Effect of antenatal betamethasone on fetal heart rate short-term variability in growth restricted fetuses
-
Tjaša Šikonja
, Ivan Verdenik
, Renata Košir Pogačnik
, Miha Lučovnik
, Gabrijela Bržan Šimenc
Abstract
Objectives
The study aimed to evaluate the magnitude and duration of the effect of antenatal betamethasone on fetal heart rate short-term variability (STV) in growth-restricted (FGR) fetuses in comparison with appropriate-for-gestational age (AGA) fetuses.
Methods
A prospective observational study was conducted at the UMC Ljubljana between June 2023 and June 2024, including 21 FGR and 20 AGA fetuses. We measured STV before applying betamethasone and at regular intervals for seven days after the first application or until delivery. Confounding variables were fetal and maternal demographic and clinical characteristics. Analysis was done using linear regression, paired-sample t-tests and one-way and two-way analysis of variance.
Results
The increase in STV 6–12 h after the first application was significant compared to baseline in both groups (p < 0.001). STV remained significantly elevated the first 24 h after the first application in the FGR group (p=0.018) but not in the AGA group. There was no significant difference in STV between baseline and 48 and 72 h after the first application in either group. When adjusted for gestational age, STV was significantly lower in the group of FGR compared to AGA fetuses at all times of cCTG recordings (p=0.031).
Conclusions
Following the initial increase in STV after the first dose of betamethasone, STV declines and returns to levels that doesn’t differ significantly from baseline after 24 h in AGA and 48 h in FGR fetuses. Longer-lasting response of FGR fetuses to betamethasone merits further investigation.
Introduction
Preterm birth remains a leading cause of perinatal morbidity and mortality, with risk increasing with the earlier onset of the birth. Birth before 32 weeks of gestation requires prolonged neonatal intensive care, primarily due to pulmonary immaturity. When birth before 34 weeks of gestation is imminent, antenatal corticosteroid (GCS) therapy is administrated in order to reduce the risks of neonatal respiratory distress syndrome [1], 2]. One of the most common causes of medically indicated preterm delivery is fetal growth restriction (FGR). We proceed with delivery when fetal status is assessed as critically compromised, posing a threat of irreversible damage of intrauterine death. In early gestation, the aim is to prolong pregnancy to improve the chances of healthy survival. The decision to deliver due to suspected fetal hypoxia is based on the assessment of fetal status, which relies mainly on indirect methods, including ultrasound and cardiotocography (CTG). CTG recording interpretation is at least partly subjective and particularly challenging in early gestation due to the gestational age-dependent maturity of the autonomic nervous system (ANS), which coordinates the fetal heart rate. For this reason, the use of computerized CTG (cCTG) and short-term (beat-to-beat) variability (STV) of the fetal heart rate is recommended in cases of FGR [3], 4]. Low STV values of the fetus, considering gestational age, can indicate fetal compromise as a result of hypoxia and is an indication for immediate delivery regardless of gestation [5]. STV is regulated by the autonomic nervous system (ANS) through a negative feedback loop, in which sympathetic precedes parasympathetic maturation. During fetal hypoxia, sympathetic dominance occurs. GCS have a similar effect on the ANS as hypoxia, which can lead to misinterpretation of the STV and possibly an inappropriate decision to deliver. The effect of maturation therapy with corticosteroids on STV is not fully understood, and data on the effect of GCS on FGR fetuses are limited. An initial transient increase in STV following GCS administration is documented; however, findings regarding a subsequent decrease in STV after completing the therapy are inconsistent [6], 7]. Moreover, short-term variability (STV) thresholds indicative of fetal hypoxia – and thus indicating the need for immediate delivery – vary across established international guidelines [3], 8].
Our study aimed to evaluate the magnitude and duration of the effect of antenatal GCS therapy with betamethasone on STV in FGR fetuses and to compare these findings with appropriate for gestational age (AGA) fetuses. Stress-related metabolism resulting from impaired oxygenation and/or nutrient supply in FGR may accelerate the maturation of the fetal autonomic nervous system, which could lead to a different physiological response to exogenous corticosteroid administration compared to AGA fetuses, therefore contributing to observed differences in treatment response between both groups. A better understanding of the impact of antenatal betamethasone on STV could improve clinical interpretation of fetal status during and after GCS administration, which could help to avoid misclassification of fetuses as hypoxic – leading to potentially unnecessary early delivery – as opposed to those whose condition still permits expectant management aimed at prolonging gestation and increasing fetal maturity.
Subjects and methods
Study design
This prospective observational cohort study was conducted at the Department of Perinatology, University Medical Centre Ljubljana, between June 2023 and June 2024. The study included fetuses of pregnant women who, based on the decision of the attending physician, required standard clinical management with betamethasone for either threatened spontaneous or medically indicated preterm birth between 24 and 34 weeks of gestation. All pregnant women gave written informed consent following verbal and written explanations. The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (No. 0120–516/2022/17, June 5th, 2023) and conducted in accordance with the Declaration of Helsinki (as revised in 2013) The study was funded by research grants from University Medical Centre Ljubljana (No. TP2022128) and the Public Agency for Scientific Research and Innovation of the Republic of Slovenia (No. P3-0124).
Subjects
We included 41 fetuses with gestational age less than 34 weeks. Of these, 14 fetuses were twins, eight from bichorionic biamniotic, four from monochorionic biamniotic, and two from monochorionic monoamniotic pregnancies. None of the monochorionic pregnancies were complicated by twin-to-twin transfusion syndrome or twin anemia-policitemia sequence. Of the 41 fetuses, 21 (including 11 twin fetuses–two from monochorionic monoamniotic, four from monochorionic biamniotic and five from bichorionic biamniotic pregnancies) were classified as FGR, and 20 (including three twin fetuses from bichorionic pregnancies) were classified as AGA.
We determined gestational age by measuring crown-rump length (CRL) on first-trimester ultrasound up to 13 weeks and six days in spontaneous conceptions and by the date of oocyte retrieval or embryo transfer (adjusted for embryo age at transfer) in pregnancies conceived via assisted reproductive technology [9]. FGR was determined using Delphi consensus criteria without structural anomalies [3], 10]. AGA was defined as the fetal weight for gestational age between the 25th and 75th centiles. The Hadlock growth chart was used to assess fetal weight and adequacy for gestational age [11]. The indication for antenatal corticosteroid therapy in the FGR group was threatened preterm delivery or planned preterm delivery due to the worsening of the fetal wellbeing. The indication for antenatal corticosteroid therapy in the AGA group was threatened preterm delivery, which was anticipated based on cervical insufficiency in nine cases and preterm premature rupture of membranes without clinical and/or laboratory signs of intrauterine infection in four cases. In the remaining seven AGA fetuses, other indications were present, including preeclampsia, gestational diabetes with preeclampsia or gestational hypertension, hepatopathy and low-lying placenta. Cervical insufficiency was defined as progressive cervical shortening to≤1 cm with cervical dilatation≥1 cm in the absence of uterine contractions.
In the study, women who did not understand the Slovenian explanation or had signs of inflammation (clinical or laboratory) were not invited. We also did not include fetuses whose fetal growth did not meet the previously described criteria, whose gestational age was unclear, who had a structural or genetic developmental abnormality, or who did not have a cCTG recording before the first application of betamethasone due to in utero transport from another maternity hospital. No pregnant woman invited refused to participate.
Variables
The study’s observed dependent variable was STV before the application of betamethasone, 6–12 h after the first application, on the first day (>12 h), and then at 24-h intervals 7 days after the first application or until delivery.
The independent variable was the antenatal application of betamethasone intramuscularly in two doses of 14 mg 24 h apart.
Among confounding variables we consider fetal baseline characteristics: estimated fetal weight EFW (g), abdominal circumference AC (mm) measured via ultrasound, umbilical artery pulsatility index (UA-PI), and the UA-PI percentile. Maternal confounding variables included body mass index BMI (kg/m2) measured upon inclusion in the study, presence or absence of chronic arterial hypertension, pregestational diabetes mellitus, and ongoing treatments with antihypertensive agents, anxiolytics, antidepressants and/or low-dose aspirin (100 mg). In addition, the presence or absence of gestational diabetes mellitus (GDM), gestational hypertension, and preeclampsia was determined. Maternal biometric measurements were also obtained prior to each cCTG recording, including body temperature (°C), heart rate (beats per minute), and mean arterial pressure (mmHg). Gestational diabetes mellitus was defined as any diabetes diagnosed in the first or second trimester of pregnancy, according to established diagnostic criteria of the International Association of Diabetes and Pregnancy Study Groups [12]. Gestational hypertension was defined as new-onset systolic blood pressure≥140 mmHg and/or diastolic blood pressure≥90 mmHg occurring after 20 weeks of gestation without proteinuria or biochemical and hematological abnormalities. Preeclampsia was defined as gestational hypertension accompanied by proteinuria (≥1+, 3 g/L) and/or dysfunction of at least one organ system [13].
Mean arterial pressure (MAP) was calculated using the following formula: MAP = [systolic blood pressure + (2 × diastolic blood pressure)]/3
cCTG recording
All fetuses for whom we decided to undergo maturation therapy with betamethasone had a 40–60 min cCTG recorded before the first application, 6–12 h after, on the first day (>12 h), and then at 24-h intervals 7 days after the first application or until delivery. All cCTGs were recorded in the morning until noon, except for the cCTG before application and 6–12 h after it, which were tied to the time of application at any time of the day between 9 am and 5 pm. STV in milliseconds (ms) was recorded and analyzed automatically by the cCTG using the Dawes/Radman algorithm [14]. Upon inclusion in the study, we measured maternal weight, and BMI was calculated from body weight and height. Before each cCTG recording, the pregnant woman’s arterial blood pressure, pulse, and body temperature were measured. The SonicaidFM800 Encore and Sonicaid Team3 Series computerized cardiotocographs (Huntleigh Healthcare Ltd., Cardiff, United Kingdom) were used for all cCTG recordings.
Statistics
Descriptive statistics of maternal and fetal baseline characteristics were presented as frequencies and percentages for categorical variables and as means with standard deviations for continuous variables. Differences in baseline characteristics between groups were tested using Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Maternal biometric parameters at the time of cCTG recordings, within and between groups, were analyzed using a repeated measures test. Associations between short-term variability (STV) and maternal biometric measurements, gestational age, fetal growth type, and fetal characteristics were analyzed using linear regression with simple coding applied to categorical variables. Differences in STV between FGR and AGA fetuses at specific cCTG recording time points were assessed using a one-way analysis of variance (ANOVA). Differences in STV before and after betamethasone administration were analyzed using paired-sample t-tests. Group differences in STV across recording periods, adjusted for gestational age, were assessed using a two-way ANOVA (between-subject effects).
Results
A total of 41 fetuses who received betamethasone therapy for the risk of preterm delivery were included in the study. Twenty-one of them were FGR, and 20 were AGA. Among the 21 FGR fetuses, 11 had an EFW and/or AC below the 3rd percentile, 10 had an EFW and/or AC between the 3rd and 10th percentile accompanied by an umbilical artery pulsatility index (UA-PI) above the 95th percentile. Of the 205 planned cCTG recordings, 188 were completed and analyzed. The 17 missing recordings included one recording 6–12 h after the first betamethasone dose (AGA fetus who subsequently underwent the next scheduled recording), two recordings on the day after the first dose (both in FGR fetuses), five recordings on the day after the second dose (three in FGR fetuses and two in AGA fetuses); and nine recordings two days after the second dose, which were missed due to delivery (seven in FGR and two in AGA fetuses).
FGR fetuses had, as expected, significantly lower weight (1310.90 ± 431.26 g vs. 1717.47 ± 449.06 g; p=0.006) and a smaller AC for gestational age (236.73 ± 29.95 mm vs. 253.10 ± 60.07; p=0.016) and the average AU-PI was significantly higher (1.31 ± 0.38 vs. 1.02 ± 0.19; p=0.002). Similarly, the percentile values of the measurements were also significantly lower in FGR (Table 1). The average gestational age did not differ significantly between them. The groups did not differ significantly in maternal characteristics (Table 1).
Basic fetal and maternal characteristics at the time of enrollment in the study.
| Variable | Group of FGR fetuses n=21 |
Group of AGA fetuses n=20 |
p-Value |
|---|---|---|---|
| Maternal characteristics | |||
| BMI (kg/m2) | 29.10 (6.59) | 27.66 (3.95) | 0.406 |
| Chronic arterial hypertension | 1 (4.8) | 2 (10.0) | 0.606 |
| Natural conception | 18 (85.7) | 18 (90.0) | 0.173 |
| Preeclampsia | 3 (14.3) | 1 (5.0) | 0.606 |
| Gestational hypertension | 5 (23.8) | 1 (5.0) | 0.184 |
| Gestational diabetes | 8 (38.1) | 8 (40.0) | 1,000 |
| Low-dose aspirin | 3 (14.3) | 5 (25.0) | 0.454 |
| Antihypertensive medication | 4 (19.0) | 2 (10.0) | 0.663 |
| Fetal characteristics | |||
| Gestational age (days) | 218.38 (15.22) | 216.8 (16.45) | 0.751 |
| Estimated fetal weight (g) | 1310.90 (431.26) | 1717.47 (449.06) | 0.006 |
| Estimated fetal weight percentile | 4.90 (5.63) | 47.58 (19.27) | < 0.001 |
| Abdominal circumference (mm) | 236.73 (29.95) | 253.10 (60.07) | 0.016 |
| Abdominal circumference percentile | 3.00 (5.69) | 47.37 (19.45) | < 0.001 |
| AU-PI | 1.31 (0.38) | 1.02 (0.19) | 0.002 |
| AU-PI percentile | 85.20 (20.84) | 59.41 (29.10) | 0.002 |
-
Results are shown as mean value (SD) for continuous variables and as number (%) for categorial variables. FGR, fetal growth restriction; AGA, appropriate-for-gestational age; BMI, Body mass index; AU-PI, umbilical artery pulsatility index.
Maternal body temperature (p=0.252), arterial pulse (p=0.401) and mean arterial pressure (p=0.072) did not differ significantly between FGR and AGA fetuses at all times of cCTG recordings, and none of them received antidepressants or anxiolytics (Figure 1).
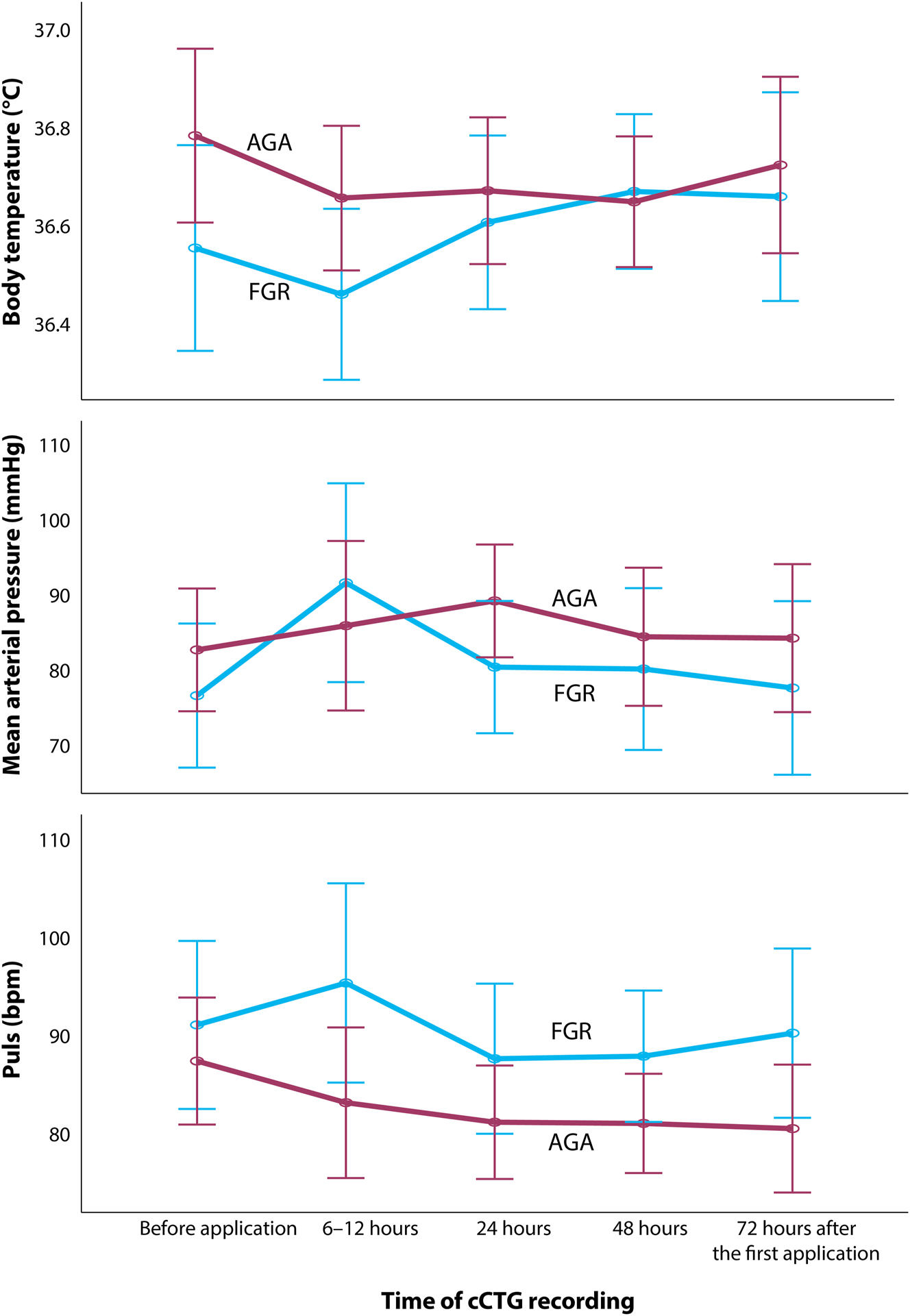
Maternal biometric measurements during the time of cCTG (computerized cardiotocogram) recording in the group of growth restricted (FGR) and appropriate-for-gestational age (AGA) fetuses considering betamethasone application.
Before the first dose of betamethasone, the STV value was significantly affected by the type of fetal growth (p=0.038) and the gestational age of the fetus (p=0.001) in the regression model, independently of other confounding variables (Table 2) FGR fetuses had an average of 4.3 m s lower STV values at any gestation compared with fetuses with normal growth. With each day of gestation, the STV value was, on average, 0.12 m s higher, regardless of the type of fetal growth.
Association of fetal heart rate short-term variability with fetal growth, gestational age, and maternal characteristics before the first application of betamethasone and cCTG recording (n=41).
| Variable | Non-standard. coefficient beta | Standard error coefficient | Standard. coefficient beta | Regression t-test | p-Value |
|---|---|---|---|---|---|
| Gestational age (days) | 0.115 | 0.032 | 0.555 | 3,623 | 0.001 |
| Fetal growth (normal vs. growth restriction) | 4,288 | 1,948 | 0.647 | 2,201 | 0.038 |
| Body mass index (kg/m2) | – 0.028 | 0.115 | – 0.040 | – 0.248 | 0.807 |
| Arterial pulse (beats/min) | – 0.037 | 0.042 | – 0.142 | – 0.886 | 0.385 |
| Body temperature (oC) | – 0.499 | 1,840 | – 0.046 | – 0.271 | 0.789 |
| Mean arterial pressure (mmHg) | – 0.043 | 0.052 | – 0.142 | – 0.831 | 0.415 |
| Estimated fetal weight percentile | – 0.060 | 0.051 | – 0.444 | – 1,166 | 0.255 |
| Abdominal circumference percentile | 0.005 | 0.057 | 0.037 | 0.086 | 0.932 |
-
Dependent variable: Fetal heart rate short-term variability (ms).
STV increased 6–12 h after the first dose of betamethasone by an average of 5.05 m s in the FGR group and 4.52 m s in the AGA group. Following this transient elevation, STV decreased by 4.00 m s, 0.81 m s, and 1.06 m s in the FGR group and by 3.24 m s, 1.48 m s, and 0.95 m s in the AGA group. STV values did not differ significantly between the two groups at any time (Table 3).
Fetal heart rate short-term variability of before and after application of betamethasone in growth-restricted and appropriate-for-gestational age fetuses, regardless of gestational age.
| FGR fetuses | AGA fetuses | p-Value | Eta squared effect size | |||
|---|---|---|---|---|---|---|
| Time period of cCTG recording | n | STV (ms) (SD) | n | STV (ms) (SD) | ||
| Before drug application | 21 | 8.43 (3.10) | 20 | 9.93 (3.12) | 0.132 | 0.232 |
| 6–12 h after the first dose | 21 | 13.48 (7.18) | 18 | 14.45 (3.59) | 0.605 | 0.134 |
| 24 h after the first dose | 19 | 9.48 (2.57) | 16 | 11.21 (4.74) | 0.179 | 0.243 |
| 48 h after the first dose | 15 | 8.67 (4.02) | 16 | 9.73 (3.69) | 0.454 | 0.192 |
| 72 h after the first dose | 13 | 7.61 (2.89) | 15 | 8.78 (3.65) | 0.360 | 0.231 |
-
Statistical method: one-way analysis of variance (ANOVA). FGR, fetal growth restriction; AGA, appropriate-for-gestational age; cCTG, computerized cardiotocograph; SVT, sfetal heart rate hort-term variability (ms).
The increase in STV observed 6–12 h after the first dose of betamethasone was statistically significant compared to baseline in both groups (p < 0.001). STV remained significantly elevated one day after the first dose in the FGR group (p=0.018). Meanwhile, it was not significantly elevated in the AGA group. The difference between the two groups was not statistically significant at that time point. Furthermore, there was no significant difference in STV between baseline (before the first dose of betamethasone) and the measurements taken on the second day after the first dose (48 h) or the third day after the second dose (72 h) in either group (Table 4, Figure 2).
Differences in fetal heart rate short-term variability before and after betamethasone application.
| Group | cCTG pairs by recording period | Paired differences | Statistical signif | |||||
|---|---|---|---|---|---|---|---|---|
| Mean value | SD | SE | 95-% confidence interval | t-test | df | p-Value | ||
| FGR fetuses | STV1 - STV2 | −5.05 | 5.62 | 1.23 | −7.61 to −2.49 | −4,114 | 20 | < 0.001 |
| STV1 - STV3 | −1.25 | 2.08 | 0.48 | −2.25–0.24 | −2,613 | 18 | 0.018 | |
| STV1 - STV4 | −0.51 | 3.18 | 0.82 | −2.28–1.25 | −0.625 | 14 | 0.542 | |
| STV1 - STV5 | 1.48 | 2.85 | 0.79 | −0.24–3.21 | 1,878 | 12 | 0.085 | |
| AGA fetuses | STV1 - STV2 | −4.55 | 3.81 | 0.90 | −6.44 to −2.66 | −5,072 | 17 | < 0.001 |
| STV1 - STV3 | −1.18 | 4.73 | 1.18 | −3.70–1.35 | −0.993 | 15 | 0.337 | |
| STV1 - STV4 | 0.28 | 3.29 | 0.82 | −1.48–2.03 | 0.334 | 15 | 0.743 | |
| STV1 - STV5 | 1.38 | 4.12 | 1.06 | −0.90–3.66 | 1,299 | 14 | 0.215 | |
-
FGR, fetal growth restriction; AGA, appropriate-for-gestational age; cCTG =computerized cardiotocogram; SD, standard deviation; SE, standard error; df=number of fetuses; STV, fetal heart rate short-term variability; STV1 =before the application of betamethasone, STV2 = 6–12 after the first application of betamethasone, STV3=24 after the first application, STV4=48 h after the first application, STV5=72 h after the first application.
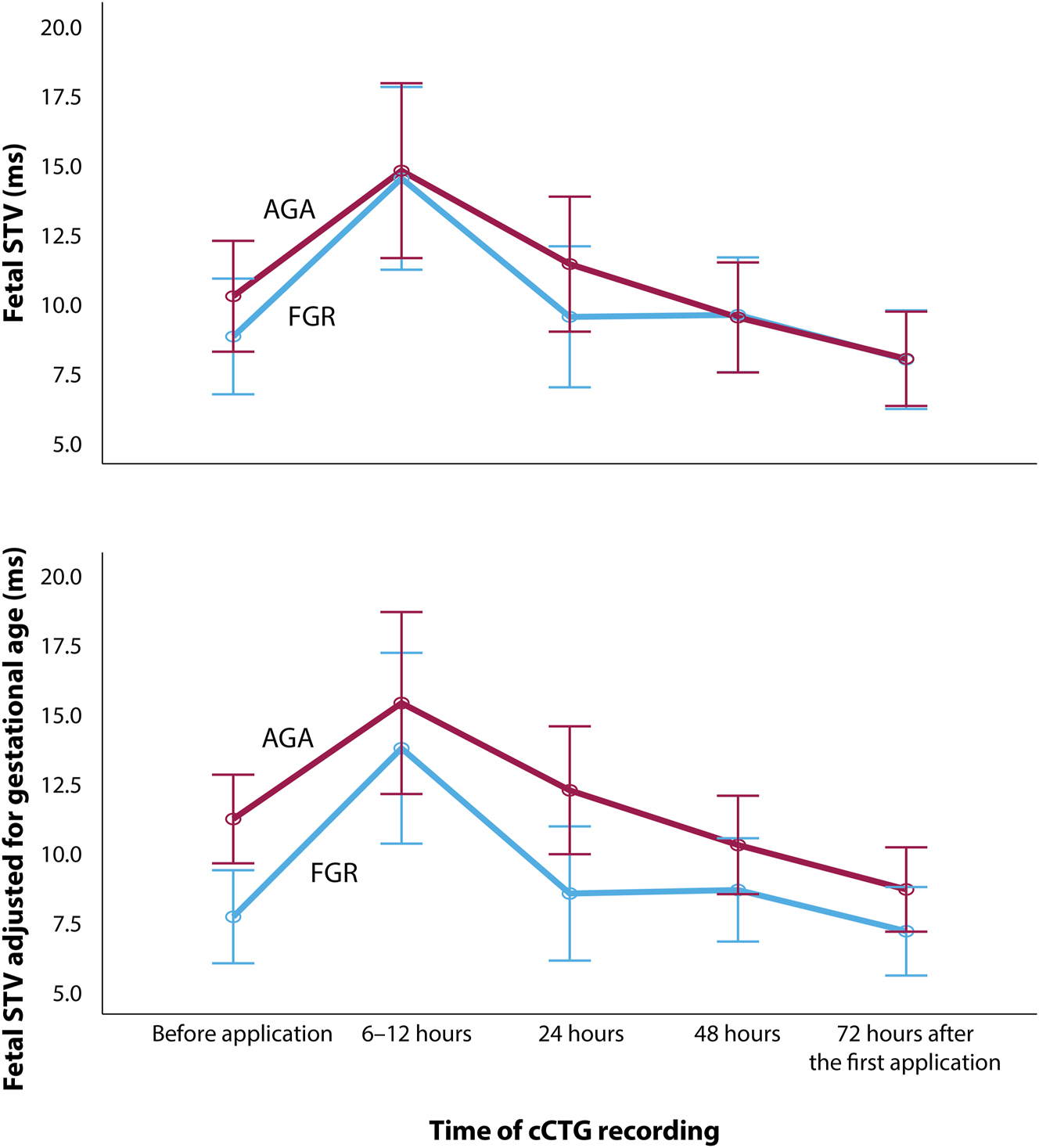
Fetal heart rate short-term variability (STV) before and after two doses of Betamethasone 24 h apart in growth restricted (FGR) (blue line) and appropriate-for-gestational age (AGA) fetuses (red line) before and after adjustement for gestational age. STV showed a significant increase in both groups 6–12 h after the first application, which remained increased in FGR fetuses after 24 h. After 24 and until 72 h, STV decreased to levels not significantly different from before application. Adjusted for gestational age, STV was significantly lower in FGR fetuses at all times of cCTG recordings (two-way analysis of variance; p=0.031).
When adjusted for gestational age, which was significantly associated with STV, STV differed significantly between the two groups and was lower in the FGR compared to the AGA group at all times of cCTG recordings (p=0.031) (Figure 2).
Discussion
The study aimed to assess the magnitude and duration of the effect of antenatal betamethasone on STV in FGR fetuses and to compare this response with that of AGA fetuses. In our study, antenatal betamethasone significantly affected STV in both fetal groups. Following the initial increase in STV after the first dose of betamethasone, STV declined – at different rates in the two groups – and returned to levels that did not differ significantly from baseline. The elevation in STV persisted longer in FGR fetuses than in AGA fetuses.
One possible explanation for the rapid increase of STV in FGR following the first dose of betamethasone is its effect on glucocorticoid receptors in the brain, which, via the hypothalamic suprachiasmatic nucleus, contributes to the suppression of the normal fetal diurnal rhythm. The inhibitory effect of betamethasone predominantly targets the sympathetic component of autonomic heart rate regulation, increasing STV [15], 16]. An increase in STV within 24 h after the first dose of betamethasone has been previously described in other studies, both in FGR fetuses and mixed cohorts. However, in contrast to our findings, several studies have reported a significant decrease in STV following the initial transient rise within the first 24 h, with the suppression lasting up to 72 h after the first dose of betamethasone [7], 17], 18]. Schneider et al. similarly observed suppression of overall fetal heart rate variability, not specifically short-term variability, following betamethasone administration using magnetocardiotocography [15]. Knaven et al. reported, in contrast to the present study’s results, that the STV increase was limited to the first 24 h after the initial dose in both the preeclampsia and FGR groups, as well as in the remaining study population [17]. The observed discrepancies between our findings and previous reports may reflect heterogeneity in fetal and maternal characteristics, differences in study design, and variability in corticosteroid protocols. In particular, the gestational age at administration and the degree of placental dysfunction in FGR fetuses likely influence the duration and direction of STV changes after antenatal corticosteroid administration. That is to say, in the study of the association between fetal heart rate variability and FGR, Zizzo et al. [19] found that while during fetal respiratory movements, FGR fetuses preserved vagal activity at similar levels as seen in AGA fetuses, during quiescence, they presented lower RMSSD and HF power, which is consistent with a suppressed cardiac vagal activity and an increased sympatho‐vagal balance, implying an activated stress response due to chronic hypoxia. The addition of exogenous GCS to an environment with elevated levels of endogenous corticosteroids may result in a prolonged initial suppressive effect on the sympathetic component of autonomic heart rate regulation. Due to the iatrogenically increased STV in FGR fetuses that persists longer than in AGA fetuses, cCTG cannot be a reliable measure for assessing fetal wellbeing during the first 48 h after the first application of betamethasone.
Based on the findings of our study, STV values in FGR fetuses measured 48 h after the first dose of betamethasone likely reflect the actual fetal condition rather than a residual pharmacologic effect of betamethasone. These results are in line with the conclusions of Knaven et al., who reported that the decline in STV 24 h after betamethasone administration was less than 1 m s, suggesting that any observed decrease in STV should be interpreted as a sign of fetal compromise rather than a persistent corticosteroid effect [17]. The decrease in STV values after 24 h from the first dose of betamethasone in our study ranged between 0.81 and 1.06 m s in FGR and between 1.48 and 0.95 m s in AGA fetuses. Based on these results, STV may be considered a reliable parameter for fetal assessment 48 h after betamethasone administration in FGR and 24 h after administration in AGA fetuses. In this context, STV threshold values should still be interpreted primarily as indicators of fetal hypoxia rather than as transient effects of antenatal corticosteroids.
The present study also found that the STV was significantly lower in FGR at any gestational age than in AGA fetuses. Ghi et al. observed a similar finding in a study on the association between fetal size and the incidence of reduced STV after antenatal betamethasone administration. They found that compared with fetuses with normal STV values, fetuses with persistently lower STV values had lower body weight estimates and birth weight [20]. Nijhuis et al. found that in FGR fetuses, the basal heart rate was generally higher, and the fetal heart rate variability was lower compared with the control group [21]. The lower fetal heart rate variability may be caused by an altered hypothalamic-pituitary-adrenal axis (HPA) characteristic of fetuses with growth retardation. Changes in HPA axis function lead to, among other things, reduced fetal and placental growth [22], 23].
Strengths and limitations
A strength of our study was the rigorous design. Why our findings differ from those of previous studies may be partly explained by differences in the definition of FGR. Notably, other studies did not consistently exclude fetuses from pregnancies complicated by maternal infection. The prospective design of our study allowed us to apply the currently recommended criteria for FGR and to exclude fetuses with signs of infection or elevated inflammatory markers during pregnancy, as well as pregnant women taking medications that may influence ANS function. Also, we checked for maternal physiological parameters that might influence fetal STV at the start of every cCTG recording. Moreover, most previous studies exploring the effects of antenatal corticosteroids in FGR populations did not conduct a direct comparison with AGA fetuses [24], 25]. We, however, took an approach by narrowing the boundaries of the AGA definition, enabling a comparison of FGR fetuses with an optimal group of normally growing fetuses.
The relatively small cohort and the early delivery that affected our monitoring could impact our conclusions. We intended to monitor changes in STV for another 7 days after the first betamethasone application, but clinical reasons led to early delivery. The time between cCTG recording before the first application of betamethasone and the first day after was not exactly 24 h but ranged from>12 to 24 h. Pregnant women who required betamethasone therapy for fetal lung matuartion received it upon decision-making or hospital admission, which could be at any time of the day. If this was after noon, the second cCTG after the first betamethasone administration, as well as all subsequent ones, was recorded in the morning hours to reduce the potential influence of the fetal circadian rhythm. The inclusion of monochorionic twins could be considered a limitation of the study due to the specific hemodynamic changes observed in AGA fetuses in selective FGR [26]. However, in our study, all fetuses from monochorionic pregnancies were FGR fetuses, which were presumably not burdened by cardiac overload, unlike AGA fetuses in selective FGR, leading to chronic stress in the latter. We also recognize some missing cCTG records as a limitation.
Conclusions
Our study confirms that the increased STV observed in FGR fetuses during the first 48 h after the initial dose of betamethasone does not reflect actual fetal condition. This transient elevation in STV can be considered corticosteroids’ direct pharmacologic effect. Therefore, alternative monitoring methods should be used to assess fetal well-being accurately during this period. The reliability of STV as an indicator of fetal well-being in FGR fetuses is restored 48 h after the first application of betamethasone when values no longer differ significantly from baseline measurements obtained before betamethasone administration. In AGA fetuses, STV is restored to baseline measurements after 24 h. At all times of cCTG recordings, FGR fetuses have significantly lower STV in comparison to AGA fetuses. Our findings should be verified in a larger cohort, possibly with different doses of betamethasone.
Funding source: University Medical Centre Ljubljana, Slovenia
Award Identifier / Grant number: TP2022128
Funding source: Public Agency for Scientific Research and Innovation of the Republic of Slovenia
Award Identifier / Grant number: P3-0124
Acknowledgments
We want to thank the midwives and nurses who diligently measured physiological parameters in pregnant women, recorded cCTGs, and encouraged pregnant women.
-
Research ethics: The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (No. 0120–516/2022/17, June 5th, 2023).
-
Informed consent: All pregnant women gave written informed consent following verbal and written explanation.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. TPS and GBŠ conceived the study. RKP, ZS and TPS contributed to data collection. TŠ, ZS, TPS and IV contributed to data analysis. TŠ and ZS wrote the first version of the manuscript. TPŠ, RKP, GBŠ and ML contributed to project supervision and manuscript critical revision.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: Research grants No. TP2022128 of the University Medical Centre Ljubljana and No. P3-0124 of the Public Agency for Scientific Research and Innovation of the Republic of Slovenia.
-
Data availability: The research data is stored on the secure web application REDCap of the University Medical Centre Ljubljana and available upon reasonable request.
References
1. Mayo Clinic. Premature birth [internet]. Rochester: Mayo Foundation for Medical Education and Research. [updated 2022 Jan 15; cited 2024 Aug 21]. Available from: https://www.mayoclinic.org/diseases-conditions/premature-birth/symptoms-causes/syc-20376730.Suche in Google Scholar
2. Daskalakis, G, Pergialiotis, V, Domellöf, M, Ehrhardt, H, Di Renzo, GC, Koç, E, et al.. European guidelines on perinatal care: corticosteroids for women at risk of preterm birth. J Matern Fetal Neonatal Med 2023;36:2160628. https://doi.org/10.1080/14767058.2022.2160628.Suche in Google Scholar PubMed
3. Lees, CC, Stampalija, T, Baschat, A, da Silva Costa, F, Ferrazzi, E, Figueras, F, et al.. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020;56:298–312. https://doi.org/10.1002/uog.22134.Suche in Google Scholar PubMed
4. Melamed, N, Baschat, A, Yinon, Y, Athanasiadis, A, Mecacci, F, Figueras, F, et al.. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet 2021;152:3–57. https://doi.org/10.1002/ijgo.13522.Suche in Google Scholar PubMed PubMed Central
5. Visser, GH, Bekedam, DJ, Ribbert, LS. Changes in antepartum heart rate patterns with progressive deterioration of the fetal condition. Int J Gynaecol Obstet 1990;25:239–46. https://doi.org/10.1016/0020-7101(90)90027-r.Suche in Google Scholar PubMed
6. Fratelli, N, Prefumo, F, Wolf, H, Hecher, K, Visser, GHA, Giussani, D, TRUFFLE Group authors, TRUFFLE Group collaborating authors, et al.. Effects of antenatal betamethasone on fetal Doppler indices and short term fetal heart rate variation in early growth restricted fetuses. Ultraschall Med 2021;42:56–64. https://doi.org/10.1055/a-0972-1098.Suche in Google Scholar PubMed
7. Kouskouti, C, Jonas, H, Levidou, G, Regner, K, Kainer, F. Alterations of the short-term variation of the fetal heart rate after antenatal maternal betamethasone administration: validation with two different computational algorithms. Z Geburtshilfe Neonatol 2020;224:26–30. https://doi.org/10.1055/a-0873-2058.Suche in Google Scholar PubMed
8. Melamed, N, Baschat, A, Yinon, Y, Athanasiadis, A, Mecacci, F, Figueras, F, et al.. FIGO (International federation of gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet 2021;152:3–57. https://doi.org/10.1002/ijgo.13522.Suche in Google Scholar
9. Salomon, LJ, Alfirevic, Z, Bilardo, CM, Chalouhi, GE, Ghi, T, Kagan, KO, et al.. ISUOG practice guidelines: performance of first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013;41:102–13. https://doi.org/10.1002/uog.12342.Suche in Google Scholar PubMed
10. Gordijn, SJ, Beune, IM, Thilaganathan, B, Papageorghiou, A, Baschat, AA, Baker, PN, et al.. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. https://doi.org/10.1002/uog.15884.Suche in Google Scholar PubMed
11. Hadlock, FP, Harrist, RB, Sharman, RS, Deter, RL, Park, SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol 1985;151:333–7. https://doi.org/10.1016/0002-9378(85)90298-4.Suche in Google Scholar PubMed
12. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger, BE, Gabbe, SG, Persson, B, Buchanan, TA, Catalano, PA, Damm, P, et al.. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia inpregnancy. Diabetes Care 2010;33:676–82. https://doi.org/10.2337/dc09-1848.Suche in Google Scholar PubMed PubMed Central
13. Brown, MA, Magee, LA, Kenny, LC, Karumanchi, SA, McCarthy, FP, Saito, S, International Society for the Study of Hypertension in Pregnancy (ISSHP), et al.. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018;72:24–43. https://doi.org/10.1161/hypertensionaha.117.10803.Suche in Google Scholar PubMed
14. Dawes, GS, Visser, GH, Goodman, JD, Redman, CW. Numerical analysis of the human fetal heart rate: the quality of ultrasound records. Am J Obstet Gynecol 1981;141:43–52. https://doi.org/10.1016/0002-9378(81)90673-6.Suche in Google Scholar PubMed
15. Schneider, U, Fiedler, A, Schröder, B, Jaekel, S, Stacke, A, Hoyer, D, et al.. The effect of antenatal steroid treatment on fetal autonomic heart rate regulation revealed by fetal magnetocardiography (fMCG). Early Hum Dev 2010;86:319–25. https://doi.org/10.1016/j.earlhumdev.2010.05.018.Suche in Google Scholar PubMed
16. Verdurmen, KM, Renckens, J, van Laar, JO, Oei, SG. The influence of corticosteroids on fetal heart rate variability: a systematic review of the literature. Obstet Gynecol Surv 2013;68:811–24. https://doi.org/10.1097/ogx.0000000000000007.Suche in Google Scholar
17. Knaven, O, Ganzevoort, W, de Boer, M, Wolf, H. Fetal heart rate variation after corticosteroids for fetal maturation. Eur J Obstet Gynecol Reprod Biol 2017;216:38–45. https://doi.org/10.1016/j.ejogrb.2017.06.042.Suche in Google Scholar PubMed
18. Weyrich, J, Setter, A, Müller, A, Schmidt, G, Brambs, CE, Ortiz, JU, et al.. Longitudinal progression of fetal short-term variation and average acceleration and deceleration capacity after antenatal maternal betamethasone application. Eur J Obstet Gynecol Reprod Biol 2017;212:85–90. https://doi.org/10.1016/j.ejogrb.2017.03.025.Suche in Google Scholar PubMed
19. Zizzo, AR, Hansen, J, Peteren, OB, Mølgaard, H, Uldbjerg, N, Kirkegaard, I. Growth-restricted human fetuses have preserved respiratory sinus arrhythmia but reduced heart rate variability estimates of vagal activity during quiescence. Phys Rep 2022;10:e15458. https://doi.org/10.14814/phy2.15224.Suche in Google Scholar PubMed PubMed Central
20. Ghi, T, Dall’Asta, A, Saccone, G, Bellussi, F, Frusca, T, Martinelli, P, et al.. Reduced short- term variation following antenatal administration of betamethasone: is reduced fetal size a predisposing factor? Eur J Obstet Gynecol Reprod Biol 2017;216:74–8. https://doi.org/10.1016/j.ejogrb.2017.07.010.Suche in Google Scholar PubMed
21. Nijhuis, IJ, ten, HJ, Mulder, EJ, Nijhuis, JG, Narayan, H, Taylor, DJ, et al.. Fetal heart rate in relation to its variation in normal and growth retarded fetuses. Eur J Obstet Gynecol Reprod Biol 2000;89:27–33. https://doi.org/10.1016/s0301-2115(99)00162-1.Suche in Google Scholar PubMed
22. Xiong, F, Zhang, L. Role of the hypothalamic–pituitary–adrenal axis in developmental programming of health and disease. Front Neuroendocrinol 2013;34:27–46. https://doi.org/10.1016/j.yfrne.2012.11.002.Suche in Google Scholar PubMed PubMed Central
23. Braun, T, Challis, JR, Newnham, JP, Sloboda, DM. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr Rev 2013;34:885–916. https://doi.org/10.1210/er.2013-1012.Suche in Google Scholar PubMed
24. Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org, Martins, JG, Biggio, JR, Abuhamad, A. Society for maternal-fetal medicine consult series #52: diagnosis and management of fetal growth restriction: (replaces clinical guideline number 3, April 2012). Am J Obstet Gynecol 2020;223:B2–17. https://doi.org/10.1016/j.ajog.2020.05.010.Suche in Google Scholar PubMed
25. Frusca, T, Soregaroli, M, Valcamonico, A, Scalvi, L, Bonera, R, Bianchi, U. Effect of betamethasone on computerized cardiotocographic parameters in preterm growth- restricted fetuses with and without cerebral vasodilation. Gynecol Obstet Invest 2001;52:194–7. https://doi.org/10.1159/000052972.Suche in Google Scholar PubMed
26. Munoz, JL, Furtun, BY, Buskmiller, C, Sanz Cortes, M, Donepudi, RV, Belfort, MA, et al.. Cardiac structural and functional assessment of monochorionic twin pregnancies complicated by type II and type III selective fetal growth restriction. Fetal Diagn Ther 2025;5:1–7. https://doi.org/10.1159/000545880.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

