivum L.) seed color and hardness genes on the consumption preference of the house mouse (Mus musculus L.)
- Short Notes
- New records of Isothrix (Wagner 1845) (Rodentia: Echimyidae) from Ecuador
- Notes on the distribution of the genus Andalgalomys (Rodentia, Cricetidae), with the first record of A. pearsoni (Myers 1978) from Argentina
- Reviewer acknowledgement Mammalia volume 80 (2016)

