To the Editor,
This report describes a case of ambiguous pharmacogenetic test results from an oral swab specimen in a patient with previous bone marrow transplantation (BMT). These results indicate chimerism of donor and recipient genotypes in the DNA isolated from the patient’s oral samples. Many laboratories do not accept oral samples for genetic testing of BMT recipients, as chimerism has been shown to be present in these samples [1]. In order to identify the recipient genotype, practice guidelines have suggested the use of fibroblast cultures, which are time-consuming to obtain and require specialized equipment that may not be available in a routine genetic testing laboratory [2]. Hair follicles have recently been used as a source of recipient DNA in one study but chimerism with donor-derived DNA has been observed in another study [2], [3]. In our report, we used trio analysis and donor genotyping of oral samples to infer the recipient genotype in pharmacogenes.
In this case, the patient (male, aged 8) was diagnosed with leukemia at the age of 2 and was treated by an allogeneic bone marrow transplant with his father as the donor. Millennium Health received an oral swab sample from the patient for its polymerase chain reaction (PCR)-based pharmacogenetics test (14 genes: CYP2B6 (*1, *4, *6, *9, *18), CYP2C9 (*1, *2, *3, *5, *8, *11), CYP2C19 (*1, *2, *3, *4, *8, *17), CYP2D6 (*1, *2, *2A, *3, *4, *4N, *5, *6, *9, *10, *17, *29, *35, *36, *41, allele-specific gene duplication), CYP3A4 (*1, *22), CYP3A5 (*1, *3), DRD2 (rs1799732 Ins/Del), HLA-B*15:02, HTR2C (rs3813929C/T), VKORC1 (rs9923231A/G), COMT (rs4680A/G), OPRM1 (rs1799971A/G), MTHFR (rs1801131A/C, rs1801133C/T) and UGT2B15 (*1, *2). The patient’s lab test report indicated that results for nine pharmacogenes were unable to be determined. After the initial test results were inconclusive, the patient was tested twice again. A total of three separate oral samples were collected (the first two were collected using the DNA Genotek (Ottawa, Canada) ORAcollect OC-100 oral swab device and the third sample was saliva collected using the DNA Genotek OrageneDx OGD-510 device). Genotyping was performed using real-time PCR in a multiplex assay. The patient’s test results failed laboratory quality control standards in all three attempts, prompting further investigation upon request from the treating physician. At this time, it was discovered that the patient was a BMT recipient, and a study protocol was developed and received Institutional Review Board (IRB) approval (Aspire IRB, Santee, CA) as per the Declaration of Helsinki. Samples from the mother and father (who was also the BMT donor) were also obtained.
Genotyping of the patient’s (proband) three samples revealed a mix of interpretable and uninterpretable results, consistent with chimerism. Some results clearly aligned with genotype clusters and thus were interpretable, and other results were found outside of genotype clusters, which were uninterpretable (Figure 1A, B). The uninterpretable results from each of the three samples had slightly different positions compared to the genotype clusters, ranging from slightly out-of-cluster to substantially out-of-cluster. Comparison with the father’s genotype revealed that for markers where the genotype of the proband matched that of the father, the proband results had high genotype confidence and were interpretable. For markers where the genotype of the proband mismatched that of the father, the results were shifted outside of the clusters and the genotype confidence was low (Figure 1C). As such, genotyping results were obtained for the proband at COMT, CYP3A4, DRD2, HLA-B*15:02 and MTHFR genes (Table 1). Of these, abnormal predicted phenotypes were found for COMT and MTHFR [4], [5], [6], [7].
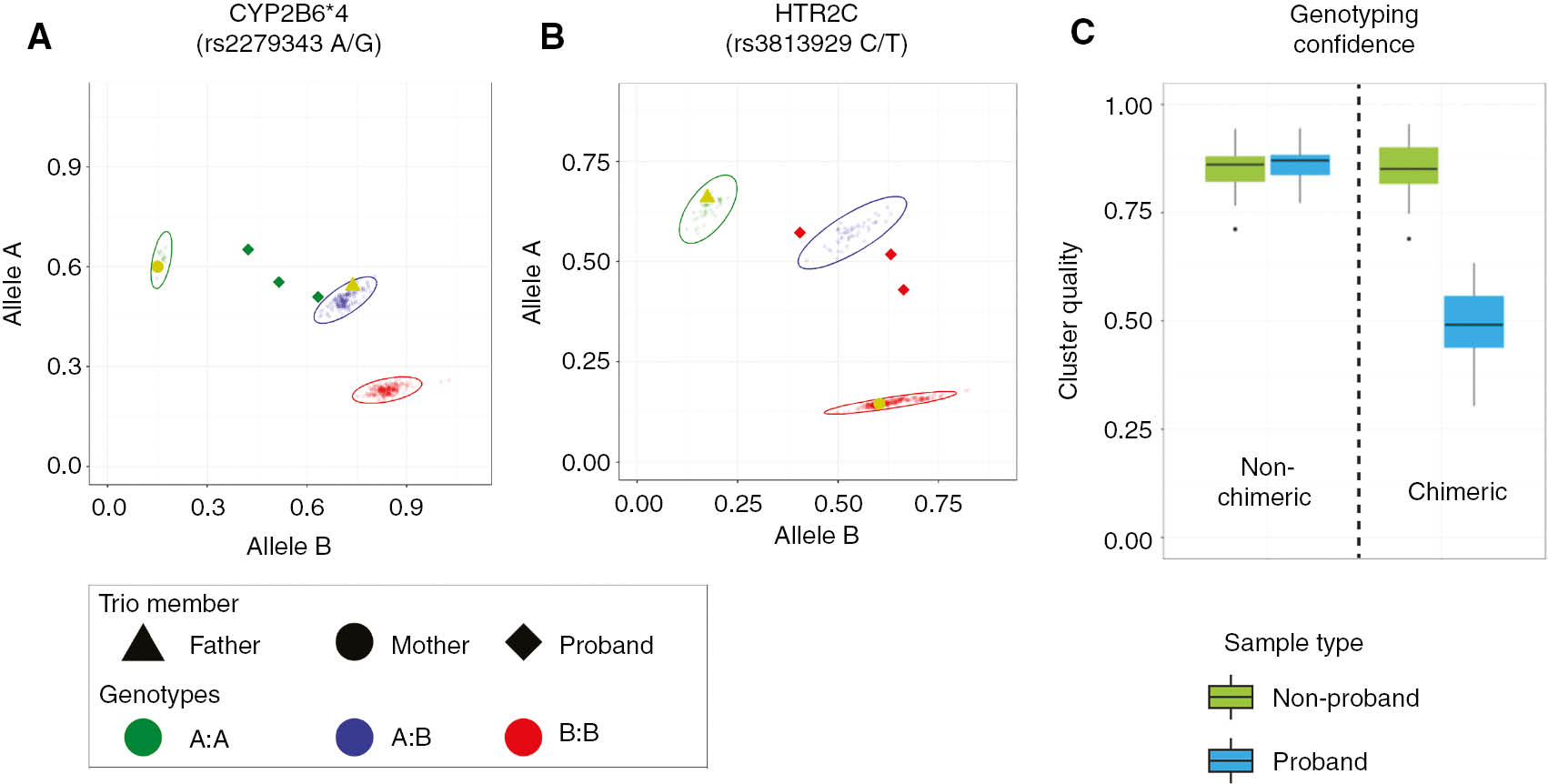
(A, B) Scatter plots for SNP genotyping results with mismatched patient and donor genotypes. Results are shown for each of the two representative markers from five genotyping runs which contained the mother, father (BMT Donor) and three separate patient (proband) samples. All other samples included in the runs are displayed as small semi-transparent circles. Genotypes were assigned based on cluster profiles. The ellipses represent the genotype cluster, within which a genotyping call would be made (points outside the cluster have low confidence). The patient’s signal (diamond) is a consistent outlier from the genotyping clusters, and as such would not be interpreted. The patient’s genotypes at these markers were presumptively assigned by trio analysis as shown in Table 1. (C) Box-and-whisker plot of genotyping quality scores for proband and non-proband samples. Genotyping result quality is shown for markers in which the genotype matched (non-chimeric) or did not match (chimeric) the donor genotype. Proband genotypes are evaluated separately from non-proband genotypes to show the relationship of cluster confidence for patient results when similar or different to parent/donor results. Cluster quality is computed by subtracting the average distance of a point to members of its own cluster from the average distance of a point to members of the next closest cluster and dividing this difference by the larger of the two distances. A score of 1 identifies a correct unambiguous assignment and a score of 0 is interpreted as an arbitrary assignment.
Genotypes and predicted phenotypes.
| Gene | Mother | Father/donor | Proband | |||
|---|---|---|---|---|---|---|
| Genotype | Predicted phenotype | Genotype | Predicted phenotype | Genotype | Predicted phenotype | |
| COMT | A/A | Normal activity | A/G | Reduced activity | A/G | Reduced activity |
| CYP2B6 | *6/*6 | Poor metabolizer | *1/*4 | Normal metabolizer | *4/*6 | Intermediate metabolizer |
| CYP2C19 | *1/*17 | Ultrarapid metabolizer | *1/*1 | Normal metabolizer | *1/*17 | Ultrarapid metabolizer |
| CYP2C9 | *1/*1 | Normal metabolizer | *1/*3 | Intermediate metabolizer | *1/*1 | Normal metabolizer |
| CYP2D6 | *1/*35 | Normal metabolizer | *1/*5 | Normal metabolizer | *5/*35 | Normal metabolizer |
| CYP3A4; CYP3A5 | *1/*1; *3/*3 | Intermediate metabolizer | *1/*1; *1/*3 | Extensive metabolizer | *1/*1; *3/*3 | Intermediate metabolizer |
| DRD2 | C/C | Normal responder | C/C | Normal responder | C/C | Normal responder |
| HLA-B*15:02 | Negative | Typical risk of hypersensitivity | Negative | Typical risk of hypersensitivity | Negative | Typical Risk of hypersensitivity |
| HTR2C | C/C | Normal expressor | T/– | Variant expressor | C/– | Normal expressor |
| MTHFR | C/T (C677T); A/A (A1298C) | Reduced activity | T/T (C677T); A/A (A1298C) | Greatly reduced activity | T/T (C677T); A/A (A1298C) | Greatly reduced activity |
| OPRM1 | A/G | Reduced expressor | A/G | Reduced expressor | A/A | Normal expressor |
| UGT2B15 | *1/*1 | Normal metabolizer | *1/*2 | Intermediate metabolizer | *1/*1 | Normal metabolizer |
| VKORC1 | G/G | Normal activity | A/A | Reduced activity | A/G | Reduced activity |
Genotypes and predicted phenotypes were assigned as per standard clinical genotyping based on cluster profiles. Additionally, proband genotypes at HTR2C and VKORC1 were predicted using Mendelian inheritance using parental genotypes. Shaded fields identify presumptive genotypes, which were inferred by the donor genotype as well as the location of the patient’s raw genotyping results relative to other samples within each assay.
With genotypes from both the mother and father, we were able to predict additional proband genotypes at HTR2C and VKORC1 using Mendelian inheritance (Table 1). Finally, using the parent genotypes along with the patient’s raw genotyping results relative to other samples, we were able to infer presumptive genotypes at CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4/CYP3A5, OPRM1 and UGT2B15. Notably, these included genotypes that are associated with increased (ultrarapid) metabolism for CYP2C19 and poor metabolism for CYP2B6 [8]. While these assignments are presumptive, other phenotypes can be ruled out; for example, poor metabolizer phenotype for CYP2C19 can be ruled out based on the parent genotypes.
With regard to clinical relevance, the patient’s response to medications is likely to be impacted by both donor and recipient phenotypes. Many of the genes tested code for medication metabolism enzymes produced by liver hepatocytes. Mature hepatocytes can develop from bone marrow-derived donor cells [9], [10], [11], and the patient’s liver is likely to be a chimeric mix of donor-derived and recipient hepatocytes. Donor hepatocytes have been shown to account for 5% of total hepatocytes as early as 13 days after transplantation [10], and in some cases have been found at higher levels (as much as 40% of total hepatocytes) months after transplantation [9]. When both donor and recipient phenotypes are the same, the clinical relevance is straight-forward. Normal phenotypes were predicted for CYP2D6 for both donor and recipient, indicating that the patient may not experience a genetic influence on CYP2D6-metabolized medications such as codeine or risperidone [8]. When donor and recipient phenotypes are different, then the impact on medication response is less clear but can still be informative; e.g. for CYP2C19, the donor phenotype is normal while the recipient phenotype is ultrarapid metabolizer. As the extent of chimerism in the patient’s liver is unknown, the patient’s ability to metabolize CYP2C19 substrates, such as sertraline [8], cannot be predicted. However, the patient is unlikely to poorly metabolize such medications. With regard to pharmacodynamic genes, both the donor and recipient phenotypes were abnormal at COMT, MTHFR and VKORC1, and thus the patient may not respond to medications associated with those genes [7], [12], [13]. On the other hand, both the donor and recipient had normal responder phenotypes for DRD2, which may impact the patient’s response to antipsychotics [14]. Similarly, the patient does not have the HLA-B*15:02 allele in either donor or recipient genotypes, and thus does not have increased risk of severe hypersensitivity in response to carbamazepine [15].
In conclusion, our results indicate that bone marrow transplants can confound genotyping even when oral samples are used for testing. Our data illustrate that donor cells can be obtained with oral specimens from both oral swab and saliva collections, and can interfere with the proper determination of a patient’s genotype. Unlike the current practice of culturing fibroblasts to obtain patient genotypes, this method incorporates both recipient and donor genotypes. While many laboratories do not accept samples from BMT patients, we have shown that genotyping of the patient along with the donor and parents may allow for identification of test results than can be regarded as conclusive, as well as potentially useful presumptive information about other pharmacogenes. With this process, BMT patients and their doctors may be able to access genetic data that can be used to guide medication selection.
Author contributions: FE: sample processing, data collection and analysis; MD: data analysis and drafting the manuscript; AD: interpretation, drafting and critical revision of the manuscript; TM: interpretation, data analysis and critical revision of the manuscript; JV: conception, design, interpretation, drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: All authors were full-time employees or consultants of Millennium Health, San Diego, CA, USA during the course of this study.
References
1. Thiede C, Prange-Krex G, Freiberg-Richter J, Bornhauser M, Ehninger G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplant 2000;25:575–7.10.1038/sj.bmt.1702170Search in Google Scholar PubMed
2. Skerl P, Krajc M, Blatnik A, Novakovic S. Genetic testing and counseling of a recipient after bone marrow transplant from a sibling harboring a germline BRCA1 pathogenic mutation. Oncol Rep 2017;38:279–82.10.3892/or.2017.5703Search in Google Scholar PubMed
3. Jacewicz R, Lewandowski K, Rupa-Matysek J, Jedrzejczyk M, Brzezinski PM, Dobosz T, et al. Donor-derived DNA in hair follicles of recipients after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:1638–44.10.1038/bmt.2010.27Search in Google Scholar PubMed
4. Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics 2012;13:1719–40.10.2217/pgs.12.152Search in Google Scholar PubMed
5. Rakvag TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, et al. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain 2005;116:73–8.10.1016/j.pain.2005.03.032Search in Google Scholar PubMed
6. Landau R, Liu SK, Blouin JL, Carvalho B. The effect of OPRM1 and COMT genotypes on the analgesic response to intravenous fentanyl labor analgesia. Anesth Analg 2013;116:386–391.10.1213/ANE.0b013e318273f2c7Search in Google Scholar PubMed
7. Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene 2014;533:11–20.10.1016/j.gene.2013.09.063Search in Google Scholar PubMed
8. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009;41:89–295.10.1080/03602530902843483Search in Google Scholar PubMed
9. Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology 2000;32:11–6.10.1053/jhep.2000.9124Search in Google Scholar PubMed
10. Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002;346:738–46.10.1056/NEJMoa3461002Search in Google Scholar PubMed
11. Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422:901–4.10.1038/nature01539Search in Google Scholar PubMed
12. Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther 2017;102:397–404.10.1002/cpt.668Search in Google Scholar PubMed PubMed Central
13. Candiotti KA, Yang Z, Buric D, Arheart K, Zhang Y, Rodriguez Y, et al. Catechol-o-methyltransferase polymorphisms predict opioid consumption in postoperative pain. Anesth Analg 2014;119:1194–200.10.1213/ANE.0000000000000411Search in Google Scholar PubMed
14. Lopez-Rodriguez R, Cabaleiro T, Ochoa D, Roman M, Borobia AM, Carcas AJ, et al. Pharmacodynamic genetic variants related to antipsychotic adverse reactions in healthy volunteers. Pharmacogenomics 2013;14:1203–14.10.2217/pgs.13.106Search in Google Scholar PubMed
15. Leckband SG, Kelsoe JR, Dunnenberger HM, George Jr AL, Tran E, Berger R, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther 2013;94:324–8.10.1038/clpt.2013.103Search in Google Scholar PubMed PubMed Central
Article note
Presented in poster form at the 2018 Pharmacogenomics Research Network – American Society for Human Genetics meeting San Diego CA, October 15–16, 2018.
©2019 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Laboratory Management
- Recommended changes of the current version of the German Rili-BAEK
- Analysis of a 6-year pilot external quality assurance survey of free light chain using Sigma metrics
- Allergy and Autoimmunity
- Different vitamin D status in common multiorgan autoimmune disease patients
- Investigation of the dual cascade algorithm in the diagnosis of antinuclear antibodies
- Neurology Laboratory
- Ischemia-modified albumin (IMA) and dynamic thiol-disulfide homeostasis in patients with postherpetic neuralgia
- Endocrinology
- Effect of hemoglobin F and A2 on hemoglobin A1c determined by cation exchange high-performance liquid chromatography
- Original Article
- A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes
- Short Communication
- Sparing the control arm using well-characterized diagnostic approaches – the Gart and Buck prevalence estimator for efficacy estimation in single-arm trials
- Letter to the Editor
- Ambiguous pharmacogenetic genotyping results in a patient with bone marrow transplantation
Articles in the same Issue
- Frontmatter
- Laboratory Management
- Recommended changes of the current version of the German Rili-BAEK
- Analysis of a 6-year pilot external quality assurance survey of free light chain using Sigma metrics
- Allergy and Autoimmunity
- Different vitamin D status in common multiorgan autoimmune disease patients
- Investigation of the dual cascade algorithm in the diagnosis of antinuclear antibodies
- Neurology Laboratory
- Ischemia-modified albumin (IMA) and dynamic thiol-disulfide homeostasis in patients with postherpetic neuralgia
- Endocrinology
- Effect of hemoglobin F and A2 on hemoglobin A1c determined by cation exchange high-performance liquid chromatography
- Original Article
- A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes
- Short Communication
- Sparing the control arm using well-characterized diagnostic approaches – the Gart and Buck prevalence estimator for efficacy estimation in single-arm trials
- Letter to the Editor
- Ambiguous pharmacogenetic genotyping results in a patient with bone marrow transplantation

