Ureaplasma parvum detected in umbilical cord tissues diagnosed with funisitis associated with adverse pregnancy outcomes and neonatal pneumonia
-
Wen Lei
, Hongyi Gao
and Hua Deng
Abstract
Objectives
Existing studies yielded conflicting evidence regarding the associations between genital tract microbial and funisitis, chorioamnionitis and adverse pregnancy outcomes. This study aims to provide additional evidence for their association through systematic investigation.
Methods
A total of 98 FFPE umbilical cord specimens confirmed as funisitis and chorioamnionitis through histopathological examination were tested for seven genital tract microorganisms using quantitative polymerase chain reaction (qPCR). Electronic medical records of mothers and neonates were retrieved to analyze the risk associations between microorganism-positive cases and chorioamnionitis as well as adverse pregnancy outcomes. The umbilical cord samples with Ureaplasma parvum positive had been sequenced for serovars analysis.
Results
Ureaplasma parvum (UP), Ureaplasma urealyticum (UU), Group B Streptococcus (GBS) and Mycoplasma homini s (MH) were all detected in the study with prevalence of 36.5 %, 7.9 %, 18.6 %, and 5.8 %, respectively, while Mycoplasma genitalium (MG), Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) were not detected. Ureaplasma spp. were identified as the predominant microorganisms detected in 98 umbilical cord cases by using qPCR, demonstrating concordance with clinical vaginal swab findings from pregnant women. Genital microorganisms infection was associated with high stage chorioamnionitis (p = 0.0254) and adverse pregnant outcomes (p = 0.0053). In addition, the prevalence of U. parvum demonstrated a strong significant association with neonatal pneumonia (p = 0.0037).
Conclusions
Umbilical cord specimens tested positive for U. parvum demonstrated a significant association with adverse perinatal outcomes and neonatal pneumonia. Additional studies are warranted to investigate the determinants enabling commensal U. parvum in the genital tract to ascend and induce intrauterine infection, thereby leading to adverse clinical outcomes.
Introduction
Genital colonization, a unique group of microorganisms that reside primarily in the genital mucosa, have garnered significant attention in recent years due to their association with numerous of adverse outcomes of pregnancy [1]. Among various genital microorganisms, Ureaplasma spp. – particularly the two clinically significant species Ureaplasma parvum (UP) and Ureaplasma urealyticum (UU) – along with Mycoplasma hominis (MH) demonstrate particularly high prevalence [2], 3]. These pathogens have been clinically associated with multiple obstetric and gynecological complications, including spontaneous abortion, stillbirth, low birth weight, preterm delivery, chorioamnionitis, intra-amniotic infection, premature rupture of membranes (PROM), postpartum endometritis, pyelonephritis, pelvic inflammatory disease, and sepsis [4], [5], [6], [7], [8].
Intrauterine inflammation, with or without infection, is commonly associated with various adverse pregnancy outcomes and neonatal complications [9], 10]. Inflammation can lead to pathological changes in the placenta, umbilical cord, and even various fetal organs. Clinically common chorioamnionitis is defined by neutrophilic infiltration within the chorioamniotic membranes (fetal membranes) or chorionic plate, while funisitis refers to inflammation of the umbilical cord [11]. Chorioamnionitis represents as evidence of a maternal (host) inflammatory response, whereas funisitis indicates a fetal inflammatory reaction [12]. The placenta and umbilical cord serve as critical physiological structures bridging the maternal and fetal systems, facilitating the transport and exchange of nutrients and blood while enabling the elimination of metabolic waste products. Intrauterine infection can transmit pathogens to the fetus via the placental or umbilical cord route, potentially leading to adverse clinical outcomes [13]. Funisitis is a recognized higher risk factor for adverse neonatal outcomes than chorioamnionitis [14].
Chorioamnionitis and funisitis may occur either due to microbial infection or in the absence of detectable microorganisms (i.e., “sterile inflammation”) [15], 16]. The predominant bacterial pathogens associated with intrauterine inflammation are primarily Ureaplasma species, followed by Fusobacterium species, Streptococcus species, Gardnerella species, and Mycoplasma species [17], [18], [19]. Clinical studies demonstrate that Ureaplasma species (U. parvum and U. urealyticum) emerge as the most common microbial isolated from amniotic fluid, umbilical cord and placental tissues [2], 20]. How do genital microorganisms traverse the chorioamniotic barrier to infect the placenta, umbilical cord and fetus? The most widely accepted route is that lower genital tract microorganisms migrate through the cervix into choriodecidual space, and then reaching the amnoitic fluid and fetus [21], 22]. Infected fetus may face a series of health problems, including pneumonia, enteritis, encephalitis, feeding difficulties and sepsis [23]. While other authors have reported that genital tract Ureaplasma colonization has no statistically significant differences with the chorioamnionitis and funisitis [24], [25], [26].
Still, the impact of genital microorganisms on funisitis and chorioamniotic is conflicting. Understanding the relationship among them is crucial for preventing and treating related diseases and ensuring the health of mothers and infants. This study investigated seven genital tract microorganisms in FFPE cord tissue samples from cases of funisitis using qPCR. Combining this result with maternal prenatal examination data, pregnancy outcomes, and neonatal clinical symptoms, it analyzed the associated risks between these microorganisms and these clinical outcomes.
Materials and methods
Inclusion and exclusion criteria
We collected paraffin-embedded tissue samples of umbilical cord with funisits at Guangdong Women and Children Hospitalduring February 2018 to December 2023 retrospectively. Inclusion Criteria: umbilical cord FFPE specimens with histopathologically confirmed funisits. Exclusion Criteria: the umbilical cord of IVF (In vitro Fertilization) and induced abortion.
Demographic data
Clinical data from 98 pregnant women whose umbilical cord underwent qPCR testing were analyzed. Pregnancy outcomes included 5 stillbirths, 19 abortions, and 74 live births, all of which were incorporated into the final analysis. Gestational age was determined by a combination of the last menstrual period and ultrasonographic evaluation. Data regarding age, parity, vaginal swab test, previous obstetric history related to adverse pregnant, gestational week at delivery and the fetus with diseases diagnosed during hospitalization were obtained from the medical charts.
Specimen collection
The fetal end and placental end of the umbilical cord should be sectioned at a thickness of 4–5 μm. Each sample underwent gross description and was fixed in 10 % formalin for 3–5 days prior to paraffin embedding. The specimens were dehydrated through graded ethanol solutions, cleared in xylene, and embedded in paraffin. Additionally, samples were preserved in 1.5 mL sterile universal tubes at −20 °C for subsequent nucleic acid extraction.
Histological examination of umbilical cord samples
The pathologists, blinded to clinical outcomes, performed standard histopathological examinations on each umbilical cord and placental specimen, and then categorized the presence or absence of funisitis (the inflammatory process involves the umbilical cord or vessels) and chorioamnionitis (defined by characteristic inflammatory patterns in the chorionic plate or fetal membranes) [13]. For the objectives of this study, only the histological analysis results of the umbilical cord, chorion and amnion are reported here.
DNA extraction and quantitative PCR
DNA was extracted using the instrument TIANamp FFPE DNA Kit (Tiangen Biochemical Technology Co., Ltd., Beijing, China), in accordance with the manufacturer’s instructions and stored frozen at −20 °C until testing.
Detection reagents were provided by a diagnostic company (Guangdong Bright-Innovation BioMed Co., Ltd., Shunde, China). The target genes including: U. urealyticum and U. parvum urease genes; M. hominis 16s rRNA gene; Mycoplasma genitalium MgpB gene; GBS sip gene; Chlamydia trachomatis16s rRNA; N. gonorrhoeae 16s rRNA. Final reaction volumes of 20 µl were made up with 2 µl nucleic acid extract and 18 µl mastermix. The procedure of DNA detection were performed using SLAN-96P Real-Time System (HONGSHI, Shanghai, China) with the following cycling conditions: 95 °C for 5 min, followed by 45 cycles of 95 °C for 15 s and 59 °C for 45 s. Human ACTB gene as the internal control was included in each qPCR run to monitor normal progression of the qPCR reaction, positive and negative controls specific to each qPCR assay were included in each run to monitor performance. Results were analyzed using SLAN-96P Real-Time System software, (HONGSHI)and recorded as cycle threshold (Ct) values; reactions which failed to produce a Ct value after 45 cycles were recorded as negative. The ACTB gene served as a reference gene and samples with a cycle threshold (Ct) value of ACTB above 35 were deemed “low quality” and excluded. Ct values of target gene <40 were judged positive.
Sequencing-based serotyping analysis of Ureaplasma parvum
To serotype UP positive samples, PCR amplicons were generated by using of the same assay [27]. PCR reactions were checked for successful amplification on an 2 % agarose gel; amplicons subsequently sent to Shenggong Biotech (Guangzhou, China) for sequencing. Serovar identity was determined with the NCBI nucleotide Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi), an online database that checks for nucleotide sequence homology.
Statistical analysis
A two-sample t-test was used to compare the mean age (presented as mean±standard deviation). Pearson’s Chi-square test or Fisher’s exact test were used to compare the frequencies. A p-value ≤ 0.05 was considered statistically significant. Graphs were generated using GraphPad Prism 5 software (version 5.0). Odds risk calculation refer to this software (MedCalc Software Ltd.) URL: https://www.medcalc.org/calc/odds_ratio.php (Version 23.3.4).
Results
Study population
A total of 120 umbilical cord samples from women diagnosed with funisitis and chorioamnionitis were analyzed in the present study and 22 cases were excluded for the following reasons: induced abortion (n=16), in vitro fertilization (IVF) (n=6) (Figure 1). The remaining 98 subjects were categorized into two groups according to perinatal outcomes. Fifty one (52.0 %) pregnant women were included in the adverse pregnancy outcomes group, comprising five fetal deaths, 19 miscarriages, and 27 preterm births. Forty seven (48.0 %) pregnant women who gave birth at or beyond 37 weeks of gestation served as a term birth group (Figure 1).
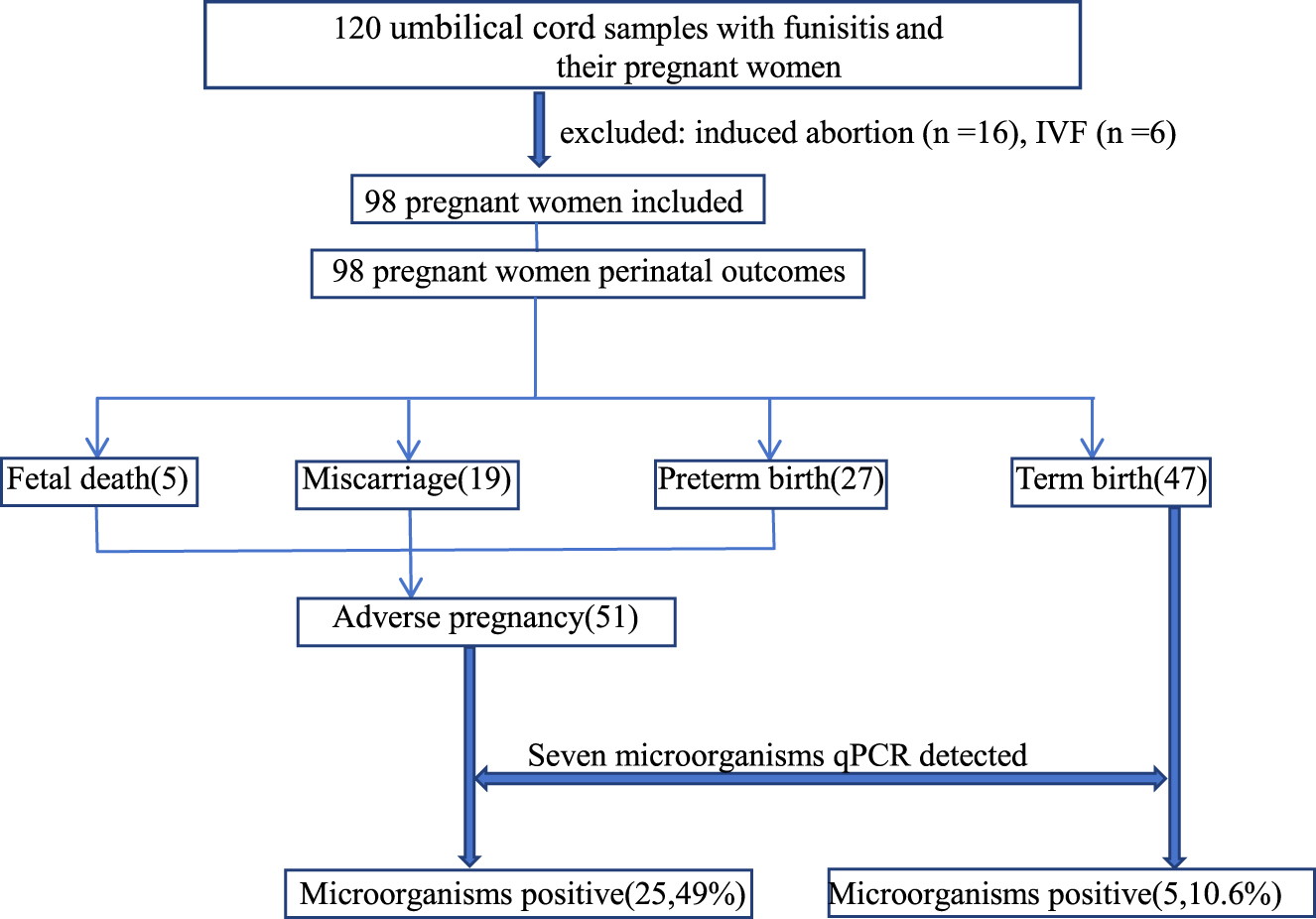
Flowchart of this study.
Characterization of participants
A comparative analysis was conducted on demographic data and laboratory results extracted from obstetric patients’ clinical electronic medical records. There were no significant differences in maternal age, number of women aged less than 25 years, vaginal cleanliness≥Ⅲ, BMI<19.8, abnormal pregnancy history between the two groups divided by the length of gestation (Table 1). Among 98 pregnant women, 48 underwent Ureaplasma spp. culture testing, and 24 (50 %) tested positive. Through various clinical testing methods, Candida, BVsialidase, GBS and M. Hominis were detected in 13.3 %, 9.2 %, 7.1 %, and 6.1 % of the 98 pregnant women, respectively. The prevalences of Candida, BV sialidase and M. hominis showed no significant difference between the two groups, while Ureaplasma spp. exhibited a significant difference between the adverse pregnancy group and term birth group (60% vs. 23 %, p = 0.0304). Concurrently, GBS positive result showed a marginal difference between the two groups, with a higher detection rate in the term group. (p = 0.0457).
Clinical and microbiological features in two pregnant groups with distinct outcomes.
| Characteristic | Mean±SD (%) | |||
|---|---|---|---|---|
| Total | Adverse pregnancy | Term birth | p-Valuea | |
| No. of women | 98 | 51 | 47 | – |
| Age (year) | 30.10±4.86 | 30.67±4.44 | 29.49±5.25 | 0.2324 |
| No. with age of <25 years | 13 (13.3 %) | 7 (13.7 %) | 6 (12.8 %) | 0.8888 |
| Vaginal cleanliness≥Ⅲ | 43 (43.9 %) | 20 (35.1 %) | 23 (48.9 %) | 0.3335 |
| BMI at entry of <19.8b | 16 (16.3 %) | 9 (17.6 %) | 7 (14.9 %) | 0.7128 |
| Abnormal pregnancy history | 11 (11.2 %) | 9 (17.6 %) | 2 (4.3 %) | 0.0523 |
| Vaginal colonization with: | ||||
| Ureaplasma spp.e | 24/48 (50 %) | 21/35 (60 %) | 3/13 (23 %) | 0.0304 |
| Candida c | 13 (13.3 %) | 8 (15.7 %) | 5 (10.6 %) | 0.4642 |
| BV sialidased | 9 (9.2 %) | 3 (5.9 %) | 6 (12.8 %) | 0.2492 |
| Group B Streptococcus (GBS)e | 7 (7.1 %) | 0 (0 %) | 7 (14.9 %) | 0.0457 |
| M. Hominis e | 6 (6.1 %) | 6 (15.7 %) | 0 (0 %) | 0.0785 |
-
aComparison between the adverse pregnancy and term birth groups; bBMI, body weight/body height squared (kg/m2); cManual microscopy; dEnzymatic method; eCultivation method.
Microorganisms identified in umbilical cord tissues via qPCR
The medical records of 98 patients (Table 1) revealed that the infection rate of Ureaplasma spp. in adverse pregnancy outcomes was significantly higher than that in term birth. Pathologists have observed inflammatory changes in the umbilical cord with funisitis, but traditional immunohistochemical (IHC) analysis cannot confirm the presence of suspected microorganisms. We aimed to investigate whether the culture results from vaginal swabs are consistent with the molecular detection in the umbilical cord. Because microbiological culture is unable to distinguish between the two Ureaplasma subtypes, we applied qPCR to identify the dominant subtypes of Ureaplasma spp. and other common genital tract microorganisms in umbilical cord tissues.
Seven genital colonizing microbiological species were detected by qPCR in umbilical cord samples. The prevalence of microorganisms in two groups were for U. parvum (19/51 vs. 0/47, p = 0.0053); for U. urealyticum (3/51 vs. 1/47, p = 0.3680); for GBS (3/51 vs. 5/47, p = 0.3967) and for M. hominis (3/51 vs. 0/47, p = 0.2070) respectively (Table 2). Compared to culture results, the positivity rate of microorganisms detected by qPCR in the placentas was basically consistent, except for GBS (Tables 1 and 2). while M. genitalium (MG), Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) were not detected.
Comparison of seven species detected in umbilical cord samples between adverse pregnancy and term birth.
| qPCR-positive | p-Value | OR (95 % CI)a | ||
|---|---|---|---|---|
| Adverse pregnancy (15–37 weeks) | Term birth (after 37 weeks) | |||
| No. of samples | 51 | 47 | – | – |
| U. parvum | 19 (36.5 %) | 0 (0 %) | 0.0053 | 57.00 (3.32–977.95) |
| SV1 | 6 | 0 | 0.0785 | 13.57 (0.74–247.91) |
| SV3 | 6 | 0 | 0.0785 | 13.57 (0.74–247.91) |
| SV6 | 7 | 0 | 0.0602 | 16.01 (0.89–288.62) |
| SV14 | 0 | 0 | – | – |
| U. urealyticum | 3 (5.8 %) | 1 (2.1 %) | 0.3680 | 2.86 (0.29–28.65) |
| GBS | 3 (5.8 %) | 5 (12.8 %) | 0.3967 | 0.53 (0.12–2.33) |
| M. hominis | 3 (5.8 %) | 0 (0 %) | 0.2070 | 6.86 (0.34–136.35) |
-
aOR, odds ratio; CI, confidence interval.
As the dominant species, U. parvum further underwent serotyping via genomic sequencing to characterize its strain specificity. We found 6 (31.6 %)cases with serovar 1, 6 cases with serovar 3 (31.6 %), and 7 cases with serovar 6 (36.8 %) (Table 2). No serovar 14-positive samples were identified.
Genital tract micro-organisms infection in umbilical cord linked to chorioamnionitis severity
Based on pathological results, the severity of chorioamnionitis in the placenta can be classified into three stages (Ⅰ/Ⅱ/Ⅲ). Stage II and III chorioamnionitis cases with inflammatory cells penetrating the basement membrane were regarded as the severe disease group. Among the 98 umbilical cord samples, the prevalence of genital tract microorganisms detected in two groups for U. parvum, U. urealyticum, GBS, and M. hominiswere 3/28 vs. 16/70, 0/28vs. 4/70, 2/28 vs. 6/70, and 0/28 vs. 3/70, respectively (Table 3). Although U. parvum demonstrated the highest positivity rate in the severe group (stage Ⅱ/Ⅲ), the prevalence of single pathogen did not exhibit significant difference between the two groups (p > 0.05). However, the total number of cases positive for genital tract pathogens demonstrated a significant difference between the two groups (14.3% vs. 38.6 %, p = 0.0254). These findings suggest that microbial infection links to the severity of chorioamnionitis.
Detection rates of genital tract microorganisms in two chorioamnionitis groups.
| Chorioamnionitis | p-Value | OR (95 % CI) | ||
|---|---|---|---|---|
| Ⅰ | Ⅱ–Ⅲ | |||
| No. of samples | 28 | 70 | – | – |
| U.parvum, n (%) | 3 (10.7 %) | 16 (22.9 %) | 0.1799 | 2.47 (0.66–9.25) |
| U.urealyticum, n (%) | 0 (0 %) | 4 (5.7 %) | 0.3705 | 3.86 (0.20–74.03) |
| GBS,n (%) | 2 (7.1 %) | 6 (8.6 %) | 0.8157 | 1.22 (0.23–6.44) |
| M. hominis, n (%) | 0 (0 %) | 3 (4.3 %) | 0.4783 | 2.96 (0.15–59.09) |
| Total no. of positive micro-organism, n (%) | 4 (14.3) | 27 (38.6) | 0.0254 | 3.77 (1.18–12.05) |
Genital microorganism present in the umbilical cord associated with adverse clinical phenotypes
Figure 2 shows the association between four genital tract microorganisms detected in the umbilical cord and adverse clinical phenotypes. Here, preterm birth and pneumonia correlated with infection by the four positive microorganisms (p < 0.05).U. parvumwas identified by qPCR in 19 specimens, while GBS, U. urealyticum and M. hominis were detected in 8, 4, and 3 samples, respectively. Three umbilical cord samples with U. urealyticum positive were co-infected with M. hominisor or GBS (Supplementary Table S1). In 19 cases with U. parvum positive, the corresponding pregnant women exhibited clinical symptoms of varying severity in their pregnancy outcomes, including premature rupture of membranes (PROM), miscarriage, and fetal death (Supplementary Table S1). We compared the associated risks of different pregnancy outcomes and fetal symptoms with positive vs. negative detection of U. parvum in umbilical cord specimens. In the fetal death and miscarriage group (p = 0.4011) and the premature rupture of membranes (PROM) group (p = 0.3891), there was no significant difference in U. parvum detection (positive vs. negative). However, in the preterm birth group (p < 0.0001) and the neonatal pneumonia group (p = 0.0021), positive detection of U. parvum showed a significant association with these two outcomes (Figure 2).
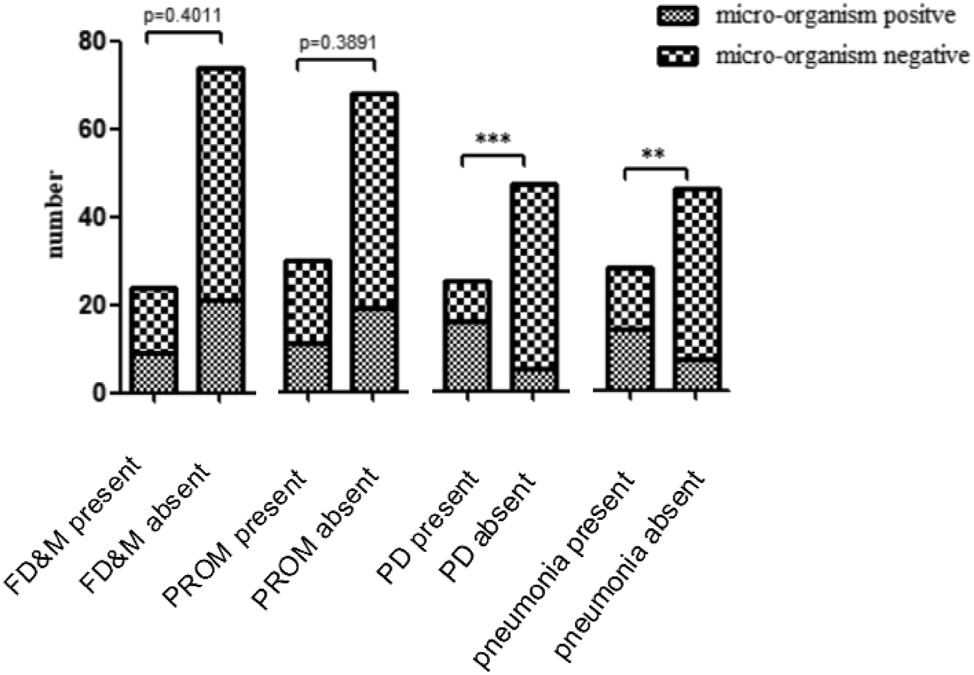
Correlation between adverse clinical phenotypes and microorganisms identified positive in umbilical cord samples. FD&M, fetal death and miscarriage; PROM, premature rupture of the membranes; PD, preterm birth;***p < 0.0005;**p < 0.005.
Correlation between U. parvumin fection in the umbilical cord and neonatal pneumonia
The study cohort comprised 98 pregnant women, with a live birth rate of 75.5 % (74/98). Among the 74 newborn infants, 28 (28/74) were diagnosed with pneumonia. The prevalence of U. parvum were higher in present of pneumonia group compared to non-pneumonia group, demonstrating a strong significant association with neonatal pneumonia (p = 0.0037). U. urealyticum, GBS and M. hominis did not appear linked to pneumonia in Table 4.
Microorganisms detected in umbilical cord samples associated with pneumonia in 74 neonates.
| Pneumonia | p-Value | OR (95 % CI) | ||
|---|---|---|---|---|
| Present | Absent | |||
| No. of samples | 28 | 46 | ||
| U.parvum, n (%) | 10 (35.7 %) | 3 (6.5 %) | 0.0037 | 7.96 (1.96–32.38) |
| U.urealyticum, n (%) | 3 (10.7 %) | 0 (0 %) | 0.0964 | 12.76 (0.63–256.99) |
| GBS, n (%) | 1 (3.6 %) | 4 (8.7 %) | 0.4094 | 0.39 (0.04–3.67) |
| M. Hominis, n (%) | 0 (0 %) | 0 (0 %) | – | – |
Discussion
Among 98 umbilical cord tissue samples, 30 (30.6 %) tested positive for seven microorganisms via qPCR, with U. parvum (UP) identified as the predominant species. This finding aligns with the dominant microbial profile detected in clinical vaginal swabs from pregnant women.
The results of this retrospective study inferred that pregnant women with genital tract microorganisms infection, especially U. parvum, were at increased risk for adverse clinical outcomes, including fetal death, miscarriage, preterm birth and neonatal pneumonia. This finding matches with a recent case-control study by Maria et al. In that study, U. parvum was significantly associated with histological acute chorioamnionitis but not with pneumonia [28]. While our research findings were partly contrary to that conclusion. The significant relation between the detection of Ureaplasma spp. and pneumonia is also supported by previous studies. Sobouti et al. reported that U. parvum can be detected in the genital tract of pregnant women and can be vertically transmitted to the fetus, potentially causing infections in the newborn [29]. These findings suggest a significant transmission of U. parvum from mothers to newborns. Abe et al. collected gastric fluid samples from 47 newborns in the NICU immediately after birth and tested using PCR assays targeting Ureaplasma spp.The subspecies of nine positive samples were U. parvum [30]. In a similar study of ours, U. parvum was detected with the highest frequency in the gastric fluid of newborns, with an odds ratio (OR) of up to six for bronchopulmonary dysplasia (BPD) [31]. Previous studies have shown that the detection of U. urealyticum in the umbilical cord is associated with funisitis [2], 32], while reports linking U. parvum to funisitis remain scarce. Our findings revealed a 19.4 % detection rate of U. parvum in umbilical cord tissues with confirmed funisitis, suggesting its potential role as the predominant pathogenic bacterium underlying the inflammatory process.
The incidence rates of funisitis and chorioamnionitis are not consistent, and they may occur independently [12], 33]. In the cases examined in this study, those diagnosed with funisitis also had a concurrent of chorioamnionitis. These discrepant findings may be attributed to variations in the primary disease focus across different research studies. Chorioamnionitis and funisitis can be caused by non-infectious factors or infectious pathogens. Common pathogens such as U. parvum and marginally U. urealyticum, GBS and other Gram-negative bacteria have been implicated in the development of funisitis and chorioamnionitis [13]. These organisms can gain access to the amniotic cavity by ascending infections from the lower genital tract through the cervix into the intraamninotic space [13], 21]. Our study demonstrated a significant association between genital tract microorganisms and chorioamnionitis severity (p = 0.0254), showing higher prevalence in stages II/III compared to stage I.
Traditionally Ureaplasma spp. classified as biovar 1 and biovar 2, these microorganisms underwent taxonomic reclassification in 2002 based on genomic evidence. Current nomenclature designates the former biovar 2 as Ureaplasma urealyticum and biovar 1 as Ureaplasma parvum [8], 34], 35]. Although previous studies have linked U. urealyticum to adverse pregnancy outcomes including stillbirth, spontaneous abortion, chorioamnionitis, and preterm labor, most investigations neither differentiated between the two clinically relevant species (U. urealyticum and U. parvum) nor employed sufficiently sensitive detection methodologies [36]. In Kataoka et al. study shown that vaginal colonization with U. parvum, but not U. urealyticum, is associated with late abortion or early preterm birth [37]. This result was also confirmed in present study. According the phenotype and genotype, serovars 1, 3, 6, and 14 subspecies are classified as U. parvum, while 2, 4, 5, 7–13 subspecies are classified as U. urealyticum [38], 39]. Rittenschober-Bohm et al. serotyped 1316 samples that colonization with U. parvum serovar 3, but not serovar 1 or serovar 6, in early pregnancy is associated with preterm delivery at very and extremely low gestational age [40]. In a cohort of Australian pregnant women, Payne et al. identified U. parvum serovar 6 (SV6) as a genotype demonstrating particular clinical significance in preterm birth outcomes, according to their molecular epidemiological study [41]. But our result, the serovar sequencing of U. parvum showed that the detection rates of the three main serotypes were close, which inconsistent with above conclusion.
Although numerous clinical observational studies have been conducted over a period of over 30 years, the clinical significance of Ureaplasma spp. infection is still under debate [42]. There is a viewpoint that the Ureaplasma spp. is a commensal in the female genital tract and considered to have of low virulence, because Ureaplasma spp. lacks a cell wall, resulting in weak immunogenicity [43]. Pavlidis et al. have shown that cervical epithelial damage facilitates ascending UP infection into the uteri of pregnant mice, with accompanying PTB and elevation of pro-inflammatory cytokines in the myometrium, fetal membranes and placenta [44]. Previous studies have demonstrated that Ureaplasma spp. are strongly associated with funisitis and chorioamnionitis. Dose a positive vaginal swab test require antibiotic treatment? Studies have shown that there is no difference in the colonization rate of Ureaplasma spp. in the endocervix between fertile women with and without symptoms of genital tract infection [45], 46]. Additionally, the pathogenic role of Ureaplasma spp. is often unclear because most of these infections are clinically asymptomatic. U. parvum infections can persist asymptomatically in humans for up to 2 months [47]. Ureaplasma spp. are not always suspected as causative agents of funisitis and chorioamnionitis. Coinfection with multiple microorganisms may require more than one antibiotic to successfully eradicate the infection [48]. Additionally, pregnant women face limited treatment options due to their concerns about the teratogenic and harmful effects of antibiotic use on the fetus. Antibiotic therapy is not recommended on a broad scale unless there is evidence of intra-amniotic infection.
Although it is currently unclear why some women infected with U. parvum experience adverse pregnancy outcomes while others do not, some researchers attribute these differences sequelae into the toxicity of the infecting serotype and bacterial load [49], [50], [51]. In our study, the Ct values, which serve as an indicator of bacterial load, has no correlation with adverse perinatal outcomes. We further recommend that molecular pathology testing be performed to identify the pathogen when pathological tissue definitively confirms funisitis or chorioamnionitis but the etiology remains undetermined. Compared to traditional cultivation methods, molecular detection, especially multiplex qPCR, can detect multiple pathogens simultaneously.
There are several limitations in our study. Firstly, the relatively small sample size due to the retrospective inclusion of FFPE tissue samples diagnosed with funisitis from archival collections within the past five years. Secondly, the lack of consistent detection time points and methods for the clinical test results of pregnant women may cause certain biases in the conclusions. Thirdly, as this is a retrospective study, vaginal swabs and neonatal oral swabs were not preserved, which hindered the verification of the correlation between vaginal colonization infection and the occurrence of preterm birth and pneumonia. Fourthly, this study only involved seven colonizing bacterias, and the impact of other bacteria on adverse pregnancy outcomes was not covered. Fifthly, the unavailability of data about other well-known factors associated with preterm birth, pneumonia, funisitis, and chorioamnionitis.
Conclusions
Genital mycoplasmas was associated increased inflammation of the chorioamnionitic membranes. The detection of U. parvum in umbilical cord tissue was significantly associated with pretem labor and neonatal pneumonia. Larger prospective studies with adjusted analyses are needed to confirm these findings and clarify mechanistic roles. By understanding and addressing this relationship, healthcare providers can improve the health outcomes for both mothers and newborns. Regular vaginal swab screening for pathogens in early pregnancy may helps in formulating treatment plans. However, further prospective studies are needed to confirm this hypothesis.
Acknowledgments
The authors acknowledge the Pathology team for their support and data collection for this study.
-
Research ethics: The study involving human participants was reviewed and approved by the Guangdong Women and Children Hospital Ethics Committee (Number: 202401422).
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. HYG and HD designed the study and edited the manuscript. WL and LJH was a major contributor in writing the manuscript. ML, XCZ and JLZ collected the clinical samples. QPC and YXL performed the experiments. SZ and LZ analyzed the data.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare no conflict of interest.
-
Research funding: None declared.
-
Data availability: Data are available upon reasonable request to the corresponding author.
References
1. Larsen, B, Hwang, J. Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: a fresh look. Infect Dis Obstet Gynecol 2010;2010:521921. https://doi.org/10.1155/2010/521921.Search in Google Scholar PubMed PubMed Central
2. Egawa, T, Morioka, I, Morisawa, T, Yokoyama, N, Nakao, H, Ohashi, M, et al.. Ureaplasma urealyticum and Mycoplasma hominis presence in umbilical cord is associated with pathogenesis of funisitis. Kobe J Med Sci 2007;53:241–9.Search in Google Scholar
3. Martinez, MA, Ovalle, A, Santa-Cruz, A, Barrera, B, Vidal, R, Aguirre, R. Occurrence and antimicrobial susceptibility of Ureaplasma parvum (Ureaplasma urealyticum biovar 1) and Ureaplasma urealyticum (Ureaplasma urealyticum biovar 2) from patients with adverse pregnancy outcomes and normal pregnant women. Scand J Infect Dis 2001;33:604–10. https://doi.org/10.1080/00365540110026782.Search in Google Scholar PubMed
4. Capoccia, R, Greub, G, Baud, D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 2013;26:231–40. https://doi.org/10.1097/qco.0b013e328360db58.Search in Google Scholar
5. Sweeney, EL, Kallapur, SG, Gisslen, T, Lambers, DS, Chougnet, CA, Stephenson, SA, et al.. Placental infection with ureaplasma species is associated with histologic chorioamnionitis and adverse outcomes in moderately preterm and late-preterm infants. J Infect Dis 2016;213:1340–7. https://doi.org/10.1093/infdis/jiv587.Search in Google Scholar PubMed PubMed Central
6. Choi, SJ, Park, SD, Jang, IH, Uh, Y, Lee, A. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann Lab Med 2012;32:194–200. https://doi.org/10.3343/alm.2012.32.3.194.Search in Google Scholar PubMed PubMed Central
7. Leli, C, Mencacci, A, Latino, MA, Clerici, P, Rassu, M, Perito, S, et al.. Prevalence of cervical colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in childbearing age women by a commercially available multiplex real-time PCR: an Italian observational multicentre study. J Microbiol Immunol Infect 2018;51:220–5. https://doi.org/10.1016/j.jmii.2017.05.004.Search in Google Scholar PubMed
8. Taylor-Robinson, D. Mollicutes in vaginal microbiology: mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res Microbiol 2017;168:875–81. https://doi.org/10.1016/j.resmic.2017.02.009.Search in Google Scholar PubMed
9. Higgins, RD, Saade, G, Polin, RA, Grobman, WA, Buhimschi, IA, Watterberg, K, et al.. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol 2016;127:426–36. https://doi.org/10.1097/aog.0000000000001246.Search in Google Scholar
10. Shim, SS, Romero, R, Hong, JS, Park, CW, Jun, JK, Kim, BI, et al.. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45. https://doi.org/10.1016/j.ajog.2004.06.085.Search in Google Scholar PubMed
11. Pacora, P, Chaiworapongsa, T, Maymon, E, Kim, YM, Gomez, R, Yoon, BH, et al.. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25. https://doi.org/10.1080/jmf.11.1.18.25.Search in Google Scholar PubMed
12. Jain, VG, Parikh, NA, Rysavy, MA, Shukla, VV, Saha, S, Hintz, S, et al.. Funisitis increases the risk of death or cerebral palsy in extremely preterm infants. Am J Obstet Gynecol 2025;233:197.e1–197.e13. https://doi.org/10.1016/j.ajog.2025.02.038.Search in Google Scholar PubMed PubMed Central
13. Kim, CJ, Romero, R, Chaemsaithong, P, Chaiyasit, N, Yoon, BH, Kim, YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52. https://doi.org/10.1016/j.ajog.2015.08.040.Search in Google Scholar PubMed PubMed Central
14. Lau, J, Magee, F, Qiu, Z, Hoube, J, Von Dadelszen, P, Lee, SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol 2005;193:708–13. https://doi.org/10.1016/j.ajog.2005.01.017.Search in Google Scholar PubMed
15. Romero, R, Miranda, J, Chaemsaithong, P, Chaiworapongsa, T, Kusanovic, JP, Dong, Z, et al.. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409. https://doi.org/10.3109/14767058.2014.958463.Search in Google Scholar PubMed PubMed Central
16. Romero, R, Miranda, J, Chaiworapongsa, T, Korzeniewski, SJ, Chaemsaithong, P, Gotsch, F, et al.. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74. https://doi.org/10.1111/aji.12296.Search in Google Scholar PubMed PubMed Central
17. DiGiulio, DB, Romero, R, Amogan, HP, Kusanovic, JP, Bik, EM, Gotsch, F, et al.. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056. https://doi.org/10.1371/journal.pone.0003056.Search in Google Scholar PubMed PubMed Central
18. Romero, R, Miranda, J, Chaiworapongsa, T, Chaemsaithong, P, Gotsch, F, Dong, Z, et al.. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58. https://doi.org/10.1111/aji.12189.Search in Google Scholar PubMed PubMed Central
19. Oh, KJ, Lee, SE, Jung, H, Kim, G, Romero, R, Yoon, BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med 2010;38:261–8. https://doi.org/10.1515/jpm.2010.040.Search in Google Scholar PubMed PubMed Central
20. Namba, F, Hasegawa, T, Nakayama, M, Hamanaka, T, Yamashita, T, Nakahira, K, et al.. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res 2010;67:166–72. https://doi.org/10.1203/pdr.0b013e3181c6e58e.Search in Google Scholar
21. Kim, MJ, Romero, R, Gervasi, MT, Kim, JS, Yoo, W, Lee, DC, et al.. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36. https://doi.org/10.1038/labinvest.2009.49.Search in Google Scholar PubMed PubMed Central
22. Romero, R, Gomez-Lopez, N, Winters, AD, Jung, E, Shaman, M, Bieda, J, et al.. Evidence that intra-amniotic infections are often the result of an ascending invasion - a molecular microbiological study. J Perinat Med 2019;47:915–31. https://doi.org/10.1515/jpm-2019-0297.Search in Google Scholar PubMed PubMed Central
23. Bonnin, F, Petitjean, J, Guillois, B, Laloum, D, Fretignet, M, Freymuth, F. Prospective study of neonatal genital mycoplasma colonization and infection. Arch Pediatr 1995;2:636–42. https://doi.org/10.1016/0929-693x-96-81217-6.Search in Google Scholar
24. Kwak, DW, Cho, HY, Kwon, JY, Park, YW, Kim, YH. Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes. J Perinat Med 2015;43:409–15. https://doi.org/10.1515/jpm-2014-0142.Search in Google Scholar PubMed
25. Romero, R, Miranda, J, Chaiworapongsa, T, Chaemsaithong, P, Gotsch, F, Dong, Z, et al.. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2015;28:1343–59. https://doi.org/10.3109/14767058.2014.954243.Search in Google Scholar PubMed PubMed Central
26. Gomez, R, Romero, R, Nien, JK, Medina, L, Carstens, M, Kim, YM, et al.. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–7. https://doi.org/10.1080/14767050500217863.Search in Google Scholar PubMed
27. Payne, MS, Tabone, T, Kemp, MW, Keelan, JA, Spiller, OB, Newnham, JP. High-resolution melt PCR analysis for genotyping of Ureaplasma parvum isolates directly from clinical samples. J Clin Microbiol 2014;52:599–606. https://doi.org/10.1128/jcm.03036-13.Search in Google Scholar
28. Latino, MA, Botta, G, Badino, C, Maria, D, Petrozziello, A, Sensini, A, et al.. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. J Perinat Med 2018;46:503–8. https://doi.org/10.1515/jpm-2016-0305.Search in Google Scholar PubMed
29. Sobouti, B, Fallah, S, Mobayen, M, Noorbakhsh, S, Ghavami, Y. Colonization of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women and their transmission to offspring. Iran J Microbiol 2014;6:219–24.Search in Google Scholar
30. Abe, Y, Inoue, M, Sekiguchi, K, Nakano, S, Tomaru, Y, Maeda, T, et al.. Clinical characteristics of preterm and term infants with Ureaplasma in gastric fluid. Pediatr Neonatol 2024;65:170–6. https://doi.org/10.1016/j.pedneo.2023.04.016.Search in Google Scholar PubMed
31. Yan, L, Deng, H, Chen, J, Liu, Y, Duan, S, Wang, Z, et al.. Ureaplasma in neonatal gastric fluid contributing to bronchopulmonary dysplasia. BMC Pulm Med 2025;25:127. https://doi.org/10.1186/s12890-025-03579-z.Search in Google Scholar PubMed PubMed Central
32. Li, J, Yin, Y, Bendon, R, Tao, X, Li, J, Sun, X, et al.. Necrotizing funisitis associated with Ureaplasma urealyticum infection: a clinicopathologic analysis of 14 cases. Placenta 2022;126:12–16. https://doi.org/10.1016/j.placenta.2022.06.001.Search in Google Scholar PubMed
33. Grossman, TB, Heller, DS, Baergen, RN. Isolated acute funisitis in the absence of acute chorioamnionitis: what does it mean? Placenta 2019;75:42–4. https://doi.org/10.1016/j.placenta.2018.12.002.Search in Google Scholar PubMed
34. Xiao, L, Glass, JI, Paralanov, V, Yooseph, S, Cassell, GH, Duffy, LB, et al.. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol 2010;48:2715–23. https://doi.org/10.1128/jcm.01877-09.Search in Google Scholar
35. Robertson, JA, Stemke, GW. Expanded serotyping scheme for Ureaplasma urealyticum strains isolated from humans. J Clin Microbiol 1982;15:873–8. https://doi.org/10.1128/jcm.15.5.873-878.1982.Search in Google Scholar PubMed PubMed Central
36. Paralanov, V, Lu, J, Duffy, LB, Crabb, DM, Shrivastava, S, Methe, BA, et al.. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol 2012;12:88. https://doi.org/10.1186/1471-2180-12-88.Search in Google Scholar PubMed PubMed Central
37. Kataoka, S, Yamada, T, Chou, K, Nishida, R, Morikawa, M, Minami, M, et al.. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 2006;44:51–5. https://doi.org/10.1128/jcm.44.1.51-55.2006.Search in Google Scholar PubMed PubMed Central
38. Robertson, JA, Stemke, GW, Davis, JW, Harasawa, R, Thirkell, D, Kong, F, et al.. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol 2002;52:587–97. https://doi.org/10.1099/00207713-52-2-587.Search in Google Scholar PubMed
39. Kim, M, Kim, G, Romero, R, Shim, SS, Kim, EC, Yoon, BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med 2003;31:146–52. https://doi.org/10.1515/jpm.2003.020.Search in Google Scholar PubMed
40. Rittenschober-Bohm, J, Waldhoer, T, Schulz, SM, Pimpel, B, Goeral, K, Kasper, DC, et al.. Vaginal Ureaplasma parvum serovars and spontaneous preterm birth. Am J Obstet Gynecol 2019;220:591–4. https://doi.org/10.1016/j.ajog.2019.01.237.Search in Google Scholar PubMed
41. Payne, MS, Furfaro, LL, Tucker, R, Tan, LY, Mokany, E. One-step simultaneous detection of Ureaplasma parvum and genotypes SV1, SV3 and SV6 from clinical samples using PlexPCR technology. Lett Appl Microbiol 2017;65:153–8. https://doi.org/10.1111/lam.12755.Search in Google Scholar PubMed
42. Sung, TJ. Ureaplasma infections in pre-term infants: recent information regarding the role of Ureaplasma species as neonatal pathogens. Korean J Pediatr 2010;53:989–93. https://doi.org/10.3345/kjp.2010.53.12.989.Search in Google Scholar PubMed PubMed Central
43. Bento, G, Richardson, LS, Da, SM, Tantengco, O, Menon, R. Modeling an ascending infection by Ureaplasma parvum and its cell signaling and inflammatory response at the feto-maternal interface. Am J Reprod Immunol 2023;90:e13770. https://doi.org/10.1111/aji.13770.Search in Google Scholar PubMed PubMed Central
44. Pavlidis, I, Spiller, OB, Sammut, DG, MacPherson, H, Howie, S, Norman, JE, et al.. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun 2020;11:199. https://doi.org/10.1038/s41467-019-14089-y.Search in Google Scholar PubMed PubMed Central
45. Marovt, M, Kese, D, Kotar, T, Kmet, N, Miljkovic, J, Soba, B, et al.. Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis 2015;34:1237–45. https://doi.org/10.1007/s10096-015-2351-8.Search in Google Scholar PubMed
46. Hunjak, B, Sabol, I, Vojnovic, G, Fistonic, I, Erceg, AB, Persic, Z, et al.. Ureaplasma urealyticum and Ureaplasma parvum in women of reproductive age. Arch Gynecol Obstet 2014;289:407–12. https://doi.org/10.1007/s00404-013-2980-z.Search in Google Scholar PubMed
47. Cassell, GH, Davis, RO, Waites, KB, Brown, MB, Marriott, PA, Stagno, S, et al.. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302.Search in Google Scholar
48. Sweeney, EL, Dando, SJ, Kallapur, SG, Knox, CL. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev 2017;30:349–79. https://doi.org/10.1128/cmr.00091-16.Search in Google Scholar PubMed PubMed Central
49. Naessens, A, Foulon, W, Breynaert, J, Lauwers, S. Serotypes of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol 1988;26:319–22. https://doi.org/10.1128/jcm.26.2.319-322.1988.Search in Google Scholar PubMed PubMed Central
50. Jacobsson, B, Aaltonen, R, Rantakokko-Jalava, K, Morken, NH, Alanen, A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand 2009;88:63–70. https://doi.org/10.1080/00016340802572646.Search in Google Scholar PubMed
51. Kasper, DC, Mechtler, TP, Reischer, GH, Witt, A, Langgartner, M, Pollak, A, et al.. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis 2010;67:117–21. https://doi.org/10.1016/j.diagmicrobio.2009.12.023.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0321).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

