The incidence and outcomes of perinatal asphyxia in spontaneous extreme preterm birth: a retrospective cohort study
-
Zohal Faiz
, Eline van ’t Hof
Abstract
Objectives
To determine the incidence and outcome of perinatal asphyxia (PA) in spontaneous extreme preterm birth (PTB) between 24 and 28 weeks gestation.
Methods
We conducted a retrospective cohort study (2010–2019) at a Dutch tertiary center, including singleton spontaneous PTBs with active neonatal management. PA was defined as a 5-min Apgar score ≤5, umbilical artery pH≤7.00, base deficit >12 mmol/L, and need for ongoing resuscitation. Cases were classified as proven PA (all criteria met), suspected PA (Apgar≤5 only if blood gas data missing), or no PA. Neurodevelopment was assessed at 2 years corrected age. Multiple imputation addressed missing data. Logistic regression, adjusted for gestational age (GA), was used to evaluate associations with infant mortality, cerebral injury, and neurodevelopmental impairment (NDI).
Results
Among 138 neonates, 84 % had no PA, 12.3 % suspected PA, and 3.6 % proven PA. After imputation, the estimated incidence of PA was 5.0 %. PA was not significantly associated with infant mortality (aOR 1.07; 95 % CI 0.13–8.87; p=0.95) or intraventricular hemorrhage (aOR 1.62; 95 % CI 0.26–10.39; p=0.61). NDI occurred in two infants with PA.
Conclusions
PA occurred more frequently in this extreme preterm cohort than in term neonates, yet did not independently influence mortality or morbidity. A major limitation is the use of ACOG criteria, originally developed for term births, which may have led to over- or underestimation of PA’s true incidence and impact. Although based on few confirmed cases, findings suggest PA, may not be a major independent predictor of adverse outcome in extreme PTB.
Introduction
The survival of extremely preterm born infants has improved and can be attributed to advancements in obstetric and neonatal care. This improvement ensured that the threshold for active management was lowered worldwide. In the Netherlands, this threshold was changed in 2010 from 25 to 24 weeks gestational age (GA), while in other countries 22 weeks GA is currently practiced [1], [2], [3]. Despite these advancements, extreme preterm birth (PTB), defined as birth before the completion of 28 weeks gestation, has a significant impact with a high risk on neonatal mortality and neurodevelopmental impairment (NDI) in later life [4], 5].
Another cause for adverse neonatal outcome is perinatal asphyxia (PA). PA at term, with an incidence of 0.5 %, is a major contributor to neonatal mortality and long-term neurological sequelae, such as cerebral palsy (CP) or severe NDI [6], 7]. There is wide variability in terminology and definitions to describe hypoxia or acidosis in birth [8]. The American College of Obstetricians and Gynecologists (ACOG) defines PA as a marked impairment of gas exchange and blood flow which, if prolonged, leads to progressive hypercapnia and significant metabolic acidosis [9].
Importantly, the ACOG definition applies only to term neonates and is not validated for preterm infants. For preterm births no universally accepted definition of PA is currently available. This lack of consensus complicates both clinical interpretation and research comparisons in this vulnerable group.
Intrapartum cardiotocography (CTG) is used to detect fetal hypoxia and prevent PA by timely interventions to improve neonatal outcome. Although the use of intrapartum CTG monitoring is common practice, the positive predictive value of intrapartum CTG in term fetus is relatively low. Obstetric intervention rates have significantly increased since the introduction of intrapartum CTG but no improvements have been observed in the incidence of CP nor NDI [10].
The clinical value of intrapartum CTG in cases of spontaneous extreme PTB remains a matter of debate. Its application is hindered by poor signal quality, substantial inter- and intra-observer variability, and the absence of evidence-based guidelines tailored to this population [11], [12], [13]. The true incidence of PA in extreme PTB, as well as its contribution to neonatal mortality, hypoxic brain injury, and long-term neurodevelopmental outcomes, remains largely unclear. Although some studies have addressed this topic, they predominantly rely on umbilical cord blood gas values as the sole criterion for diagnosing PA. As such, these studies do not meet the full diagnostic criteria outlined in the ACOG definition, limiting their applicability and comparability [14], 15].
Therefore, the primary aim of this study is to determine the incidence of PA in a cohort of extreme PTB with a spontaneous onset of birth. The secondary outcomes include the impact of PA on outcomes such as infant mortality, hypoxic brain injury, and long-term prognosis in this group.
Materials and methods
Study design
A retrospective cohort study was performed at the Amsterdam University Medical Centre, a tertiary hospital. Maternal and infant data was collected by reviewing electronic medical records. Ethical approval was obtained from the Medical Ethics Review Committee (file number 2020–4,306). Informed parental consent was obtained by opting out. Participants had six weeks to opt out by e-mail or mail.
Participants
All spontaneous extreme PTBs between 24 and 28 weeks GA admitted from January 2010 to December 2019, with documented active neonatal management, were eligible for inclusion. Spontaneous birth was defined as start of labour with contractions or spontaneous rupture of membranes. Active neonatal management was defined as actively facilitating neonatal (pulmonary) transition. The threshold for neonatal cardiac massage was ≥26 0/7 weeks [1]. Exclusion criteria included multiple pregnancy, severe congenital anomaly, primary caesarean section (CS) and antepartum fetal demise.
Outcome measures
The primary outcome was the incidence of PA, defined by the ACOG as a 5-min Apgar score ≤5, an umbilical cord blood gas pH ≤7.00, and a base deficit (BD) >12 mmol/L, with the need for continued neonatal resuscitation (defined as the need for intubation, invasive ventilatory support, and/or cardiac massage directly after birth) [9]. PA was classified based on fulfilled criteria, see Table 1. Infants were diagnosed with proven PA if all criteria were met. In case of missing umbilical cord blood gas, PA was suspected if the Apgar score at 5-min was ≤5 with a need for continued neonatal resuscitation.
Criteria perinatal asphyxia.
| Criteria | Suspected or proven PA |
|---|---|
| 1) Apgar score at 5-min | ≤5 |
| 2) Umbilical artery cord blood gas pH | ≤7.00 |
| 3) Umbilical artery cord blood gas base deficit | >12 mmol/L |
| 4) Need for resuscitation | Yes |
| Suspected PA | Two or three criteria met. Or Apgar ≤5 with resuscitation when blood gas data missing. |
| Proven PA | All four criteria met |
-
Criteria are based on PA, perinatal asphyxia; AS, apgar score. Need for resuscitation is defined as the need for intubation, invasive ventilatory support, and/or cardiac massage directly after birth.
Secondary outcomes included mode of delivery, categorized as either vaginal delivery or secondary CS and cerebral (hypoxic) injuries. Short term infant cerebral (hypoxic) injuries were diagnosed by either cerebral Magnetic Resonance Imaging (MRI), or by bedside cerebral ultrasound (CUS). Severe cerebral injuries were defined as an intraventricular haemorrhage (IVH) ≥grade 2 on CUS or MRI or periventricular leukomalacia (PVL) ≥grade 2 as detected by CUS or MRI. CUS was performed at standardized time intervals up until 28 days postnatal (according to national clinical protocol) [16]. MRI was performed mostly to confirm or specify abnormalities seen on CUS. All brain-imaging findings were reviewed by a skilled pediatric neuro-radiologist. IVH grading was performed according to the Volpe classification [17]. PVL assessments and grading (I- III) according to published standards [18].
Long term outcomes were infant mortality and long-term NDI. Infant mortality was defined as mortality before the age of 2 years. NDI assessment was standardized with the Bayley Scales of Infant and Toddler development (3rd edition, Dutch language [BSID-III-NL]). If applicable, the level of CP was classified using the Gross Motor Function Classification System (GMFCS). Adverse outcome in survivors was defined as a test score of ≥1 standard deviation (SD) below the reference mean on the BSID-III-NL composite cognitive score and/or composite motor score and/or a GMFCS of ≥2, and/or hearing loss requiring hearing aids and/or severe visual impairment (blind of abnormal vision) at 24 months. For the statistical analyses, the binary outcomes of interest at 24 months were used, e.g. a mild impairment was a score ≤85 and >70, and a moderate to severe impairment a score ≤70 [19], 20].
Covariables
Characteristics related to pregnancy and birth were collected for assessment of baseline characteristics. Maternal variables included maternal age, administration of antenatal corticosteroid therapy (a full course: two doses of Betamethasone 24 h apart), diabetes, hypertensive disorders, colonization of group B Streptococcus (GBS), fever >38.0 °C, intrapartum CTG and placental abruption. Neonatal variables were GA in weeks, birth weight in grams, sex and GBS in neonatal blood culture.
Statistical analyses
All data was systematically recorded in an electronic case report form (CRF) on a cloud-based clinical data management platform, Castor EDC (Castor Electronic Data Capture, version 2021.1, Amsterdam, the Netherlands). Data were analysed using SPSS Statistics version 26.0 and R Studio (integrated development for R, Boston, 2015) (R Studio Desktop version 4.2.0). Data was grouped according to Table 1. Descriptive statistics were used to report demographics. For normal distributed continuous variables, the mean and SD was used and analysis was performed using the unpaired t-test. Median with interquartile range (IQR) was used for not normally distributed variables and the Mann-Whitney U test was performed to compare the distribution. Categorical variables were reported as number (n) and percentage and differences in distribution were analysed using the Chi-square test. Missing data were handled using multiple imputation (25 imputed datasets) to enable the use of partial information on missing variables from observed related variables. Analysed results were pooled using Rubin’s rules [21]. Relevant variables used in the multiple imputation process were the following: GA, CTG monitoring, Apgar score at 5 min, umbilical cord blood gas (pH and BD), IVH and PVL on CUS. Missing values for these variables were imputed, for each missing variable the relationship with other variables is considered.
If possible, logistic regression was performed to assess the association of secondary outcomes with the presence of PA quantified with odds ratio (OR) including 95 % confidence interval (CI) adjusted for GA. As part of a sensitivity analysis, we also explored the association between components of the asphyxia definition (5-min Apgar score <5, umbilical cord blood gas pH, umbilical cord blood gas BD, need for resuscitation) and secondary outcomes in relation to infant mortality adjusted for maternal age, maternal diabetes, hypertensive disorders, placental abruption, positive GBS in vaginal swab, maternal fever, antenatal corticosteroids, GA, birthweight, GBS positive blood culture and fetal sex. Statistical significance was defined as a p-value < 0.05.
Results
Baseline characteristics
Of the 254 singleton deliveries, 138 spontaneous singleton PTB were included, see Figure 1. Infants born without PA were born at a later GA and had a higher birthweight compared to infants with suspected PA. No differences were found in maternal risk factors including diabetes and hypertensive disorders, see Table 2.
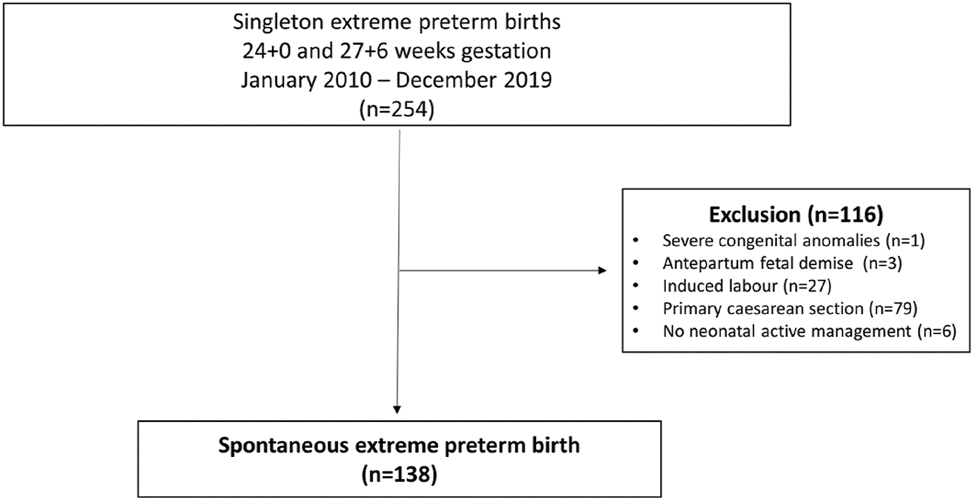
Study exclusion flowchart.
Baseline characteristics.
| Study group | Total (n=138) | No PA (n=116) | Suspected PA (n=17) | Proven PA (n=5) | p-Value |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
|
|
|||||
| Maternal age, mean years ± SD | 30 ± 7 | 30 ± 7 | 30 ± 7 | 32 ± 3 | 0.56a/0.32b/0.92c |
| Maternal diabetes, n (%) | 7 (5) | 6 (5) | 1 (6) | 0 | 0.86 |
| Hypertensive disorders, n (%) | 8 (6) | 7 (6) | 1 (6) | 0 | 0.85 |
| Placental abruption, n (%) | 12 (9) | 10 (9) | 1 (6) | 1 (20) | 0.61 |
| GBS positive swab, n (%) | 35 (25) | 30 (26) | 5 (29) | 0 | 0.39 |
| Maternal fever (>38.0 °C), n (%) | 2 (3) | 2 (2) | 0 | 0 | 0.82 |
| Antenatal corticosteroids, n (%) | 78 (57) | 66 (57) | 8 (47) | 4 (80) | 0.33 |
| Intrapartum CTG, n (%) | 103 (75) | 91 (78) | 9 (53) | 3 (60) | 0.45 |
|
|
|||||
| Neonatal characteristics | |||||
|
|
|||||
| GA groups | 0.09 | ||||
| 24+ 0–24+6 weeks, n (%)z | 34 (25) | 25 (22) (73.5) | 8 (47) (23.5) | 1 (20) (2.9) | |
| 25 +0–25+6 weeks, n (%)z | 28 (20) | 22 (19) (78.6) | 3 (18) (10.7) | 3 (60) (10.7) | |
| 26+ 0–26+6 weeks, n (%)z | 36 (26) | 33 (28) (91.7) | 3 (18) (3, 8) | 0 (0,0) | |
| 27+ 0–27+6 weeks, n (%)z | 40 (29) | 36 (31) (90) | 3 (18) (7.5) | 1 (20) (2.5) | |
| GA in weeks, median (IQR) | 26 +1 (2) | 26 + 1 (2) | 25 + 3 (2) | 25+4 (3) | 0.34a/0.60b/0.05c |
| Birth weight (g), mean ± SD | 900 ± 182 | 916 ± 174 | 823 ± 221 | 792 ± 141 | 0.12a/0.78b/0.05c |
| GBS positive blood culture, n (%) | 17 (12) | 16 (14) | 1 (6) | 0 | 0.55 |
| Infant sex male, n (%) | 68 (49) | 55 (47) | 10 (59) | 3 (60) | 0.60 |
-
PA, perinatal asphyxia; SD, standard deviation; GBS, group B Streptococcus; GA, gestational age. The T-test and the Mann-Whitney U test was employed to assess the differences in maternal age (mean), gestational age (median) and birth weight (mean) across three groups. The Chi-square test was used to investigate differences in distribution of dichotomous variables across the three groups. P-Valuea presents differences between proven perinatal asphyxia and no perinatal asphyxia. p-Valueb presents differences between proven perinatal asphyxia and suspected perinatal asphyxia. p-Valuec presents differences between no perinatal asphyxia and suspected perinatal asphyxia. zNumber of cases (n), followed by the percentage of the total population in parentheses, and then the breakdown by gestational age subgroups within a second set of parentheses. The gestational age subgroups are presented as percentages within each row per PA category (No PA, Suspected PA, Proven PA).
The incidence of perinatal asphyxia (Table 3)
Umbilical cord blood gas pH and base deficit was available in 80 cases (58 %). In total, 116 (84 %) infants were not diagnosed with PA, in 17 infants (12 %) PA was suspected. The remaining 5 infants fulfilled all criteria of PA. Before multiple imputation, the incidence of PA was 6.3 % (5/80). Following imputation, the overall incidence of PA was adjusted to 5.0 % (173/3450).
The overall infant mortality rate before the age of 2 years was 25 % (34/138). Mortality rates for without PA, suspected PA and proven PA were respectively 21 , 47 and 40 %. Before multiple imputation, proven PA was observed in three out of 104 surviving infants (2.9 %) and two out of 34 deceased infants (5.9 %). The incidence of proven PA in imputed data was 4.0 % in surviving infants (104/2600) and 7.7 % in deceased infants (65/850). After adjusting for GA, infant mortality was not significantly associated with proven PA in imputed data (OR 1.99, 95 % CI 0.31–12.50, p-value 0.47, aOR 1.07, 95 % CI 0.13–8.87, p-value 0.95), see Table 3.
Primary outcome perinatal asphyxia.
| Study group | Available cases | Total | Without PA (n=116) | Suspected PA (n=17) | Proven PA (n=5) | p-Value |
|---|---|---|---|---|---|---|
| Apgar score at 5 min<5, n (%) | 138 | 39 (28) | 17 (15) | 17 (100) | 5 (100) | NA |
| Umbilical cord blood gas pH <7.00, n (%) | 80 | 5 (6) | 0/73 | 0/2 | 5 (100) | NA |
| Umbilical cord blood gas base deficit >12 mmol/L, n (%) | 80 | 5 (4) | 0/73 | 0/2 | 5 (100) | NA |
| Need for resuscitation, n (%) | 138 | 78 (57) | 56/106 (52) | 17 (100) | 5 (100) | NA |
| Infant mortality, n (%) | 138 | 34 (25) | 24 (21) | 8 (47) | 2 (40) | 0.95 |
|
|
||||||
| Cerebral (hypoxic) injuries | ||||||
|
|
||||||
| Severe IVH>grade 2 on CUS, n (%) | 124 | 33(27) | 26/109 (24) | 5/11 (45) | 2/4 (50) | 0.61 |
| IVH on MRI, n (%) | 29 | 16 (76) | 12/24 (50) | 3/4 (75) | 1/1 (100) | NA |
| PVL on CUS, n (%) | 124 | 6 (5) | 4/109 (4) | 2/11 (18) | 0/4 | NA |
| PVL on MRI, n (%) | 29 | 5 (24) | 4/24 (17) | 1/4 (25) | 0/1 | NA |
|
|
||||||
| NDI | ||||||
|
|
||||||
| CP, n (%) | 64 | 2 (3) | 2/57 (4) | 0/5 | 0/2 | NA |
| Mild mental impairment, n (%) | 49 | 6 (12) | 5/44 (11) | 1/5 (20) | 0 | NA |
| Moderate to severe mental impairment, n (%) | 49 | 1 (2) | 1/44 (2) | 0/5 | 0 | NA |
| Mild motor impairment, n (%) | 44 | 2 (5) | 0/39 | 2/5 (40) | 0 | NA |
| Moderate to severe motor impairment, n (%) | 44 | 1 (2) | 1/39 (3) | 0/5 | 0 | NA |
|
|
||||||
| Secondary outcomes | ||||||
|
|
||||||
| Secondary CS delivery, n (%) | 138 | 40 (30) | 35 (30) | 5 (29) | 0 | NA |
-
Criteria for categories can be found in Table 1. Logistic regression was performed to assess p-values and odds ratio’s, corresponding associations are found in text. The p-value presented is obtained from imputed data and is adjusted for gestational age. PA, perinatal asphyxia; IVH, intraventricular hemorrhage; CUS, cerebral ultrasound; PVL, periventricular leukomalacia; NDI, neurodevelopmental impairment at the corrected age of 2 years; CP, cerebral palsy. A mild impairment according to Bayley State of Infant development III-NL is defined as a score >70 and <85. A moderate to severe impairment is defined as a score ≤70. BE, base excess; CTG, cardiotocography; CS, caesarean section; NA, not applicable.
Apgar score at 5 min<5 was observed in 39 out of 138 (28 %) infants. In deceased infants, Apgar score at 5 min <5 was more common in comparison to surviving infants, respectively 53 and 20 %, (OR 4.45, 95 % CI 1.96 to 10.31, p-value<0.001). After adjustment for several covariables this effect decreased and lost significance (aOR 2.5, 95 % CI 0.83 to 7.73, p-value 0.10).
Umbilical cord blood gas pH and BD was obtained in 80 out of 138 infants (58 %). Five infants had an umbilical arteria pH below 7.00 and base deficit >12.0 mmol/L. Three infants survived until follow up. A low umbilical cord blood gas pH and high BD were not significantly associated with infant mortality (OR 2.04, 95 % CI 0.39 to 8.78, p-value 0.35; aOR 3.12, 95 % CI 0.17 to 55.90, p-value 0.42 and OR 2.67, 95 % CI 0.32 to 17.52, p-value 0.31, aOR 3.12, 95 % CI 0.166 to 55.9, p-value 0.42, respectively).
Neonatal resuscitation was performed in 78 infants (57 %). In Infants who received neonatal resuscitation, 27 (35 %) infants had died before follow up. No significant associations were found between performing neonatal resuscitation and infant mortality after adjustment for covariates. (OR 4.68, 95 % CI 1.88 to 13.40, p-value <0.001; aOR 1.88 95 % CI 0.52 to 7.66, p-value 0.35).
All live born neonates underwent a CUS during hospitalization. Sixteen infants were diagnosed with severe IVH (confirming CUS diagnosis) and MRI was the primary means of diagnosis of IVH in five infants. Neonates with proven PA suffered severe IVH (>grade 2) twice as much as compared with neonates without PA. In the non-imputed data, severe IVH occurred in 2/4 (50 %) infants with proven PA, 5/11 (45 %) with suspected PA, and 26/109 (24 %) without PA. The risk for development of severe IVH in infants with proven PA was not increased in imputed data (OR 2.10, 95 % CI 0.34–12.81 p-value 0.42, aOR 1.62, 95 % CI 0.26 to 10.39, p-value 0.61). MRI was performed in 29 infants, however in only one infant with proven PA.
Of the 33 infants with severe IVH, ten (30 %) infants died before the corrected age of 2 years. No association between severe IVH and infant mortality was found (OR 1.32, 95 % CI 0.53–3.20, p-value 0.54, aOR 1.09 95 % CI 0.21–3.02, p-value 0.87). PVL diagnosed on CUS was noted more often in neonates with suspected PA as compared to neonates without PA.
A total of 104 out of 138 (75 %) infants survived up until the corrected age of 2 years, of these 104 infants 64 (62 %) visited the outpatient clinic for neurodevelopmental assessment. CP was diagnosed in 2 out of 104 (3 %) infants, both did not have a history of PA nor was there a suspicion for PA.
Cognitive impairment was mild in six infants and moderate to severe in one infant. For psychomotor impairment the incidence was lower; two infants suffered from moderate to severe motor impairment and one from mild motor impairment. BSID neurodevelopmental assessments were not performed in the three infants with proven PA. However, in one infant with proven PA a normal development was seen. Of the other two infants, one infant suffered from mental impairment at the corrected age of 24 months and one infant, with data until 12 months of age, hearing loss-impairment and general developmental impairment was observed.
Secondary CS was performed in 40 out of 138 (29 %) infant and in 95 % CTG was performed during delivery. Suspected fetal hypoxia was the reason for CS delivery in almost 50 %, see supplemental data. After secondary CS delivery, no infants were diagnosed with proven PA, in five out of 40 (13 %) infants PA was suspected and 35 out of 40 (88 %) infants did not fulfill criteria for PA (p-value 0.35). Infant mortality after CS delivery was reported in 9 out of 40 (23 %) infants and after vaginal delivery in 25 out of 98 (26 %). The risk on infant mortality remained non-significant after adjustment for co-variables in non-imputed data (OR 0.85, 95 % CI 0.34 to 1.97, p-value 0.71, aOR 3.50, 95 % CI 0.79 to 18.3, p-value 0.11).
Intrapartum CTG application was not associated with proven PA after adjustment for GA in imputed data (OR 0.50 95 % CI 0.08 to 2.08, p-value 0.46, aOR 0.44, 95 % CI 0.05 to 3.78, p-value 0.45).
In surviving infants, CTG was employed in 77 out of 103 cases (75 %), and in deceased infants, CTG was performed in 26 out of 34 cases (76 %). No significant associations arose between mortality and CTG employment after adjustment for co-variables in non-imputed data (OR 0.25, 95 % CI 0.11 to 0.58, p-value <0.001, aOR 1.51, 95 % CI 0.32 to 7.56, p-value 0.60).
Discussion
Main findings
In this retrospective cohort study, the incidence of PA in spontaneous extreme PTB between 24 and 28 weeks was 5.0 %. The incidence of suspected PA was 12.3 %. Although the incidence of proven PA was nearly twice as high in deceased infants and in infants with IVH compared to surviving and healthy infants, no significant differences were found. The lack of significance may be due to the small sample size, which limits the statistical power to detect differences in outcomes. NDI was present in two infants with proven PA.
Interpretations in light of evidence
There is limited literature describing perinatal PA specifically in the setting of PTB. In contrast, for term infants, PA has a reported incidence of approximately 0.5 %. [6] In our cohort, the incidence was significantly higher, approximately tenfold, when using criteria developed for term neonates. Because there are no validated definitions for PA in preterm infants, we used the American College of Obstetricians and Gynecologists (ACOG) criteria as the most suitable alternative. However, this approach may overestimate the true incidence of PA in extremely preterm infants. A low Apgar score and metabolic acidosis, which form the basis of the ACOG criteria, can occur in preterm neonates for reasons unrelated to hypoxia. For instance, in the absence of uterine contractions, extremely preterm infants may still present with low Apgar scores due to physiological immaturity, including diminished respiratory drive, poor tone, and delayed adaptation to extrauterine life.
In cases where contractions are present, the combination of cord compression and a reduced amount of Wharton’s jelly [13], which normally protects the umbilical vessels, may indeed increase the risk of impaired blood flow and oxygenation. This could lead to lower Apgar scores and acidosis, more closely resembling true PA. Yet, in many preterm births, especially those without labor, similar clinical signs may simply reflect gestational immaturity rather than hypoxic insult.
The weak association between PA (as defined by term-based criteria) and adverse outcomes in our study supports the notion that these criteria may lack specificity in preterm populations. While prematurity may increase susceptibility to hypoxia under certain conditions, the clinical picture often attributed to PA may, in reality, be an expected consequence of immaturity.
The impact of PA on perinatal outcomes in PTB are not evident. According to a review with mostly animal studies, the preterm (sheep) fetus can combat longer periods of severe hypoxia compared to the term (sheep) fetus and is tri-phasic. Primary compensation with peripheral vasoconstriction, ensures central perfusion. Secondly, adaptation to longer periods of hypoxia results in controlled reperfusion of peripheral organs while maintaining central perfusion. At last, a fall in blood pressure results in reduced cerebral perfusion. Preterm fetus seem to be more adaptive in phase 2 and 3, maintaining longer periods of adequate cerebral perfusion according to their metabolic needs and a slower fall in blood pressure. This leads to a paradox. Their ability to withstand long periods of hypoxia can also contribute to a longer cerebral hypoperfusion. Additionally, the antenatally compromised fetus, e.g. intra uterine infections and placental insufficiency, are more likely to suffer from severe injury. Fetal sheep models are used and differences in species should be taken into account, it does provide valuable insights when human research is scare [22], 23].
Two studies in extreme PTB report on metabolic acidosis. The first study examined 3979 live born extreme low birth weight (ELBW) infants (401–1,000 g), with mean GA of 26+6 weeks and mean birth weight of 730 g. Metabolic acidosis, defined as umbilical cord blood gas pH <7.00 or BD <-12 mmol/L, was observed in 6.3 %. ELBW infants might be more at risk for metabolic acidosis due to placental insufficiency [24]. Results were limited by missing data in umbilical cord blood gas. Infants with these missing data had a lower GA, were smaller for GA, with lower Apgar scores and were less likely to have received antenatal steroids or antibiotics. Moreover cases with missing data on pH demonstrated a higher mortality and NDI rate. This could have resulted in bias and an understatement of the effect of acidosis on outcome. Additionally, the study showed a two times increased risk in death and NDI in ELBW infants with metabolic acidosis. In contrast, our study could not find this association. This might be due to our relative “healthy” population.
Another cohort study examined intrapartum hypoxia with acidosis in extreme PTB between 22 and 26 weeks of gestation. Acidosis, defined as umbilical cord blood gas pH <7.08 (corresponding to the <10th percentile), was observed in nearly 10 % of cases. This relatively high incidence may be explained by the broader definition of acidosis used, as well as by the inclusion of extremely premature infants, particularly those between 22 and 24 weeks of gestation. Similar to our study, they did not find a significant increased risk on mortality nor in NDI. They suggested that acidosis specifically is not an evident contributing risk factor for infant mortality or NDI in extreme PTB. We agree it is plausible that outcomes of extreme PTB are more related to extreme prematurity than to PA [15]. Brain injuries are common in extreme preterm neonates, with around 20 % developing NDI such as motor issues. [25], 26]. Long-term sequelae were rare among infants with proven PA in our cohort. However, not all children underwent formal neurodevelopmental assessment, and BSID-III-NL scores were not available for all cases. Two infants with NDI and prior PA were identified. While we found an increased risk for IVH in neonates with proven PA, results were not significant. Low umbilical cord pH was not associated with NDI, consistent with previous studies [14], 15].
Our secondary CS rate was 30 % and half were performed due to suspicion of fetal hypoxia, yet none of the infants with proven PA were born via CS delivery. CS delivery might be beneficial, potentially protecting the preterm fetus from developing severe acidosis. Another possibility, based on abnormal CTG, the decision to operatively intervene was withheld in case of suspected poor outcome.
The lack of evidence-based guidelines to interpret preterm FHR can lead to inappropriate assessment and timing of obstetric interventions. Establishing proper indications is important since the procedure can cause substantial maternal harm and potential complications in future pregnancies [27], [28], [29]. Nevertheless, cesarean delivery may still be warranted in preterm births for indications beyond suspected fetal hypoxia, as clinical decision-making is often multifactorial. Similar to term pregnancies, maternal indications, breech presentation, and fetal growth restriction, among others, can justify a CS in the preterm setting. Even though no PA occurred in babies born by CS, it’s unclear if the procedure improved outcome. In the absence of definitive evidence, some CS may have occurred preemptively based on ambiguous CTG findings [30], 31]. Importantly, it may be appropriate to re-evaluate the current threshold for performing CS in preterm labor. This could potentially support a more conservative approach in selected cases.
The diagnostic value of intrapartum CTG is unclear, especially in the preterm period. In our study, intrapartum CTG was performed in 75 %, analysis did not reveal a significant beneficial effect. Other studies are limited in both number and quality. A Cochrane review did not ascertain the benefits of intrapartum CTG, however, the studies included do not reflect current practices and results are not specified for extreme PTB [10].
Limitations and strengths
This is the first study to investigate the incidence of PA in spontaneous extreme PTB between 24 and 28 weeks of gestation. Only a limited number of previous studies have reported on the occurrence of metabolic acidosis in this population [14], 15]. In contrast to earlier work, PA in this study was defined using the full criteria outlined by the ACOG [9]. Although the ACOG definition provides a structured and clinically relevant framework, it was originally developed for term neonates. At present, no universally accepted definition for PA exists specifically for preterm infants, which limits cross-study comparability and may lead to under- or overestimation of the true incidence.
The ACOG definition is intentionally broad and includes not only umbilical cord blood gas values indicating metabolic acidosis, but also clinical signs as the need for neonatal resuscitation. Interestingly, in our cohort, only a minority of infants required resuscitation at birth, which may reflect differences in intrapartum pathophysiology or neonatal vulnerability in this specific population.
Another notable strength of this study is the comprehensive assessment of both short- and long-term neonatal outcomes.
An important limitation is that the study includes older data and that the relatively low incidence of the primary outcome restricts the statistical power to detect robust associations with morbidity and mortality. However, spontaneous extreme PTB with active neonatal management remains rare, and assembling large, contemporary cohorts in this population is inherently difficult. As a result, inclusion of older data is often necessary to reach a sufficient sample size for meaningful analysis, especially for rare outcomes such as PA.
Secondly, our cohort exclusively includes spontaneously initiated deliveries, encompassing both vaginal births and intrapartum secondary CSs. This aligns with the study’s focus on intrapartum mechanisms contributing to PA. Uniquely, the majority of international cohorts in this gestational age range involve planned (primary) cesarean delivery. Our cohort, with a secondary CS rate of approximately 30 %, offers valuable insights into intrapartum hypoxia specifically in the context of attempted vaginal birth, a scenario that remains underrepresented in the literature.
Thirdly, the study was limited by missing data on umbilical cord arterial blood gas values, which were not available in all cases. This may be due to technical challenges in obtaining samples from extremely preterm cords, which contain minimal Wharton’s jelly [13], as well as the urgency of neonatal management in acute cases. To address this, multiple imputation techniques were applied, allowing for integration of available data and enabling the use of combined criteria for PA diagnosis. Although this approach improved data completeness, a formal sensitivity analysis comparing results from imputed and non-imputed datasets was not included. Nevertheless, further review of the data suggests that imputation did not materially alter the study’s conclusions. For example, the incidence of proven PA decreased slightly after imputation (from 6.3 to 5.0 %), and associations between severe IVH and proven PA, and between CS and mortality, remained non-significant and directionally consistent across both imputed and non-imputed datasets. These observations support the robustness of the findings despite the use of imputed data and the absence of a formal sensitivity analysis.
Another limitation is the variation in how active care was applied. Although obstetric management was consistently active, the initial focus was mainly on neonatal care. As neonatal outcomes improved over time, this influenced how active care decisions were made. Intrapartum CTG monitoring is generally considered a marker of active obstetric management; however, in this cohort, only about three-quarters of cases received active obstetric care as indicated by CTG monitoring, which may have impacted outcomes and the generalizability of findings.
Conclusions
Practical and research recommendations
The incidence of proven PA in spontaneous extreme PTB below 28 weeks GA is 5.0 %, which is approximately ten times higher than in term births. Preterm fetus may be more susceptible to developing PA due to their limited physiological compensation mechanisms. However, the exact impact of PA on perinatal outcomes in this population remains uncertain, as other complications associated with extreme prematurity; such as infection, immaturity of organ systems, or IVH, may have a more dominant role in determining prognosis. A major limitation of this study is the use of ACOG criteria to define PA, as these were designed for term neonates. Applying these criteria to extreme preterm infants may lead to significant over- or underestimation of PA’s true incidence and clinical impact.
Emergency CS may reduce the incidence of proven PA, yet any potential benefit must be carefully weighed against the risks of operative delivery, particularly when fetal hypoxia is the sole indication. The clinical utility and predictive accuracy of intrapartum CTG in extreme PTB also require further investigation, as its effectiveness remains unproven and inized guidelines for interpretation in this GA group are lacking. Developing and validating CTG interpretation guidelines specifically for (extreme) preterm birth should therefore be a future research priority.
Given the absence of a GA specific definition for PA in preterm infants and in light of the differing compensatory capacities of the preterm fetus, we propose the development of GA specific PA criteria, as an important future research priority. Such tailored criteria could enhance diagnostic accuracy, enable more meaningful comparisons across studies, and inform more targeted clinical interventions. Moreover, they could serve as a foundation for a large-scale randomized controlled trial to validate these findings.
-
Research ethics: Ethical approval was obtained from the Medical Ethics Review Committee in Amsterdam University Medical Centre in September 2020 (file number 2020–4,306). The study was conducted in accordance with the Declaration of Helsinki.
-
Informed consent: Informed parental consent was obtained by opting out. Participants had six weeks to opt out by e-mail or mail.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Rijken, M, van Heijst, AFJ, de Boer, M, Bol, SP, Duvekot, JJ, Evers, A, et al.. ENG: Guideline perinatal management of extremely preterm delivery. Perintaal beleid bij extreme vroeggeboorte. 2024.Suche in Google Scholar
2. Wilkinson, D, Verhagen, E, Johansson, S. Thresholds for resuscitation of extremely preterm infants in the UK, Sweden, and Netherlands. Pediatrics 2018;142:S574–84. https://doi.org/10.1542/peds.2018-0478i.Suche in Google Scholar
3. Domellöf, M, Blomberg, M, Engström, E, Farooqi, A, Hafström, O, Herbst, A, et al.. ENG: management of threatened preterm births and newborns at the limit of viability. Nationella riktlinjer, utarbetade av en arbetsgrupp “konsensusgruppen” utsedd av Svenska Neonatalföreningen och Perinatal-ARG inom Svensk Förening för Obstetrik och Gynekologi. 2016.Suche in Google Scholar
4. World Health Organization. Born too soon: decade of action on preterm birth. Geneva: World Health Organization; 2023.Suche in Google Scholar
5. Morgan, AS, Mendonça, M, Thiele, N, David, AL. Management and outcomes of extreme preterm birth. BMJ 2022;376:e055924. https://doi.org/10.1136/bmj-2021-055924.Suche in Google Scholar PubMed PubMed Central
6. Gillam-Krakauer, M, Shah, M, Gowen, CWJr. Birth asphyxia. In: StatPearls [Online]. Treasure Island (FL). StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK430782/ [Accessed 1 Dec 2024].Suche in Google Scholar
7. Ahearne, CE, Boylan, GB, Murray, DM. Short and long term prognosis in perinatal asphyxia: an update. World J Clin Pediatr 2016;5:67–74. https://doi.org/10.5409/wjcp.v5.i1.67.Suche in Google Scholar PubMed PubMed Central
8. Vayssière, C, Yli, B, Ayres-de-Campos, D, Ugwumadu, A, Loussert, L, Hellström-Westas, L, et al.. European association of perinatal medicine (EAPM). Eur J Obstet Gynecol Reprod Biol 2024;294:55–7.10.1016/j.ejogrb.2024.01.006Suche in Google Scholar PubMed
9. Executive summary. Neonatal encephalopathy and neurologic outcome, second edition. Report of the American college of obstetricians and gynecologists ’task force on neonatal encephalopathy. Obstet Gynecol 2014;123:896–901.10.1097/01.AOG.0000445580.65983.d2Suche in Google Scholar PubMed
10. Alfirevic, Z, Devane, D, Gyte, GM, Cuthbert, A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017;2:CD006066. https://doi.org/10.1002/14651858.CD006066.pub3.Suche in Google Scholar PubMed PubMed Central
11. Faiz, Z, Van ’t Hof, EM, Colenbrander, GJ, Lippes, R, Bakker, P. The quality of intrapartum cardiotocography in preterm labour. J Perinat Med 2022;50:74–81. https://doi.org/10.1515/jpm-2021-0214.Suche in Google Scholar PubMed
12. Schiermeier, S, Westhof, G, Leven, A, Hatzmann, H, Reinhard, J. Intra- and interobserver variability of intrapartum cardiotocography: a multicenter study comparing the FIGO classification with computer analysis software. Gynecol Obstet Invest 2011;72:169–73. https://doi.org/10.1159/000327133.Suche in Google Scholar PubMed
13. Afors, K, Chandraharan, E. Use of continuous electronic fetal monitoring in a preterm fetus: clinical dilemmas and recommendations for practice. J Pregnancy 2011;2011:848794. https://doi.org/10.1155/2011/848794.Suche in Google Scholar PubMed PubMed Central
14. Randolph, DA, Nolen, TL, Ambalavanan, N, Carlo, WA, Peralta-Carcelen, M, Das, A, et al.. Outcomes of extremely low birthweight infants with acidosis at birth. Arch Dis Child Fetal Neonatal Ed 2014;99:F263–8. https://doi.org/10.1136/archdischild-2013-304179.Suche in Google Scholar PubMed PubMed Central
15. Zaigham, M, Källén, K, Maršál, K, Olofsson, P. Hypoxia with acidosis in extremely preterm born infants was not associated with an increased risk of death or impaired neurodevelopmental outcome at 6.5 years. Acta Paediatr 2020;109:85–92. https://doi.org/10.1111/apa.14925.Suche in Google Scholar PubMed
16. Leijser, LM, Meijler, G, Mulder-de Tollenaer, SM. ENG: national recommendations neonatal follow-up – neuroimaging. Landelijke aanbeveling neonatale follow-up – Neuroimaging 2014. https://neonatology.eu/sites/default/files/neonatale_neuroimaging_versie_1_5_feb_2015.pdf [Accessed 1 Dec 2024].Suche in Google Scholar
17. Volpe, JJ. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr Neurol 2019;95:42–66. https://doi.org/10.1016/j.pediatrneurol.2019.02.016.Suche in Google Scholar PubMed
18. Chao, CP, Zaleski, CG, Patton, AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics 2006;26:S159–72. https://doi.org/10.1148/rg.26si065504.Suche in Google Scholar PubMed
19. De Proost, L, Verweij, EJT, Ismaili, M, Hamdi, H, Reiss, IKM, Steegers, EAP, et al.. The edge of perinatal viability: understanding the Dutch position. Front Pediatr 2021;9:634290. https://doi.org/10.3389/fped.2021.634290.Suche in Google Scholar PubMed PubMed Central
20. Burakevych, N, McKinlay, CJ, Alsweiler, JM, Wouldes, TA, Harding, JE. Bayley-III motor scale and neurological examination at 2 years do not predict motor skills at 4.5 years. Dev Med Child Neurol 2017;59:216–23. https://doi.org/10.1111/dmcn.13232.Suche in Google Scholar PubMed PubMed Central
21. van Buuren, S. Flexible imputation of missing data, 2nd ed. Boca Raton (FL): Chapman & Hall/CRC; 2018.10.1201/9780429492259Suche in Google Scholar
22. Bennet, L. Sex, drugs and rock and roll: tales from preterm fetal life. J Physiol 2017;595:1865–81. https://doi.org/10.1113/jp272999.Suche in Google Scholar
23. Miller, SL. The paradox of the preterm fetus. J Physiol 2017;595:1851–2. https://doi.org/10.1113/jp274001.Suche in Google Scholar PubMed PubMed Central
24. Engineer, N, Kumar, S. Perinatal variables and neonatal outcomes in severely growth restricted preterm fetuses. Acta Obstet Gynecol Scand 2010;89:1174–81. https://doi.org/10.3109/00016349.2010.501370.Suche in Google Scholar PubMed
25. Serenius, F, Källén, K, Blennow, M, Ewald, U, Fellman, V, Holmström, G, et al.. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. Jama 2013;309:1810–20. https://doi.org/10.1001/jama.2013.3786.Suche in Google Scholar PubMed
26. van Beek, P, Groenendaal, F, Broeders, L, Dijk, PH, Dijkman, D, van den Dungen, FAM, et al.. Survival and causes of death in extremely preterm infants in the Netherlands. Arch Dis Child Fetal Neonatal Ed 2021;106:251–7. https://doi.org/10.1136/archdischild-2020-318978.Suche in Google Scholar PubMed PubMed Central
27. Garry, N, Farooq, I, Milne, S, Lindow, SW, Regan, C. Trends in obstetric management of extreme preterm birth at 23 to 27 weeks’ gestation in a tertiary obstetric unit: a 10-year retrospective review. Eur J Obstet Gynecol Reprod Biol 2020;253:249–53. https://doi.org/10.1016/j.ejogrb.2020.08.034.Suche in Google Scholar PubMed
28. Angolile, CM, Max, BL, Mushemba, J, Mashauri, HL. Global increased cesarean section rates and public health implications: a call to action. Health Sci Rep 2023;6:e1274. https://doi.org/10.1002/hsr2.1274.Suche in Google Scholar PubMed PubMed Central
29. Stoll, BJ, Hansen, NI, Bell, EF, Walsh, MC, Carlo, WA, Shankaran, S, et al.. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. Jama 2015;314:1039–51. https://doi.org/10.1001/jama.2015.10244.Suche in Google Scholar PubMed PubMed Central
30. Blanc, J, Resseguier, N, Goffinet, F, Lorthe, E, Kayem, G, Delorme, P, et al.. Association between gestational age and severe maternal morbidity and mortality of preterm cesarean delivery: a population-based cohort study. Am J Obstet Gynecol 2019;220:399.e1-.e9. https://doi.org/10.1016/j.ajog.2019.01.005.Suche in Google Scholar PubMed
31. Alfirevic, Z, Milan, SJ, Livio, S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev 2013;2013:Cd000078. https://doi.org/10.1002/14651858.cd000078.pub3.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

