Abstract
Chronic and recurrent obstruction of the inferior vena cava by the gravid uterus may be asymptomatic for the mother and yet contribute to preeclampsia, fetal growth restriction, preterm birth, dysfunctional labor and uterine atony. This previously unrecognized possible cause of chronic and recurrent fetal, placental and myometrial hypoxia might be detected and corrected using “cardiac output-guided maternal positioning,” since positional obstruction of the inferior vena cava causes a positional decrease in maternal cardiac output (CO). These positional decreases in CO may therefore constitute an actionable warning signal for a pregnant woman to change her body position and thereby restore optimal uterine perfusion and fetal oxygenation. Continuous, non-invasive and hands-free trending of maternal CO is now feasible in real time in order to detect this warning signal in real time. Further research is required to evaluate this hypothesis, and if it is valid, the approach and therapy proposed might constitute a breakthrough for preventing some complications of pregnancy.
Background
For tens of thousands of years, human beings have undoubtedly tried to protect their developing babies from harm and to provide them with the best possible opportunities for healthy and productive lives after birth. These earlier humans probably tried to achieve this goal through dietary, behavioral and religious means. In much more recent times, pregnant women and their caregivers have attempted to prevent maternal infections which injure the fetus and have also learned that specific dietary inputs such as iron, folic acid and essential amino acids are important for fetal and maternal health. In the second half of the twentieth century, we have discovered that pregnant women should not smoke or drink alcohol, and that maternal ingestion of certain other drugs – both legal and illegal – causes devastating fetal injuries.
In terms of more active interventions to protect the developing fetus, we now control maternal blood glucose much more carefully than in the past in order to avoid birth defects and administer antenatal steroids and magnesium sulfate to pregnant women in order to reduce pulmonary and neurological complications in infants born prematurely. Hence, we can see that the goal of protecting the fetus while still in the womb – the goal of treating the fetus as a patient – has a very long and quite successful history.
This review attempts to stimulate research into another possible opportunity for improving fetal and maternal pregnancy outcomes. The goal of the treatment would be to actively protect – and even ensure – a continuously adequate supply of oxygen for the fetus, placenta and myometrium throughout gestation, and especially during its second half, when the gravid uterus is thought to be large enough to obstruct its own venous return at the inferior vena cava (IVC).
This highly ambitious goal may now be attainable using well-understood physiological and hemodynamic principles together with currently available non-invasive technology. The possibility of improving lifelong fetal and maternal outcomes by ensuring adequate fetal, placental and myometrial oxygenation throughout gestation may represent “low-hanging fruit” in our efforts to improve both short- and long-term outcomes for fetuses and mothers.
The fetal oxygen supply
Until approximately 12 weeks of gestation, the embryo and fetus develop in a relatively low oxygen environment, but between the 12th and 16th weeks of gestation the placental intervillous circulation is established, which progressively increases intervillous perfusion to supply the progressively increasing oxygen needs of the developing fetus [1]. Failure of appropriate dilation (“remodeling”) of the maternal spiral arteries is often associated with both fetal growth restriction (FGR) and preeclampsia (PE) and this suggests that a lack of oxygen has something to do with the causation of both disorders. This review suggests that a similar inadequacy of intervillous perfusion and fetal and placental oxygenation could be imposed by obstruction of the venous side of the uterine circulation by the gravid uterus in certain maternal positions. But it is also the contention of this review that venous obstruction of intervillous space (IVS) perfusion may be much more harmful for fetal and placental oxygenation than an “equivalent” degree of arterial obstruction, because venous obstruction increases IVS pressure as well as “merely” decreasing IVS flow. This increased pressure may then unleash several harmful hemodynamic mechanisms which are not called into play when the obstruction to intervillous perfusion is only located on the arterial side.
The 1970s saw the introduction of continuous electronic fetal heart rate monitoring in an attempt to reduce fetal injuries due to hypoxic episodes during labor. Hence, for many decades, the fetus’ need for a continuously adequate supply of oxygen during gestation has been well recognized. But few proposals have been made for actively protecting and ensuring an adequate fetal oxygen supply.
Practitioners did recognize that both maternal anemia and residence at high altitude could inhibit fetal growth, but – with the exception of the treatment of maternal anemia – it seemed that most practitioners believed that the fetus was “on its own” in terms of the oxygen it received from the mother. If the fetus’ oxygen supply were restricted or eliminated – due to a placental abruption or a knotted umbilical cord, for example – it was often thought that nothing could have been done to prevent the resulting stillbirth. The beginnings of a more active “hemodynamic approach” to improving fetal oxygenation appeared in 1953, when the “supine hypotensive syndrome of pregnancy” was first described using that specific term [2], and it was soon recognized that the syndrome could be accompanied by placental abruption [3].
The supine hypotensive syndrome of pregnancy
Once the supine hypotensive syndrome of pregnancy was widely recognized as a danger to the mother, the avoidance of aortocaval compression by the gravid uterus became a fundamental principle for the hemodynamic management of pregnant patients. But most discussions of the syndrome emphasized only three points: 1) the syndrome affects the mother, 2) it has a sudden and dramatic onset, involving maternal lightheadedness, tachycardia, nausea, vomiting, and even loss of consciousness or maternal death, and 3) it is caused by a sudden reduction of maternal blood pressure (BP) caused by a decrease in maternal venous return and cardiac output (CO). Hence, in most discussions of the syndrome, it was treated as a maternal problem initiated by a deficiency of arterial perfusion.
Although the recognition, description and prevention of this syndrome have greatly improved the care of pregnant women, the traditional description of the syndrome is simplistic and incomplete since it pays no attention to the increased pressure which it causes on the venous side of the maternal and uterine circulations. Descriptions of the syndrome also do not usually discuss the welfare of the fetus, except, perhaps, to mention that the syndrome is sometimes accompanied by placental abruption and fetal death. In other words, discussions of the supine hypotensive syndrome of pregnancy usually treat the uterus only as a bothersome mass – a “black box” – which can cause problems for the mother, with little attention being paid to the harm that such obstruction might cause to the fetus, placenta and myometrium, particularly if the obstruction were prolonged rather than transitory.
When the supine hypotensive syndrome of pregnancy was first described, this lack of mention of the fetus was understandable, since the syndrome was considered only as a very short-term phenomenon which had to be corrected immediately by turning the supine pregnant patient onto her side to restore maternal venous return and BP.
But what if the gravid uterus can obstruct the IVC and its own venous return on a chronic and recurrent basis in a woman who has an adequate BP? What if that woman feels comfortable in a body position which is – unbeknownst to her – harmful to the fetus? What might happen to the fetus, placenta and myometrium under those circumstances?
Chronic positional obstruction of maternal and uterine venous return may be an unrecognized cause of pregnancy complications
There is now abundant circumstantial evidence that chronic and recurrent positional obstruction of maternal and uterine venous return by the gravid uterus might contribute to FGR, PE, preterm birth (PTB), dysfunctional labor (DL) and postpartum uterine atony, and that detection and prevention of such obstruction might prevent – or at least mitigate – some of these pregnancy complications, but further research is needed to prove or disprove this hypothesis
Recent MRI studies have yielded quantitative data and general insights regarding vascular compression during pregnancy, as follows:
Compared to the left lateral decubitus (LLD) position, the supine position is associated with a 16.4 % decrease in CO, a 32.3 % decrease in abdominal aortic blood flow at the bifurcation, an 85.3 % decrease in IVC flow at its origin, and a 44.4 % decrease in IVC flow at the level of the renal veins [4]. This result, and others [5], suggest that a relatively modest reduction in maternal CO may serve as a warning signal for greatly reduced lower body perfusion, accompanied by venous congestion.
Symptomatic and asymptomatic pregnant women who adopt the supine position experience similar decreases in CO [4], 6]. This fact is consistent with the concept that when pregnant women experience a position-related decrease in CO, some women avoid hypotension and its associated symptoms by increasing their SVR, whereas other women do not increase their SVR and therefore experience hypotensive symptoms [4], 6]. In the past, the lack of maternal symptoms when a pregnant woman adopts the supine position has been seen as a favorable compensatory circumstance, but the supine hypotensive syndrome of pregnancy actually appears to protect the fetus since it forces a change in maternal position. The “compensatory” prevention of maternal symptoms by the sympathetic nervous system (SNS) when it increases the SVR and maintains normal maternal BP may actually injure the fetus since it allows venous obstruction and fetal hypoxia to persist for prolonged periods of time.
The supine position and the presence of FGR are independently associated with decreased fetal oxygenation, and the effects are additive, so that the supine position may be especially harmful in patients with growth restricted fetuses [5].
In 23 % of patients the right lateral decubitus position is associated with a higher CO than the LLD position [7].
The supine position is associated with variable compensatory increases in collateral flow through the lumbar, vertebral venous and azygos system [8].
Beyond the supine hypotensive syndrome of pregnancy
Figure 1 shows the favorable situation for the fetus, placenta and myometrium which occurs when maternal and uterine venous return is not obstructed, and CO, systemic vascular resistance (SVR) and maternal BP are all normal. As shown in Figure 1, venous return from the uterus and the rest of the lower body is free and unobstructed, and – as a consequence – maternal CO and a normal SVR create a normal maternal BP. This normal maternal BP combined with low uterine venous pressure allows abundant uteroplacental and fetoplacental perfusion, and these two vigorous but separate blood streams create adequate flow shear stress within their respective circulations to enable adequate future placental growth.
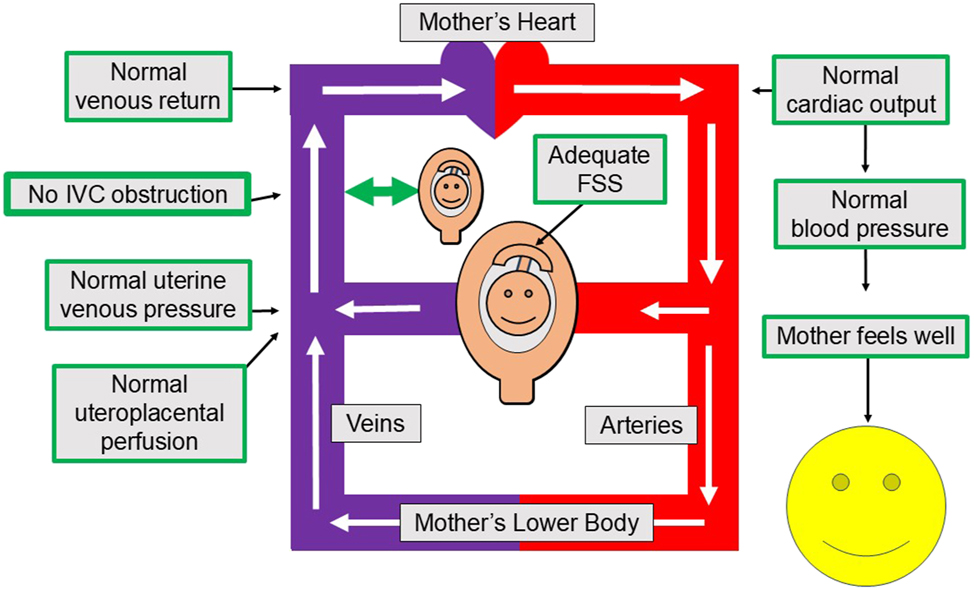
Fetal, placental and myometrial perfusion with an unobstructed inferior vena cava. This Figure shows how an unobstructed IVC allows for adequate current fetal, placental and myometrial oxygenation, as well as future placental growth. Specifically, maternal venous return and cardiac output are adequate, uterine venous and intervillous space pressures are low and uteroplacental perfusion and oxygenation are adequate. These favorable conditions allow for current oxygen needs, while vigorous but non-violent flow shear stress within and around the chorionic villi encourages the placenta to grow to serve the fetus’ future needs. IVC, inferior vena cava; FSS, flow shear stress.
In contrast, Figure 2 shows the hemodynamics of the supine hypotensive syndrome of pregnancy. The gravid uterus obstructs the IVC and, consequently, maternal venous return and CO decrease. This decreased CO results in decreased maternal BP because the maternal SNS and the renin angiotensin aldosterone system (RAAS) do not adequately increase the maternal SVR. The decreased maternal BP then causes the pregnant woman to suffer the supine hypotensive syndrome of pregnancy to a greater or lesser degree – until the obstruction of the IVC by the gravid uterus is relieved by a maternal position change.
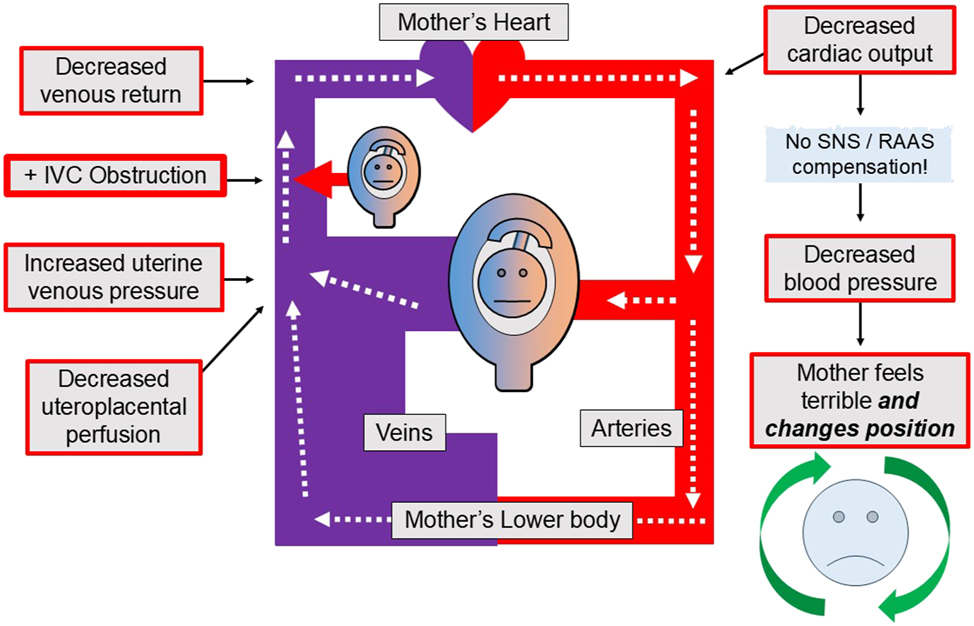
The maternal circulation during the “supine hypotensive syndrome of pregnancy”. The supine hypotensive syndrome of pregnancy occurs when the uterus obstructs maternal venous return and reduces maternal cardiac output and blood pressure. As a result, the pregnant woman experiences severe hypotensive symptoms or signs, such as light-headedness, nausea, vomiting, loss of consciousness or even death. But the original description of this syndrome did not consider the possible effects of this complex and unfavorable hemodynamic situation on the fetus, placenta or myometrium that would occur if some of those effects were allowed to persist for longer than a few minutes. Ironically – and fortunately – the supine hypotensive syndrome probably protects the fetus since it forces the mother (or her caregivers) to quickly change her body position, which then restores her normal venous return, cardiac output and blood pressure. The fetus, placenta and myometrium also benefit, of course, because their adequate oxygenation is promptly restored along with the mother’s circulation. The curved green arrows surrounding the mother’s blue face in the lower right-hand corner suggests this fortunate position change, which then produces recovery for both the mother and the fetus. Also, note that the supine hypotensive syndrome of pregnancy is theorized to occur because of a failure of the SNS and the RAAS to maintain normal blood pressure in the face of a decreased CO. This “fortunate failure” can be characterized as a lack of maternal cardiovascular compensation for a suddenly decreased BP. BP, arterial blood pressure; CO, cardiac output; IVC, inferior vena cava; SNS, sympathetic nervous system; RAAS, renin angiotensin aldosterone system.
But the very appearance of severe symptoms causes the pregnant woman (or her caregivers) to change her body position, thereby relieving the obstruction of the IVC and restoring adequate maternal and fetoplacental perfusion and oxygenation. Hence the supine hypotensive syndrome of pregnancy must be a very time-limited process which promptly forces its own resolution, thereby protecting the fetus from prolonged hypoxia.
Cardiovascular compensation for reduced cardiac output – convenient for the mother but bad for the fetus?
Figure 3 shows what is theorized to occur when maternal cardiovascular (CV) compensation for decreasing venous return and BP increases maternal SVR and thereby maintains the mother’s BP and sense of well-being. In this situation the pregnant woman is comfortable in her current body position and is unaware that her position has created a “silent” but dangerous hemodynamic situation which threatens her developing baby’s supply of oxygen!

Fetal, placental and myometrial perfusion and oxygenation with an obstructed inferior vena cava and “successful” cardiovascular compensation. If obstruction of the IVC with a reduction of CO can occur and persist without triggering the supine hypotensive syndrome of pregnancy, the following unfavorable events may be occurring, as shown in the Figure: 1) compensation by the SNS and the RAAS to maintain maternal blood pressure, 2) decreased uteroplacental perfusion, 3) increased uterine venous and intervillous space pressure, 4) fetal, placental and myometrial hypoxia, and 5) decreased flow shear stress within and around the chorionic villi – thus hampering placental growth. The potential tragedy of this “successful” maternal cardiovascular compensation is that it allows the mother to feel well even though her developing baby, placenta and myometrium are being deprived of oxygen! CO, cardiac output; FSS, flow shear stress; IVC, inferior vena cava; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system.
Mechanisms of cardiovascular compensation for decreasing blood pressure
In the short term the SNS maintains an approximately constant and adequate BP despite postural variations in venous return. It does this by varying the SVR, venous tone, heart rate and cardiac contractility. The SNS “targets” BP, not CO, but a reduction in CO causes a decrease in BP in the absence of CV compensation.
Decreases in venous return due to obstruction of the IVC will decrease maternal CO, and this decreased CO will either lower the BP or else trigger an increase in SVR via the SNS and RAAS. This CV compensation for obstructed venous return may be one cause of the “low volume circulation” discussed by Gyselaers and Lees [11].
The RAAS system normally has increased activity during pregnancy, and over the longer term, with chronic and recurrent obstruction of the IVC by the gravid uterus, the RAAS may increase SVR via secretion of renin and angiotensin, and increase sodium retention via aldosterone secretion. The antidiuretic hormone (ADH) system then retains more water in response to the increased serum osmolality accompanying sodium retention, with the net result being increased total body water with a normal sodium concentration. This increased total body water may also be accompanied by an increase in maternal blood volume, which – as we know – does occur during pregnancy.
Another possible long-term compensatory mechanism for obstruction of the IVC may be the development during pregnancy of lumbar, vertebral venous and azygos collaterals to the IVC, which allow blood from the lower part of the body – including the uterus – to return to the heart when the IVC is obstructed. The fact that PE is more common in first pregnancies than in subsequent pregnancies is compatible with the theory that a first pregnancy with obstruction of the IVC may encourage the gradual development of such collaterals, which may then be more extensive and efficacious in subsequent pregnancies.
Other hemodynamic considerations
1) Cardiac output is a global, systemic and non-specific measure
Cardiac output is a global systemic measure which does not localize the source of preload reduction. For this reason, attempts to define “hemodynamic profiles” associated with FGR and PE (or other disorders) are problematic unless the possible occurrence of IVC obstruction is explicitly taken into consideration as a cause of the abnormal profile. Several investigators have maintained that mothers with growth restricted fetuses tend to have lower CO and higher SVR than mothers with normally grown fetuses, but some of these studies may have involved the measurement of maternal hemodynamics in a “standard” supine semi-recumbent position [11], 12]. This position probably causes positional IVC obstruction and a consequent decrease in CO, which then provokes a compensatory increase in SVR, thereby promoting the creation of the “abnormal profile”. Therefore, in these studies, it is not clear whether the “low CO, high SVR” circulation observed is caused by a global and “intrinsic” defect in the maternal CV system, or whether it is the result of maternal positioning which causes a low CO and a high SVR due to obstruction of the IVC. This has led to the suggestion that future examinations of “hemodynamic profiles” should be performed in that maternal position in which CO is maximized, so that the effects of concurrent IVC obstruction on maternal hemodynamics are minimized or eliminated altogether.
2) Does chronic or recurrent inferior vena cava obstruction “train” or “encourage” the maternal cardiovascular system to “behave badly” or “go bad”?
An additional possibility exists that chronic and recurrent obstruction of the IVC by the gravid uterus “trains” the maternal CV system to chronically increase its SVR, which then leads to chronic and recurrent decreases in CO, in order to maintain maternal BP at a normal level (without hypertension). Another way of formulating this idea is that chronic and recurrent obstruction of the IVC may cause the SNS and RAAS to be chronically hyperactive (or “upregulated”) because they are frequently called into action. From this perspective as well, the “guilty party” in a “low-volume circulation” situation may be the obstruction of the IVC by the gravid uterus, rather than some “intrinsic” or “global” defect in the maternal CV system. With this in mind, prevention of IVC obstruction during the second half of pregnancy might prevent the process of “SNS and RAAS hypertrophy” which could lead not only to CV problems during the current pregnancy, but which might also lead to lifelong CV damage and dysfunction. In other words, could IVC obstruction by the gravid uterus contribute not only to PE in a current pregnancy but also lead to the lifelong CV dysfunction and disease which are known to develop in many previously preeclamptic women?
3) A decrease in CO may result from factors unrelated to uterine venous obstruction, such as dehydration, hemorrhage, or anesthesia
This fact requires that for any use of electrical cardiometry (EC) or any other technology for trending CO it is necessary to examine the entire clinical picture. For example, OUVR at the IVC will tend to exacerbate the hypotensive effects of dehydration, hemorrhage or anesthesia.
4) Hemodynamic threats to the fetus, placenta and myometrium work in combination
An example of this principle is that a patient with impaired spiral artery remodeling may be at increased risk of fetal hypoxia due to venous obstruction to uteroplacental perfusion. Another example is that extrauterine venous obstruction may be particularly dangerous in patients with large (“overdistended”) uteruses because – by Laplace’s Law – a large uterus will tend to have higher intramyometrial tissue pressure than a smaller uterus, and this higher pressure will present an increased obstacle to uteroplacental perfusion within the uterine wall itself. This principle – that barriers to fetal oxygenation work “in series” – is shown in Figure 4.
![Figure 4:
Four components of a successful uterine perfusion system. A healthy pregnancy requires at least four components for adequate uterine perfusion: 1) an adequate maternal cardiac output, 2) well-remodeled maternal spiral arteries abundantly perfusing the intervillous spaces, 3) an adequately low myometrial tissue pressure which allows adequate uteroplacental perfusion, and 4) unobstructed uterine venous return. This review focuses on component 4). Figure modified from [13].](/document/doi/10.1515/jpm-2025-0333/asset/graphic/j_jpm-2025-0333_fig_004.jpg)
Four components of a successful uterine perfusion system. A healthy pregnancy requires at least four components for adequate uterine perfusion: 1) an adequate maternal cardiac output, 2) well-remodeled maternal spiral arteries abundantly perfusing the intervillous spaces, 3) an adequately low myometrial tissue pressure which allows adequate uteroplacental perfusion, and 4) unobstructed uterine venous return. This review focuses on component 4). Figure modified from [13].
In summary, OUVR should be seen as one additional potential stress to fetal oxygenation which can be exacerbated by any of the other “uterine perfusion factors,” as shown in Figure 4. Additionally, the measurement or trending of maternal CO with any technology must be undertaken by taking the entire and dynamic clinical picture into account, including the possible occurrence of OUVR.
Multiple possible mechanisms of fetal, placental and myometrial injury from obstruction of uterine venous return (see also Table 1)
Obstruction of the IVC in the absence of adequate collateral pathways for venous return will tend to decrease maternal CO and BP, which will tend to decrease total uteroplacental perfusion “from the arterial side”.
Increased uterine venous and IVS pressure will decrease uteroplacental perfusion even further, since that perfusion is determined by the difference between uterine arterial and venous pressures.
Decreased intervillous perfusion, in the presence of continuing oxygen uptake by intact chorionic villi, will decrease intervillous oxygen tension, which may cause direct and immediate injury to both the chorionic villi and the fetus.
Increased IVS pressure compresses chorionic villi and the fetal chorionic vessels contained within them, thereby increasing the afterload on the fetal heart.
The compressed fetal chorionic vessels will also tend to pick up less oxygen for fetal needs. It should be noted that this “compression” mechanism for decreased fetoplacental perfusion is distinct from the HFPV-mediated “hypoxia” mechanism mentioned in point 4).
Decreased beneficial uteroplacental flow shear stress on the syncytiotrophoblast (“exterior”) surface of the chorionic villi may reduce placental invasion of endometrium and placental growth “from the outside” [16].
Decreased beneficial fetoplacental flow shear stress within fetal chorionic vessels may decrease branching angiogenesis and placental growth “from the inside” [17].
In addition to all of the preceding dangers of chronic and recurrent OUVR, it is necessary to recognize that excessive variation in placental oxygenation (apart from its absolute average level) can damage both placental and fetal tissues [1].
A complex pathogenesis of fetal injury: both rapid and gradual injury caused by both flow- and pressure-related mechanisms
It should be noted that these proposed mechanisms of fetal, placental and myometrial injury involve both flow- and pressure-related mechanisms and that they may cause injury both rapidly and gradually. The rapid injury may be caused by hypoxia of existing structures, while the longer-term injuries may arise from placental injury and deficient placental growth, as well as “programmed” subsequent lifelong injuries to the fetus caused by an adverse and hypoxic intrauterine environment.
The complexity of possible fetal injury continues: interactions between the flow-related mechanisms of injury
Figure 5 shows how decreased uteroplacental perfusion may have multiple interacting consequences. First of all, decreased uteroplacental perfusion – in the presence of normal oxygen uptake from the IVS – will lower the oxygen tension within the IVS. This decreased oxygen tension may cause direct hypoxic injury to the fetus or placenta, but it may also trigger global HFPV throughout the placenta. HFPV is thought to perform a useful local function by restricting fetal perfusion of individual hypoxic intervillous spaces, such as might occur with a localized intervillous thrombosis. But HFPV may be disastrous when it occurs globally throughout all the intervillous spaces of a placenta, the function of which has already been globally impaired by OUVR.
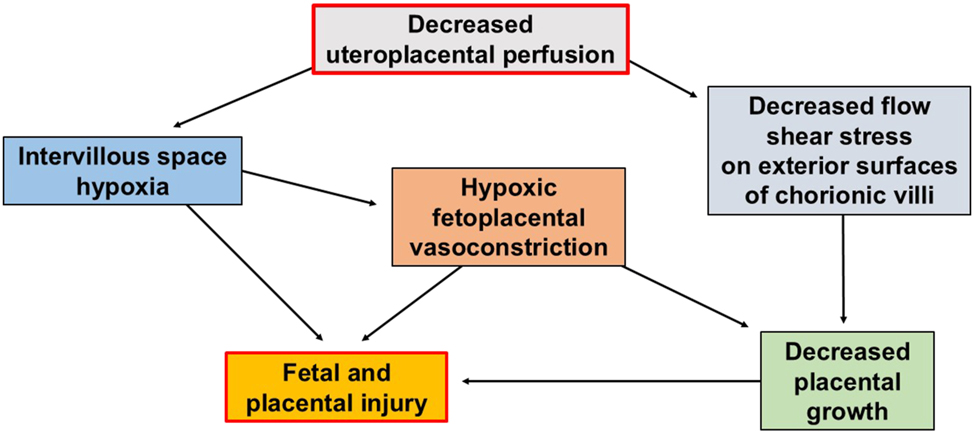
Decreased uteroplacental perfusion may cause fetal and placental injury, by both fast and slow mechanisms. Decreased uteroplacental perfusion in the presence of intact chorionic villi and normal oxygen uptake capacity will promptly cause a decrease in intervillous oxygen tension. This hypoxia may injure the fetus and placenta outright or by triggering generalized HFPV throughout the placenta in an indiscriminate and counterproductive way which decreases total fetoplacental perfusion. It is possible that nitroglycerin, which is known to interfere with HFPV, may be beneficial in ameliorating fetal growth restriction because it interferes with this counterproductive global role of HFPV throughout the placenta. HFPV is normally a beneficial response when it acts locally within the placenta to direct the fetal cardiac output away from hypoxic chorionic villi and towards chorionic villi which have access to oxygen. Decreased intervillous (uteroplacental) perfusion may also inhibit placental growth slowly over time by reducing flow shear stress on the exterior surfaces of the chorionic villi, which are in direct contact with flowing maternal blood. HFPV, hypoxic fetoplacental vasoconstriction.
For this reason, generalized HFPV could be a threat to the fetus, limiting fetal oxygen delivery and placental growth, as well as being a local mechanism for directing fetal CO away from a small number of individual hypoxic intervillous spaces. This possible injurious and counterproductive role of generalized HFPV in further reducing fetal oxygenation may explain why nitroglycerin (NTG) administration, together with oral fluid volume loading, appears to mitigate FGR [18], [19], [20], [21], since NTG appears to inhibit HFPV [22], [23], [24].
Flow shear stress within the two placental circulations promotes the growth of chorionic villi “from the outside” and “from the inside”
Another potentially harmful result of decreased uteroplacental perfusion shown in Figure 5 is that it may reduce the beneficial flow shear stress needed by the syncytiotrophoblast covering of the chorionic villi to successfully invade the endometrium. This would be a mechanism by which decreased uteroplacental perfusion could impair placental growth “from the outside” [16].
In contrast, Figure 6 shows how increased uterine venous and IVS pressure may hydrostatically compress existing chorionic villi and the fetal chorionic vessels within them, causing the decreased fetoplacental perfusion sometimes called “sluice flow.” This sluice flow, in turn, may directly and immediately limit fetal oxygen uptake and thereby injure the fetus, but it may also limit necessary future placental growth because it limits the beneficial flow shear stress which is needed for the branching angiogenesis of fetal chorionic vessels – thereby limiting placental growth “from the inside”.
![Figure 6:
Increased uterine venous pressure may cause fetal and placental injury, by both fast and slow mechanisms. Increased uterine venous pressure “backs up” into the intervillous spaces and compresses fetal chorionic vessels, decreasing fetoplacental perfusion in a process which has been termed “sluice flow” [10]. This reduced fetoplacental perfusion may have both rapid and delayed consequences. Immediately, fetal oxygen uptake and delivery are reduced and the afterload on the fetal heart is increased. Decreased fetoplacental perfusion may also reduce flow shear stress on the endothelium of the chorionic vessels and thereby reduce the future growth of those vessels by branching angiogenesis. This would reduce placental growth and the ability of the placenta to meet the growing fetus’ growing needs for oxygen.](/document/doi/10.1515/jpm-2025-0333/asset/graphic/j_jpm-2025-0333_fig_006.jpg)
Increased uterine venous pressure may cause fetal and placental injury, by both fast and slow mechanisms. Increased uterine venous pressure “backs up” into the intervillous spaces and compresses fetal chorionic vessels, decreasing fetoplacental perfusion in a process which has been termed “sluice flow” [10]. This reduced fetoplacental perfusion may have both rapid and delayed consequences. Immediately, fetal oxygen uptake and delivery are reduced and the afterload on the fetal heart is increased. Decreased fetoplacental perfusion may also reduce flow shear stress on the endothelium of the chorionic vessels and thereby reduce the future growth of those vessels by branching angiogenesis. This would reduce placental growth and the ability of the placenta to meet the growing fetus’ growing needs for oxygen.
“Low volume circulation”
Gyselaers and Lees [11] have used the term “low volume circulation” to describe the decreased CO and increased SVR that are associated with some cases of FGR and PE, and it would appear that one possible cause of this “low volume circulation” could be obstruction of the IVC with a reduction in CO combined with an compensatory increase in SVR due to increased activity of both the SNS and the RAAS. This observation raises another issue about the methodology of research into the hemodynamics of pregnancy.
Many of the studies of maternal hemodynamics are performed by taking hemodynamic measurements on pregnant women lying in “standard” positions, such as the supine or the supine semi-recumbent position, both of which promote obstruction of the IVC by the gravid uterus. The problem with this attempt to “standardize” maternal position for taking hemodynamic measurements is that many supine pregnant women will have varying degrees of obstruction of the IVC in the “standard position”, whereas some women may have no obstruction of venous return whatsoever. Hence, the result of this attempt to “standardize” measurements is to actually confuse them, since the hemodynamics of some women will reflect obstruction of maternal venous return in addition to the “intrinsic” or “global” hemodynamics provided by their overall CV systems, whereas other women without obstruction will manifest their “intrinsic” or “global” hemodynamics unaffected by localized venous obstruction. It would therefore seem that attempts to draw conclusions about the hemodynamic profiles associated with PE or FGR may be of doubtful value without the explicit knowledge of whether or not the position adopted for hemodynamic measurement is causing IVC obstruction or not. An alternative for future studies of the “hemodynamic profiles” associated with various conditions would be that BP, CO and SVR measurements are taken in that maternal position which maximizes CO (which is often, but not always, the LLD position).
“Bed rest” in obstetrics
Another questionable area of obstetric practice and research is the use of “bed rest” as a treatment for various pregnancy-related conditions. In 1983, Spinapolice [25] published a small series showing that maternal bed rest in the LLD position reduced the occurrence of PE, and this intervention appeared to be an early use of maternal “position therapy” to improve fetal oxygenation and fetal and maternal outcomes [26], [27], [28], [29], [30], [31], [32]. But the early paper by Spinapolice appears to have been largely ignored, and maternal “bed rest” (without reference to the maternal position in which that “rest” was taken) became a discredited modality for improving several pregnancy outcomes [33], [34], [35], [36]. It appears, however, that an important conceptual error was made by ignoring the specific maternal and uterine hemodynamics of the “bed rest”. In other words, it appears that practitioners assumed that “all bed rest was equal” and that the possible benefits of bed rest could be supplied to a mother merely by confining her to a bed! Practitioners appear not to have considered that the beneficial part of the bed rest prescribed in Spinapolice’s study might have been the adoption of a position which prevented obstruction of the IVC by the gravid uterus.
When confined to “bed rest”, pregnant women (like other adults), usually adopt the supine semi-recumbent position for reading, watching television or visiting with friends and family, and this position is likely to impair fetal oxygenation by promoting the OUVR at the IVC. But in recent years, there has been a resurgence of interest in the dangers of the maternal supine position and this has led to renewed interest in improving fetal outcomes by actively managing maternal position to ensure adequate fetal oxygenation [4], [5], [6], [7], [8], [[37], [38], [39].
Bed rest in the left lateral decubitus position may be beneficial during pregnancy
Quite recently, studies and opinions have appeared which support the utility of bed rest specifically in the LLD position for improving fetal oxygen delivery and ameliorating FGR [4], 6], 8], 12], [40], [41], [42], [43], [44], [45], [46]. This “position therapy” is used empirically and is not performed with CO guidance, but may work very well because the LLD position is likely to be the best position for optimizing uteroplacental perfusion – although it may not be the best position in 23 % of patients [7]. Studies have also supported the concept that mothers at risk of stillbirth should sleep on their sides, rather than in the supine position, but little emphasis has been placed on the systematic and long-term positional augmentation of fetal oxygen delivery while the patient is awake (except during labor). The introduction of the term “cardiac output-guided maternal positioning” was intended to draw attention to a possible technique for systematically ensuring adequate fetal oxygenation during the second half of pregnancy – when it appears to be the most precarious.
Can nitroglycerin mitigate fetal growth restriction?
Two groups have had promising results using NTG patches and oral volume loading for the mitigation of FGR [18], [19], [20], [21]. But NTG is a very potent venodilator which will tend to reduce maternal venous return and CO, especially in patients with obstruction of the IVC which is already impeding maternal venous return. Following this logic, it is possible that oral volume loading compensates for the generalized venodilation caused by NTG and that NTG exerts its beneficial effect on fetal oxygenation by preventing generalized – and hence counterproductive – HFPV throughout the placenta. Future studies are warranted in which NTG and oral volume loading are used in combination with COGMP in order to optimize maternal venous return while also blocking generalized and counterproductive HFPV.
The global uterine perfusion system
So far in this review I may have created the impression that OUVR accounts for all of the possible causes of fetal and placental hypoxia. Of course, this is not the case and Figure 4 shows that there are at least four components of a successful uterine perfusion system: 1) a normal maternal heart which produces an adequate CO, 2) well-remodeled maternal spiral arteries which allow adequate intervillous perfusion, 3) adequately low myometrial tissue pressure which minimizes intramural vascular obstruction to placental and myometrial perfusion, and 4) unobstructed maternal and uterine venous return, which allows adequate CO, low uterine venous pressure and adequate uteroplacental and fetoplacental perfusion. Despite the importance of all four of these components of a successful uterine perfusion system, this review is limited to considering the possible role of OUVR in causing or exacerbating pregnancy complications by causing uteroplacental hypoxia, acidosis and edema and this focus is appropriate and timely, and related research is urgent, because such obstruction may be both detectable and preventable by using COGMP.
Cardiac output-guided maternal positioning
By now, I hope I have convinced the reader that the systematic prevention of obstruction to maternal and uterine venous return throughout gestation might be a way of ensuring adequate fetal, placental and myometrial oxygenation throughout an entire pregnancy. This review further proposes that achieving such a goal might prevent or mitigate some cases of PE, FGR, PTB, DL, and uterine atony. But how might we systematically prevent obstruction of uterine and overall maternal venous return throughout gestation?
Years ago, the practical achievement of this goal would have been impossible, but due to new technology and a simple hemodynamic insight, such prevention may be possible. The fundamental insight is that a positional decrease in maternal CO can serve as a marker or warning signal that the pregnant woman should change her current body position to one which restores adequate fetoplacental and myometrial oxygenation, as shown in Figure 7. This is the fundamental idea behind COGMP.
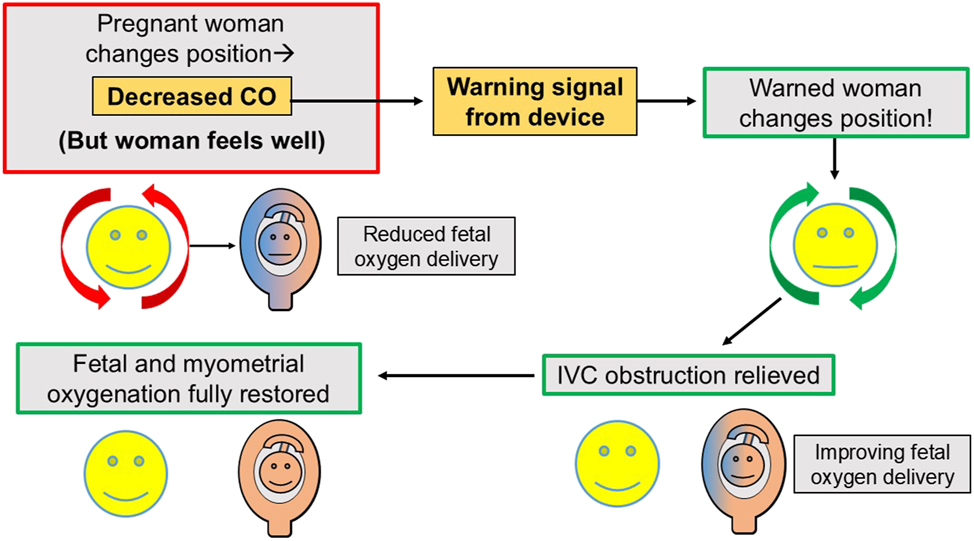
Cardiac output-guided maternal positioning: A positional decrease in maternal cardiac output serves as an actionable marker or warning signal for positional obstruction of maternal venous return. The idea of “cardiac output-guided maternal positioning” is to use positional decreases in maternal cardiac output to warn a pregnant woman – who may be feeling entirely comfortable – that her current body position may injure the fetus and to urge her to change her body position in order to restore a normal cardiac output with its associated low uterine venous pressure and optimal fetal oxygenation. It should be noted that the isolated beneficial effect of restoring maternal cardiac output per se to normal, may be difficult to determine in this scenario, since OUVR both decreases maternal CO and increases uterine venous pressure, and relief of OUVR by position change has the double benefit of both increasing maternal CO and decreasing uterine venous pressure. The important practical insight is that a positional decrease in maternal CO identifies an unfavorable hemodynamic situation which should be promptly corrected. This is the idea of cardiac output-guided maternal positioning. CO, cardiac output; OUVR, obstruction of uterine venous return.
Positional decreases in maternal cardiac output – cause of injury or marker of injury?
It is important for the reader to understand that, while this review proposes that positional reductions in maternal CO can be used to identify and change maternal body positions which are dangerous for both fetus and mother, this review does not claim that it is the reduction of maternal CO which necessarily or solely causes the fetal or maternal injury. As noted in Table 1, OUVR is associated with multiple serious hemodynamic derangements due to decreased blood flow and increased pressure, only one of which is the reduction of maternal CO. In the author’s opinion, the local uterine effects of increased uterine venous pressure are probably more injurious to the fetus and placenta than the associated decrease in maternal CO. A metaphor which may make this distinction clear is that – as we attempt to think about this issue – a wily “pathogenetic magician” who wants to confuse us, is focusing our attention on something that is relatively unimportant, but visible, (such as a mildly decreased maternal cardiac output) so that we don’t notice how the “trick” (fetal growth restriction) is really “performed” (which is by means of increased uterine venous pressure)! Hence, the decrease in maternal CO which drives the concept of COGMP, should perhaps be regarded more as a “marker” or “signal” of impaired oxygenation, rather than the primary cause of that impairment.
Possible mechanisms of fetal, placental and myometrial injury from obstruction of uterine venous return.
|
-
CO, cardiac output; IVS, intervillous space; HFPV, hypoxic fetoplacental vasoconstriction.
The trending of maternal cardiac output is sufficient for cardiac output-guided maternal positioning. Accurate absolute cardiac output measurements are not required to detect obstruction of the inferior vena cava
The distinction between the accurate trending of maternal CO – that is, the correct identification of the direction of change of CO and its approximate magnitude – and its accurate absolute measurement is important, since accurate trending of CO is much easier to achieve non-invasively than the acquisition of “correct” absolute values. For this reason, investigators should not spend time trying to confirm that the CO measurements they obtain are “correct” in terms of the absolute values. Definite and reproducible directional changes will probably be adequate for COGMP to be therapeutically effective and, as mentioned previously, relatively “small” decreases in CO may be associated with (but not necessarily cause) surprisingly “large” decreases in abdominal and pelvic perfusion.
Which technology should we use for trending maternal cardiac output for this research?
Transthoracic echocardiography, the USCOM device, and various types of impedance cardiography (IC) have all been used in pregnancy hemodynamic research. The author performed his published research with the “Electrical Cardiometry” IC system manufactured by Osypka Medical GmbH, Berlin, Germany [47]. The advantage of this system (and other IC systems) is that hemodynamic data can be obtained in a continuous, unobtrusive and hands-free manner which does not require the presence of an operator, whereas both transthoracic echocardiography and the USCOM device provide only intermittent data and require the close presence of operators whenever measurements are required. The protracted presence of a stranger can be bothersome to the patient, especially during labor, and such a labor-intensive practice would obviously be impossible for continuous outpatient monitoring. Another potential continuous and hands-free option for trending maternal CO would be photoplethysmography, but – to the author’s knowledge – this technology is not as well developed for trending CO as IC.
Is Electrical Cardiometry reliable enough for use in pregnancy hemodynamic research?
We will consider three aspects of the question of the “reliability” of EC.
1) “Positional reliability” (lack of artifactual measurements due to maternal position change alone)
Based on my clinical research experience, both published and unpublished, my opinion is that EC reliably identifies the direction of change of CO which occurs with a change in maternal position. But it is possible that maternal position change alone changes the CO which is reported by EC, even if there is no corresponding true change in CO. My way of expressing this idea is to say that EC has not been “positionally validated”. The reason for this need for “positional validation” is that EC measures changes in thoracic impedance during the cardiac cycle using electrode patches applied above and below the left side of the chest wall, and for this reason it is possible that any shift in the position of the thoracic organs occurring with maternal position change might artifactually affect the reported CO value. To my knowledge, this possibility has not been examined and this possible source of measurement artifact in positional hemodynamic studies during pregnancy must be carefully evaluated if EC is to be utilized. If this “positional question” is not settled at the outset of this research, skeptics can always assert that the apparent positional CO changes detected with EC are not “real,” but rather are merely artifacts of measurement caused by the position change itself, rather than by true changes in CO. Hence, the first study which I propose in order to eliminate the possibility of significant positional artifact from EC would utilize USCOM as the reference standard and is explained in the “Future research – Study #1” section below.
2) Signal quality
EC has robust signal quality at rest and absolute immobility is not required for the acquisition of good data. Each set of measurements includes a “signal quality index”, or “SQI”, allowing the post hoc rejection of measurements with an SQI below a user-defined limit (usually 70). For investigating the efficacy of COGMP, what are needed are reliable measurements at rest, and in our six years’ experience at UCSD, obtaining such measurements was straightforward.
3) Sampling parameters may be changed with electrical cardiometry
The investigator chooses how often hemodynamic values are recorded, ranging from every 10 s or less to every 10 min or longer, and each recorded value is the average of a user-selected number of previous heartbeats, ranging from a “beat-to-beat” mode in which the values from one heartbeat are recorded, to the use of the average of a very large number of heartbeats to generate one recorded value. For example, for routine clinical use at UCSD we recorded values every 10 s, with each value being based on the average of the preceding 10 heartbeats. Hence, the sampling parameters can be changed in order to “smooth out” changes due to motion.
The methodological limitations of electrical cardiometry – summary
The method does not give correct absolute values for CO in pregnant patients, but reliable trending of CO in one maternal position and reliable detection of true positional change of CO are all that is required for investigating the efficacy of COGMP.
Cardiac output cannot be measured during significant patient motion, such as walking or changing position in bed, but measurements at rest are robust and measurements with a good “signal quality index” (SQI) greater than or equal to 70 are obtained again rapidly after the patient stops moving.
The “positional reliability” of EC (as defined above) must be established beyond the shadow of a doubt before further positional CO studies are performed in pregnancy with EC. USCOM would be a suitable reference standard for this key initial study, since it is almost certainly resistant to positional artifact due to its methodology of obtaining the velocity-time integral (VTI) from the ascending aorta through the sternal notch acoustic window.
The “positional validation” study of EC would measure maternal CO in a variety of positions (e.g. supine, supine semi-recumbent and left and right lateral positions) with both USCOM and EC, and the positional changes in the CO values measured by the two technologies would be compared. If EC correctly identifies the maternal position in which CO is maximized, then EC would be considered to be “positionally validated”. If EC does not correctly identify the maternal position in which maternal CO is maximal (as determined by USCOM), further positional CO research would have to be conducted using USCOM or another suitable alternative.
When maternal position is unchanged during the measurement period, EC appears to reliably identify trends in maternal CO during a) autotranfusion caused by uterine contractions during labor [32], 48], 49], b) “fetal distress” occurring during medical procedures performed with the mother in the supine position [13], 50], 51], c) oxytocin administration [52], d) magnesium sulfate administration [13], e) spinal and epidural anesthesia [53] and f) rapid administration of indigo carmine [54].
A clinical example of maternal cardiac output trends during labor
Figure 8 shows one example of the use of EC for the trending of maternal CO during pregnancy [32], 48], 50], 51].
![Figure 8:
Trending maternal cardiac output during labor identifies favorable and unfavorable maternal body positions. This is a hemodynamic record from a preeclamptic patient in painful labor who has just received epidural analgesia for labor pain. Seventy minutes of data are shown, from 1550 until 1700. On the left side of the record, with the patient in right-side-down positions, the maternal blood pressure and cardiac output are low, and vasopressors are required to maintain adequate blood pressure. After turning the patient into the full left lateral decubitus position (at “L90”) the blood pressure and cardiac output increase without any need for vasopressors, and each uterine contraction is now accompanied by corresponding increases in maternal cardiac output due to autotransfusion in the presence of unobstructed uterine venous return. It should be noted that during this entire 70 min episode of care the fetal heart rate and variability were normal and the patient was comfortable once the epidural relieved her labor pain. These two facts suggest that the detection of a positional decrease in maternal cardiac output during labor can serve as an “early warning signal” prompting an immediate change in maternal position, which then preemptively avoids more overt and serious problems later on. Further research is required to determine whether this type of non-invasive hemodynamic monitoring during labor could reduce the incidence of “fetal distress,” dysfunctional labor with cesarean delivery and postpartum uterine atony and hemorrhage. Figure from [48], 50], 51]. AT, autotransfusion “waves” of increased maternal cardiac output; CI, cardiac index; EPH10 one dose of intravenous ephedrine 10 mg; IUPC, intrauterine pressure measured by intrauterine catheter; L90, patient turned into full left-lateral decubitus position; MAP, mean maternal arterial pressure; PE50 × 2, two intravenous doses of phenylephrine 50 mg; R30/R90, patient turned with right side down either 30 or 90° from supine; 1550, 3:50 PM; 1700, 5:00 PM.](/document/doi/10.1515/jpm-2025-0333/asset/graphic/j_jpm-2025-0333_fig_008.jpg)
Trending maternal cardiac output during labor identifies favorable and unfavorable maternal body positions. This is a hemodynamic record from a preeclamptic patient in painful labor who has just received epidural analgesia for labor pain. Seventy minutes of data are shown, from 1550 until 1700. On the left side of the record, with the patient in right-side-down positions, the maternal blood pressure and cardiac output are low, and vasopressors are required to maintain adequate blood pressure. After turning the patient into the full left lateral decubitus position (at “L90”) the blood pressure and cardiac output increase without any need for vasopressors, and each uterine contraction is now accompanied by corresponding increases in maternal cardiac output due to autotransfusion in the presence of unobstructed uterine venous return. It should be noted that during this entire 70 min episode of care the fetal heart rate and variability were normal and the patient was comfortable once the epidural relieved her labor pain. These two facts suggest that the detection of a positional decrease in maternal cardiac output during labor can serve as an “early warning signal” prompting an immediate change in maternal position, which then preemptively avoids more overt and serious problems later on. Further research is required to determine whether this type of non-invasive hemodynamic monitoring during labor could reduce the incidence of “fetal distress,” dysfunctional labor with cesarean delivery and postpartum uterine atony and hemorrhage. Figure from [48], 50], 51]. AT, autotransfusion “waves” of increased maternal cardiac output; CI, cardiac index; EPH10 one dose of intravenous ephedrine 10 mg; IUPC, intrauterine pressure measured by intrauterine catheter; L90, patient turned into full left-lateral decubitus position; MAP, mean maternal arterial pressure; PE50 × 2, two intravenous doses of phenylephrine 50 mg; R30/R90, patient turned with right side down either 30 or 90° from supine; 1550, 3:50 PM; 1700, 5:00 PM.
Future research – general approach
Future research projects should be performed on the basis of initial pilot studies to demonstrate feasibility and to learn the approximate range of values that will be obtained, followed by more structured studies and randomized therapeutic trials.
Future research – specific study outlines
Study #1: Does EC have “positional reliability?”
Determine whether EC detects the true direction and approximate percentage magnitude of changes in CO which accompany changes in maternal position, using USCOM as the reference standard. This study is very important and should not take a long time to perform, since all women in late pregnancy would be eligible for the study and the total measurement period per woman would be relatively brief (perhaps 20–30 min), depending on team experience.
As mentioned previously, this first study should determine whether EC reliably identifies the maternal position in which CO is maximized. The reference standard would be USCOM. Because of its specific methodology (continuous wave ultrasound acquisition of the VTI in the ascending aorta utilizing the sternal notch acoustic window), USCOM should be resistant to positional artifact, and hence should be a suitable reference standard for the evaluation of EC. It is possible that maternal position change per se may cause an artifactual change in the CO recorded by EC in the absence of a true change in CO, but as long as the direction of change (and, ideally, the approximate percentage magnitude of the change) agree with USCOM, small positional artifacts while using EC should not disqualify EC for clinical use in positional studies.
Study #1 would utilize a sample of women in late pregnancy (perhaps 30–50 women) whose CO will be measured in 3–6 recumbent positions using EC and USCOM simultaneously. Ideally – to acquire the most data – the positions will be supine, supine semi-recumbent, 45-degree left lateral, 90-degree left lateral (full LLD position), 45-degree right lateral and 90-degree right lateral (full right lateral decubitus position). But if all these positions caused an excessive workload, the number of positions could be reduced. There would be no need to select any special type of patient for this study. In fact, a broad range of women in late pregnancy of varying BMI, maternal age, gestational age and other diagnoses (e.g. diabetes or hypertension) might make the study results more robust and might even allow new hypothesis generation.
If EC agrees with USCOM in identifying the maternal position associated with the highest CO, then EC could be used in future studies rather than USCOM, since EC – unlike USCOM – is continuous, operator-independent, hands-free and requires very limited training and practice before it can be usefully implemented.
Study #2: Pilot studies with cardiac output-guided maternal positioning in laboring patients
Labor involves dramatic changes in CO, SVR and BP due to pain, analgesia, uterine contractions with autotransfusion, changes in maternal position and administration of vasoactive medications such as vasopressors, oxytocin and magnesium sulfate. Hence it provides a rich environment for learning about maternal hemodynamics and the equipment needed for studying it. Besides the existence of this rich learning environment, it makes sense to begin maternal hemodynamic research in labor, since the results of such research will be acquired relatively quickly – either during labor itself or during the immediate postpartum period (e.g. if hemorrhage, neonatal intensive care unit admission or endometritis occur).
Suggested outcomes to be analyzed for the pilot studies are the 1) rate of cesarean delivery (CD) plus indications, 2) rate of operative delivery (forceps or vacuum), 3) duration of labor, 4) frequency of “fetal distress” episodes, 5) positional variation and behavior of “autotransfusion waves,” 6) frequency of hypotonic DL, 7) frequency of oxytocin use to augment labor, 8) frequency of maternal hypotension with epidural analgesia requiring vasopressors, 9) frequency of postpartum hemorrhage, and 10) rate of postpartum endometritis (possibly due to poor endometrial oxygenation during labor).
Study #3: Therapeutic trials of cardiac output-guided maternal positioning in labor
Based on the results of pilot studies and the existing literature, investigators would compare two randomized groups of patients who were at high risk of experiencing the unfavorable outcomes being studied, such as obese and short patients who are at high risk of CD, DL, or postpartum hemorrhage. The two randomized groups would receive either routine care or COGMP. The clinical outcomes compared between the two groups would be those listed for Study #2.
Study #4: Observational association studies on hospitalized patient not in labor
This study would seek associations between 1) large positional variations in maternal CO and 2) the presence of PE, FGR or preterm labor. The hypothesis would be that OUVR may contribute to the causation of any of the three conditions listed. A positive result using odds ratios would be the finding of an association between large positional decreases in CO and any of the three diagnoses listed, in comparison to healthy controls matched for maternal age, gestational age, BMI and other known risk factors for the three conditions. Even if greater positional variation of CO were found in patients with disease than in controls without disease, causality would not be proved, but such an association might shed light on effect sizes and would justify randomized therapeutic trials with COGMP to determine whether reducing the amount of time a mother spent with low maternal CO would lead to improved fetal and maternal outcomes.
Study #5: Therapeutic trials of cardiac output-guided maternal positioning in hospitalized patients not in labor
The patients for randomization to COGMP or routine care would be patients with 1) normal labor, 2) non-indicated preterm labor, 3) FGR or 4) PE. The goal of this study would be to determine whether associations based on odds ratios between highly positional CO and pregnancy complications were causal. That is, if by preventing OUVR by means of COGMP we could reduce the occurrence or severity of the complications, in comparison to the controls who would receive routine care. The goals for COGMP would be to avoid the problems previously discussed in normal labor, prolong intrauterine gestation, increase birthweight and reduce neonatal intensive care unit admissions.
Needless to say, the timing of the intervention with COGMP would probably be of key importance, since therapy applied too late might not have the desired effect, whereas therapy applied sufficiently early in the course of a disease might have positive preventive or therapeutic effects. In this regard, it is possible that once fetal distress, dysfunctional labor, PE, FGR or preterm labor had progressed irreversibly, COGMP might be “too late.”
Study #6: Home-based “position therapy” in the left lateral decubitus position, without or with cardiac output-guided maternal positioning
This study would consist of repeating and extending the work of Spinapolice [25] and, much more recently, DeVore [41], [42], [43], [44], [45], [46] in prescribing maternal rest in the left lateral position to prevent PE or mitigate FGR, respectively. It appears that DeVore and his colleagues are not using CO to guide their prescription of the LLD position for maternal rest, but are doing so empirically, in the justified belief that – for most patients – this will be the position of most benefit.
In my opinion, additional research in the prevention of pregnancy complications by “position therapy” should be guided by COGMP, if feasible, in order to personalize the therapy and hopefully maximize fetal oxygenation for each patient. Such systematic and “time-stamped” individual hemodynamic measurements might also lead to the demonstration of a dose-response relationship, in which increasing time spent in favorable maternal positions would have increasingly beneficial effects on fetal growth and health. The demonstration of such a dose-response relationship would greatly increase confidence in an emerging therapy. In this regard, it has been demonstrated that in 23 % of patients the right lateral decubitus position is associated with higher resting CO than the LLD position [7], and in these patients the empirical prescription of rest in the LLD position might lead to suboptimal decompression of the IVC and submaximal fetal oxygenation. Hence, “in a perfect world” (and eventually) COGMP might be indicated in one form or another during the entire second half of pregnancy in order to ensure maximum fetal oxygen delivery.
Study #7: Twenty-four hour per day ambulatory cardiac output-guided maternal positioning with wearable devices
A “futuristic fantasy” would be that many women would wear a device, much like a wristwatch, ring or necklace, which would provide feedback in real time regarding the suitability of her current body position for optimal fetal oxygen delivery. The feedback could be given in the form of colored lights or sounds (or both) in order to prompt the woman to change her body position because her current position might not be supplying adequate oxygen to her baby.
More questions about cardiac output-guided maternal positioning
Which pregnant patients would benefit the most from COGMP?
This is, of course, completely unknown, since no benefit from COGMP has been proven so far – and no one has looked! But one might theorize that “high risk” patients with obesity, short stature, advanced maternal age, chronic hypertension and diabetes might be amongst the greatest beneficiaries. Also, as suggested by Figure 4, patients might benefit who have other hemodynamic risk factors such as known heart disease, a history or early indications of PE, or a large (“overdistended”) uterus.
When in pregnancy should COGMP begin?
This is completely unknown, but pilot studies should perhaps be done starting as early as 16–20 weeks of gestation, to document how positional obstruction of the IVC progressively becomes more severe as the uterus enlarges. Traditionally, it is taught that the uterus is large enough to obstruct the IVC at 20 weeks of gestation, but perhaps the uterus sometimes obstructs its own venous return in a more limited and local manner before it is large enough to fully obstruct the entire IVC. In this regard we also need to remember the MRI findings which show that a relatively small reduction in CO is associated with dramatic decreases in blood flow in the abdominal IVC and aorta [4].
Ideally – to document the gradual development of positional IVC obstruction – the same patients would have positional CO measurements taken at each prenatal visit until delivery. This research would be labor intensive and would require that the positional measurements be made as quickly and easily as possible. This is only one of many scenarios in which EC would be much easier to use than USCOM.
How much of a positional decrease in maternal CO warrants maternal position change?
The answer to this question is unknown. It is impressive and thought-provoking, however, to see that turning from the LLD position to the supine position is associated with a relatively modest 16.4 % reduction in maternal CO, but an 85.3 % reduction of IVC flow at its origin and a 32.3 % reduction in abdominal aortic flow at its bifurcation [4]. These findings show that a relatively “small” decrease in CO can be associated with a “large” decrease in lower abdominal and pelvic perfusion, and therefore suggest that even “small” positional reductions in maternal CO might be associated with “large” problems for uterine and pelvic perfusion.
Other reasons why this question cannot be answered at present are that 1) the “correct” threshold for intervention may vary from patient to patient because of coexisting risk factors, 2) the average appropriate threshold for intervention may vary from one type of pregnancy complication to another, 3) the positional reduction of maternal CO may not be the actual primary cause of fetal or placental injury, but rather may only be the most detectable (or “visible”) part of an entire unfavorable hemodynamic condition created by OUVR at the IVC, as shown in Table 1. A fourth reason why the question cannot be answered is that the results of prolonged suboptimal fetal oxygenation may be both immediate (e.g. fetal distress or death) or delayed (e.g. learning disabilities, or diabetes or chronic hypertension in later life), in keeping with the “fetal origins of adult disease” hypothesis.
Obstacles to hemodynamic research and treatment in pregnancy
The obstacles we may encounter as we attempt to perform positional hemodynamic studies or treatments in pregnant women will depend heavily on whether the investigators are physically present with the woman when measurements are being taken, and on the intrusiveness and discomfort that might be associated with the taking of those measurements. Hence, for any specific research project (or therapeutic activity) we must answer two key questions:
Does the investigator have to continuously touch the patient while all measurements are being acquired, or are the measurements taken continuously in a hands-free, “automatic” and discreet manner which does not interfere with the woman’s privacy and autonomy?
Is the patient expected to manage her own positioning in response to device-provided advice, or is maternal positioning managed by the investigator and/or the healthcare treatment team?
Non-invasive and time-limited hemodynamic studies could be performed easily during prenatal care appointments, but the amount of extra time required would have to be minimized, for the convenience of both the patient and the health care staff. This consideration suggests that EC would be far superior to both USCOM and TTE for the acquisition of hemodynamic data. Similarly, hemodynamic measurements on patients who are hospitalized for PE, FGR or preterm labor would also be relatively easy to obtain using EC.
At-home “position therapy” such as DeVore has investigated raises additional questions, such as “How do we know that the patient is complying with the LLD position bed rest which has been prescribed?” and “How many hours per day is the patient remaining in the prescribed position?” Solving this problem will require the use of spatial orientation sensors which track the orientation of the device with respect to the “down” position (i.e. towards the center of the earth). Such sensors are available but would have to be applied to the mother’s chest wall or abdomen in some acceptable fashion which could be tolerated for many days at a time before it was moved and re-applied in another location.
Complete, real-time COGMP would require the combined use of two devices which communicated with one another: a spatial orientation sensor and a device for trending maternal CO in all body positions. The combined device would inform the pregnant woman in real time when her current body position was decreasing her CO below its normal resting level and would tactfully suggest to her that she change her body position in order to restore maximal fetal oxygenation.
Obviously, this last concept of operator-independent, autonomous and real time positioning advice being given to an ambulatory pregnant woman is – at this point – a futuristic dream, and we can foresee that there would be many problems and potentially embarrassing mistakes that would arise as the device was being developed, such as: a) false alarms, b) unrealistic alarm thresholds, c) maternal annoyance and anxiety produced by the frequent occurrence of false alarms, d) inability to turn the alarms off, e) difficulty in communicating promptly with the healthcare team, f) dissatisfaction with the cosmetic and comfort-related aspects of the device, and g) false expectations of the perfect elimination of pregnancy risk by the use of the device.
Despite these problems, if such new technology were shown to reduce pregnancy complications it might be readily adopted and would offer new marketing and profit opportunities for investigators, inventors, medical device companies and patent holders.
Is there a risk from prolonged exposure to low-amplitude high frequency alternating current when impedance cardiography is used for cardiac output-guided maternal positioning?
Electrical cardiometry is FDA-approved for use in adults, children and babies, but there may be unknown risks from prolonged or even 24 h per day exposure to low amplitude high frequency electric current. When diagnostic ultrasound was introduced in past decades, the issue was raised that prolonged exposure to ultrasound energy might damage tissue, including the fetus. The current consensus for both ultrasound and IC seems to be that for time-limited use, the benefits of diagnostic ultrasound and IC far outweigh the risks. Whether or not this same principle will apply to the prolonged use of low amplitude high frequency electric current with impedance cardiography, remains to be seen.
More possible problems with research and therapy
The idea of COGMP is new, potentially disruptive, and even threatening to established norms and habits. In my experience, comfortable laboring women with an epidural are often positioned with a slight 5-to-10-degree tilt to the left side on an empirical basis, or even allowed to lie supine, and as long as the fetal heart rate and variability are acceptable. The patient may then stay in that position for relatively long periods of time since it facilitates nursing care and the laboring mother can see visitors on both sides of her bed. The justification for this routine positioning practice seems to be that an acceptable fetal heart rate means that “all is well” with labor.
Within the model of COGMP, acceptable fetal heart rate and variability are of course still necessary, but they would not be considered sufficient for acceptable positioning. Unlike current labor practice – which focuses on the fetal heart rate and variability – COGMP would treat the positional maximization of maternal CO during labor as the “summum bonum” (greatest good), which must be achieved for therapy to be considered as being properly administered. Obviously, a given maternal position that was “best” from the point of view of COGMP might cause compression of the umbilical cord or other severe problems, but situations obviously demanding an immediate position change would be considered as completely understandable exceptions to the rule.
Paying attention to CO, as well as fetal heart rate and variability, would increase the staff’s workload and might thereby cause confusion, anxiety and resentment. The way to avoid this resentment and ill-feeling would be to:
Fully explain what the research team is doing and why.
Ensure the discreet presence and availability of investigators in the labor room during COGMP, to explain to the nurses what to do, and to explain the rationale for therapy to the patients.
Complete each study quickly so that the staff – once oriented to the research protocol – became accustomed to a new routine and did not forget it over the course of a prolonged study period.
Potential impact of successful preventive therapy on health economics and society
If COGMP were able to substantially reduce pregnancy complications – including cesarean deliveries – it might reduce revenue for health care institutions. The potential disruption might even conceivably reduce the incomes of obstetricians and neonatologists, and this threat might – consciously or unconsciously – arouse resistance to adopting new methods. Additionally, the fact that this new therapy would not be based on the development of new medications might make it difficult to receive financial support for research from pharmaceutical companies.
On the other hand, of course, the successful reduction of pregnancy complications without new drug development would be of immense value for individuals and society as a whole. One can hope that COGMP might decrease the incidence of PE, FGR, PTB, DL, CD and postpartum hemorrhage and infection. One can also hope that COGMP might decrease the incidence of neonatal brain damage and later learning disabilities, as well as decrease the incidence of diabetes and chronic hypertension in later life, in keeping with the theory of the “fetal origins of adult disease.” The fact that the new method did not involve medications, but rather simply restored adequate uterine blood flow by “natural” means (maternal positioning), might make the method very attractive and “empowering” to many people, once the therapy was understood, and education regarding the therapy could be facilitated by the development of animations and simulations, since the concept is easily understood visually.
Of course, this is an idealized vision, but I do believe that this idea offers promise and that some of these dreams may come true. Of course, many of the ideas presented in this review are unproven theories, but these ideas and theories deserve prompt and thorough investigation.
Summary
Efforts to “treat the fetus as a patient” in order to optimize short- and long-term fetal outcomes have probably existed as long as human beings have existed, with important objective successes being achieved since the 19th century.
This review proposes a new “hemodynamic approach” to protecting and ensuring adequate fetal oxygenation during the second half of gestation when the pregnant uterus is large enough to obstruct its own venous return, perfusion and oxygenation.
There is substantial circumstantial evidence to suggest that OUVR may contribute to PE, FGR, PTB, DL or postpartum uterine atony and hemorrhage, but further research is required to systematically and objectively evaluate this theory.
Positional OUVR at the inferior vena causes a positional decrease in maternal CO and this decrease can be used as a marker or warning signal in real time to the pregnant woman to change her body position and thereby restore optimal uterine perfusion and fetoplacental oxygenation.
The reduction in maternal CO by itself may not be the primary cause of fetal, placental or myometrial injury caused by OUVR at the IVC. Several other concomitants of the decreased CO may be equally or more dangerous to fetal health, such as increased uterine venous pressure (see Table 1).
The author has coined the phrase “cardiac output-guided maternal positioning” (COGMP) to describe this preventive and therapeutic approach. In practice, this approach could be carried out either as a screening procedure performed at prenatal visits, and/or as an outpatient monitoring and warning system in patients known to be at risk for significant positional obstruction of the IVC. Ultimately, complete outpatient therapy might require a wearable device for continuously trending maternal CO with a real-time feedback feature that would warn the wearer of the device to change her body position in order to restore optimal fetal oxygenation.
Should this approach be shown to decrease pregnancy complications, the specific practical devices for widespread use could be developed using only the accurate trending of maternal CO, since correct absolute values of CO are not required in order to identify positional reductions in CO. This ability to use trends rather than requiring correct absolute values may be important, since trends (or directions of change) are much easier to determine non-invasively and continuously than correct absolute values. In this approach each pregnant woman serves as her own control, and what is important is the direction of change of CO rather than its absolute magnitude.
The theory of COGMP presented in this review deserves prompt evaluation, since – if it is valid – it could provide a conceptually simple and highly “physiological” approach to protecting and ensuring adequate fetal oxygenation, which would not require the development and risks of new drugs. Instead, this approach would achieve its benefits by the restoration of a normal and healthy hemodynamic state in pregnancy.
Outlook
Despite the dramatic advances in obstetric and perinatal care of the last several decades, the incidence of PE, FGR, PTB, DL and postpartum uterine atony and hemorrhage have not decreased, and this lack of progress may be related to 1) increasing obesity, 2) increased sedentary lifestyle, 3) older age at pregnancy, and 4) increased concurrent maternal disease. The supine hypotensive syndrome of pregnancy has been well-understood as a risk to pregnant women since the 1950s, but the possibility that the same fundamental hemodynamic impairment as found in the syndrome – but with maternal CV compensation to prevent hypotension – could occur on a chronic, recurrent and unrecognized basis and injure the fetus, placenta and myometrium, has not been adequately investigated. Prevention of this possible cause of fetal injury could be “low-hanging fruit” in our attempts to improve pregnancy outcomes for both fetuses and mothers. Research to evaluate this possible advance in care is urgently required, since the possible solution is biologically plausible and conceptually simple, since it merely restores optimal pregnancy hemodynamics.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author knows Markus Osypka, PhD, the owner of Osypka Medical, Inc. and inventor of the Electrical Cardiometry (EC) impedance cardiography system. The author has communicated with Dr. Osypka since 2009 about technical and medical issues and has recently received an invitation from his company to attend a free Webinar about the use of EC in neonates. The author has proposed to Dr. Osypka that he (Dr. Osypka) hold an educational event regarding the use of EC in obstetrics. The author has sent a copy of his 2024 book “Threats to Fetal, Placental and Myometrial Oxygenation: A Unified Hemodynamic Approach” to Dr. Osypka as a gift, but the author does not have and has never had any financial or business relationships with Dr. Osypka.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Burton, GJ, Cindrova-Davies, T, Yung, HW, Jauniaux, E. Hypoxia and reproductive health: oxygen and development of the human placenta. Reproduction 2021;161:F53–65. https://doi.org/10.1530/REP-20-0153.Suche in Google Scholar PubMed
2. Howard, BK, Goodson, JH, Mengert, WF. Supine hypotensive syndrome in late pregnancy. Obstet Gynecol 1953;1:371–7.Suche in Google Scholar
3. Sullivan, WMJ, Grossbard, P. Supine hypotensive syndrome producing abruptio placentae. J Med Soc N J 1954;51:470–1.Suche in Google Scholar
4. Humphries, A, Mirjalili, SA, Tarr, GP, Thompson, JMD, Stone, P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med 2019;32:3923–30. https://doi.org/10.1080/14767058.2018.1478958.Suche in Google Scholar PubMed
5. Jani, D, Clark, A, Couper, S, Thompson, JMD, David, AL, Melbourne, A, et al.. The effect of maternal position on placental blood flow and fetoplacental oxygenation in late gestation fetal growth restriction: a magnetic resonance imaging study. J Physiol 2023;601:5391–411. https://doi.org/10.1113/JP284269.Suche in Google Scholar PubMed
6. Humphries, A, Mirjalili, SA, Tarr, GP, Thompson, JMD, Stone, P. Hemodynamic changes in women with symptoms of supine hypotensive syndrome. Acta Obstet Gynecol Scand 2020;99:631–6. https://doi.org/10.1111/aogs.13789.Suche in Google Scholar PubMed
7. Fujita, N, Higuchi, H, Sakuma, S, Takagi, S, Latif, MAHM, Ozaki, M. Effect of right-lateral versus left-lateral tilt position on compression of the inferior vena cava in pregnant women determined by magnetic resonance imaging. Anesth Analg 2019;128:1217–22. https://doi.org/10.1213/ANE.0000000000004166.Suche in Google Scholar PubMed
8. Humphries, A, Stone, P, Mirjalili, SA. The collateral venous system in late pregnancy: a systematic review of the literature. Clin Anat 2017;30:1087–95. https://doi.org/10.1002/ca.22959.Suche in Google Scholar PubMed
9. Farsetti, D, Barbieri, M, Magni, E, Zamagni, G, Monasta, L, Maso, G, et al.. The role of umbilical vein blood flow assessment in the prediction of fetal growth velocity and adverse outcome: a prospective observational cohort study. Am J Obstet Gynecol 2025;233:66.e1–14. https://doi.org/10.1016/j.ajog.2025.01.001.Suche in Google Scholar PubMed
10. Power, GG, Longo, LD. Sluice flow in placenta: maternal vascular pressure effects on fetal circulation. Am J Physiol 1973;225:1490–6. https://doi.org/10.1152/ajplegacy.1973.225.6.1490.Suche in Google Scholar PubMed
11. Gyselaers, W, Lees, C. Maternal low volume circulation relates to normotensive and preeclamptic fetal growth restriction. Front Med (Lausanne) 2022;9:902634. https://doi.org/10.3389/fmed.2022.902634.Suche in Google Scholar PubMed PubMed Central
12. Vasapollo, B, Novelli, GP, Farsetti, D, Pometti, F, Gagliardi, G, Picone, S, et al.. Maternal hemodynamics in early and late fetal growth restriction. Best Pract Res Clin Obstet Gynaecol 2025. https://doi.org/10.1016/j.bpobgyn.2025.102618.Suche in Google Scholar PubMed
13. Archer, TL, Conrad, BE. Electrical velocimetry follows the hemodynamics of drug therapy and aortocaval compression in preeclampsia. Int J Obstet Anesth 2011;20:91–2. https://doi.org/10.1016/j.ijoa.2010.08.001.Suche in Google Scholar PubMed
14. Howard, RB, Hosokawa, T, Maguire, MH. Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am J Obstet Gynecol 1987;157:1261–6. https://doi.org/10.1016/s0002-9378(87)80307-1.Suche in Google Scholar PubMed
15. Ramasubramanian, R, Johnson, RF, Downing, JW, Minzter, BH, Paschall, RL. Hypoxemic fetoplacental vasoconstriction: a graduated response to reduced oxygen conditions in the human placenta. Anesth Analg 2006;103. https://doi.org/10.1213/01.ane.0000222468.76942.d8.Suche in Google Scholar PubMed
16. Lee, TC, Moulvi, A, James, JL, Clark, AR. Multi-scale modelling of shear stress on the syncytiotrophoblast: could maternal blood flow impact placental function across gestation? Ann Biomed Eng 2023;51:1256–69. https://doi.org/10.1007/s10439-022-03129-2.Suche in Google Scholar PubMed PubMed Central
17. Morley, LC, Debant, M, Walker, JJ, Beech, DJ, Simpson, NAB. Placental blood flow sensing and regulation in fetal growth restriction. Placenta 2021;113:23–8. https://doi.org/10.1016/j.placenta.2021.01.007.Suche in Google Scholar PubMed PubMed Central
18. Valensise, H, Vasapollo, B, Novelli, GP, Giorgi, G, Verallo, P, Galante, A, et al.. Maternal and fetal hemodynamic effects induced by nitric oxide donors and plasma volume expansion in pregnancies with gestational hypertension complicated by intrauterine growth restriction with absent end-diastolic flow in the umbilical artery. Ultrasound Obstet Gynecol 2008;31:55–64. https://doi.org/10.1002/uog.5234.Suche in Google Scholar PubMed
19. Vasapollo, B, Novelli, GP, Farsetti, D, Pometti, F, Frantellizzi, R, Maellaro, F, et al.. NO donors on top of anti-hypertensive therapy reduces complications in chronic hypertensive pregnancies with hypodynamic circulation. Eur J Obstet Gynecol Reprod Biol 2023;291:219–24. https://doi.org/10.1016/j.ejogrb.2023.10.033.Suche in Google Scholar PubMed
20. Farsetti, D, Pometti, F, Vasapollo, B, Novelli, GP, Nardini, S, Lupoli, B, et al.. Nitric oxide donor increases umbilical vein blood flow and fetal oxygenation in fetal growth restriction. A pilot study. Placenta 2024;151:59–66. https://doi.org/10.1016/j.placenta.2024.04.014.Suche in Google Scholar PubMed
21. Abdelrazek, MM, Ahmed, MA, Ibrahim, MEM. Effect of nitric oxide donor and plasma volume expansion on pregnancies with early onset fetal growth restriction: a randomized controlled trial. Arch Gynecol Obstet 2025;311:1435–44. https://doi.org/10.1007/s00404-025-07988-7.Suche in Google Scholar PubMed PubMed Central
22. Byrne, BM, Howard, RB, Morrow, RJ, Whiteley, KJ, Adamson, SL. Role of the L-arginine nitric oxide pathway in hypoxic fetoplacental vasoconstriction. Placenta 1997;18:627–34. https://doi.org/10.1016/s0143-4004(97)90003-5.Suche in Google Scholar PubMed
23. Hampl, V, Jakoubek, V. Regulation of fetoplacental vascular bed by hypoxia. Physiol Res 2009;58:S87–94. https://doi.org/10.33549/physiolres.931922.Suche in Google Scholar PubMed
24. Bednov, A, Espinoza, J, Betancourt, A, Vedernikov, Y, Belfort, M, Yallampalli, C. L-arginine prevents hypoxia-induced vasoconstriction in dual-perfused human placental cotyledons. Placenta 2015;36:1254–9. https://doi.org/10.1016/j.placenta.2015.08.012.Suche in Google Scholar PubMed
25. Spinapolice, RX, Feld, S, Harrigan, JT. Effective prevention of gestational hypertension in nulliparous women at high risk as identified by the rollover test. Am J Obstet Gynecol 1983;146:166–8. https://doi.org/10.1016/0002-9378(83)91047-5.Suche in Google Scholar PubMed
26. Archer, TL. Position therapy in pregnancy: can trending maternal cardiac output put it on a more objective foundation? Acta Obstet Gynecol Scand 2021;100:1539–40. https://doi.org/10.1111/aogs.14167.Suche in Google Scholar PubMed
27. Archer, TL. Cardiac output-guided maternal position therapy for preterm labor-it’s time for a trial. Acta Obstet Gynecol Scand 2022;101:931–2. https://doi.org/10.1111/aogs.14365.Suche in Google Scholar PubMed PubMed Central
28. Archer, TL. Cardiac output-guided maternal positioning in pregnancy – can it improve outcomes? Best Pract Res Clin Obstet Gynaecol 2025;100:102596. https://doi.org/10.1016/j.bpobgyn.2025.102596.Suche in Google Scholar PubMed
29. Archer, TL. Obstructed uterine venous return: a preventable cause of dysfunctional labor? Reproduction 2021;161:V15–17. https://doi.org/10.1530/REP-21-0002.Suche in Google Scholar PubMed
30. Archer, TL, Suresh, P, Ballas, J. Don’t forget aortocaval compression when imaging abdominal veins in pregnant patients. Ultrasound Obstet Gynecol 2011;38:479–81. https://doi.org/10.1002/uog.10065.Suche in Google Scholar PubMed
31. Archer, TL, Shapiro, AE, Suresh, PJ. Maximisation of maternal cardiac output during labour might help to prevent not only foetal hypoxaemia but also myometrial ischaemia, dysfunctional labour, uterine atony and postpartum endometritis. Anaesth Intensive Care 2011;39:774–5.Suche in Google Scholar
32. Archer, TL, Suresh, P, Shapiro, AE. Cardiac output measurement, by means of electrical velocimetry, may be able to determine optimum maternal position during gestation, labour and caesarean delivery, by preventing vena caval compression and maximising cardiac output and placental perfusion pressure. Anaesth Intensive Care 2011;39:308–11.Suche in Google Scholar
33. Grobman, WA, Gilbert, SA, Iams, JD, Spong, CY, Saade, G, Mercer, BM, et al.. Activity restriction among women with a short cervix. Obstet Gynecol 2013;121:1181–6. https://doi.org/10.1097/AOG.0b013e3182917529.Suche in Google Scholar PubMed PubMed Central
34. McCall, CA, Grimes, DA, Lyerly, AD. “Therapeutic” bed rest in pregnancy: unethical and unsupported by data. Obstet Gynecol 2013;121:1305–8. https://doi.org/10.1097/AOG.0b013e318293f12f.Suche in Google Scholar PubMed
35. Maloni, JA. Lack of evidence for prescription of antepartum bed rest. Expert Rev Obstet Gynecol 2011;6:385–93. https://doi.org/10.1586/eog.11.28.Suche in Google Scholar PubMed PubMed Central
36. Biggio, JRJr. Bed rest in pregnancy: time to put the issue to rest. Obstet Gynecol 2013;121:1158–60. https://doi.org/10.1097/AOG.0b013e318294480d.Suche in Google Scholar PubMed
37. Couper, S, Clark, A, Thompson, JMD, Flouri, D, Aughwane, R, David, AL, et al.. The effects of maternal position, in late gestation pregnancy, on placental blood flow and oxygenation: an MRI study. J Physiol 2021;599:1901–15. https://doi.org/10.1113/JP280569.Suche in Google Scholar PubMed PubMed Central
38. Rossi, A, Cornette, J, Johnson, MR, Karamermer, Y, Springeling, T, Opic, P, et al.. Quantitative cardiovascular magnetic resonance in pregnant women: cross-sectional analysis of physiological parameters throughout pregnancy and the impact of the supine position. J Cardiovasc Magn Reson 2011;13:31. https://doi.org/10.1186/1532-429X-13-31.Suche in Google Scholar PubMed PubMed Central
39. Hughes, EJ, Price, AN, McCabe, L, Hiscocks, S, Waite, L, Green, E, et al.. The effect of maternal position on venous return for pregnant women during MRI. NMR Biomed 2021;34:e4475. https://doi.org/10.1002/nbm.4475.Suche in Google Scholar PubMed
40. Valensise, H, Novelli, GP, Vasapollo, B. Restricted physical activity and maternal rest improve fetal growth: should we look for the reason in the cardiovascular modifications? Am J Obstet Gynecol 2025;232:e61–2. https://doi.org/10.1016/j.ajog.2024.08.002.Suche in Google Scholar PubMed
41. DeVore, GR, Polanco, B. Maternal rest improves fetal growth in small-for-gestational age and growth restricted fetuses defined by the delphi consensus protocol. J Clin Ultrasound 2025;53:1045–61. https://doi.org/10.1002/jcu.23980.Suche in Google Scholar PubMed
42. DeVore, GR, Polanco, B, Lee, W, Fowlkes, JB, Peek, EE, Putra, M, et al.. Maternal rest improves growth in small-for-gestational-age fetuses (<10th percentile). Am J Obstet Gynecol 2025;232:118.e1–118.e12. https://doi.org/10.1016/j.ajog.2024.04.024.Suche in Google Scholar PubMed PubMed Central
43. DeVore, GR, Polanco, B. Improved fetal growth associated with maternal rest in fetuses diagnosed with an estimated fetal weight and an abdominal circumference growth velocity less than the 10th percentile. J Clin Ultrasound. https://doi.org/10.1002/jcu.70002.Suche in Google Scholar PubMed
44. DeVore, GR. Could maternal rest improve adverse outcome in fetuses defined by abnormal growth trajectory? Ultrasound Obstet Gynecol 2025;65:393–4. https://doi.org/10.1002/uog.29152.Suche in Google Scholar PubMed
45. DeVore, GR, Polanco, B. Fetuses with deceleration of growth improve their growth following maternal rest. J Clin Ultrasound 2025;53:103–12. https://doi.org/10.1002/jcu.23832.Suche in Google Scholar PubMed
46. DeVore, GR. Maternal rest improves fetal growth. Am J Obstet Gynecol 2024;231:e233. https://doi.org/10.1016/j.ajog.2024.06.035.Suche in Google Scholar PubMed
47. https://www.osypkamed.com/.Suche in Google Scholar
48. Ballas, J, Mantell, K, Archer, TL. Electrical cardiometry demonstrates the hemodynamics of aortocaval compression and autotransfusion during labor. Poster and oral presentation at Society for Obstetric Anesthesia and Perinatology (SOAP) meeting, May 2–5, 2012, Monterey, California.Suche in Google Scholar
49. Archer, TL. Transthoracic echocardiography and electrical cardiometry elucidate the hemodynamics of autotransfusion during labor under epidural analgesia. Int J Obstet Anesth 2017;31:113–15. https://doi.org/10.1016/j.ijoa.2017.03.010.Suche in Google Scholar PubMed
50. Archer, TL. Obstetric anesthesia: a case-based and visual approach. Cham, Switzerland: Springer Nature Switzerland AG; 2020.10.1007/978-3-030-26478-9Suche in Google Scholar
51. Archer, TL. Threats to fetal, placental and myometrial oxygenation: a unified hemodynamic approach. Cham, Switzerland: Springer Nature Switzerland AG; 2024.10.1007/978-3-031-60364-8Suche in Google Scholar
52. Archer, TL, Conrad, BE, Suresh, P, Tarsa, M. Electrical velocimetry demonstrates the increase in cardiac output and decrease in systemic vascular resistance accompanying cesarean delivery and oxytocin administration. J Clin Anesth 2012;24:79–82. https://doi.org/10.1016/j.jclinane.2011.02.014.Suche in Google Scholar PubMed
53. Liu, Y, Pian-Smith, MC, Leffert, LR, Minehart, RD, Torri, A, Cote, C, et al.. Continuous measurement of cardiac output with the electrical velocimetry method in patients under spinal anesthesia for cesarean delivery. J Clin Monit Comput 2015;29:627–34. https://doi.org/10.1007/s10877-014-9645-8.Suche in Google Scholar PubMed
54. Archer, TL. Electrical velocimetry elucidates the hemodynamics of hypertension caused by indigo carmine. J Clin Anesth 2011;23:166–8. https://doi.org/10.1016/j.jclinane.2010.02.013.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

