Abstract
Monochorionic placentas occur in two-thirds of identical (monozygotic) twin pregnancies and typically feature inter-fetal vascular anastomoses on their surface. These anastomoses can cause very specific complications including twin-to-twin transfusion syndrome (TTTS) in about 10 %, TAPS in 5 %, twin reversed arterial perfusion (TRAP) sequence in 2.5 % and spontaneous intrauterine fetal death (IUFD) in 2 % of monochorionic-diamniotic twin pregnancies. Moreover, unequal sharing of placental territories can lead to selective fetal growth restriction (sFGR) in about 15 %. Monochorionic multiples affected by such complications are at increased risk of mortality, brain abnormalities and neurodevelopmental disorders. This narrative review provides insight into prenatal detection and management of specific complications of monochorionic twin pregnancies.
Introduction
Monochorionic placentas occur in two-thirds of identical (monozygotic) twin pregnancies and typically feature inter-fetal vascular anastomoses on their surface. [1], [2], [3]. Three types of anastomose are distinguished including arterio-arterial (AA), veno-venous (VV), and arterio-venous (AV) connections. While AA and VV anastomoses represent direct connections between arteries or veins allowing bidirectional blood flow, AV anastomoses enable blood to flow in only one direction, from the artery to the vein. On average, there are about eight anastomoses in a monochorionic placenta [4]. These anastomoses can cause very specific complications including twin-to-twin transfusion syndrome (TTTS) in about 10 %, TAPS in roughly 5 %, twin reversed arterial perfusion (TRAP) sequence in about 2.5 % and spontaneous intrauterine fetal death (IUFD) in approximately 2 % of monochorionic-diamniotic twin pregnancies [5], [6], [7], [8]. Moreover, unequal sharing of placental territories can lead to selective fetal growth restriction (sFGR) in about 15 %. Monochorionic multiples affected by such complications are at increased risk of mortality, brain abnormalities and neurodevelopmental disorders [9], [10], [11].
Monochorionic twin pregnancies can be identified reliably before 14 weeks of gestation by sonographic examination of the intertwin septum featuring two thin amniotic layers, which may be referred to as the “empty lambda sign” (Figure 1) [12]. These pregnancies require regular monitoring to detect the onset of specific complications [12], 13]. Evaluation should begin at 16 weeks of gestation and be continued in at least biweekly intervals. During each examination, the amount of amniotic fluid in both sacs should be measured and doppler ultrasound should be performed. If discrepancy in amniotic fluid volumes, fetal weight estimation or doppler anomalies are present, transfer to an experienced center and closer monitoring intervals are indicated (Table 1).
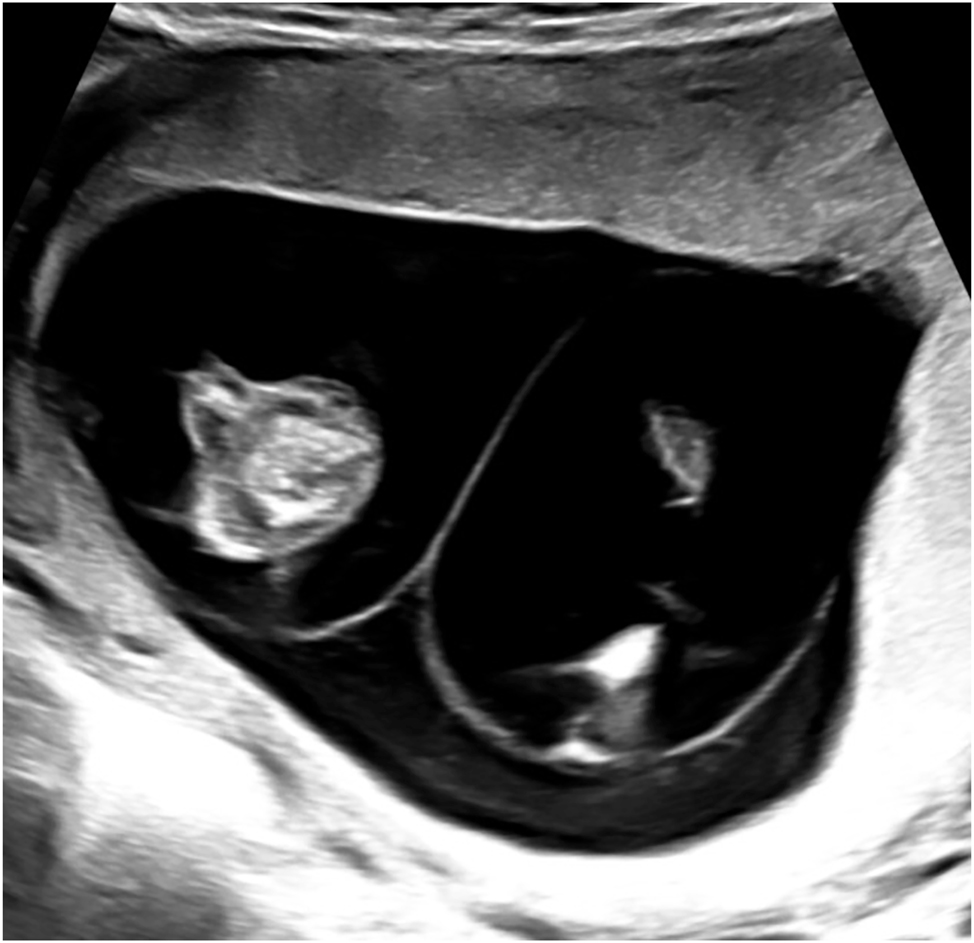
Sonographic image showing the interfetal membrane consisting of two amniotic layers appearing as “false lambda sign” in a monochorionic diamniotic twin pregnancy at 12 + 0 weeks of gestation.
Management algorithm of monochorionic diamniotic twin pregnancies.
| Gestational age, weeks | Task |
|---|---|
| 11–14 |
|
| 16–20 |
|
| 20–22 |
|
| 22–36 |
|
| 36 + 0–37 + 0 |
|
-
In case of suspicious findings: referral to experienced center.
This narrative review provides insight into prenatal detection and management of specific complications of monochorionic twin pregnancies.
Twin-to-twin transfusion syndrome (TTTS)
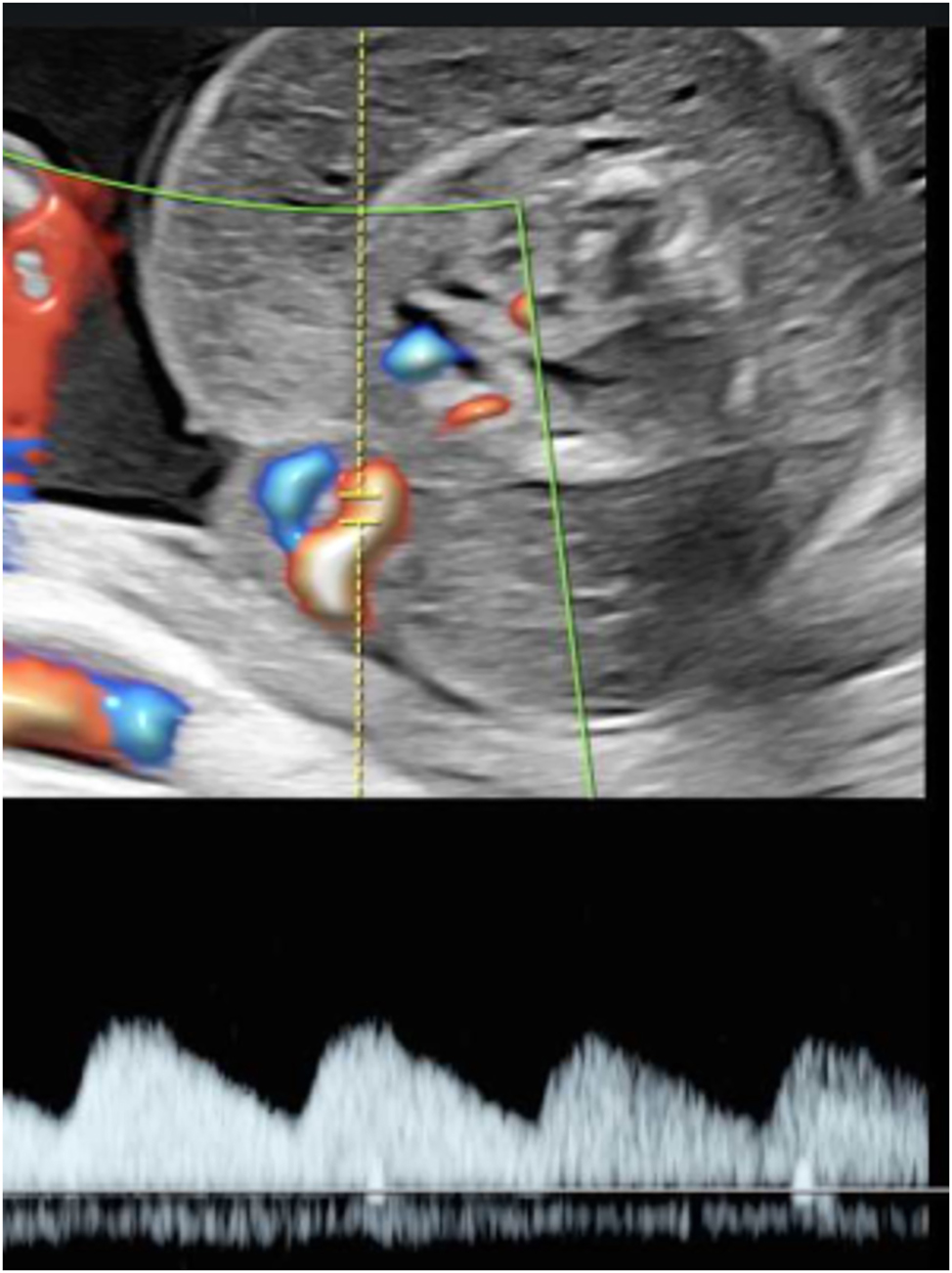
Twin-to-twin transfusion syndrome (TTTS) affects about 10 % of MCDA twin pregnancies and typically manifests between the 16th and 26th week of gestation [2]. It is sonographically defined by oligohydramnios in the donor and polyhydramnios in the recipient, with different cut-offs depending on gestational age. If the deepest vertical amniotic fluid pocket before 20 weeks of gestation meets the criteria of >8 cm in the recipient and <2 cm in the donor, this is referred to as Quintero stage 1 (Figure 2). After 20 weeks the threshold of >10 cm is used for polyhydramnios in the recipient [13], 14]. If such amniotic fluid discordance is accompanied by the absence of bladder filling in the donor, this is referred to as Quintero stage 2. Doppler sonography should be used to assess the hemodynamic stress on both fetuses and is also applied for staging according to Quintero. Typically, the recipient’s cardiac volume overload is reflected in abnormal flow patterns (absent or reversed flow in the a-wave) in the ductus venosus (Figure 3) or pulsatile flow in the umbilical vein, while the donor may present with absent or reversed end-diastolic flow in the umbilical artery. As soon as such signs of hemodynamic compromise occur, this is referred to as Quintero stage 3, although the stage progression does not necessarily have to occur in chronological order [13], 15]. If hemodynamic compromise progresses this can lead to fetal hydrops (Quintero Stage 4, Figure 3) or death (Quintero Stage 5). While the inclusion of further cardiovascular parameters can stratify additional characteristics of fetal pathophysiological changes, this does not improve the prediction of outcome after intrauterine interventions. Despite certain limitations [16], the Quintero stages remain the most practicable classification for TTTS [12], 13].
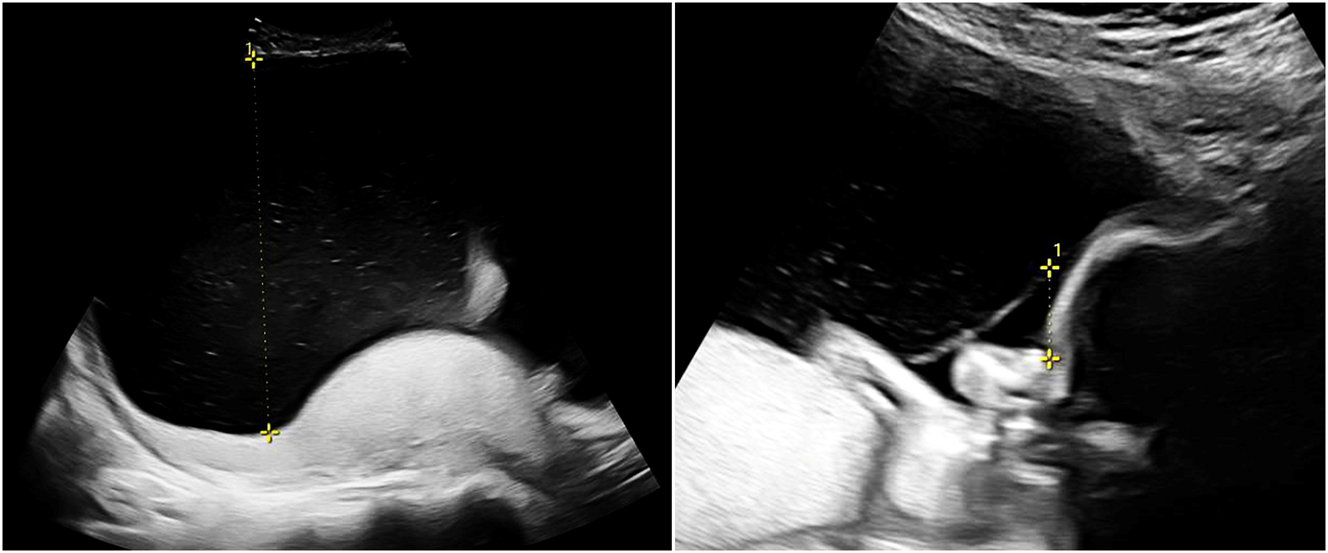
Sonographic image of TTTS (22 + 0 weeks) with polyhydramnios of 14 cm in the recipient (left) and oligohydramnios (1.6 cm) in the donor (right).
Sonographic image of a recipient (22 + 0 weeks, same as in Figure 2) with TTTS stage IV with abnormal ductus venosus flow (left) and skin edema (right).
Treatment should be initiated from Quintero stage 2 while in Quintero stage 1 a conservative approach under close monitoring is also possible [12], 13], 17]. However, progression to a higher stage, an increase in polyhydramnios causing maternal complaints and cervical shortening should be considered as an indication for an active therapeutic approach. Such progression affects roughly 30–60 % of pregnancies with TTTS stage 1 [17], [18], [19]. If left untreated, TTTS is associated with high mortality and morbidity.
The only causal treatment for TTTS is fetoscopic laser ablation of the placental vascular anastomoses with the creation of two independent placental territories [20], 21]. In brief, after percutaneous insertion of the fetoscope, the entire placental surface is examined, the two umbilical cord insertions are visualized, and the vascular equator is specifically searched for anastomoses. All anastomoses are then treated with laser energy until complete cessation of blood flow occurs (Figure 4) [22], 23]. Once all visible anastomoses have been closed, amniotic drainage is performed to reduce obstetric complications.
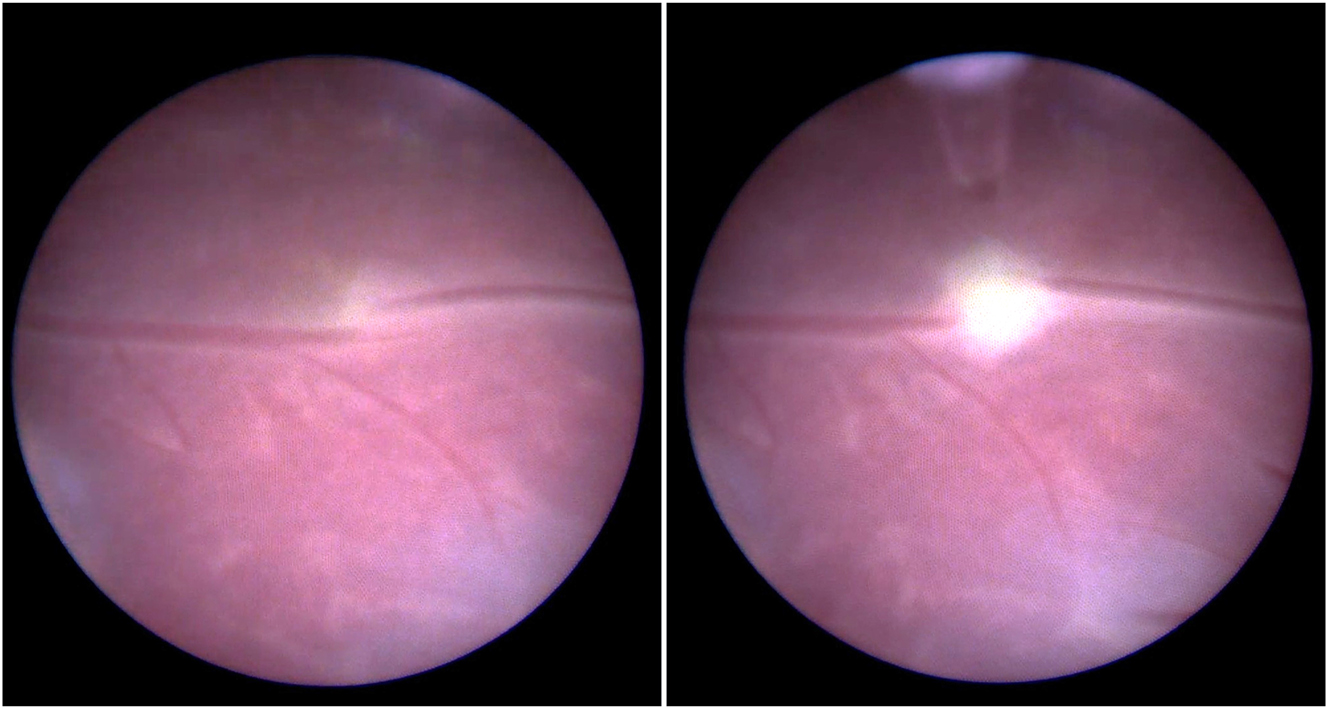
Fetoscopic image of an arterio-venous anastomosis before (left) and after (right) laser ablation. The donor artery originates from the right and connects into a recipient vein that leads to the left.
The efficacy and superiority of fetoscopic laser therapy over amniotic drainage was demonstrated in a multicenter randomized study [14]. Laser therapy resulted in better survival rates and reduced neurological morbidity compared to amniotic drainage, so that overall survival rates of 75–90 % are assumed today [24], [25], [26]. Demise of one or both fetuses occurs in 13–33 %, predominantly within the first week after the procedure [27], [28], [29]. Mortality is associated with cardiac overload of the recipient, unequal placental sharing or incomplete laser ablation leaving residual anastomoses [30], 31]. The latter can lead to a recurrence of TTTS or TAPS which has been described in up to 14 % of cases [32], 33]. These two complications may increase the risk of cerebral lesions, which occur in around 2 % of cases after laser therapy [34], 35]. The most common maternal complication of laser therapy is preterm premature rupture of membranes in 6–10 %.
Neurodevelopmental outcome is significantly better after laser therapy than after amniodrainage, and neurological development at the age of two years largely corresponds to that of dichorionic twins of the same age [36]. In general, long-term developmental delay is described in up to 15 % of survivors [9], [37], [38], [39], [40], [41] and is considered to be a consequence of TTTS, laser therapy and the higher rate of preterm delivery [42]. At the age of 6 years, around 9 % of children after TTTS are affected by severe neurological deficit [43].
Monitoring after laser therapy includes regular weekly ultrasound checks for the first 4–6 weeks and biweekly thereafter until delivery. Amniotic fluid volumes, fetal growth and Doppler of the umbilical artery, the ductus venosus and the middle cerebral artery – to rule out fetal anemia – should be checked [12], 13], 44]. After successful treatment, the amniotic fluid volume and cardiac dysfunction usually normalize within two to four weeks, usually earlier so in the donor than in the recipient [45], 46]. In the further course, cervical length should also be examined for early detection of an imminent premature birth and the fetal extremities should be examined for possible amniotic bands, which have been observed somewhat more frequently after intrauterine interventions [47]. Due to the above-mentioned rate of prenatally diagnosable brain lesions after TTTS of 2 %, parents can be offered fetal magnetic resonance imaging at 30–32 weeks of gestation in order to be able to terminate the pregnancy in cases with severe cerebral damage [48]. It is also noteworthy that around 8 % of all children after laser therapy have pulmonary artery stenosis at the age of 10 years, with former recipients appearing to be affected more frequently. [49], [50], [51].
There is little evidence on the optimal time and mode of delivery after intrauterine laser therapy, but some experts recommend delivery at 34 weeks of gestation [12], 52]. However, it also appears acceptable to deliver at 37 weeks’ gestation if the course of the pregnancy is otherwise perfectly normal [12], 13].
Twin-anemia-polycythemia sequence (TAPS)
Another specific problem associated with monochorionic placenta is twin anemia polycythemia sequence (TAPS), which occurs spontaneously in around 5 % of monochorionic diamniotic twins, but has been observed significantly more frequently after conventional laser therapy [53]. It is assumed that very small diameter arteriovenous anastomoses are responsible for this condition, which – in contrast to TTTS – lead to chronic blood loss and anemia in the donor and polycythemia in the recipient. This condition can only be detected in a timely manner by measuring the MCA-PSV of both fetuses demonstrating MCA-PSV ≥1.5 multiples of the median (MoM) in the donor and ≤0.8 MoM in the recipient or delta MCA-PSV between the twins of ≥1.0 MoM [12], 54]. However, the correlation between low MCA-PSV and polycythemia in the recipient is relatively poor [55]. It has therefore been suggested that the difference in MCA PSV>0.5 MoM between the fetuses may be a more sensitive parameter for diagnosing TAPS, while other studies suggested an MCA-PSV difference of 0.373 as the optimal cut-off [56], 57]. In the case of an increasing delta-MCA-PSV with still normal individual MCA-PSV values in the fetuses, the development or presence of TAPS should be expected. If such serial Doppler investigations are not performed routinely, affected fetuses may only be noticed when fetal hydrops or intrauterine demise become apparent. It is therefore essential to perform these Doppler examinations routinely from 20 weeks of gestation onwards and regularly after laser surgery until delivery [12], 13]. Additional sonographic signs may include differences in the thickness and echogenicity of both placental areas. In the donor, the placenta is usually hyperechoic and thickened (hydropic), while in the recipient it appears narrow and hypoechoic. Furthermore, a “starry sky” appearance may be noticeable in the recipient’s liver, which is due to reduced echogenicity of the liver parenchyma with increased echogenicity of the venous portal vessels (Figure 5) [58].
Ultrasound findings detectable in TAPS: Placental dichotomy with hyperechoic hydropic placenta of the donor in the back and hypoechoic flat placenta of the recipient in the front at 20 + 0 weeks of gestation (left). Cross section through abdomen of a recipient featuring a starry sky liver at 19 + 0 weeks of gestation (right).
Postnatal diagnosis is based on the presence of chronic anemia in the donor with reticulocytosis and polycythemia in the recipient. Diagnostic criteria therefore include hemoglobin differences between the neonates of more than 8 g/dl and, in addition, either a reticulocyte count ratio of more than 1.7 or the presence of only minuscule vascular anastomoses (<1 mm diameter) on the placental surface [53]. Such small anastomoses can easily be overlooked during prenatal laser therapy, which explains the increased rate of TAPS cases after laser therapy. A multicenter randomized trial reported that the incidence of TAPS can be significantly reduced by additional superficial coagulation of the connecting areas between the individual coagulation points on the placental surface [59]. This method, known as the “Solomon technique,” has since become the gold standard in fetoscopic laser therapy for TTTS. Depending on the gestational age, treatment options for TAPS include intrauterine transfusion in the donor, potentially with hemodilution in the recipient, fetoscopic laser ablation of the anastomoses, or delivery [33], [60], [61], [62].
The outcome of pregnancies with TAPS is variable. Severe TAPS can lead to demise of one or both fetuses, whereas mild TAPS can result in the birth of two neonates who, apart from the significant hemoglobin difference, show no morbidity. Studies suggest that surviving twins after TAPS have neurological developmental problems in about 30 % of cases, with the risk being about four times higher for the donor than for the recipient [33], 61], 63]. As a precautionary measure, the fetal brains should be examined by ultrasound or MRI in the third trimester, and a neurological assessment of the children should be performed at the age of 2 years. However, spontaneous single intrauterine death appears to be associated with low risk of mortality and severe neonatal cerebral injury in the surviving co-twin [64].
Twin-reversed-arterial-perfusion (TRAP)
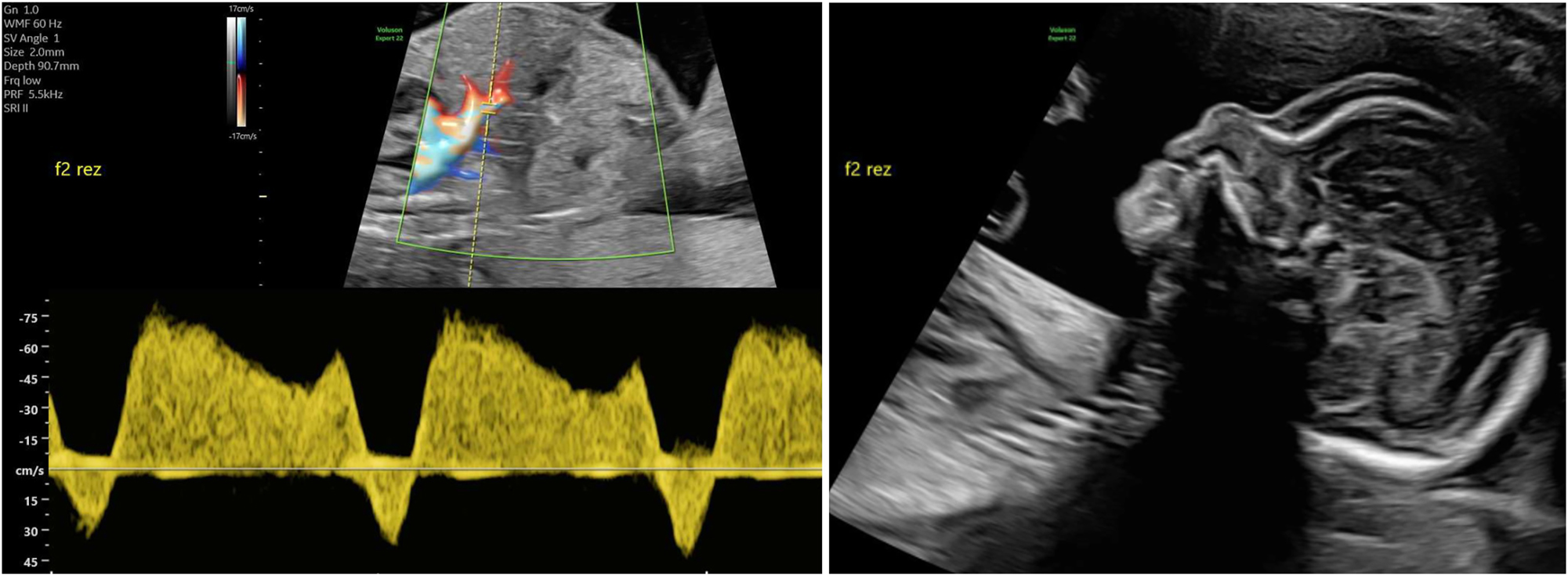
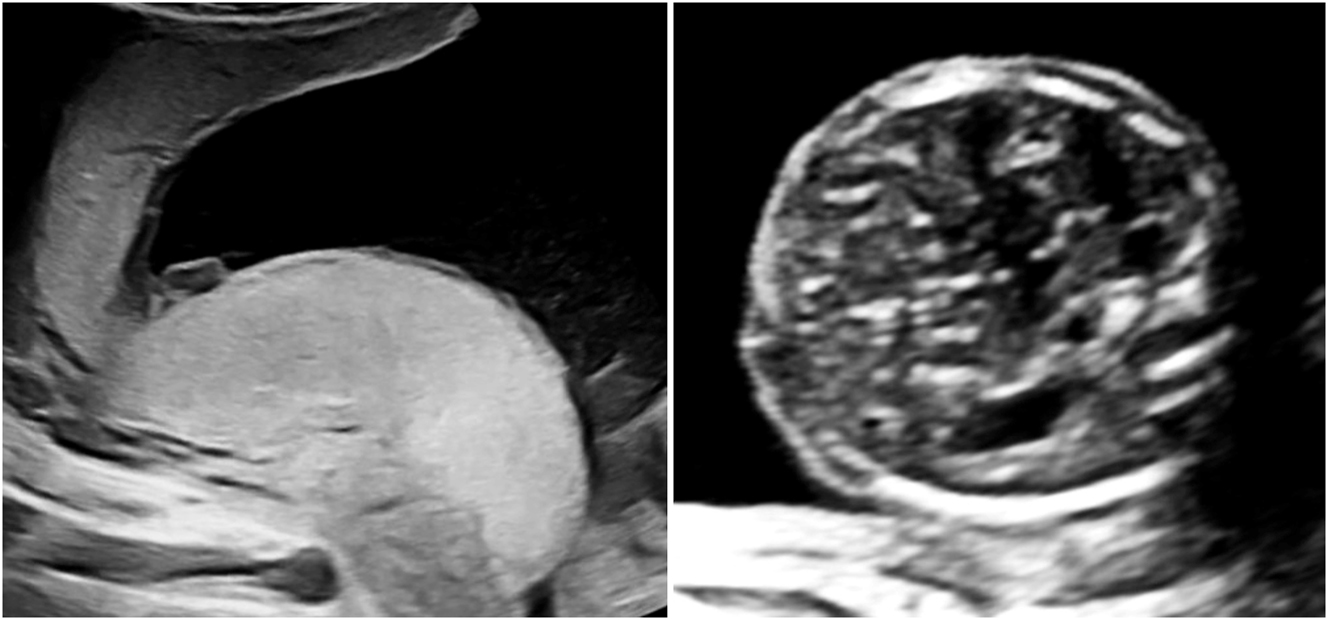
A rare variant of interfetal transfusion in monochorionic twins is twin reversed arterial perfusion (TRAP) sequence, which occurs in approximately 2.5 % of monozygotic twins [7], 8]. This involves reversed perfusion of the commonly singular umbilical artery of the affected twin via a large-caliber arterio-arterial anastomosis, resulting in disrupted development of the heart and the upper body (Figure 6). While the affected fetus has no chance of survival, the healthy pump twin is also at risk due to excessive strain on the heart [65].
Sonographic image of a rudimentary TRAP fetus (22 + 4 weeks of gestation) with typical reversed arterial perfusion in the singular umbilical artery.
Diagnosis can be made by demonstrating reversed perfusion in the umbilical artery of the TRAP fetus using Doppler sonography. Monitoring the ductus venosus of the pump twin can be used to detect cardiac decompensation in a timely manner.
To increase survival rates of the pump twin, the feeding umbilical vessel is usually closed using laser energy or other occlusive techniques like radiofrequency ablation [66], 67]. Since sudden death of the pump twin can occur despite close monitoring, it may be reasonable to perform such a procedure early in pregnancy [12], 68]. A multicenter study is currently investigating whether this should be done at 16 weeks of gestation or as early as 12–14 weeks of gestation (TRAPIST trial, ClinicalTrials.gov Identifier: NCT02621645).
Selective fetal growth restriction (sFGR)
Diagnostic criteria for selective growth restriction in monochorionic twin pregnancies combine various parameters, including the estimated weight and abdominal circumference of both fetuses, the difference in estimated weights, and the Doppler values of the umbilical artery of the growth restricted fetus. The weight difference is calculated as: (Weight of the larger fetus – weight of the smaller fetus) x 100/weight of the larger fetus.
Diagnostic criteria for sFGR in monochorionic twins [12], 69]:
Estimated weight <3rd percentile
or 2 of 4 parameters:
Estimated weight of one fetus <10th percentile
Difference in estimated weight ≥25 %
Umbilical artery PI >95th percentile in FGR fetuses
Abdominal circumference of one fetus <10th percentile
In monochorionic twins sFGR is mainly due to discordant placental supplying territories and may be detected already before 20 weeks of gestation by the occurrence of an absent or reversed end diastolic flow pattern (AREDF) in the umbilical artery of the growth restricted fetus. However, it should be considered that in monochorionic twins with AREDF there is a significantly longer average latency period (10–11 weeks) until fetal decompensation than is the case in singletons or dichorionic twins [70], 71].
Classification of sFGR in monochorionic twins is based on the pattern of Doppler flow in the umbilical artery of the smaller twin, which rarely changes during pregnancy, thus allowing prognostic assessment [72]. In about two-thirds of cases the smaller twin has consistently positive end-diastolic flow in umbilical artery Doppler (type 1), while the remaining third has absent or reversed end-diastolic flow that can be persistent (type 2) or intermittent (type 3).
Type 1 sFGR is the most favorable subgroup with survival rates of >90 % and low risk for intrauterine mortality (2–4%). Usually, there is moderate discordance of the placental territories resulting in a favorable clinical course in most cases. Therefore, a conservative approach can be adopted in most cases. Nevertheless, Doppler ultrasound checks should be performed at least weekly to detect (rare) progression to type 2. On average, these pregnancies can be prolonged to 34–36 weeks of gestation, and the rate of neurological complications in the larger child is low [73].
Type 2 sFGR is associated with high risk of intrauterine fetal death of the smaller twin and early preterm birth with associated risk of neurodevelopmental delay in the co-twin. Prospectively, the risk of IUFD is up to 29 % and the risk of neurological sequelae is up to 15 % in preterm births <30 weeks of gestation [72], 74]. The average gestational age at birth is 28–33 weeks [73]. Placentas in type 2 sFGR usually present with significant discordance of supplying territories and a lack of compensatory anastomoses. In this subgroup there is placental dysfunction in the smaller twin, so that type 2 twins behave similarly to singletons with FGR, and the progression of the Doppler parameters indicates progressive placental insufficiency. This type is usually progressive, but outcome can be predicted based on the typical cascade of signs of decompensation (abnormal ductus venosus, abnormal CTG), so that close monitoring allows intervention before IUFD occurs. Management depends primarily on the clinical condition, gestational age, and the preferences of the parents, and may include iatrogenic premature birth, laser ablation, or selective feticide [13], 75], 76].
Type 3 sFGR presents with unique flow pattern and is often only recognizable when the umbilical Doppler flow is observed over a longer period and the measurement is taken at close proximity to the placental cord insertion site (Figure 7). The typical alternation of positive phases with AREDF usually occurs cyclically and indicates the presence of a large arterioarterial anastomosis, which transfers the systolic flow pattern of the larger twin to the umbilical artery of the smaller twin. In type 3, the placental supply area of the FGR twin is typically significantly smaller than that of its co-twin. Survival is primarily ensured by large interfetal anastomoses and the resulting blood exchange [8]. This subgroup is associated with a 10–20 % risk of IUFD of the growth-restricted fetus, which, unlike type 2, is not preceded by the typical sequential cascade of decompensation and is therefore unpredictable and can even occur in situations with apparently stable ultrasound findings. Due to the large AA anastomoses, there is also a significantly increased risk of up to 20 % of neurological sequelae in the surviving co-twin [77], 78]. Depending on the clinical findings, technical possibilities, and parental wishes, an expectant approach or a prenatal intervention in the form of laser ablation or selective feticide may be chosen [75], 76], 79]. The average gestational age at birth is between 29 and 34 weeks [73].
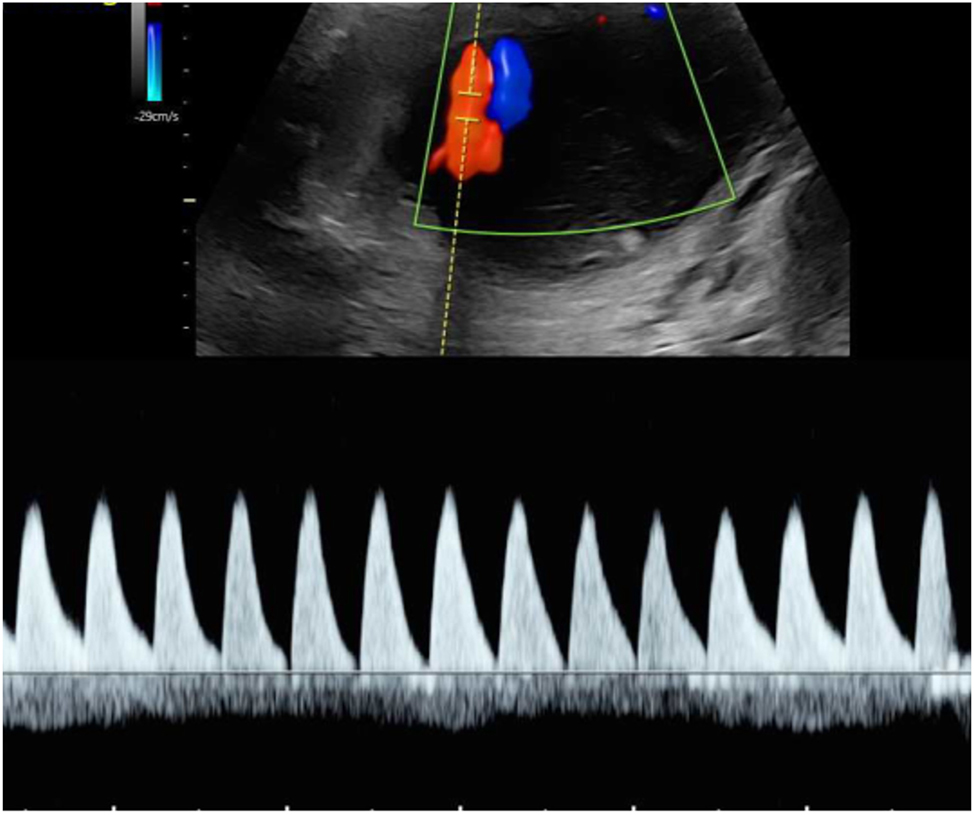
Umbilical artery Doppler flow pattern in sFGR type 3 at 22 + 6 weeks of gestation. Demonstration of intermittent end-diastolic positive flow, absent flow and negative flow.
Monoamniotic twins
Around 5 % of monochorionic pregnancies are sharing a common amniotic sac and are therefore referred to as monoamniotic [12], 13]. These twins have an increased risk of fetal death and neonatal morbidity, which is mainly due to congenital anomalies, preterm birth, and complications from entanglement of the fetal umbilical cords. Indeed, discordant structural anomalies occur in up to 20 % of monoamniotic pregnancies making careful screening examinations mandatory [80], 81]. While almost all monoamniotic twin pregnancies are affected by umbilical cord entanglement (Figure 8), studies have demonstrated that the mere presence of cord entanglement as well as the presence of notching in the umbilical Doppler flow do not contribute to increased perinatal morbidity and mortality [82], [83], [84]. In monoamniotic pregnancies, TTTS occurs significantly less frequently and can be diagnosed through discordant bladder fillings and polyhydramnios in the shared amniotic sac.
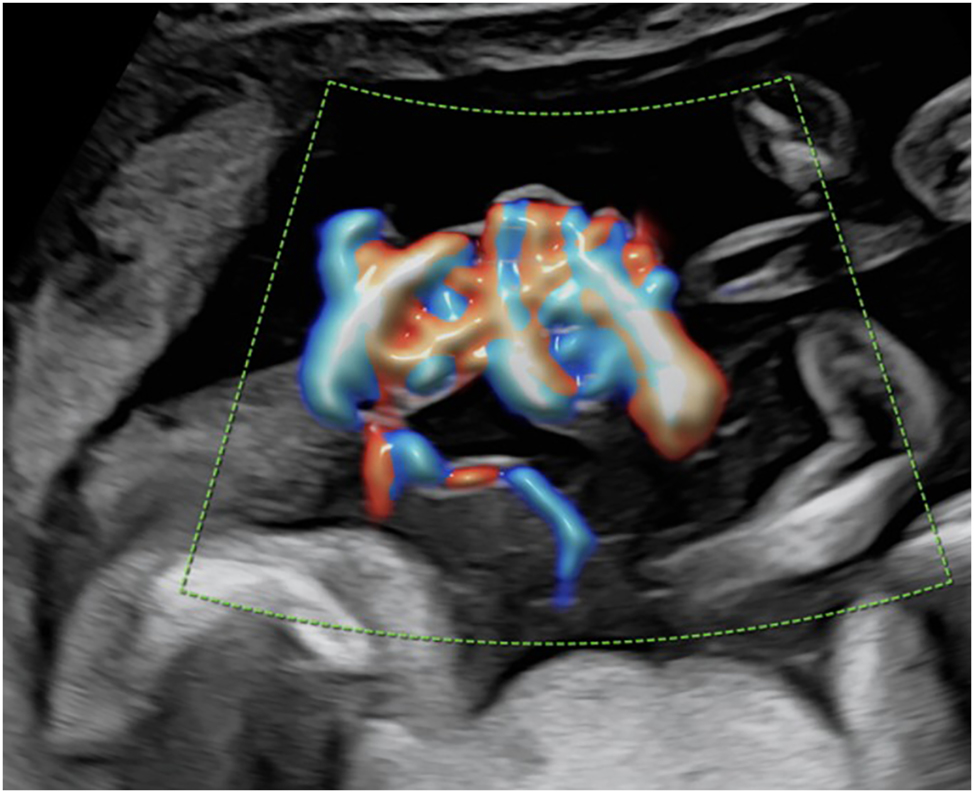
Color Doppler image of typical umbilical cord entanglement in a monochorionic-monoamniotic twin pregnancy at 17 + 2 weeks of gestation.
Obstetric management of monoamniotic twin pregnancies includes prophylactic antenatal corticosteroids and continuous inpatient or intensive outpatient monitoring from approximately 26 to 28 weeks of gestation until planned cesarean section between 32 + 0 and 33 + 0 weeks of gestation [13], 85], 86].
Single intrauterine fetal death (sIUFD)
Single intrauterine fetal death (sIUFD) in monochorionic twins may lead to acute blood loss of the surviving co-twin into the fetal-placental unit of the deceased twin resulting in severe anemia, hypovolemia, and ischemia. The co-twin may therefore be affected by subsequent IUFD in 15 %, premature delivery in 68 %, pathological postnatal brain morphology in 34 %, and long-term neurological morbidity in 26 % [12], [87], [88], [89]. If sIUFD occurs, the pregnant woman should be referred to an experienced center and should be counseled about potential complications. Immediate delivery should be avoided before 34 weeks of gestation and close monitoring for signs of fetal anemia should be performed instead [13], 90]. If evidence of fetal anemia is detected, intrauterine transfusion can be performed [91], [92], [93]. If MCA-PSV remains stable over several days following sIUFD, the likelihood of developing anemia later becomes very low. Subsequently, growth monitoring and cerebral and umbilical Doppler examinations should be conducted at 2 to 4-week intervals. The surviving fetus should undergo detailed neurosonography or magnetic resonance imaging approximately 4–6 weeks after IUFD for assessment of cerebral damage. If severe cerebral damage is detected, the possibility of late termination can be discussed with the parents. Otherwise, early delivery at 34–36 weeks of gestation may be considered. Surviving infants should undergo neurodevelopmental evaluation at the age of two years [12], 13].
Selective feticide in complicated monochorionic twin pregnancies
Selective feticide may be requested by the parents in the event of discordant anomalies associated with severe illness or neurological impairment, or lethal anomalies that pose an increased risk of intrauterine mortality, which could endanger the healthy co-twin. Occasionally, selective feticide may also be considered in severely compromised fetuses in TTTS, TAPS, or sFGR to avoid sIUFD in the previable period. Conditions that only become lethal after birth could also be managed conservatively, thereby avoiding the risks and emotional burden of selective feticide. In monochorionic multiples conventional feticide is not an option due to the presence of placental anastomoses, as potassium chloride could pass via the anastomoses or the co-twin could exsanguinate into the demised twin. Selective feticide in monochorionic multiples must therefore ensure that no anastomoses remain patent between the twins. Ultrasound-guided bipolar umbilical cord occlusion, radiofrequency ablation, or laser ablation of the umbilical cord have proven effective for this purpose. Regardless of the method used, the survival rate of the unaffected fetus is approximately 80–90 %. The most common complications of the procedures include premature rupture of membranes (26 %) and preterm birth before 34 + 0 weeks of gestation (37 %) [94]. In monoamniotic twin pregnancies, selective feticide should be performed by umbilical cord occlusion followed by fetoscopic laser transection of the umbilical cord to avoid complications by umbilical cord torsion [95]. Timing and technique of selective feticide depend on the individual case and local legislation. In most cases, procedures are performed between 18 and 20 weeks of pregnancy or, if legally possible, as a late reduction after 28 weeks, when the risk of premature birth is lower.
Neurodevelopmental follow-up in complicated monochorionic twins
Although the majority (80 %) of all MCDA twin pregnancies result in two healthy children, there is a substantial risk for the subgroup of fetuses from pregnancies affected by specific complications as stated above. While TTTS is the most important cause of unfavorable outcome, also infants surviving TAPS, sFGR or sIUFD may experience long-term neurodevelopmental impairment [9], 41], 61], 63], 73], 74], 77], [87], [88], [89]. Therefore, all surviving children following complicated monochorionic pregnancies should undergo regular neurological assessment, at least at the age of two and ideally until school age, in order to detect any neurological developmental disorders and treat them if necessary. Follow-up may include parental questionnaires on infant development and standardized neurodevelopmental assessment techniques like Bayley Scales of Infant Development. In addition, infants should undergo physical and neurological examinations. Possible protocols are described in the studies by Bolch et al. or Ortibus et al. [96], 97].
Emerging technologies and future directions
Several studies investigated the use of artificial intelligence in supporting diagnosis and treatment of TTTS [98], [99], [100], [101]. Moreover, learning-based frameworks for in vivo fetoscopy as well as automatic placental pose estimation in fetoscopic images were proposed [102], [103], [104]. Other groups investigated the use of high-intensity focused ultrasound (HIFU) for noninvasive occlusion of placental vasculature in pregnancies with TTTS and anterior placenta [105], 106]. However, the benefit of such technologies in improving the outcome of TTTS with regards to the most important parameters being intact survival and near term birth remains to be demonstrated.
-
Research ethics: Not applicable.
-
Informed consent: Not required.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Denbow, ML, Cox, P, Taylor, M, Hammal, DM, Fisk, NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182:417–26. https://doi.org/10.1016/s0002-9378(00)70233-x.Suche in Google Scholar PubMed
2. Lewi, L, Jani, J, Blickstein, I, Huber, A, Gucciardo, L, Van Mieghem, T, et al.. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199:514 e1–8. https://doi.org/10.1016/j.ajog.2008.03.050.Suche in Google Scholar PubMed
3. Fisk, NM, Duncombe, GJ, Sullivan, MH. The basic and clinical science of twin-twin transfusion syndrome. Placenta 2009;30:379–90. https://doi.org/10.1016/j.placenta.2009.02.005.Suche in Google Scholar PubMed
4. Zhao, DP, de Villiers, SF, Slaghekke, F, Walther, FJ, Middeldorp, JM, Oepkes, D, et al.. Prevalence, size, number and localization of vascular anastomoses in monochorionic placentas. Placenta 2013;34:589–93. https://doi.org/10.1016/j.placenta.2013.04.005.Suche in Google Scholar PubMed
5. Lewi, L, Gucciardo, L, Van Mieghem, T, de Koninck, P, Beck, V, Medek, H, et al.. Monochorionic diamniotic twin pregnancies: natural history and risk stratification. Fetal Diagn Ther 2010;27:121–33. https://doi.org/10.1159/000313300.Suche in Google Scholar PubMed
6. Lewi, L, Deprest, J, Hecher, K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am J Obstet Gynecol 2013;208:19–30. https://doi.org/10.1016/j.ajog.2012.09.025.Suche in Google Scholar PubMed
7. van Gemert, MJ, van den Wijngaard, JP, Vandenbussche, FP. Twin reversed arterial perfusion sequence is more common than generally accepted. Birth Defects Res A Clin Mol Teratol 2015;103:641–3. https://doi.org/10.1002/bdra.23405.Suche in Google Scholar PubMed
8. Lewi, L. Monochorionic diamniotic twins: what do I tell the prospective parents? Prenat Diagn 2020;40:766–75. https://doi.org/10.1002/pd.5705.Suche in Google Scholar PubMed
9. Hecher, K, Gardiner, HM, Diemert, A, Bartmann, P. Long-term outcomes for monochorionic twins after laser therapy in twin-to-twin transfusion syndrome. Lancet Child Adolesc Health 2018;2:525–35. https://doi.org/10.1016/s2352-4642(18)30127-5.Suche in Google Scholar PubMed
10. Knijnenburg, PJC, Lopriore, E, Slaghekke, F, van Klink, JMM. Long-term follow-up of complicated monochorionic twin pregnancies: focus on neurodevelopment. Best Pract Res Clin Obstet Gynaecol 2022;84:166–78. https://doi.org/10.1016/j.bpobgyn.2022.03.014.Suche in Google Scholar PubMed
11. Prasad, S, Beg, S, Badran, D, Masciullo, L, Huddy, C, Khalil, A. Neurodevelopmental outcome in complicated twin pregnancy: prospective observational study. Ultrasound Obstet Gynecol 2024;63:189–97. https://doi.org/10.1002/uog.27448.Suche in Google Scholar PubMed
12. Khalil, A, Sotiriadis, A, Baschat, A, Bhide, A, Gratacos, E, Hecher, K, et al.. ISUOG practice guidelines (updated): role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol 2025;65:253–76. https://doi.org/10.1002/uog.29166.Suche in Google Scholar PubMed PubMed Central
13. von Kaisenberg, C, Klaritsch, P, Ochsenbein-Kolble, N, Hodel, ME, Nothacker, M, Hecher, K. Screening, management and delivery in twin pregnancy. Ultraschall Med 2021;42:367–78. https://doi.org/10.1055/a-1248-8896.Suche in Google Scholar PubMed
14. Senat, MV, Deprest, J, Boulvain, M, Paupe, A, Winer, N, Ville, Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004;351:136–44. https://doi.org/10.1056/nejmoa032597.Suche in Google Scholar
15. Quintero, RA, Morales, WJ, Allen, MH, Bornick, PW, Johnson, PK, Kruger, M. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19:550–5. https://doi.org/10.1038/sj.jp.7200292.Suche in Google Scholar PubMed
16. Ville, Y. Twin-to-twin transfusion syndrome: time to forget the Quintero staging system? Ultrasound Obstet Gynecol 2007;30:924–7. https://doi.org/10.1002/uog.5221.Suche in Google Scholar PubMed
17. Stirnemann, J, Slaghekke, F, Khalek, N, Winer, N, Johnson, A, Lewi, L, et al.. Intrauterine fetoscopic laser surgery versus expectant management in stage 1 twin-to-twin transfusion syndrome: an international randomized trial. Am J Obstet Gynecol 2021;224:528 e1–12. https://doi.org/10.1016/j.ajog.2020.11.031.Suche in Google Scholar PubMed
18. Khalil, A, Cooper, E, Townsend, R, Thilaganathan, B. Evolution of stage 1 twin-to-twin transfusion syndrome (TTTS): systematic review and meta-analysis. Twin Res Hum Genet 2016;19:207–16. https://doi.org/10.1017/thg.2016.33.Suche in Google Scholar PubMed
19. Washburn, EE, Sparks, TN, Gosnell, KA, Rand, L, Gonzalez, JM, Feldstein, VA. Stage I twin-twin transfusion syndrome: outcomes of expectant management and prognostic features. Am J Perinatol 2018;35:1352–7. https://doi.org/10.1055/s-0038-1627095.Suche in Google Scholar PubMed PubMed Central
20. De Lia, JE, Cruikshank, DP, Keye, WRJr. Fetoscopic neodymium:YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol 1990;75:1046–53.Suche in Google Scholar
21. Ville, Y, Hyett, J, Hecher, K, Nicolaides, K. Preliminary experience with endoscopic laser surgery for severe twin-twin transfusion syndrome. N Engl J Med 1995;332:224–7. https://doi.org/10.1056/nejm199501263320404.Suche in Google Scholar
22. Ierullo, AM, Papageorghiou, AT, Bhide, A, Fratelli, N, Thilaganathan, B. Severe twin-twin transfusion syndrome: outcome after fetoscopic laser ablation of the placental vascular equator. BJOG 2007;114:689–93. https://doi.org/10.1111/j.1471-0528.2007.01336.x.Suche in Google Scholar PubMed
23. Klaritsch, P, Albert, K, Van Mieghem, T, Gucciardo, L, Done, E, Bynens, B, et al.. Instrumental requirements for minimal invasive fetal surgery. BJOG 2009;116:188–97. https://doi.org/10.1111/j.1471-0528.2008.02021.x.Suche in Google Scholar PubMed
24. Roberts, D, Neilson, JP, Kilby, MD, Gates, S. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev 2014;2014:CD002073. https://doi.org/10.1002/14651858.cd002073.pub3.Suche in Google Scholar PubMed PubMed Central
25. Diehl, W, Diemert, A, Grasso, D, Sehner, S, Wegscheider, K, Hecher, K. Fetoscopic laser coagulation in 1020 pregnancies with twin-twin transfusion syndrome demonstrates improvement in double-twin survival rate. Ultrasound Obstet Gynecol 2017;50:728–35. https://doi.org/10.1002/uog.17520.Suche in Google Scholar PubMed
26. Stirnemann, J, Djaafri, F, Kim, A, Mediouni, I, Bussieres, L, Spaggiari, E, et al.. Preterm premature rupture of membranes is a collateral effect of improvement in perinatal outcomes following fetoscopic coagulation of chorionic vessels for twin-twin transfusion syndrome: a retrospective observational study of 1092 cases. BJOG 2018;125:1154–62. https://doi.org/10.1111/1471-0528.15147.Suche in Google Scholar PubMed
27. Huber, A, Diehl, W, Bregenzer, T, Hackeloer, BJ, Hecher, K. Stage-related outcome in twin-twin transfusion syndrome treated by fetoscopic laser coagulation. Obstet Gynecol 2006;108:333–7. https://doi.org/10.1097/01.aog.0000225945.17022.6b.Suche in Google Scholar
28. Hecher, K, Diehl, W, Zikulnig, L, Vetter, M, Hackeloer, BJ. Endoscopic laser coagulation of placental anastomoses in 200 pregnancies with severe mid-trimester twin-to-twin transfusion syndrome. Eur J Obstet Gynecol Reprod Biol 2000;92:135–9. https://doi.org/10.1016/s0301-2115(00)00437-1.Suche in Google Scholar PubMed
29. Quintero, RA, Comas, C, Bornick, PW, Allen, MH, Kruger, M. Selective versus non-selective laser photocoagulation of placental vessels in twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol 2000;16:230–6. https://doi.org/10.1046/j.1469-0705.2000.00265.x.Suche in Google Scholar PubMed
30. Cavicchioni, O, Yamamoto, M, Robyr, R, Takahashi, Y, Ville, Y. Intrauterine fetal demise following laser treatment in twin-to-twin transfusion syndrome. BJOG 2006;113:590–4. https://doi.org/10.1111/j.1471-0528.2006.00913.x.Suche in Google Scholar PubMed
31. Zikulnig, L, Hecher, K, Bregenzer, T, Baz, E, Hackeloer, BJ. Prognostic factors in severe twin-twin transfusion syndrome treated by endoscopic laser surgery. Ultrasound Obstet Gynecol 1999;14:380–7. https://doi.org/10.1046/j.1469-0705.1999.14060380.x.Suche in Google Scholar PubMed
32. Robyr, R, Lewi, L, Salomon, LJ, Yamamoto, M, Bernard, JP, Deprest, J, et al.. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2006;194:796–803. https://doi.org/10.1016/j.ajog.2005.08.069.Suche in Google Scholar PubMed
33. Tollenaar, LSA, Lopriore, E, Faiola, S, Lanna, M, Stirnemann, J, Ville, Y, et al.. Post-laser twin anemia polycythemia sequence: diagnosis, management, and outcome in an international cohort of 164 cases. J Clin Med 2020;9. https://doi.org/10.3390/jcm9061759.Suche in Google Scholar PubMed PubMed Central
34. Stirnemann, J, Chalouhi, G, Essaoui, M, Bahi-Buisson, N, Sonigo, P, Millischer, AE, et al.. Fetal brain imaging following laser surgery in twin-to-twin surgery. BJOG 2018;125:1186–91. https://doi.org/10.1111/1471-0528.14162.Suche in Google Scholar PubMed
35. Sileo, FG, Curado, J, D’Antonio, F, Benlioglu, C, Khalil, A. Incidence and outcome of prenatal brain abnormality in twin-to-twin transfusion syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2022;60:176–84. https://doi.org/10.1002/uog.24895.Suche in Google Scholar PubMed
36. Lenclen, R, Ciarlo, G, Paupe, A, Bussieres, L, Ville, Y. Neurodevelopmental outcome at 2 years in children born preterm treated by amnioreduction or fetoscopic laser surgery for twin-to-twin transfusion syndrome: comparison with dichorionic twins. Am J Obstet Gynecol 2009;201:291 e1–5. https://doi.org/10.1016/j.ajog.2009.05.036.Suche in Google Scholar PubMed
37. Maschke, C, Diemert, A, Hecher, K, Bartmann, P. Long-term outcome after intrauterine laser treatment for twin-twin transfusion syndrome. Prenat Diagn 2011;31:647–53. https://doi.org/10.1002/pd.2797.Suche in Google Scholar PubMed
38. Lopriore, E, Walther, FJ, Oepkes, D. Long-term neurodevelopmental outcome in TTTS in the eurofoetus trial. Am J Obstet Gynecol 2011;205:e15; author reply e-6. https://doi.org/10.1016/j.ajog.2011.02.065.Suche in Google Scholar PubMed
39. Salomon, LJ, Ortqvist, L, Aegerter, P, Bussieres, L, Staracci, S, Stirnemann, JJ, et al.. Long-term developmental follow-up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2010;203:444 e1–7. https://doi.org/10.1016/j.ajog.2010.08.054.Suche in Google Scholar PubMed
40. Schou, KV, Lando, AV, Ekelund, CK, Jensen, LN, Jorgensen, C, Norgaard, LN, et al.. Long-term neurodevelopmental outcome of monochorionic twins after laser therapy or umbilical cord occlusion for twin-twin transfusion syndrome. Fetal Diagn Ther 2019;46:20–7. https://doi.org/10.1159/000491787.Suche in Google Scholar PubMed
41. Hessami, K, Nassr, AA, Sananes, N, Castillo, J, Castillo, HA, Sanz, CM, et al.. Perinatal risk factors of neurodevelopmental impairment after fetoscopic laser photocoagulation for twin-twin transfusion syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2021;58:658–68. https://doi.org/10.1002/uog.23706.Suche in Google Scholar PubMed
42. Lopriore, E, Ortibus, E, Acosta-Rojas, R, Le Cessie, S, Middeldorp, JM, Oepkes, D, et al.. Risk factors for neurodevelopment impairment in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol 2009;113:361–6. https://doi.org/10.1097/aog.0b013e318195873e.Suche in Google Scholar
43. Graeve, P, Banek, C, Stegmann-Woessner, G, Maschke, C, Hecher, K, Bartmann, P. Neurodevelopmental outcome at 6 years of age after intrauterine laser therapy for twin-twin transfusion syndrome. Acta Paediatr 2012;101:1200–5. https://doi.org/10.1111/apa.12017.Suche in Google Scholar PubMed
44. Klaritsch, P, Deprest, J, Van Mieghem, T, Gucciardo, L, Done, E, Jani, J, et al.. Reference ranges for middle cerebral artery peak systolic velocity in monochorionic diamniotic twins: a longitudinal study. Ultrasound Obstet Gynecol 2009;34:149–54. https://doi.org/10.1002/uog.6436.Suche in Google Scholar PubMed
45. Van Mieghem, T, Martin, AM, Weber, R, Barrea, C, Windrim, R, Hornberger, LK, et al.. Fetal cardiac function in recipient twins undergoing fetoscopic laser ablation of placental anastomoses for stage IV twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2013;42:64–9. https://doi.org/10.1002/uog.12454.Suche in Google Scholar PubMed
46. Assaf, SA, Korst, LM, Chmait, RH. Normalization of amniotic fluid levels after fetoscopic laser surgery for twin-twin transfusion syndrome. J Ultrasound Med: Off J American Inst Ultrasound Med 2010;29:1431–6. https://doi.org/10.7863/jum.2010.29.10.1431.Suche in Google Scholar PubMed
47. Habli, M, Bombrys, A, Lewis, D, Lim, FY, Polzin, W, Maxwell, R, et al.. Incidence of complications in twin-twin transfusion syndrome after selective fetoscopic laser photocoagulation: a single-center experience. Am J Obstet Gynecol 2009;201:417 e1–7. https://doi.org/10.1016/j.ajog.2009.07.046.Suche in Google Scholar PubMed
48. Spruijt, M, Steggerda, S, Rath, M, van Zwet, E, Oepkes, D, Walther, F, et al.. Cerebral injury in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol 2012;120:15–20. https://doi.org/10.1097/aog.0b013e31825b9841.Suche in Google Scholar
49. Herberg, U, Gross, W, Bartmann, P, Banek, CS, Hecher, K, Breuer, J. Long term cardiac follow up of severe twin to twin transfusion syndrome after intrauterine laser coagulation. Heart 2006;92:95–100. https://doi.org/10.1136/hrt.2004.057497.Suche in Google Scholar PubMed PubMed Central
50. Mustafa, HJ, Jawwad, M, Iqbal Mansoor, A, Pagani, G, D’Antonio, F, Khalil, A. Right ventricular outflow tract obstruction in twin-to-twin transfusion syndrome undergoing laser surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2024;103:1513–21. https://doi.org/10.1111/aogs.14825.Suche in Google Scholar PubMed PubMed Central
51. Herberg, U, Bolay, J, Graeve, P, Hecher, K, Bartmann, P, Breuer, J. Intertwin cardiac status at 10-year follow-up after intrauterine laser coagulation therapy of severe twin-twin transfusion syndrome: comparison of donor, recipient and normal values. Arch Dis Child Fetal Neonatal Ed 2014;99:F380–5. https://doi.org/10.1136/archdischild-2013-305034.Suche in Google Scholar PubMed
52. Stirnemann, JJ, Quibel, T, Essaoui, M, Salomon, LJ, Bussieres, L, Ville, Y. Timing of delivery following selective laser photocoagulation for twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2012;207:127 e1–6. https://doi.org/10.1016/j.ajog.2012.06.042.Suche in Google Scholar PubMed
53. Slaghekke, F, Kist, WJ, Oepkes, D, Pasman, SA, Middeldorp, JM, Klumper, FJ, et al.. Twin anemia-polycythemia sequence: diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther 2010;27:181–90. https://doi.org/10.1159/000304512.Suche in Google Scholar PubMed
54. Khalil, A, Gordijn, S, Ganzevoort, W, Thilaganathan, B, Johnson, A, Baschat, AA, et al.. Consensus diagnostic criteria and monitoring of twin anemia-polycythemia sequence: delphi procedure. Ultrasound Obstet Gynecol 2020;56:388–94. https://doi.org/10.1002/uog.21882.Suche in Google Scholar PubMed
55. Lucewicz, A, Fisher, K, Henry, A, Welsh, AW. Review of the correlation between blood flow velocity and polycythemia in the fetus, neonate and adult: appropriate diagnostic levels need to be determined for twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2016;47:152–7. https://doi.org/10.1002/uog.14782.Suche in Google Scholar PubMed
56. Tollenaar, LSA, Lopriore, E, Middeldorp, JM, Haak, MC, Klumper, FJ, Oepkes, D, et al.. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound Obstet Gynecol 2019;53:788–93. https://doi.org/10.1002/uog.20096.Suche in Google Scholar PubMed PubMed Central
57. Tavares de Sousa, M, Fonseca, A, Hecher, K. Role of fetal intertwin difference in middle cerebral artery peak systolic velocity in predicting neonatal twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2019;53:794–7. https://doi.org/10.1002/uog.20116.Suche in Google Scholar PubMed
58. Tollenaar, LSA, Lopriore, E, Middeldorp, JM, Klumper, F, Haak, MC, Oepkes, D, et al.. Prevalence of placental dichotomy, fetal cardiomegaly and starry-sky liver in twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2020;56:395–9. https://doi.org/10.1002/uog.21948.Suche in Google Scholar PubMed PubMed Central
59. Slaghekke, F, Lopriore, E, Lewi, L, Middeldorp, JM, van Zwet, EW, Weingertner, AS, et al.. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet 2014;383:2144–51. https://doi.org/10.1016/s0140-6736(13)62419-8.Suche in Google Scholar
60. Genova, L, Slaghekke, F, Klumper, FJ, Middeldorp, JM, Steggerda, SJ, Oepkes, D, et al.. Management of twin anemia-polycythemia sequence using intrauterine blood transfusion for the donor and partial exchange transfusion for the recipient. Fetal Diagn Ther 2013;34:121–6. https://doi.org/10.1159/000346413.Suche in Google Scholar PubMed
61. Tollenaar, LSA, Slaghekke, F, Lewi, L, Colmant, C, Lanna, M, Weingertner, AS, et al.. Spontaneous twin anemia polycythemia sequence: diagnosis, management, and outcome in an international cohort of 249 cases. Am J Obstet Gynecol 2021;224:213 e1–11. https://doi.org/10.1016/j.ajog.2020.07.041.Suche in Google Scholar PubMed
62. Tollenaar, LSA, Slaghekke, F, Lewi, L, Ville, Y, Lanna, M, Weingertner, A, et al.. Treatment and outcome of 370 cases with spontaneous or post-laser twin anemia-polycythemia sequence managed in 17 fetal therapy centers. Ultrasound Obstet Gynecol 2020;56:378–87. https://doi.org/10.1002/uog.22042.Suche in Google Scholar PubMed PubMed Central
63. Tollenaar, LSA, Lopriore, E, Slaghekke, F, Oepkes, D, Middeldorp, JM, Haak, MC, et al.. High risk of long-term neurodevelopmental impairment in donor twins with spontaneous twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2020;55:39–46. https://doi.org/10.1002/uog.20846.Suche in Google Scholar PubMed
64. van de Sande, MJA, Lopriore, E, Lanna, M, Ville, Y, Lewi, L, Weingertner, AS, et al.. Single fetal demise in twin anemia-polycythemia sequence: perinatal outcome of surviving cotwin. Ultrasound Obstet Gynecol 2025;66:51–5. https://doi.org/10.1002/uog.29242.Suche in Google Scholar PubMed PubMed Central
65. Lewi, L, Valencia, C, Gonzalez, E, Deprest, J, Nicolaides, KH. The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am J Obstet Gynecol 2010;203:213 e1–4. https://doi.org/10.1016/j.ajog.2010.04.018.Suche in Google Scholar PubMed
66. Tonni, G, Granese, R, Incognito, GG, Grisolia, G, Lituania, M, Sepulveda, W, et al.. Outcomes of intrauterine interventions in twin reversed arterial perfusion (TRAP) sequence: a systematic review of the literature over the past 35 years. Prenat Diagn 2025;45:396–422. https://doi.org/10.1002/pd.6725.Suche in Google Scholar PubMed
67. Molina-Giraldo, S, Torres-Valencia, N, Johnson, A, Lewi, L, Ryan, G, Sepulveda, W. The management of acardiac twinning: twin reverse arterial perfusion sequence – an international survey. Fetal Diagn Ther 2023;50:446–53. https://doi.org/10.1159/000531791.Suche in Google Scholar PubMed
68. Weber, EC, Recker, F, Gottschalk, I, Strizek, B, Geipel, A, Gembruch, U, et al.. Outcome of TRAP sequence treated in the first trimester – a ten-year single-center experience. Ultraschall Med 2022;43:614–8. https://doi.org/10.1055/a-1526-1775.Suche in Google Scholar PubMed
69. Khalil, A, Beune, I, Hecher, K, Wynia, K, Ganzevoort, W, Reed, K, et al.. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol 2019;53:47–54. https://doi.org/10.1002/uog.19013.Suche in Google Scholar PubMed
70. Vanderheyden, TM, Fichera, A, Pasquini, L, Tan, TY, Wee, LY, Frusca, T, et al.. Increased latency of absent end-diastolic flow in the umbilical artery of monochorionic twin fetuses. Ultrasound Obstet Gynecol 2005;26:44–9. https://doi.org/10.1002/uog.1900.Suche in Google Scholar PubMed
71. Ishii, K, Murakoshi, T, Hayashi, S, Saito, M, Sago, H, Takahashi, Y, et al.. Ultrasound predictors of mortality in monochorionic twins with selective intrauterine growth restriction. Ultrasound Obstet Gynecol 2011;37:22–6. https://doi.org/10.1002/uog.8846.Suche in Google Scholar PubMed
72. Gratacos, E, Lewi, L, Munoz, B, Acosta-Rojas, R, Hernandez-Andrade, E, Martinez, JM, et al.. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol 2007;30:28–34. https://doi.org/10.1002/uog.4046.Suche in Google Scholar PubMed
73. El, ES, Groene, SG, Verweij, EJ, Slaghekke, F, Khalil, A, van Klink, JMM, et al.. Gestational age at birth and outcome in monochorionic twins with different types of selective fetal growth restriction: a systematic literature review. Prenat Diagn 2022;42:1094–110. https://doi.org/10.1002/pd.6206.Suche in Google Scholar PubMed PubMed Central
74. Ishii, K, Murakoshi, T, Takahashi, Y, Shinno, T, Matsushita, M, Naruse, H, et al.. Perinatal outcome of monochorionic twins with selective intrauterine growth restriction and different types of umbilical artery Doppler under expectant management. Fetal Diagn Ther 2009;26:157–61. https://doi.org/10.1159/000253880.Suche in Google Scholar PubMed
75. Chalouhi, GE, Marangoni, MA, Quibel, T, Deloison, B, Benzina, N, Essaoui, M, et al.. Active management of selective intrauterine growth restriction with abnormal Doppler in monochorionic diamniotic twin pregnancies diagnosed in the second trimester of pregnancy. Prenat Diagn 2013;33:109–15. https://doi.org/10.1002/pd.4031.Suche in Google Scholar PubMed
76. Parra-Cordero, M, Bennasar, M, Martinez, JM, Eixarch, E, Torres, X, Gratacos, E. Cord occlusion in monochorionic twins with early selective intrauterine growth restriction and abnormal umbilical artery doppler: a consecutive series of 90 cases. Fetal Diagn Ther 2016;39:186–91. https://doi.org/10.1159/000439023.Suche in Google Scholar PubMed
77. Gratacos, E, Carreras, E, Becker, J, Lewi, L, Enriquez, G, Perapoch, J, et al.. Prevalence of neurological damage in monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end-diastolic umbilical artery flow. Ultrasound Obstet Gynecol 2004;24:159–63. https://doi.org/10.1002/uog.1105.Suche in Google Scholar PubMed
78. Valsky, DV, Eixarch, E, Martinez, JM, Crispi, F, Gratacos, E. Selective intrauterine growth restriction in monochorionic twins: pathophysiology, diagnostic approach and management dilemmas. Semin Fetal Neonatal Med 2010;15:342–8. https://doi.org/10.1016/j.siny.2010.07.002.Suche in Google Scholar PubMed
79. Gratacos, E, Antolin, E, Lewi, L, Martinez, JM, Hernandez-Andrade, E, Acosta-Rojas, R, et al.. Monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end-diastolic flow (type III): feasibility and perinatal outcome of fetoscopic placental laser coagulation. Ultrasound Obstet Gynecol 2008;31:669–75. https://doi.org/10.1002/uog.5362.Suche in Google Scholar PubMed
80. Allen, VM, Windrim, R, Barrett, J, Ohlsson, A. Management of monoamniotic twin pregnancies: a case series and systematic review of the literature. BJOG 2001;108:931–6. https://doi.org/10.1111/j.1471-0528.2001.00216.x.Suche in Google Scholar PubMed
81. Baxi, LV, Walsh, CA. Monoamniotic twins in contemporary practice: a single-center study of perinatal outcomes. J Matern Fetal Neonatal Med 2010;23:506–10. https://doi.org/10.3109/14767050903214590.Suche in Google Scholar PubMed
82. Dias, T, Mahsud-Dornan, S, Bhide, A, Papageorghiou, AT, Thilaganathan, B. Cord entanglement and perinatal outcome in monoamniotic twin pregnancies. Ultrasound Obstet Gynecol 2010;35:201–4. https://doi.org/10.1002/uog.7501.Suche in Google Scholar PubMed
83. Rossi, AC, Prefumo, F. Impact of cord entanglement on perinatal outcome of monoamniotic twins: a systematic review of the literature. Ultrasound Obstet Gynecol 2013;41:131–5. https://doi.org/10.1002/uog.12345.Suche in Google Scholar PubMed
84. Aurioles-Garibay, A, Hernandez-Andrade, E, Romero, R, Garcia, M, Qureshi, F, Jacques, SM, et al.. Presence of an umbilical artery notch in monochorionic/monoamniotic twins. Fetal Diagn Ther 2014;36:305–11. https://doi.org/10.1159/000361020.Suche in Google Scholar PubMed PubMed Central
85. Van Mieghem, T, De Heus, R, Lewi, L, Klaritsch, P, Kollmann, M, Baud, D, et al.. Prenatal management of monoamniotic twin pregnancies. Obstet Gynecol 2014;124:498–506. https://doi.org/10.1097/aog.0000000000000409.Suche in Google Scholar PubMed
86. Khairudin, D, Khalil, A. Monochorionic monoamniotic twin pregnancies. Best Pract Res Clin Obstet Gynaecol 2022;84:96–103. https://doi.org/10.1016/j.bpobgyn.2022.08.004.Suche in Google Scholar PubMed
87. Ong, SS, Zamora, J, Khan, KS, Kilby, MD. Prognosis for the co-twin following single-twin death: a systematic review. BJOG 2006;113:992–8. https://doi.org/10.1111/j.1471-0528.2006.01027.x.Suche in Google Scholar PubMed
88. Hillman, SC, Morris, RK, Kilby, MD. Single twin demise: consequence for survivors. Semin Fetal Neonatal Med 2010;15:319–26. https://doi.org/10.1016/j.siny.2010.05.004.Suche in Google Scholar PubMed
89. Shek, NW, Hillman, SC, Kilby, MD. Single-twin demise: pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2014;28:249–63. https://doi.org/10.1016/j.bpobgyn.2013.11.003.Suche in Google Scholar PubMed
90. Senat, MV, Loizeau, S, Couderc, S, Bernard, JP, Ville, Y. The value of middle cerebral artery peak systolic velocity in the diagnosis of fetal anemia after intrauterine death of one monochorionic twin. Am J Obstet Gynecol 2003;189:1320–4. https://doi.org/10.1067/s0002-9378(03)00644-6.Suche in Google Scholar PubMed
91. Nicolini, U, Pisoni, MP, Cela, E, Roberts, A. Fetal blood sampling immediately before and within 24 hours of death in monochorionic twin pregnancies complicated by single intrauterine death. Am J Obstet Gynecol 1998;179:800–3. https://doi.org/10.1016/s0002-9378(98)70086-9.Suche in Google Scholar PubMed
92. Senat, MV, Bernard, JP, Loizeau, S, Ville, Y. Management of single fetal death in twin-to-twin transfusion syndrome: a role for fetal blood sampling. Ultrasound Obstet Gynecol 2002;20:360–3. https://doi.org/10.1046/j.1469-0705.2002.00815.x.Suche in Google Scholar PubMed
93. Nakata, M, Sumie, M, Murata, S, Miwa, I, Kusaka, E, Sugino, N. A case of monochorionic twin pregnancy complicated with intrauterine single fetal death with successful treatment of intrauterine blood transfusion in the surviving fetus. Fetal Diagn Ther 2007;22:7–9. https://doi.org/10.1159/000095834.Suche in Google Scholar PubMed
94. Shinar, S, Agrawal, S, El-Chaar, D, Abbasi, N, Beecroft, R, Kachura, J, et al.. Selective fetal reduction in complicated monochorionic twin pregnancies: a comparison of techniques. Prenat Diagn 2021;41:52–60. https://doi.org/10.1002/pd.5830.Suche in Google Scholar PubMed
95. Greimel, P, Csapo, B, Haeusler, M, Lang, U, Klaritsch, P. A modified technique for cord transection in monochorionic monoamniotic twin pregnancies. Fetal Diagn Ther 2018;44:236–40. https://doi.org/10.1159/000489882.Suche in Google Scholar PubMed
96. Bolch, C, Fahey, M, Reddihough, D, Williams, K, Reid, S, Guzys, A, et al.. Twin-to-twin transfusion syndrome neurodevelopmental follow-up study (neurodevelopmental outcomes for children whose twin-to-twin transfusion syndrome was treated with placental laser photocoagulation). BMC Pediatr 2018;18:256. https://doi.org/10.1186/s12887-018-1230-8.Suche in Google Scholar PubMed PubMed Central
97. Ortibus, E, Lopriore, E, Deprest, J, Vandenbussche, FP, Walther, FJ, Diemert, A, et al.. The pregnancy and long-term neurodevelopmental outcome of monochorionic diamniotic twin gestations: a multicenter prospective cohort study from the first trimester onward. Am J Obstet Gynecol 2009;200:494 e1–8. https://doi.org/10.1016/j.ajog.2009.01.048.Suche in Google Scholar PubMed
98. Casella, A, Moccia, S, Frontoni, E, Paladini, D, De Momi, E, Mattos, LS. Inter-foetus membrane segmentation for TTTS using adversarial networks. Ann Biomed Eng 2020;48:848–59. https://doi.org/10.1007/s10439-019-02424-9.Suche in Google Scholar PubMed
99. Bano, S, Vasconcelos, F, Vander Poorten, E, Vercauteren, T, Ourselin, S, Deprest, J, et al.. FetNet: a recurrent convolutional network for occlusion identification in fetoscopic videos. Int J Comput Assist Radiol Surg 2020;15:791–801. https://doi.org/10.1007/s11548-020-02169-0.Suche in Google Scholar PubMed PubMed Central
100. van der Schot, AM, Sikkel, E, Spaanderman, MEA, Vandenbussche, F. Computer-assisted fetal laser surgery in the treatment of twin-to-twin transfusion syndrome: recent trends and prospects. Prenat Diagn 2022;42:1225–34. https://doi.org/10.1002/pd.6225.Suche in Google Scholar PubMed PubMed Central
101. Khan, IU, Aslam, N, Anis, FM, Mirza, S, AlOwayed, A, Aljuaid, RM, et al.. Amniotic fluid classification and artificial intelligence: challenges and opportunities. Sensors (Basel) 2022;22. https://doi.org/10.3390/s22124570.Suche in Google Scholar PubMed PubMed Central
102. Casella, A, Bano, S, Vasconcelos, F, David, AL, Paladini, D, Deprest, J, et al.. Learning-based keypoint registration for fetoscopic mosaicking. Int J Comput Assist Radiol Surg 2024;19:481–92. https://doi.org/10.1007/s11548-023-03025-7.Suche in Google Scholar PubMed PubMed Central
103. Bano, S, Vasconcelos, F, Tella-Amo, M, Dwyer, G, Gruijthuijsen, C, Vander, PE, et al.. Deep learning-based fetoscopic mosaicking for field-of-view expansion. Int J Comput Assist Radiol Surg 2020;15:1807–16. https://doi.org/10.1007/s11548-020-02242-8.Suche in Google Scholar PubMed PubMed Central
104. Ahmad, MA, Ourak, M, Gruijthuijsen, C, Deprest, J, Vercauteren, T, Vander Poorten, E. Deep learning-based monocular placental pose estimation: towards collaborative robotics in fetoscopy. Int J Comput Assist Radiol Surg 2020;15:1561–71. https://doi.org/10.1007/s11548-020-02166-3.Suche in Google Scholar PubMed PubMed Central
105. Caloone, J, Huissoud, C, Kocot, A, Vincenot, J, Dehay, C, Giroud, P, et al.. Non-invasive high-intensity focused ultrasound treatment of the placenta: a preliminary in-vivo study using a simian model. Ultrasound Obstet Gynecol 2017;50:635–41. https://doi.org/10.1002/uog.17350.Suche in Google Scholar PubMed
106. Shaw, CJ, Civale, J, Botting, KJ, Niu, Y, Ter Haar, G, Rivens, I, et al.. Noninvasive high-intensity focused ultrasound treatment of twin-twin transfusion syndrome: a preliminary in vivo study. Sci Transl Med 2016;8:347ra95. https://doi.org/10.1126/scitranslmed.aaf2135.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

