Abstract
Background
Stillbirth remains a major public health issue with long-lasting psychological impacts. Despite advancements in prenatal diagnostics, many stillbirths remain unexplained. Slovenia has implemented a structured, centralized algorithm for stillbirth investigation and care.
Content
This mini-review analyzes a decade of clinical data (2013–2023) from the Department of Perinatology at University Medical Centre Ljubljana (UMC Ljubljana), assessing the outcomes of Slovenia’s stillbirth management algorithm. The Slovenian approach is also compared with international guidelines from ACOG, RCOG, CNGOF, PSANZ, SOGC, and FOGSI. Slovenia reports one of the lowest stillbirth rates in Europe – 2 per 1,000 births at ≥24 weeks and 1.4 per 1,000 at ≥28 weeks. At UMC Ljubljana, fetal death rates remained stable between 0.4 % and 0.6 %. The structured algorithm includes maternal history, laboratory testing, placental and fetal pathology, and genetic evaluation. Active induction is preferred over expectant management, and routine TORCH screening and centralized committee oversight are emphasized.
Summary
Slovenia’s structured, algorithm-based system has led to notably low stillbirth rates, supported by uniform clinical care and systematic investigations. Although Slovenia’s experience is encouraging, these results derive from a single-center national registry without comparative cohort analysis, limiting attribution of outcomes to specific elements of the algorithm.
Outlook
Future progress will involve the expansion of WES access and full ICD-PM implementation by 2027, enhancing data comparability and facilitating broader international research.
Introduction
Stillbirth, defined as fetal death at or after 22 weeks of gestation, remains a significant public health issue, causing profound psychological and social impacts on families. Globally, approximately 2.6 million stillbirths occur annually, with rates varying widely among countries [1], 2]. Despite advances in prenatal care and diagnostics, many stillbirths remain unexplained, with estimates ranging from 25 to 60 % [3], 4]. This highlights the urgent need for standardized, comprehensive investigation protocols and effective management strategies to reduce preventable fetal deaths.
Slovenia exemplifies a nation with low stillbirth rates, achieved through a structured, centralized approach led by the Department of Perinatology at University Medical Centre Ljubljana (UMC Ljubljana). This review examines Slovenia’s stillbirth algorithm, its outcomes over the last decade, and its alignment with major international guidelines, offering insights into best practices in stillbirth care.
Definitions and epidemiology
Stillbirth rates serve as key indicators of maternal and perinatal healthcare quality. The World Health Organization (WHO) defines stillbirth as fetal death at or beyond 22 weeks of gestation or a birthweight ≥500 g if gestational age is unknown [5]. In 2019, European stillbirth rates ranged from 2.0 to 4.7 per 1,000 births at ≥24 weeks and 2.0 to 3.7 per 1,000 births at ≥28 weeks [6].
Slovenia reports among the lowest rates globally. In 2019, the stillbirth rate was 2 per 1,000 births at ≥24 weeks and 1.4 per 1,000 at ≥28 weeks [6]. At UMC Ljubljana, fetal death rates remained between 0.4 % and 0.6 % from 2013 to 2023, with a slight peak in 2022. Termination of pregnancy (TOP) rates ranged from 0.6 to 1.0 % during this period [7]. These trends indicate effective implementation of standardized prenatal care pathways and diagnostic protocols.
Figure 1 presents the annual rates of fetal deaths (stillbirths) and terminations of pregnancy (TOP) at or beyond 22 weeks of gestation at the Department of Perinatology, UMC Ljubljana, from 2013 to 2023. These improvements underscore the effectiveness of uniform care pathways and rigorous investigation standards.
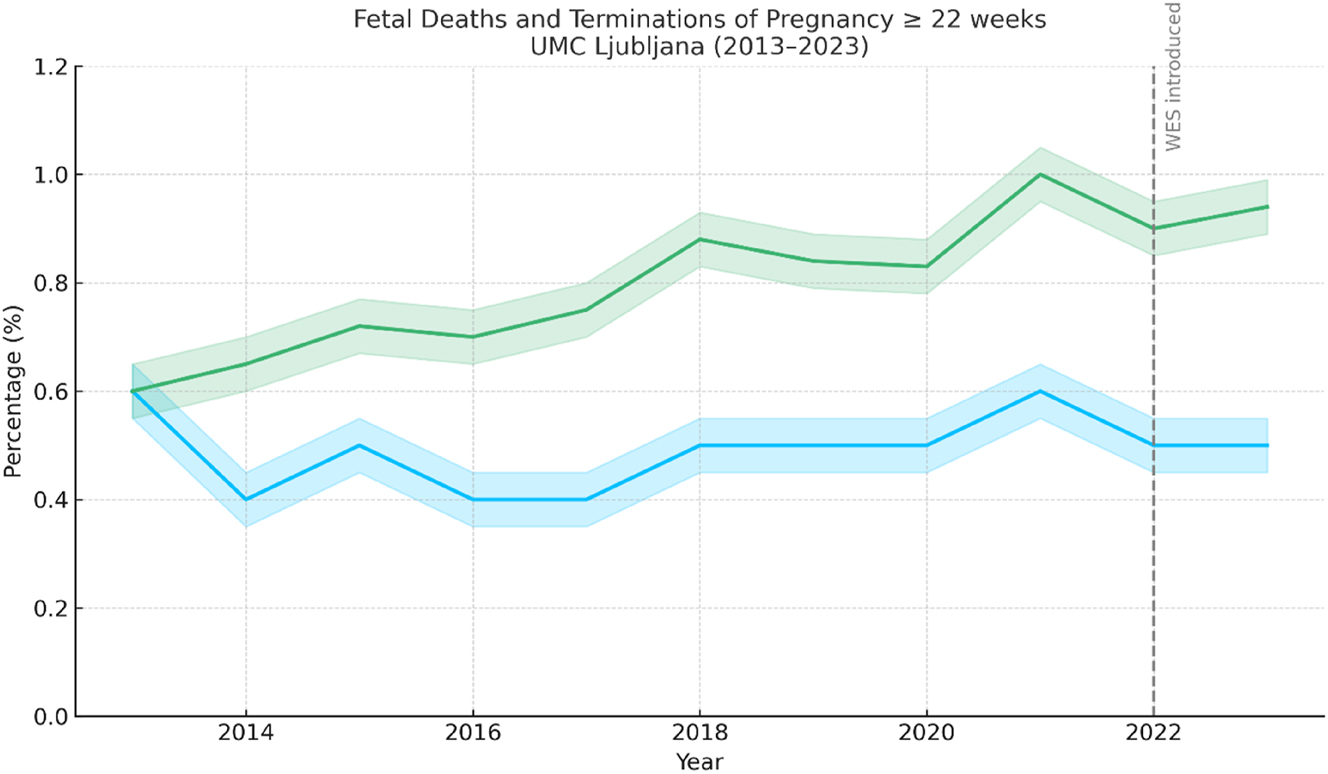
Annual rates of fetal deaths (stillbirths) and terminations of pregnancy (TOP) at or beyond 22 weeks of gestation at the Department of Perinatology, UMC Ljubljana (2013–2023). Stillbirth rates remained between 0.4 % and 0.6 %, while TOP rates gradually increased to 1.0 % in 2021, followed by a slight decline. Confidence intervals (±0.05) are shaded around the trend lines.
Decrease in combined fetal death and TOP rates, indicate overall improved prenatal care and diagnostics. These improvements underscore the effectiveness of uniform care pathways and rigorous investigation standards.
Slovenian algorithm for stillbirth management at the Department of Perinatology in Ljubljana
At the Department of Perinatology in Ljubljana, a structured algorithm guides the investigation and management of stillbirths. This algorithm ensures a standardized and multidisciplinary approach to all cases at or beyond 22 weeks of gestation. The process is coordinated by the Committee for Stillbirths and Developmental Anomalies (Slovenian – KAMRA). This Committee, which was established in 2007 at the Department of Perinatology, Ljubljana, includes maternal-fetal specialists (ob-gyn specialists), clinical geneticists, pathologists and neonatologist. KAMRA deals with all cases of fetal death during pregnancy, i.e. medically terminated pregnancies due to developmental anomalies, spontaneous abortions after the 12th week of pregnancy and all cases of stillbirth that are completed at the Department of Perinatology in Ljubljana. The committee meets monthly to review all available clinical, pathological, genetical and laboratory data to determine the likely cause of fetal death and advise on future pregnancy planning [8].
There were some implementation challenges with algorithm into practise as inconsistent parental consent and logistical gaps in multidisciplinary coordination. These barriers were addressed through support from the KAMRA committee, and development of patient communication materials. Financial investments were required for routine CMA and expansion of laboratory capabilities for WES analysis.
Figure 2 presents an algorithm flowchart of stillbirth investigation implemented at the Department of Perinatology, UMC Ljubljana, for cases occurring at or beyond 22 weeks of gestation.
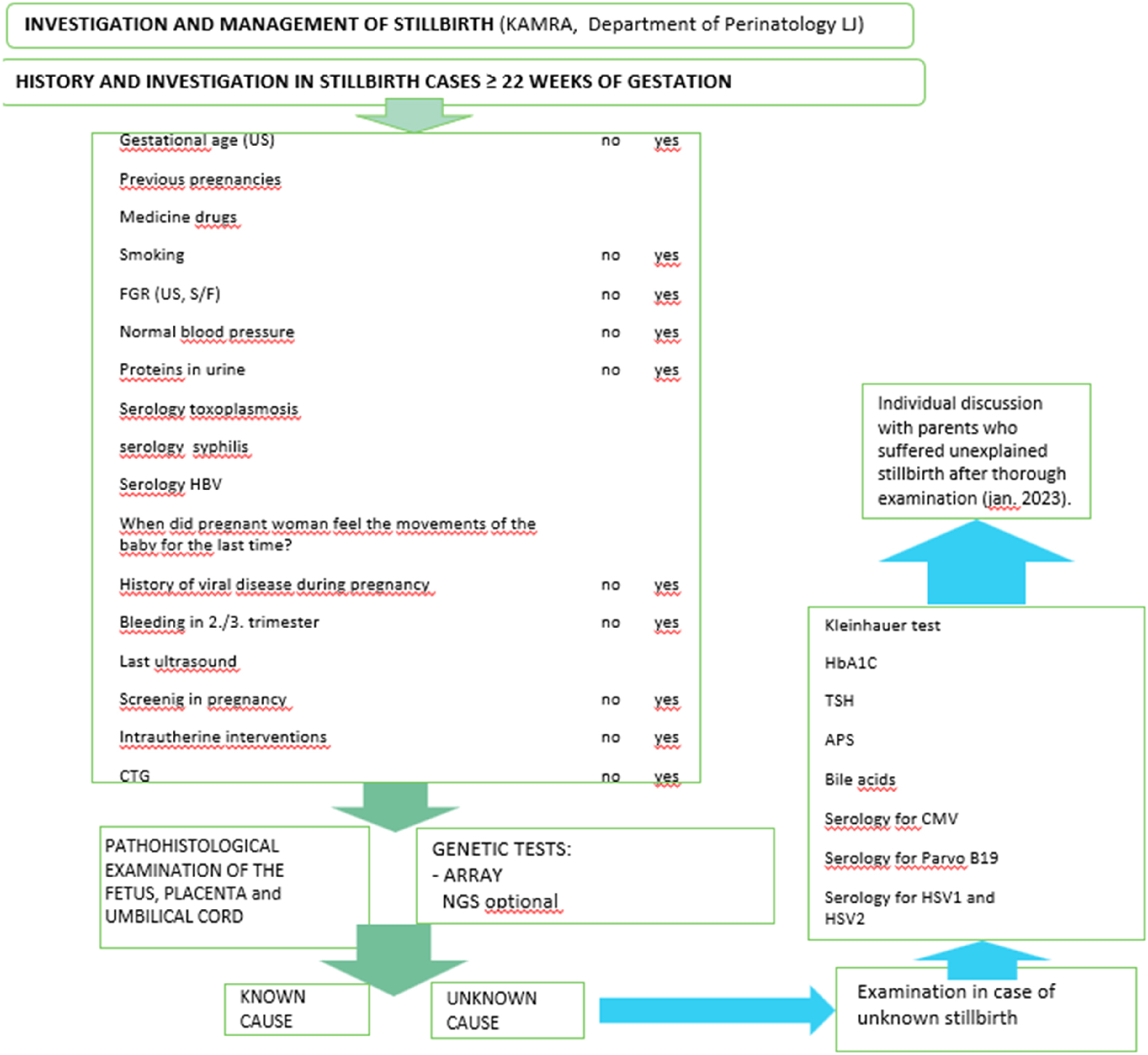
Algorithm flowchart of stillbirth investigation implemented at the Department of Perinatology, UMC Ljubljana, for cases at or beyond 22 weeks of gestation. Includes clinical, pathological, and genetic investigations, as well as individualized counselling.
Figure 3 presents a flowchart showing the multidisciplinary team structure and timeline for investigations and final opinion.
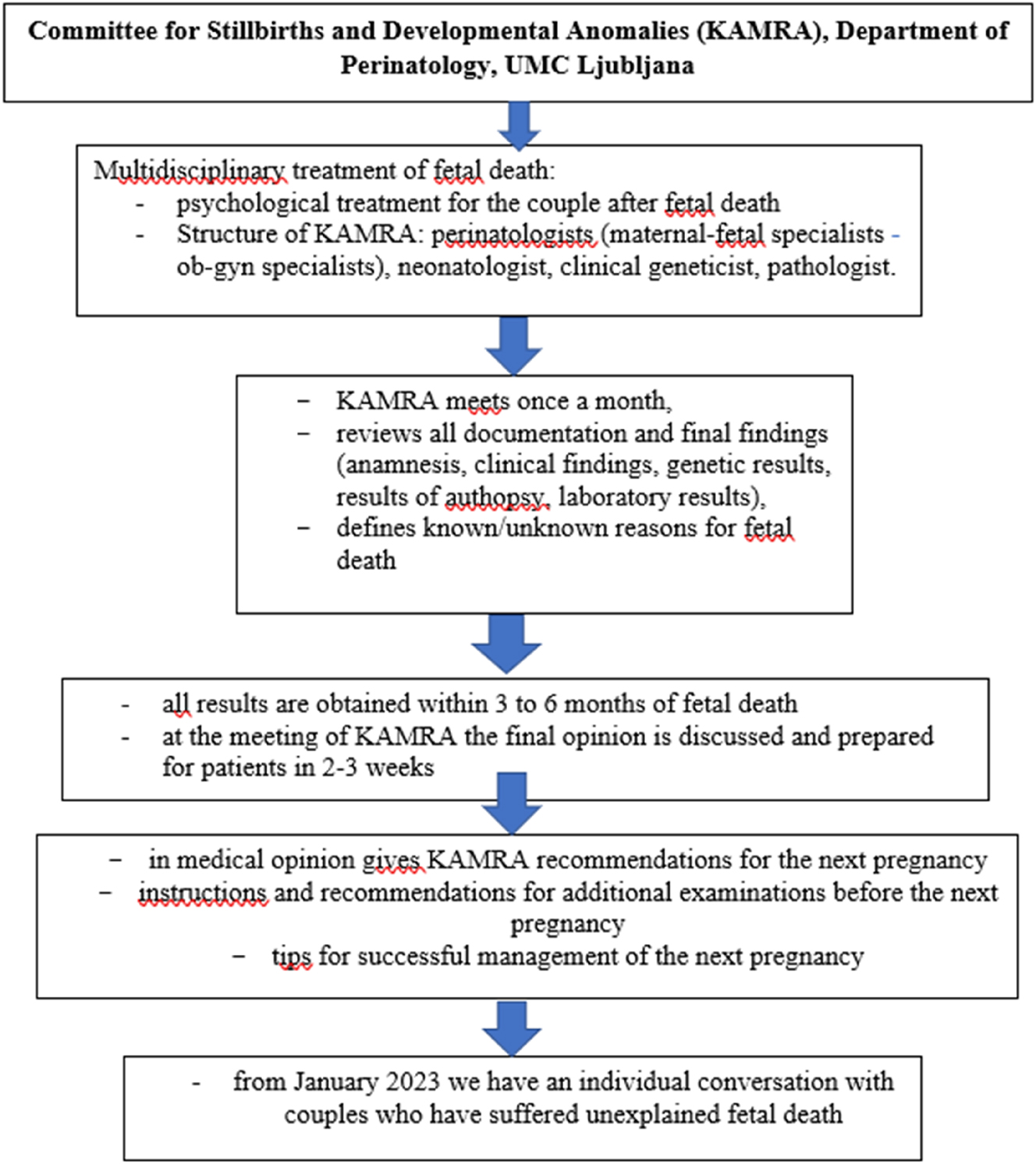
A flowchart showing the multidisciplinary team structure of KAMRA and timeline for investigations and final opinion.
The diagnostic process begins with a detailed clinical history and standardized checklist, which includes:
Confirmation of gestational age by ultrasound.
Review of obstetric history, including previous pregnancies and complications.
Documentation of maternal medication use and smoking status.
Assessment for fetal growth restriction (FGR) via ultrasound and biometric ratios.
Screening for hypertensive disorders, based on blood pressure and proteinuria or signs and symptoms of any other organ disfunction.
Serologic testing for toxoplasmosis, syphilis, and hepatitis B.
Timing of last fetal movements as reported by the mother.
Review of infectious disease history, second or third trimester bleeding, and recent ultrasound findings.
Evaluation of antenatal screening results and intrauterine interventions.
Review of cardiotocography (CTG) if available.
Following clinical evaluation, macroscopic and microscopic examinations of the fetus, placenta, and umbilical cord are performed by a perinatal pathologist. Chromosomal microarray (CMA) is conducted in all cases. Where indicated, next-generation sequencing (NGS) or whole-exome sequencing (WES) may be added. Non-invasive imaging, such as fetal MRI, is occasionally used during pregnancy, mainly in fetuses with central nervous system anomalies, also as a proxy for neuropathologic examination.
If the initial investigation fails to identify a cause, second-tier testing is initiated:
Kleihauer-Betke test for fetomaternal hemorrhage
HbA1c to screen for undiagnosed maternal diabetes
TSH for thyroid function
Antiphospholipid antibody screening (APS) for thrombophilia
Bile acids to assess for intrahepatic cholestasis of pregnancy
Expanded maternal serology, including:
Cytomegalovirus (CMV)
Parvovirus B19
Herpes simplex virus types 1 and 2 (HSV-1/HSV-2)
This approach aims to identify otherwise hidden maternal, fetal, or placental contributors to stillbirth [8].
Even after thorough examination, some cases of stillbirth remain unexplained. Parents often seek any available information about the cause of fetal death. For this reason, since January 2023, the Committee for Stillbirths and Developmental Anomalies (KAMRA) of UMC Ljubljana has formally introduced individualized consultations for families affected by unexplained stillbirth. These sessions review all findings, offer emotional support, and guide planning for future pregnancies [8]. Though formal parental satisfaction data is in development, qualitative feedback suggests strong parental support for structured debriefings. Psychological care is embedded within the algorithm, ensuring early, multidisciplinary intervention.
Management of pregnancy following stillbirth
In Slovenia, the management of pregnancies following a stillbirth emphasizes individualized care, beginning with preconception counseling and continuing with enhanced surveillance during the subsequent pregnancy. Preconception care includes a thorough review of the previous loss, optimization of maternal health (e.g., weight and chronic disease control), and genetic counseling when indicated. During pregnancy, early evaluations include a first trimester dating and nuchal translucency scan, followed by a detailed anomaly scan at 20–24 weeks. In the third trimester, serial growth ultrasounds and antepartum fetal surveillance are initiated from 32 weeks onward. Delivery is typically planned by 39 weeks, unless clinical indications warrant earlier intervention.
The Slovenian algorithm draws upon recommendations from both the American College of Obstetricians and Gynecologists (ACOG) and the Royal College of Obstetricians and Gynaecologists (RCOG), while incorporating context-specific modifications. Key differences compared to international guidelines are outlined in the Table 1 reflecting local clinical practice, resource availability, and national outcomes data [9], 10].
Comparison of stillbirth investigation and management guidelines – Slovenia vs. international bodies (with outcome data).
| Component | Slovenia | ACOG (America) | RCOG (England) | PSANZ (Australia, New Zealand) | SOGC (Canada) | CNGOF (France) | FOGSI (India) |
|---|---|---|---|---|---|---|---|
| Definition of stillbirth | ≥22 weeks | ≥20 weeks | ≥24 weeks | ≥20 weeks | ≥20 weeks | ≥22 weeks | ≥28 weeks (per WHO) |
| Core investigations | Maternal history, TORCH, thyroid, bile acids, APS, Kleihauer-Betke, HbA1c | Tiered, avoids routine TORCH unless indicated | Structured checklist, avoids non-indicated TORCH | Tiered approach, TORCH only if clinically indicated | Tiered, patient-guided | Structured panel + TORCH + thrombophilia | Full maternal and fetal work-up including TORCH |
| Placental histopathology | All cases | Recommended | Strongly encouraged | Standard component | Universal | Recommended | Strongly recommended |
| Fetal autopsy | Offered to all; performed if consented | Strongly encouraged | Recommended | Strongly encouraged | Universal offer | Systematic if consented | Advised in unexplained cases |
| Genetic testing | CMA/karyotyping; WES in select cases since 2022 | CMA encouraged, especially in unexplained or anomalous cases | Based on clinical suspicion | Based on clinical and morphological indication | CMA encouraged in all unexplained cases | CMA or karyotype recommended | Advised in anomalies or unexplained cases |
| Delivery management | Active induction; expectant management not practiced | Individualized; expectant possible if stable | Individualized, with shared decision-making | Early delivery favored; expectant sometimes considered | Individualized, with counseling | Active management preferred | Immediate induction usually recommended |
| Psychological support | Multidisciplinary, early post-loss debriefing | Strongly recommended | Standard part of care | Structured bereavement support standard | Strongly encouraged | Recommended | Emphasized and culturally sensitive support |
| Use of ICD-PM classification | Not universal; uses internal codes | Encouraged | Used alongside ReCoDe | Strongly recommended | Routinely used | Partial implementation | Recommended |
| Reported outcomes | Stillbirth rate 1.4/1,000 (≥28 wks); diagnostic yield with WES; high patient satisfaction | Variable by center; limited reporting on explained cases | Stillbirth rate ∼3.8/1,000; postmortem diagnosis 35–50 % if autopsy performed | Stillbirth rate ∼2.7/1,000; diagnosis improved with PSANZ guidelines | ∼3.5/1,000; autopsy uptake improving outcomes | 2.2/1,000; enhanced TORCH detection | >3.5/1,000; improved outcomes with guideline adherence |
-
ACOG, American College of Obstetricians and Gynecologists; RCOG, Royal College of Obstetricians and Gynaecologists; PSANZ, Perinatal Society of Australia and New Zealand; SOGC, Society of Obstetricians and Gynaecologists of Canada; CNGOF, Collège National des Gynécologues et Obstétriciens Français (French College of Obstetrics and Gynecology); FOGSI, Federation of Obstetric and Gynaecological Societies of India; TORCH, toxoplasmosis, rubella, cytomegalovirus, herpes and other agents; APS, antiphospholipid syndrome; HbA1c, glycated haemoglobin; CMA, chromosomal microarray analysis; WES, whole exome sequencing; ICD-PM, international classification of diseases-perinatal mortality; ReCoDe, relevant condition at death.
UMC Ljubljana’s implementation of the structured algorithm is reflected in improved perinatal outcomes [8].
Comparison of Slovenia’s stillbirth investigation and management protocols with international guidelines
Slovenia’s national approach to stillbirth investigation and management demonstrates considerable alignment with established international guidelines, including those issued by the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), Perinatal Society of Australia and New Zealand (PSANZ), Society of Obstetricians and Gynaecologists of Canada (SOGC), Collège National des Gynécologues et Obstétriciens Français (CNGOF), and Federation of Obstetric and Gynaecological Societies of India (FOGSI) [9], [10], [11], [12], [13], [14].
Notably, Slovenia defines stillbirth as fetal death at or after 22 weeks of gestation, consistent with definitions from CNGOF and PSANZ [11], 13], although international thresholds vary – FOGSI and WHO use a 28-week threshold [5], 14].
In terms of core investigations, Slovenia recommends a comprehensive maternal history, routine laboratory investigations (including TORCH screening, APS, thyroid function tests, and bile acids), and systematic placental histopathology in all of cases [7], 8]. Fetal autopsy is offered universally, chromosomal microarray analysis (CMA) is done in all cases, whole-exome sequencing (WES) is performed selectively [8]. This strategy closely mirrors the structured protocols of RCOG and CNGOF, which emphasize thorough placental and autopsy evaluation [10], 11]. However, it contrasts with ACOG, PSANZ, and SOGC, which generally recommend against routine TORCH screening unless clinically indicated [9], 12], 13]. Slovenia’s inclusion of TORCH screening reflects a more proactive, comprehensive approach. TORCH screening is performed routinely in Slovenia since 1995, reflecting national epidemiology and diagnostic thoroughness. Between 2005 and 2014, 222 cases of toxoplasmosis were reported in Slovenia. In the 1980s, 52 % of women were infected, but in the 1990s, the prevalence dropped to 37 %. The estimated frequency of primary toxoplasma infection during pregnancy is 0.75 %. This can be largely attributed to the routine screening of pregnant women for toxoplasmosis. National surveillance reports indicate periodic CMV and parvovirus B19 surges [15].
Genetic evaluation in Slovenia is pursued based on clinical suspicion, with increasing use of CMA and WES in unexplained cases, aligning with updated recommendations from ACOG and CNGOF supporting genetic analysis when standard investigations fail or anomalies are suspected [9], 11].
Regarding delivery management for women, who suffered a stillbirth, Slovenia practices active induction of labor and does not support expectant management, citing maternal risks. In our practical experience active induction has proven to be better from a psychological point of view and also in terms of maternal morbidity or mortality. In the future the data on psychological outcomes will be done. This practice aligns with CNGOF and FOGSI recommendations [11], 14], while diverging from ACOG, RCOG, and PSANZ, which allow for individualized decision-making, including the option for expectant management in carefully selected cases [9], 10], 13].
Psychological support in Slovenia is delivered through early, structured multidisciplinary debriefing and bereavement counseling, a practice strongly endorsed by all international guidelines and recognized as a critical component of stillbirth care [9], [10], [11], [12], [13], [14] (see Table 1).
Overall, Slovenia’s stillbirth algorithm reflects an evidence-based, multidisciplinary, and patient-centered approach. It integrates best practices from ACOG and RCOG, while preserving distinctive national elements such as routine TORCH screening and a uniform interventional delivery policy. This tailored model ensures comprehensive, consistent care, while supporting ongoing harmonization with international standards [8].
Table 1 shows comparison of stillbirth investigation and management guidelines – Slovenia vs. International bodies.
Discussion
Slovenia’s structured approach to stillbirth investigation and management shows substantial alignment with major international guidelines, particularly in areas such as comprehensive maternal and fetal assessment, selective genetic testing, and early psychological support [9], [10], [11], [12], [13]. A notable distinction is Slovenia’s routine TORCH screening, even in the absence of clinical suspicion, which aligns with CNGOF and RCOG but contrasts with ACOG, SOGC, and PSANZ, which recommend limiting TORCH testing to cases with specific indications [9], 10], 12], 13]. Additionally, expectant management is generally not practiced in Slovenia, favoring active induction of labor. This approach is consistent with CNGOF and FOGSI guidance [11], 14], underscoring Slovenia’s commitment to proactive and comprehensive management.
Slovenia’s stillbirth management algorithm – coordinated through the Department of Perinatology at UMC Ljubljana is closely associated with its low stillbirth rates and improved diagnostic clarity [7], 8]. This success can be attributed to several key factors: very good prenatal and intrapartum care in Slovenia [8]; active delivery management that limits delays after intrauterine fetal demise, reducing maternal morbidity [11], 14]; comprehensive but selective diagnostic workups, including histopathology and genetic testing, that optimize the balance between diagnostic benefit and resource utilization [9], [10], [11], [12]; and early, structured bereavement support which offers timely psychological care for grieving families, enhancing both clinical outcomes and parental experience [10], 13].
Nevertheless, challenges persist. Acceptance of postmortem examinations remains limited in some cases due to cultural, emotional, or religious factors, potentially reducing opportunities to determine a definitive cause of death. Additionally, while chromosomal microarray analysis (CMA) is routinely available, access to whole-exome sequencing (WES) – now recognized for its diagnostic utility in unexplained stillbirth – is still evolving [8], 11], 12]. Between 2015 and 2021 we performed the research about whole exome sequencing and unexplained stillbirths at the Department of Perinatology in Ljubljana. We wanted to discover some still unknown causes of unexplained stillbirths in the field of cardiomyopathies and channelopathies. In our research, we thus analyzed 98 genes in panels related to cardiomyopathies, channelopathies and sudden, unexplained death in young people, using trio exome analysis in our research. We found a rare functional variant that could be the cause of stillbirth in 89.5 % of the cases in our cohort, but we did not find a clear genetic cause. None of our variants were detected de novo. We did not confirm a clear genetic cause for cases of unexplained stillbirth with our genetic analysis. Our research is thus a springboard for further genetic tests.
Additional more extensive studies and analysis will be needed to obtain more accurate data [8].
Another area for advancement involves the adoption of standardized classification systems, such as the ICD-PM (International Classification of Diseases – Perinatal Mortality) framework, to improve international data comparability, enhance audit processes, and support epidemiological surveillance [13], 16]. Integrating Slovenia’s registry into international data networks and maintaining real-time audit and feedback mechanisms could further bolster national and global quality improvement initiatives [17], [18], [19], [20], [21].
Slovenia’s experience offers a valuable model of how centralized coordination, protocol-driven investigation, and interdisciplinary collaboration can drive improvements in stillbirth outcomes, parental care, and health system accountability.
Conclusions
Slovenia’s national framework for stillbirth investigation exemplifies a comprehensive and systematically coordinated model of perinatal care, integrating standardized clinical protocols, multidisciplinary diagnostics, and timely psychosocial support. This approach has contributed to one of the lowest stillbirth rates in Europe and provides a benchmark for evidence-based, equitable care [6], [7], [8, 10], 13], 17]. Although Slovenia’s experience is encouraging, these results derive from a single-center national registry without comparative cohort analysis, limiting attribution of outcomes to specific elements of the algorithm. Further multicenter evaluations are needed.
To further optimize outcomes, future strategies should focus on expanding access to advanced molecular diagnostics, particularly whole-exome sequencing, in cases of unexplained stillbirth [8], 11], 12]. Enhancing parental engagement in postmortem investigations and increasing uptake of internationally standardized classification systems – such as ICD-PM – will be essential for improving diagnostic granularity and enabling robust international comparisons [13], 16], 18]. Slovenia plans to expand WES access by 2026. Full ICD-PM classification adoption is expected by 2027. These steps will support international comparison, registry participation (e.g., Euro-Peristat), and further reductions in unexplained stillbirths.
Slovenia’s experience underscores the critical importance of systems-level integration in reducing perinatal mortality and improving family-centered care. As such, it offers a transferable model for other healthcare systems seeking to implement structured, scalable solutions to address the persistent global challenge of stillbirth.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Lawn, JE, Blencowe, H, Waiswa, P, Amouzou, A, Mathers, C, Hogan, D, et al.. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387:587–603. https://doi.org/10.1016/s0140-6736(15)00837-5.Suche in Google Scholar PubMed
2. World Health Organization. Making every baby count: audit and review of stillbirths and neonatal deaths. Geneva: WHO; 2020.Suche in Google Scholar
3. Flenady, V, Wojcieszek, AM, Middleton, P, Ellwood, D, Erwich, JJH, Coory, M, et al.. Stillbirths: the way forward in high-income countries. Lancet 2011;377:1703–17. https://doi.org/10.1016/S0140-6736(11)60064-0.Suche in Google Scholar PubMed
4. Heazell, AEP, Siassakos, D, Blencowe, H, Burden, C, Bhutta, ZA, Cacciatore, J, et al.. Stillbirth investigations: an evidence-based approach. BJOG 2017;124:1157–67.Suche in Google Scholar
5. World Health Organization. International statistical classification of diseases and related health problems, 10th revision (ICD-10). Geneva: WHO; 2016.Suche in Google Scholar
6. EuroPeristat Project. European perinatal health report: core indicators of the health and care of pregnant women and babies in Europe from 2015 to 2019. Paris: Inserm; 2019.Suche in Google Scholar
7. University Medical Centre Ljubljana, Department of Perinatology. Internal stillbirth data report (2013–2023). Ljubljana: UMC; 2024.Suche in Google Scholar
8. Dolanc, MM. Genomic approach in explaining and preventing stillbirths [PhD thesis]. Ljubljana: University of Ljubljana; 2025.Suche in Google Scholar
9. American College of Obstetricians and Gynecologists. Practice Bulletin No. 200: Early pregnancy loss. Obstet Gynecol 2018;132:e197–207. https://doi.org/10.1097/AOG.0000000000002899.Suche in Google Scholar PubMed
10. Royal College of Obstetricians and Gynaecologists. Greentop guideline No. 55: late intrauterine fetal death and stillbirth. London: RCOG; 2017.Suche in Google Scholar
11. Garabedian, C, Sibiude, J, Anselem, O, Attie-Bittach, T, Bertholdt, C, Blanc, J, et al.. Fetal death: expert consensus of the French College of Obstetricians and Gynecologists. Int J Gynecol Obstet 2025;168:999–1008. https://doi.org/10.1002/ijgo.16079.Suche in Google Scholar PubMed PubMed Central
12. Leduc, L, SOGC CLINICAL PRACTICE GUIDELINE. Guideline No. 394-Stillbirth investigation. J Obstet Gynaecol Can 2020;42:92–9.10.1016/j.jogc.2019.04.001Suche in Google Scholar PubMed
13. Perinatal Society of Australia and New Zealand. Clinical practice guideline: perinatal mortality. Melbourne: PSANZ; 2020.Suche in Google Scholar
14. Federation of Obstetric and Gynaecological Societies of India. FOGSI guidelines for prevention and management of stillbirths. New Delhi: FOGSI; 2021.Suche in Google Scholar
15. National Institute of Public Health, Slovenia. Center for infectious diseases. Toxoplasmosis, 2015. Internet. http://www.nijz.si/sl/toksoplazmoza.Suche in Google Scholar
16. World Health Organization. ICDPM: application of ICD10 to deaths during the perinatal period. Geneva: WHO; 2016.Suche in Google Scholar
17. Heazell, AEP, McLaughlin, MJ, Schmidt, EB, Roberts, D, Flenady, V, Ellwood, D, et al.. Improving perinatal mortality surveillance: data collection and use. BJOG 2021;128:e17–25.Suche in Google Scholar
18. Flenady, V, Wojcieszek, AM, Middleton, P, Ellwood, D, Erwich, JJH, Coory, M, et al.. Stillbirths: recall to action in highincome countries. Lancet 2016;387:691–702. https://doi.org/10.1016/s0140-6736(15)01020-x.Suche in Google Scholar
19. Draper, ES, Gallimore, ID, Kurinczuk, JJ, Smith, PW, Boby, T, Manktelow, BN, et al.. MBRRACEUK perinatal mortality surveillance report 2022. Oxford: NPEU; 2022.Suche in Google Scholar
20. Gardosi, J, Madurasinghe, V, Williams, M, Malik, A, Francis, A, Kurinczuk, JJ, et al.. Confidential enquiry into stillbirths: a systems-level approach. BMJ 2013;346:f108.10.1136/bmj.f108Suche in Google Scholar PubMed PubMed Central
21. European Perinatal Health Report. Core indicators of the health and care of pregnant women and babies in Europe from 2015 to 2019. Paris: Inserm; 2019.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

