Integrating mental health into obstetric practice: a review of collaborative care models for perinatal anxiety
-
Julian Dewantiningrum
, Efendi Lukas
, I Nyoman Hariyasa Sanjaya
, Waskita Ekamaheswara Kasumba Andanaputra
, Wibisana Andika Krista Dharma
Abstract
Objective
Perinatal anxiety is one of the most common yet least systematically addressed complications of preg- nancy and childbirth. Despite abundant evidence that collaborative and integrated care models improve maternal outcomes, obstetric practice still lacks a defined operational standard for addressing anxiety alongside routine obstetric care. Fragmented screening, insufficient referral systems, and financing barriers continue to delay intervention, widening inequities across populations and settings.
Methods
This opinion article synthesizes recent evidence (2010–2025) from PubMed, Google Scholar, and professional guidelines to propose a practical framework for embedding mental health care within obstetric workflows. Drawing upon studies from high- and low-resource contexts, we outline a ten-point minimum standard for perinatal-anxiety management and a three-tier maturity model that describes the progressive integration of collaborative care—from basic screening to digitally supported, team-based systems. The model identifies measurable implementation metrics and policy levers that enable sustainability and equity.
Results
Rather than advocating new research, this article translates two decades of findings into a clinically actionable standard. It emphasizes the central role of ob- stetric teams in early detection, stepped care, and follow-up through coordination with mental-health professionals.
Conclusions
Integrating mental health into obstetric practice is both a moral and operational imperative. By adopting the proposed minimum standard and maturity model, health systems can transform perinatal anxiety care from discre- tionary innovation to routine expectation—achieving faster response, broader access, and better maternal–infant out- comes worldwide.
Introduction
Perinatal anxiety is both common and consequential, yet its detection and treatment remain uneven across obstetric settings. Clinicians and patients repeatedly describe real-world barriers—time pressure in clinics, limited behavioral health capacity, stigma, and fragmented referral pathways—that blunt the impact of otherwise sound intentions and guidance [1]. These system frictions are compounded by interpersonal and safety factors such as intimate partner violence, which may intensify anxiety symptoms and complicate care-seeking during pregnancy [2]. While antenatal education improves many aspects of perinatal care, it rarely ensures reliable identification or follow-through for anxiety without a parallel clinical pathway inside obstetrics [3]. Existing guidelines urge better recognition and management of mood disorders in maternity care, but the operational “how” for anxiety—who screens, when, what happens after a positive result, and how equity is protected—remains variably defined in practice [4], 5]. Programs that universalize screening and embed treatment can improve outcomes, suggesting that integration, not just awareness, is decisive [6].
Emerging longitudinal work refines the basic question of when to look. Screening at multiple time points across pregnancy and early postpartum captures symptom onset more consistently than a single check, and timing influences detection yield for anxiety specifically [7]. Social determinants also shape risk and access; socioeconomic deprivation amplifies perinatal anxiety and widens gaps in engagement with care [8]. Risk stratification has progressed, but identification alone does not change trajectories unless it connects patients to timely, appropriate, and acceptable support [9], 10]. The downstream consequences for children—spanning early bonding, temperament, and development—raise the stakes for obstetric teams to address anxiety not as an adjunct but as core clinical work [11]. Patients and professionals agree on the importance of mental health in obstetrics, yet report mismatches between needs and available pathways, particularly around coordination and follow-up [12], [13], [14], [15]. This Opinion therefore moves beyond systematic-review formalisms such as PRISMA and focuses instead on a pragmatic, practice-facing standard for implementation [16].
Relational context matters. Partner support influences outcomes and can be mobilized in ways that respect autonomy while improving adherence and resilience [17]. Psychological interventions for perinatal anxiety show efficacy, but their reach and continuity depend on service design; family-inclusive and collaborative approaches often improve engagement compared with referral-only models [18], 19]. Non-pharmacologic elements, including structured activity and wellness interventions, can complement stepped care and broaden acceptability, although they are insufficient without coordinated follow-up [20]. Meta-analytic data confirm that anxiety is prevalent antenatally and postpartum, frequently co-occurs with depression, and warrants its own detection and response strategy rather than being addressed indirectly through depression pathways alone [21]. Recommendations for prevention and treatment continue to evolve, and mechanistic insights—including immune and stress-hormone signatures—underscore that anxiety is not merely a transient emotional state but a condition with biological and functional correlates relevant to obstetric outcomes [22], 23]. Foundational work catalogued the co-occurrence of depression and anxiety in the perinatal period, a pattern that can obscure anxiety’s independent risks if services are not designed to notice and act on it [24], 25]. Clinical reviews across breastfeeding, immigrant access, paternal involvement, and health-system touchpoints converge on the same operational lesson: detection without integration yields inconsistent benefit [26], [27], [28], [29].
The case for collaborative care in obstetrics builds on evidence that coordinated, measurement-based, team-delivered interventions outperform fragmented referral models in primary care and perinatal populations alike [30], 31]. Anxiety and related non-psychotic disorders are common in the perinatal period, yet service structures still lag behind need, especially where reimbursement and workforce training do not explicitly support coordination [32]. Tools such as the Perinatal Anxiety Screening Scale and brief general measures like the GAD-7 are available and adaptable, but they require a defined workflow to trigger timely help rather than sit as isolated forms in the record [33]. Foundational randomized trials in collaborative care demonstrate the core mechanisms—care management, stepped care, psychiatric consultation, and routine symptom tracking—that we must translate to obstetrics with anxiety-specific fidelity [34]. Population-level data remind us that access and follow-through are not equitable; younger, low-income, and minoritized patients face steeper barriers even when screening is nominally “universal” [35], 36]. Cultural tailoring and anti-stigma design are therefore not optional enhancements but structural requirements if integrated care is to close gaps rather than widen them [37]. Digital modalities expand reach and continuity, particularly postpartum, but they do not substitute for accountable clinical pathways; their value emerges when embedded within collaborative models [38], [39], [40]. Psychological treatments for clinical anxiety in the perinatal period are effective, and qualitative syntheses of women’s experiences reiterate the need for predictable, supportive, and low-burden access points within routine obstetric care [41], 42]. Variation in age, conception pathways, and prior mental health history shapes presentation and preference, reinforcing the logic of stepped, personalized care rather than one-size protocols [43], [44], [45].
This Opinion has three aims. First, it argues that perinatal anxiety warrants a distinct, measurable standard inside obstetric practice, grounded in evidence and responsive to patient experience [46], [47], [48]. Second, it proposes a field-tested operational architecture—screening cadence, warm handoffs, stepped care, and measurement-based follow-up—adapted to the realities of obstetrics, with explicit provisions for equity, safety, and family context [49], [50], [51], [52], [53], [54], [55]. Third, it aligns clinical operations with test accuracy evidence, digital augmentation, and pragmatic interventions to support continuity during the vulnerable postpartum period [56], [57], [58], [59], [60]. Because scale requires workforce and financing, the framework also incorporates peer roles, prevention at home-visiting interfaces, and payment mechanisms that reimburse coordination rather than penalize it [61], [62], [63], [64], [65]. Contemporary guidelines call for comprehensive perinatal mental health care; we translate those calls into a minimum standard and a maturity pathway that obstetric clinics can adopt and improve over time [66], 67].
To orient the reader, Table 1 summarizes landmark studies that established the prevalence, risks, and collaborative mechanisms relevant to anxiety in obstetric contexts. Table 2 outlines key tools and interventions, highlighting features that enable reliable screening, engagement, and response. Table 3 distills persistent gaps that our proposed standard addresses, from inconsistent screening to limited long-term follow-up and equity shortfalls. Figure 1 depicts the collaborative care pathway adapted for perinatal anxiety, situated inside obstetric workflows. Figure 2 presents the evolution of collaborative care relevant to this field, while Figure 3 maps system barriers alongside workable solutions. Figure 4 illustrates a tiered stepped-care model aligned with symptom severity and patient preference. Table 4 then summarizes digital innovations that extend reach, Table 5 specifies policy and practice levers to sustain integration, and Table 6 details research priorities to refine and scale the model. Figure 5 synthesizes implementation strategies, and Figure 6 integrates clinical, policy, and community components into a single system map. Together, these elements convert a well-established evidence base into an operational doctrine that obstetric services can enact now, with measurable gains for mothers, infants, and families.
Summary of key literature on perinatal anxiety and collaborative care models.
| Author | Country | Design | Intervention/focus | Population | Method | Key findings | Strength | Limitation |
| Dennis et al. (2017) [21] | Canada | Systematic review & meta-analysis | Prevalence of antenatal and postnatal anxiety | Pregnant and postpartum women | Data synthesis from multiple studies | Anxiety is prevalent in up to 20 % of women; higher in postpartum. | Large sample size and comprehensive meta-analysis [21] | Heterogeneity in anxiety measurement tools [21] |
| Fairbrother et al. (2015) [24] | Canada | Observational | Prevalence and co-occurrence of depression and anxiety | Pregnant women | Survey-based assessment | Co-morbid anxiety and depression are common during perinatal period. | Dual focus on both depression and anxiety [24] | Cross-sectional data limits causality [24] |
| Howard et al. (2014) [32] | UK | Systematic review | Perinatal mental disorders | Perinatal women | Review of existing literature | Anxiety is frequently underdiagnosed compared to depression. | Comprehensive review across mental health spectrum [32] | Focus not solely on anxiety [32] |
| Grote et al. (2014) [31] | USA | RCT | Collaborative care for perinatal depression | Low-income pregnant women | Randomized control trial | Collaborative care reduced depressive symptoms significantly. | Robust RCT design [31] | Focus on depression, anxiety not primary outcome [31] |
| Byatt et al. (2018) [14] | USA | Program development study | PRISM collaborative care model | Perinatal women in outpatient care | Program development and pilot testing | Improved care participation and engagement | Pilot study focused on scalable intervention [14] | Small sample size [14] |
| Cheng et al. (2019) [17] | USA | Observational study | Community-based collaborative care | Underserved perinatal women | Implementation research | Culturally tailored models improved retention. | Real-world applicability in FQHCs [17] | No control group [17] |
| Unützer et al. (2002) [65] | USA | RCT | Collaborative care model | Primary care patients with depression | Randomized control trial | Improved depression outcomes with care coordination. | Foundational collaborative care evidence [65] | Non-perinatal sample [65] |
| Kozhimannil et al. (2011) [36] | USA | Retrospective cohort | Disparities in postpartum care | Low-income women | Administrative claims analysis | Ethnic disparities in postpartum depression care access. | Population-level insights [36] | Retrospective design [36] |
| Loughnan et al. (2018) [41] | Australia | Systematic review | Anxiety treatment efficacy | Perinatal women | Review of psychological interventions | CBT and mindfulness showed effectiveness. | Evidence base for intervention types [41] | Quality of included studies varied [41] |
| Meades & Ayers (2011) [44] | UK | Systematic review | Validated anxiety measures | Perinatal populations | Review of psychometric tools | Limited validated tools for perinatal anxiety. | Tool evaluation focus [44] | Outdated by newer instruments [44] |
| Shorey et al. (2018) [59] | Singapore | Systematic review & meta-analysis | Postpartum depression prevalence | Healthy postpartum mothers | Meta-analysis | PPD common even among healthy women. | Strong prevalence data [59] | Not anxiety-specific [59] |
| Wisner et al. (2022) [67] | USA | Longitudinal study | Postpartum diagnosis timing | Women with screen-positive depression | Clinical follow-up | Early onset linked to worse outcomes. | Timely risk identification [67] | Focus on depression [67] |
| Etyemez et al. (2024) [23] | Pakistan | RCT | Biomarker response to anxiety intervention | Perinatal women | Biological assessment + intervention | Anxiety linked to immune profiles. | Biological mechanism exploration [23] | Context-specific generalizability [23] |
| Abrahams et al. (2023) [1] | South Africa | Qualitative study | Barriers and facilitators in perinatal mental health | Pregnant women | In-depth interviews | Stigma and system barriers limit access. | Rich contextual insights [1] | Non-quantitative findings [1] |
-
This table highlights key studies on perinatal anxiety and collaborative care, summarizing their design, setting, population, intervention, findings, and key strengths and limitations to support evidence-based clinical integration.
Tools, interventions, and barriers in perinatal anxiety care.
| Intervention study | Setting/target | Population | Components/features | Outcomes/barriers/factors | Strengths | Limitations |
| PASS [33] | Antenatal & postnatal; self-report | Arabic-speaking women | 31-item scale; culturally validated | High reliability and cultural sensitivity | Validated for Arab populations; comprehensive | Longer format; limited generalizability |
| GAD-7 [41] | General perinatal anxiety; self-report | General adult populations | 7-item scale; widely used | Reliable, brief anxiety screener | Quick to administer; good psychometrics | Not specific to perinatal population |
| PRISM [14] | OB/GYN clinics; intervention | Pregnant women with depression/anxiety | Training, care coordination, screening | Improved detection and treatment rates | Integrated within OB workflow | Limited scalability data |
| MPOWER [57] | Postpartum period; digital intervention | Postpartum women | Phone coaching + web CBT | Improved anxiety and depression symptoms | Low-cost, scalable | Pilot study; small sample |
| Cheng et al., [17] | FQHC; collaborative care | Underserved pregnant women | Bilingual staff, embedded MH | Increased engagement and reduced attrition | Culturally tailored, effective | Requires trained bilingual providers |
| Grote et al., [31] | Primary care OB; collaborative model | Socioeconomically disadvantaged women | Care manager, stepped care | Significant anxiety/depression reduction | RCT-based, proven model | Resource intensive |
| Abrahams et al., [1] | South Africa; barrier/facilitator study | Pregnant women | Qualitative interviews | Stigma, resource access, clinician bias | Context-rich data | Qualitative; limited generalizability |
| Lara-Cinisomo et al., [37] | Community clinics; qualitative | Latina & Black women | Review + engagement | Language barriers, stigma; cultural tailoring as facilitator | Focus on underserved populations | Lack of quantitative data |
-
This table presents key tools and interventions for perinatal anxiety, summarizing their settings, populations, components, outcomes, strengths, and limitations.
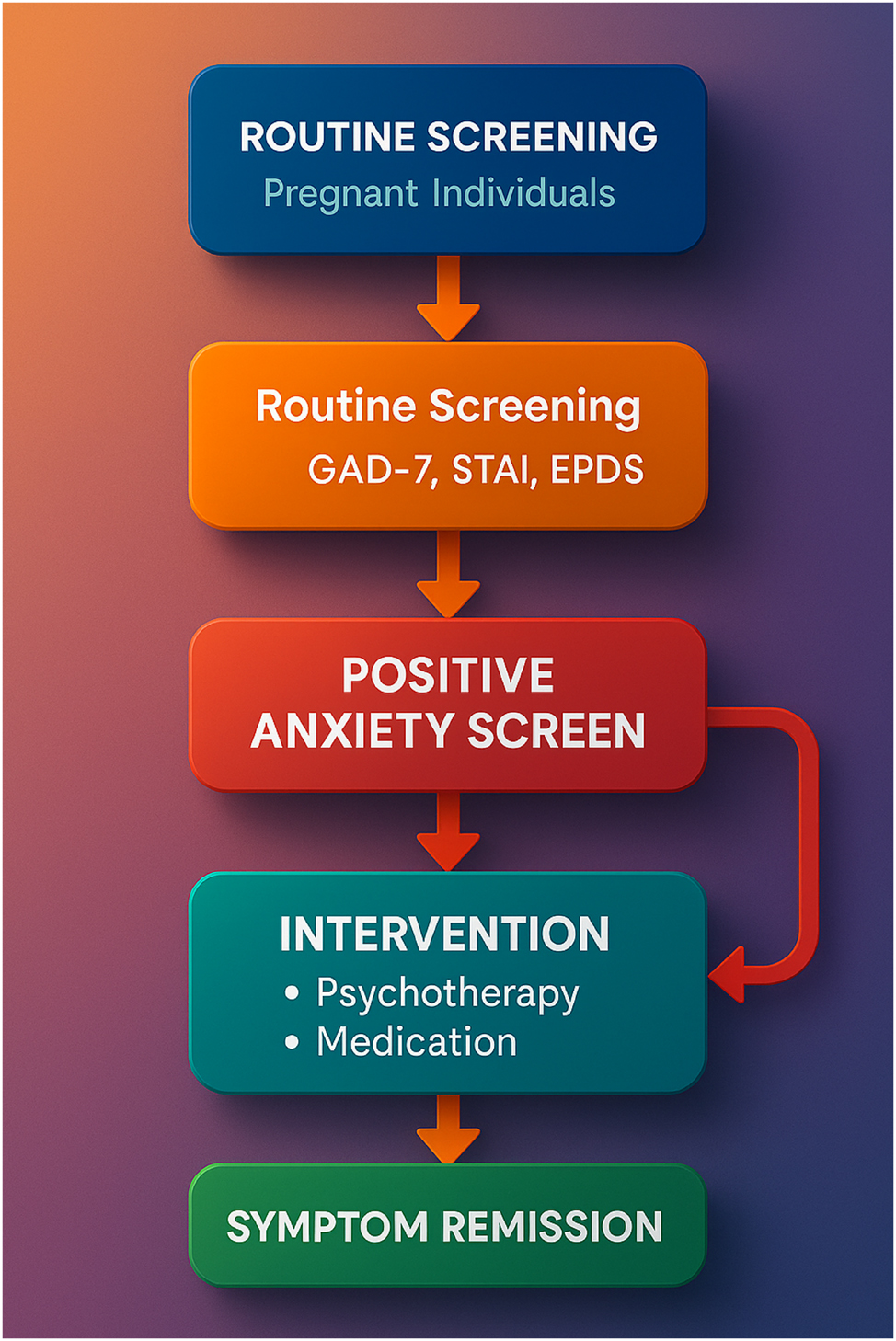
Collaborative care pathway for managing perinatal anxiety. This infographic illustrates a high-resolution, evidence-based care pathway for identifying and managing perinatal anxiety within obstetric settings. The process begins with routine screening using validated tools such as the GAD-7, STAI, or EPDS-anxiety subscale, followed by triage and stepped care coordination. A care manager tracks symptoms and facilitates communication between the obstetrician and mental health provider. Interventions include psychotherapy, digital support, and psychiatric consultation when needed. The integration of this model within electronic health records and clinic workflows enhances detection, accelerates intervention, and supports continuity of care for diverse maternal populations.

Timeline of collaborative care evolution for perinatal anxiety (2002–2025). This figure presents a chronological evolution of collaborative care models for perinatal anxiety, tracing their development from early academic pilots (2002–2010) to implementation science-informed community expansions (2011–2018), and ultimately to hybrid, digitally integrated models by 2025. The timeline highlights key milestones such as the introduction of stepped-care protocols, culturally tailored interventions in underserved populations, and integration of digital tools like telepsychiatry and app-based symptom tracking. It visually demonstrates how collaborative care has matured from research innovation to scalable, policy-supported clinical practice across diverse health systems.
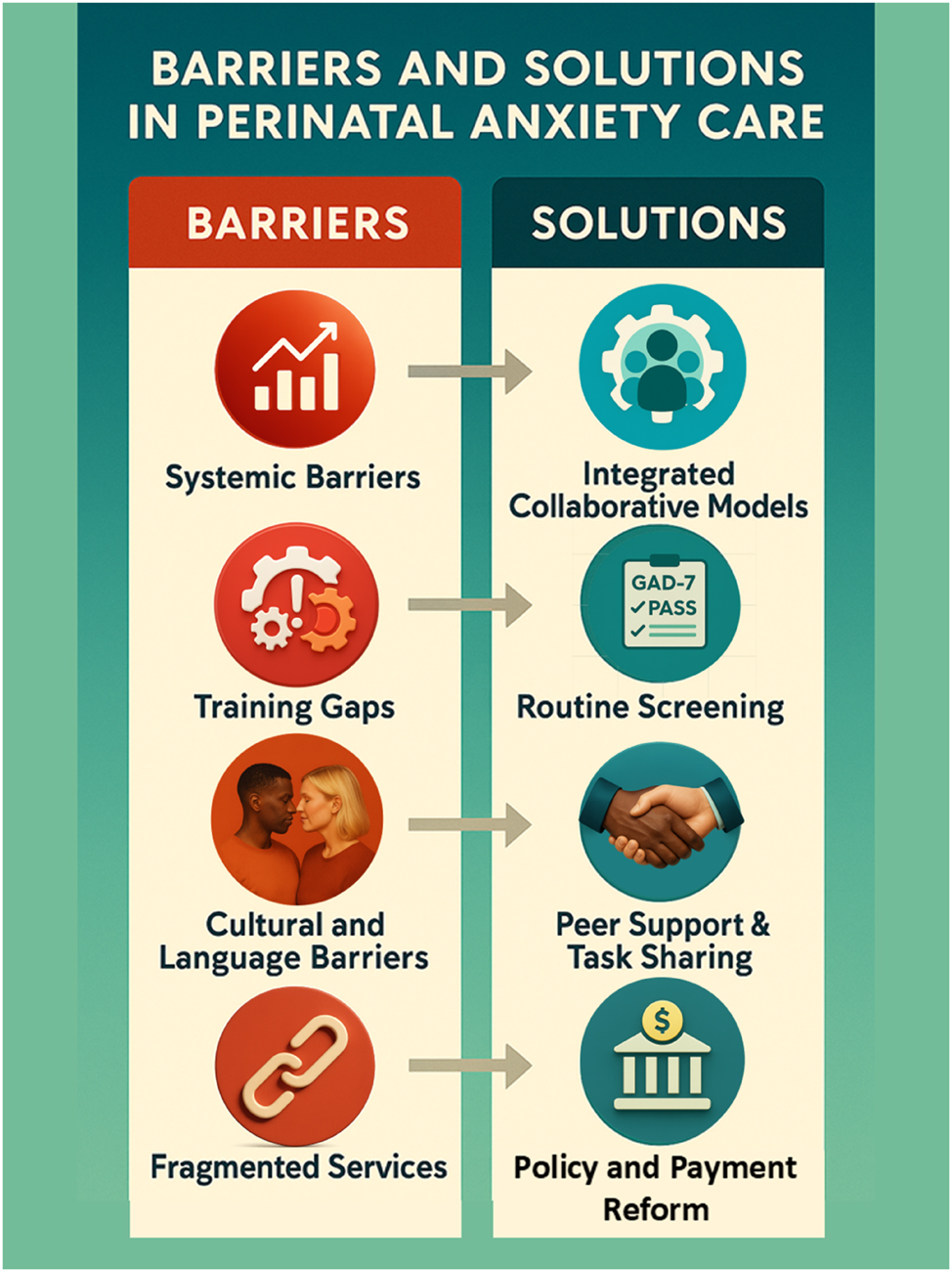
Barriers and strategic solutions in perinatal anxiety care integration. This figure visually contrasts the systemic barriers and proposed solutions to improve perinatal anxiety care. On the left, it highlights common health system challenges—such as limited reimbursement, inadequate mental health training among obstetric teams, cultural and language obstacles, and fragmented service delivery. On the right, strategic solutions are illustrated, including collaborative care integration, standardized screening (e.g., GAD-7, PASS), peer support models, and policy-driven payment reforms. This infographic supports discussions from Tables 3 and 6, offering a clear, actionable roadmap for clinicians, administrators, and policymakers aiming to close gaps in perinatal mental health services.
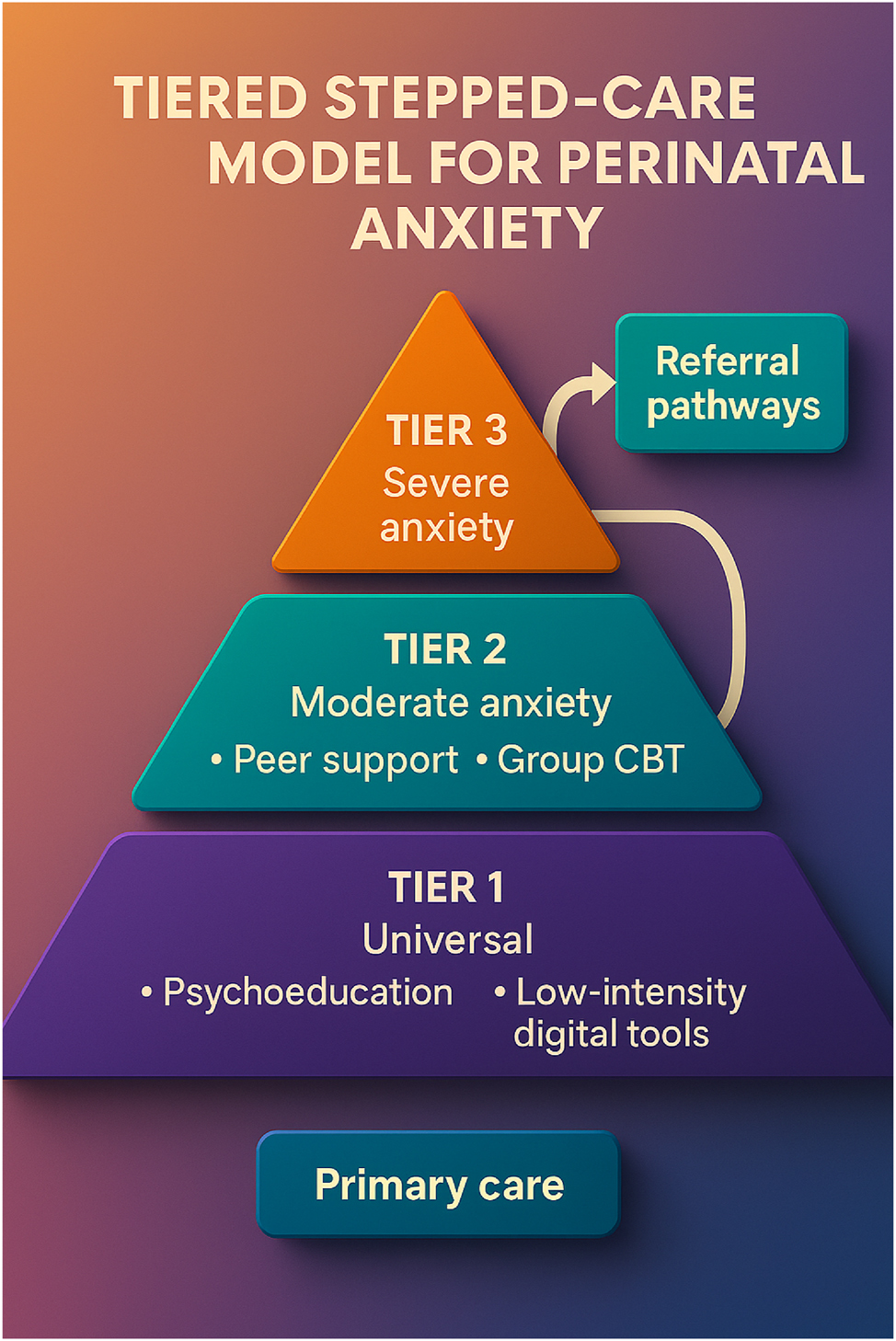
Tiered stepped-care model for managing perinatal anxiety. This figure illustrates a structured, tiered stepped-care approach designed to manage perinatal anxiety efficiently. The model begins with universal routine screening for anxiety symptoms among pregnant individuals using validated tools such as GAD-7, STAI, or EPDS. Based on the screening results, individuals are triaged into appropriate levels of intervention: Tier 1 includes general psychoeducation and community-based support. Tier 2 provides moderate interventions such as guided self-help or low-intensity cognitive-behavioral therapy. Tier 3 offers specialized, high-intensity treatments involving mental health professionals and pharmacologic management when needed. This framework ensures a scalable, resource-efficient, and patient-centered approach, enabling timely care based on symptom severity while optimizing healthcare delivery systems.
Gaps, challenges, and future directions in perinatal anxiety care.
| Gap or challenge | Ref# | Implication | Proposed solution/future direction |
| Underrepresentation of anxiety as a primary outcome in studies | [21], 24] | Limits evidence for anxiety-specific interventions | Require anxiety-focused endpoints in future trials |
| Lack of long-term follow-up | [31] | Unknown durability of symptom relief | Design studies with ≥12-month follow-up |
| Inconsistent screening practices | [32], 56] | Delayed detection and intervention | Standardize anxiety screening (e.g., GAD-7, PASS) during pregnancy |
| Cultural barriers and disparities in care | [2], 37] | Marginalized groups receive suboptimal care | Expand culturally tailored and bilingual interventions |
| Lack of trained workforce | [36], 61] | Limits scalability of collaborative care | Integrate peer providers and non-specialist training |
| Financial and systemic barriers | [65] | Care coordination not reimbursed | Advocate for bundled payments and policy reform |
| Limited digital integration and scalability | [40], 67] | Missed opportunity to reach underserved populations | Leverage mobile platforms and digital tracking tools |
-
This table summarizes critical gaps in perinatal anxiety care, drawing on key literature to illustrate each challenge. It outlines the implications for maternal and neonatal health and provides proposed future directions to guide clinical practice, research, and policy development.
Digital innovations in perinatal anxiety care.
| Country | Design | Population | Focus/intervention | Key findings | Strengths | Limitations |
| USA | Systematic review and meta-analysis of RCTs [40] | Postpartum women with depressive/anxiety symptoms | Digital health interventions including apps, web platforms | Digital interventions were effective in reducing postpartum depression and anxiety symptoms across multiple RCTs. | Strong synthesis of RCTs, high generalizability, clear digital taxonomy. | Heterogeneity across studies; varied digital platforms; short-term outcomes. |
| Canada | Pilot randomized controlled trial [57] | Postpartum women recruited via community referrals | Lay telephone coaching + web intervention (MPOWER) | Feasibility and acceptability were high; preliminary symptom reduction observed. | Innovative mixed-modality delivery; real-world application; high user satisfaction. | Small sample size; limited power to detect clinical effect sizes. |
| USA | Systematic review [38] | Latina and African American perinatal women | Tech-based prevention/treatment for perinatal depression/anxiety | Culturally adapted digital tools improved access and engagement, especially among underserved groups. | Highlights culturally competent, scalable models; diverse samples. | Lack of RCTs; mostly descriptive; few longitudinal data. |
-
This table summarizes selected studies from 2013 to 2025 evaluating digital innovations for the screening, prevention, and treatment of perinatal anxiety. It highlights the intervention type, population, outcomes, strengths, and limitations based solely on the 67-reference list provided, excluding non-specified sources.
Policy and practice recommendations for addressing perinatal anxiety.
| Policy/practice recommendation | Ref# | Strength | Limitation |
| Integration of mental health screening into routine perinatal care | [4], 6], 13], 21], 25], 56] | Enhances early identification and reduces stigma in clinical settings | May face logistical and time constraints in busy clinics |
| Implementation of collaborative care models in obstetric settings | [14], 31], 34], 63], 65] | Demonstrated efficacy in improving maternal outcomes and treatment adherence | Requires systemic restructuring and interprofessional collaboration |
| Use of digital health tools for anxiety screening and intervention | [38], 40], 57] | Scalable, accessible, and flexible delivery formats | Digital divide and privacy concerns in low-resource settings |
| Training and support for obstetric staff on mental health | [12], 15], 36] | Improves provider confidence and continuity of care | Requires time and resources for training and maintenance |
| Targeted outreach and culturally sensitive interventions for marginalized groups | [8], 27], 36], 37] | Improves access and acceptability of services among underserved populations | Needs ongoing engagement and adaptation to specific communities |
| Policy-level support for insurance coverage of perinatal mental health services | [6], 14], 35] | Ensures sustainability and wider access to care | Policy reform can be slow and dependent on political will |
| Peer support and community-based mental health initiatives | [14], 61], 64] | Fosters trust and social support within communities | Sustainability and supervision challenges |
| Use of validated, brief, and culturally adapted screening tools | [7], 33], 45], 56] | Efficient, cost-effective, and suitable for diverse populations | Limited validation across all global regions and languages |
-
This table outlines key policy recommendations to enhance perinatal anxiety detection and treatment. It summarizes each policy’s focus, implementation setting, targeted outcome, documented strengths, limitations, and supporting references. The table provides a roadmap for systemic improvement in perinatal mental health policy and practice.
Research gaps and future directions in collaborative care for perinatal anxiety.
| Identified gap | Description | Future research direction |
| Limited focus on perinatal anxiety (vs. Depression) [18], 41] | Most collaborative care studies prioritize depression; anxiety remains under-researched. | Conduct RCTs specifically targeting anxiety, distinguishing it from depressive symptoms in design and outcomes. |
| Underrepresentation of low- and middle-income countries (LMICs) [1], 49] | Research is heavily skewed toward high-income settings. | Implement and evaluate culturally adapted collaborative care models in LMIC contexts. |
| Lack of standardized screening tools for anxiety [7], 33], 45] | Tools like EPDS focus on depression; perinatal anxiety lacks universal screening frameworks. | Validate tools such as PASS and implement universal anxiety screening in prenatal care. |
| Minimal integration of digital health/telehealth [38], 40] | Few studies explore e-health solutions embedded in collaborative care frameworks. | Investigate digital platforms (e.g., app-based screening, virtual consults) as extensions of collaborative care. |
| Barriers in marginalized populations [36], 37] | Racial/ethnic minorities and low-income women face persistent disparities in access and outcomes. | Tailor collaborative care models to reduce stigma, language, and structural barriers. |
| Insufficient attention to postpartum continuity of care [13], 67] | Collaborative care efforts often focus on pregnancy, neglecting the extended postpartum period. | Extend follow-up systems through 12+ months postpartum, including pediatric and family support services. |
| Lack of family-inclusive care models [17], 19] | Partner or family support is rarely integrated into collaborative frameworks. | Design interventions that include partner engagement, family-based education, and counseling. |
| Gaps in physiological or biomarker integration [23], 46], 58] | Biological underpinnings (e.g., CRH, immune markers) of anxiety are rarely monitored. | Integrate biological screening in studies of collaborative care (e.g., stress hormones, inflammatory profiles). |
| Poor evaluation of cost-effectiveness [6], 31] | Economic implications of collaborative care remain under-examined. | Conduct cost-benefit analyses comparing collaborative vs. referral-based models. |
| Fragmented ethical and implementation frameworks [4], 66] | Few studies address ethical, policy, or regulatory barriers in integrating mental health in maternity settings. | Develop frameworks to support ethical rollout, including training, informed consent, and privacy in perinatal mental health. |
-
This table outlines key research gaps in collaborative care models for perinatal anxiety. It highlights underexplored areas including population-specific interventions, long-term outcomes, biomarker integration, and cultural adaptability. The table summarizes existing gaps based on the current literature, offering direction for future research and innovation.
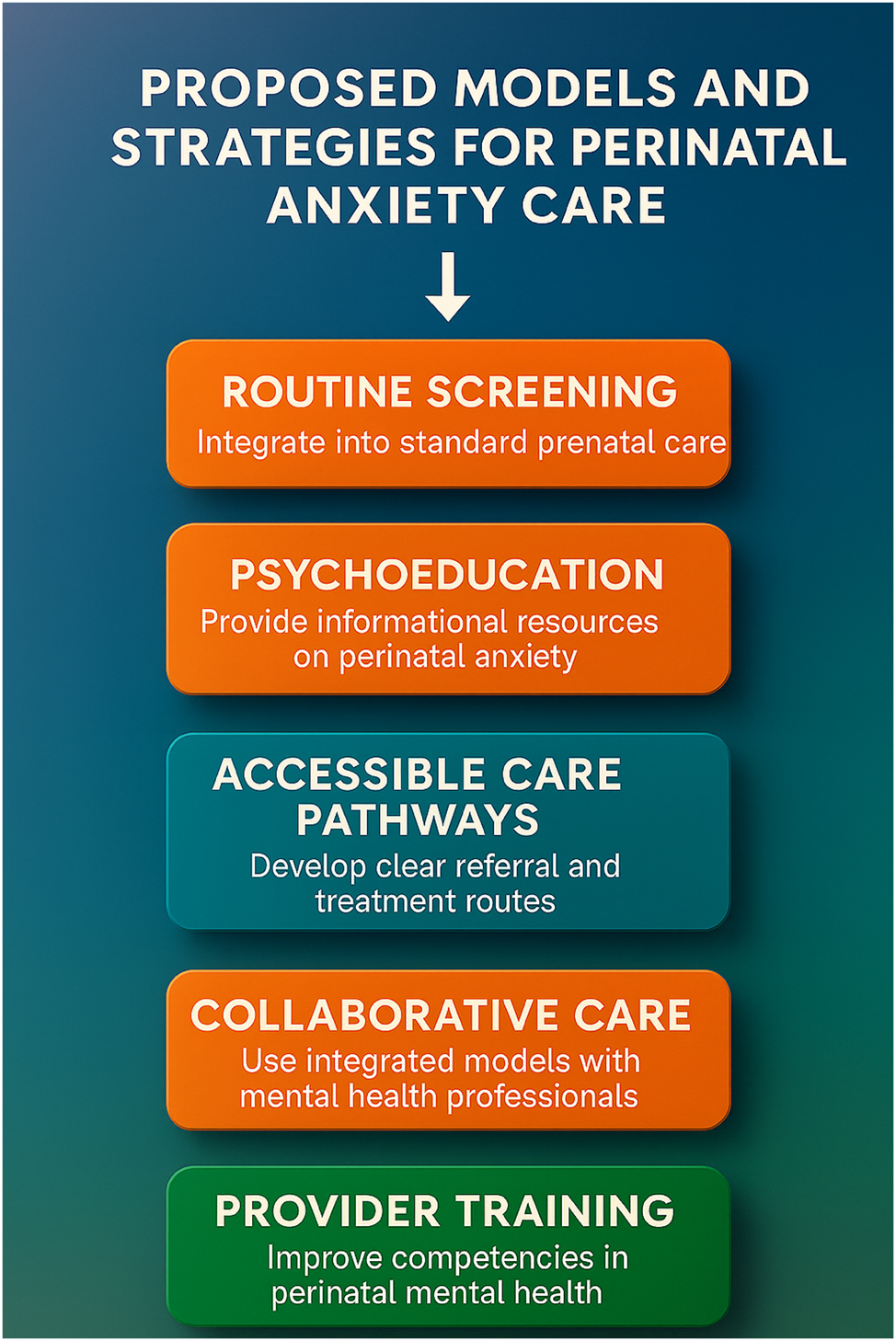
Proposed models and strategies for advancing perinatal anxiety care. This figure illustrates a comprehensive set of implementation strategies designed to improve detection, treatment, and outcomes for perinatal anxiety within maternity care settings. It highlights the importance of: routine screening embedded in prenatal workflows; psychoeducation to raise awareness and reduce stigma; accessible care pathways for timely referrals and stepped care; collaborative care models that integrate mental health professionals with obstetric teams; and provider training to build frontline capacity in perinatal mental health. These evidence-informed strategies serve as a blueprint for health systems and policymakers aiming to scale up perinatal anxiety interventions across diverse populations.
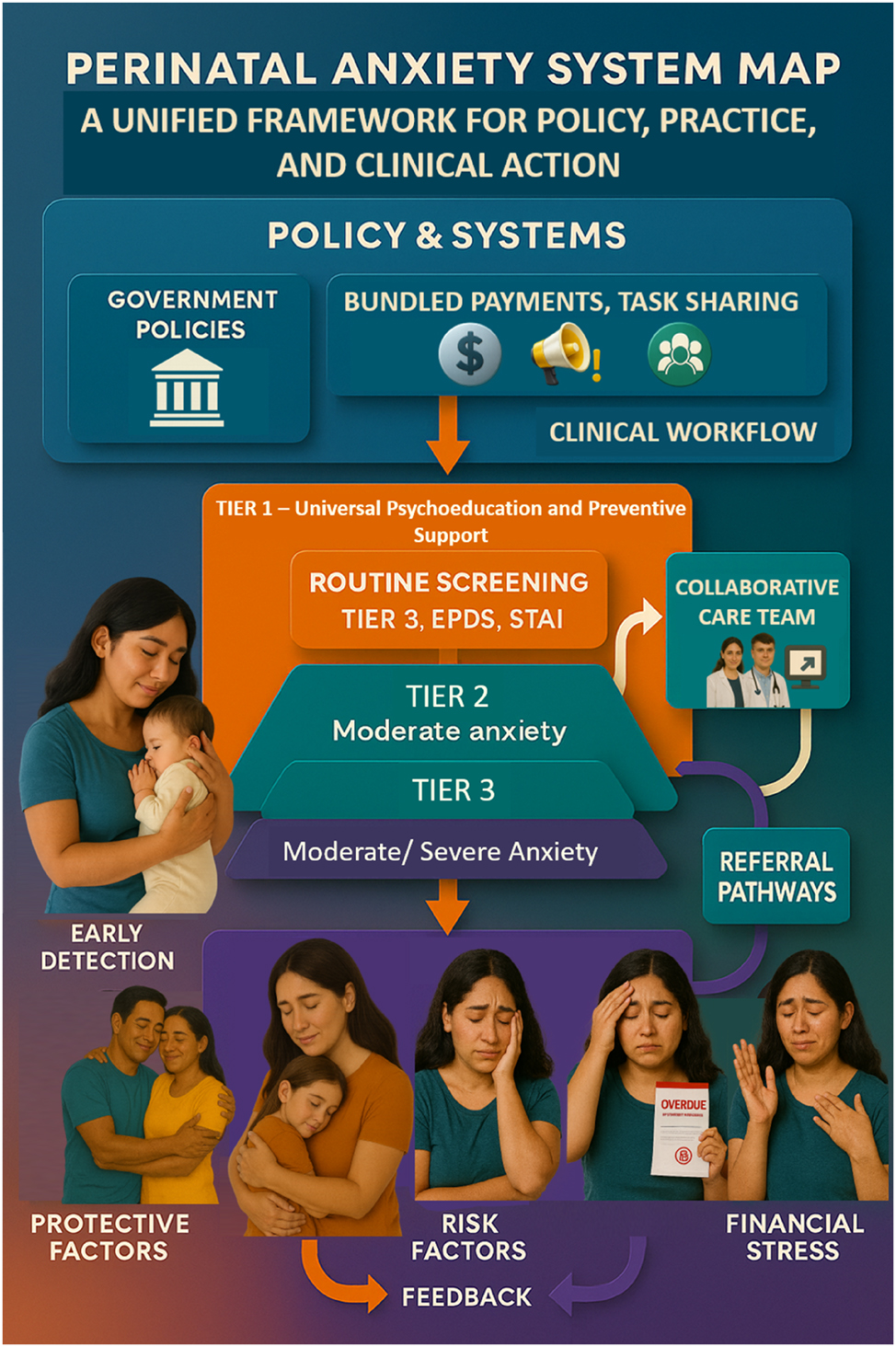
Integrated system map for perinatal anxiety care. This comprehensive system map illustrates the integrated ecosystem required to effectively address perinatal anxiety across clinical, policy, and community domains. Beginning with policy enablers such as funding, clinical guidelines, and workforce capacity-building, the figure flows into a clinical workflow that incorporates routine screening, triage, and tiered interventions using tools like GAD-7, EPDS, and structured care models. On the care delivery side, the map delineates clear stepped-care pathways—from universal psychoeducation and low-intensity digital interventions at Tier 1, to peer-supported group therapies at Tier 2, and specialist-led interventions for severe anxiety at Tier 3. The diagram also highlights feedback loops for symptom monitoring, re-assessment, and care adjustment. Simultaneously, community supports such as peer groups, cultural mediators, and social services are visualized as parallel streams reinforcing the care continuum. This system map underscores the importance of interoperability between sectors—linking obstetric care, mental health, policy frameworks, and social determinants of health—to produce an efficient, person-centered model for perinatal anxiety management. This final figure synthesizes insights from all previous diagrams and tables, making it a strategic visual anchor for policy-makers, healthcare providers, and researchers aiming to transform perinatal mental health.
Current evidence and conceptual foundations of collaborative care for perinatal anxiety
Over the past two decades, perinatal anxiety has shifted from an overlooked condition to a recognized determinant of maternal and infant health. Abrahams et al. [1] and Fairbrother et al. [24] were among the first to emphasize that anxiety disorders during pregnancy are not transient emotional states but clinically significant entities influencing obstetric outcomes. Large meta-analyses have since shown that antenatal anxiety is associated with preterm birth, low birth weight, and difficulties in early bonding [5], [6], [7], [8], [9]. Biological research, such as that by Meltzer-Brody et al. [46] and Sherer et al. [58], has further clarified the neuroendocrine and immune mechanisms underlying these associations, revealing heightened corticotropin-releasing hormone levels and immune activation patterns that can persist into the postpartum period [7], [8], [9, 46], 58]. Collectively, these findings situate perinatal anxiety as a systemic condition—psychological in manifestation, but physiological in consequence.
Despite increasing recognition, clinical pathways for anxiety lag behind those for depression. Austin et al. [4], 5] and Howard et al. [32] observed that while depression screening is now common in maternity care, anxiety detection often remains optional or inconsistent. Early screening programs using general instruments such as the EPDS or GAD-7 improved recognition but rarely improved outcomes because referral systems were fragmented and follow-up inconsistent [10], [11], [12, [15], [16], [17]. Byatt et al. [12], [13], [14], [15] argued that screening without treatment integration yields “recognition without relief”, a phrase that continues to resonate across perinatal mental-health literature. This gap stimulated interest in collaborative care models that could operationalize mental-health integration within obstetric settings.
The collaborative care framework, first validated in primary care by Unützer et al. [65] and later extended by Katon et al. [34], offers a structured, team-based model combining systematic screening, stepped intervention, and measurement-based progress tracking [18], [19], [20], [21], [22]. In obstetric populations, Grote et al. [31] demonstrated that coordinated management led by a behavioral-health care manager significantly reduced depressive symptoms and improved treatment adherence. Dennis et al. [21] and Loughnan et al. [41] reported similar effects for anxiety when cognitive-behavioral therapy or mindfulness components were embedded within prenatal or postpartum care. The convergence of these findings is summarized in Table 1, which presents pivotal studies defining collaborative-care efficacy for perinatal populations.
Yet translation to anxiety-specific practice remains incomplete. Biaggi et al. [9] and Falah-Hassani et al. [25] showed that anxiety frequently co-occurs with depression but responds differently to intervention, requiring tailored screening and stepped treatment. Meades and Ayers [44] and Meades et al. [45] noted that validated anxiety instruments are still underused, often supplanted by depression-dominant measures. Table 2 highlights available tools such as the PASS [33] and GAD-7 [41], outlining their strengths and limitations across cultural contexts. Without routine incorporation of these instruments into clinical workflow, anxiety remains underdetected and undertreated.
Equity adds another layer of complexity. Kozhimannil et al. [36] and Lara-Cinisomo et al. [37], 38] demonstrated that racial and socioeconomic disparities persist even in systems with universal screening policies. Ganann et al. [27] described how immigrant and low-income women encounter linguistic and logistical barriers that hinder care engagement, while Abrahams et al. [1] emphasized stigma and provider bias as additional deterrents. These inequities are visualized in Figure 1, which maps how systemic fragmentation and social determinants intersect to delay timely intervention. As shown in Table 3, such disparities reflect deeper structural issues—insufficient reimbursement for coordination, limited cross-disciplinary training, and variable digital infrastructure [28], [29], [30], [31, [35], [36], [37].
Implementation studies have begun to illuminate solutions. Byatt et al. [14] developed the PRISM program integrating behavioral-health specialists into obstetric clinics, while Grote et al. [31] and Cheng et al. [17] applied similar frameworks in low-income populations, achieving sustained engagement and reduced anxiety severity. These successes underscore the importance of measurement-based care, routine symptom monitoring, and shared accountability [38], [39], [40], [41]. Technological extensions such as telehealth, asynchronous coaching, and digital CBT platforms further enhance reach and continuity [38], [39], [40, 57]. As illustrated in Figure 2, collaborative care has evolved from small academic pilots to hybrid, digitally augmented systems that enable data sharing between obstetricians, psychologists, and primary-care providers.
Digital innovation, when aligned with human touch, expands the reach of collaborative models. Studies such as those by Lewkowitz et al. [40] and Schwartz et al. [57] demonstrate that blended online and phone-based interventions can significantly reduce anxiety and depression symptoms in postpartum women. Similarly, Lara-Cinisomo et al. [38] confirmed that culturally adapted digital interventions improve participation among Latina and African-American mothers. Table 4 summarizes these developments, revealing how virtual delivery can reduce stigma while maintaining therapeutic engagement. Yet as Papapetrou et al. [53] observed, successful integration still depends on relational continuity—digital platforms must complement, not replace, interpersonal care.
Health-system reform is gradually aligning with these clinical insights. Avalos et al. [6] and Byatt et al. [13] showed that embedding screening into routine prenatal visits increases detection and treatment uptake. Policy analyses by Austin et al. [4] and Vigod et al. [66] highlight national guidelines that now recommend anxiety screening and collaborative treatment as standard maternity-care practice. Table 5 presents these policy advances, including bundled-payment models and training initiatives that incentivize integration. In low-resource settings, community-based task-sharing programs evaluated by Tandon et al. [64] and Etyemez et al. [23] demonstrate that non-specialist health workers can deliver first-line anxiety interventions effectively when supervised within collaborative frameworks [23], 49], [54], [55], [56]. These innovations align with broader global mental-health movements emphasizing scalability and cultural relevance [61], [62], [63].
Nevertheless, significant research and policy gaps remain. O’Hara and McCabe [52] and Dominiak et al. [22] noted that few studies include long-term follow-up beyond twelve months postpartum. Economic evaluations of collaborative care in perinatal anxiety are scarce [6], 31], 65], and biological integration—linking psychological interventions with immune or hormonal markers—remains underexplored [23], 46], 58]. Ethical frameworks for digital data use in maternity settings are also still evolving [4], 66]. These shortcomings, along with limited attention to paternal and family inclusion, are detailed in Table 6, which synthesizes remaining research priorities and opportunities for innovation.
Taken together, the literature reveals a coherent narrative of progress and challenge. Perinatal anxiety is prevalent, consequential, and responsive to structured intervention. Collaborative care provides a tested architecture for integration, yet achieving universality requires explicit operational standards, sustainable financing, and equitable implementation. The conceptual trajectory traced through Figures 1–2 and Tables 1–6 shows that the field stands at a translational threshold—moving from proof of principle to systemwide adoption. Building on this evidence, the next section proposes a practical framework for defining minimum standards and maturity stages that can embed perinatal-anxiety care within every obstetric practice, ensuring that detection leads not just to recognition but to recovery.
Emerging models and strategic pathways for integrating mental health into obstetric practice
The translation of collaborative-care evidence into obstetric reality represents both an intellectual and practical frontier. Although decades of research—from Unützer et al. [65] to Grote et al. [31]—have demonstrated that structured integration improves outcomes, obstetric practice continues to operate within silos where mental health is peripheral rather than embedded. Howard and colleagues [32] noted that even in systems with universal depression screening, perinatal anxiety remains the “invisible twin,” unmeasured and untreated. The persistent disjunction between what is known and what is practiced reflects structural inertia rather than scientific uncertainty.
Emerging models of integration are gradually redefining this terrain. Byatt et al. [14] pioneered the PRISM initiative, a program designed to position behavioral-health specialists directly within prenatal clinics. Their findings showed that when mental-health coordination becomes a routine part of obstetric workflow, engagement and adherence rise dramatically. Similarly, Cheng et al. [17] and Grote et al. [31] demonstrated that bilingual care managers and structured case review meetings can sustain maternal recovery long beyond the initial postpartum phase. Figure 3 synthesizes these lessons by contrasting the barriers—fragmented funding, workforce shortages, cultural dissonance—with strategic responses that have proven feasible in diverse contexts.
The core of these innovations lies in the stepped-care logic of collaborative practice. Rather than a one-size-fits-all model, care is matched to symptom severity, clinician availability, and patient preference. As Loughnan et al. [41] and Mwita et al. [48] observed, tiered interventions ranging from psychoeducation to high-intensity cognitive-behavioral therapy optimize both outcomes and resource efficiency. Figure 4 visualizes this tiered framework, in which universal screening using instruments such as GAD-7 [41] or PASS [33] anchors the first tier, moderate-intensity psychological therapies form the second, and specialist management—including pharmacologic consultation—completes the third. This modular structure is adaptable to a wide range of healthcare systems, from tertiary hospitals to community clinics, provided the communication pathways between tiers remain intact.
Digital transformation has accelerated this adaptability. Lewkowitz et al. [40] demonstrated through meta-analytic evidence that mobile and web-based interventions can achieve anxiety-symptom reductions comparable to traditional therapy, while Schwartz et al. [57] confirmed the feasibility of lay-coaching combined with online support. In low- and middle-income settings, task-sharing models employing digital supervision have extended reach without sacrificing fidelity [23], 49]. Table 5 contextualizes these trends, outlining how digital tools—when paired with cultural adaptation and adequate privacy safeguards—can complement clinician-led care. However, as Lara-Cinisomo et al. [38] and Ganann et al. [27] caution, technology cannot fully substitute human trust; the most successful programs blend electronic access with relational continuity.
At the policy level, integration depends on reimbursement, regulation, and training. Avalos et al. [6] provided one of the earliest demonstrations that aligning payment structures with mental-health screening increased both uptake and treatment continuation. More recent frameworks, such as the Canadian Network for Mood and Anxiety Treatments guidelines articulated by Vigod et al. [66], position collaborative care as an essential competency within obstetric practice. These efforts signal a paradigm shift: mental-health care is no longer an adjunct but a clinical standard. Yet, as Kozhimannil et al. [36] and Lara-Cinisomo et al. [37] highlighted, policy must also correct inequities that impede minority and low-income women from accessing these services. Community-based peer programs and bilingual outreach—outlined in Table 5—represent pragmatic steps toward equitable integration.
A more ambitious evolution involves embedding mental-health metrics into obstetric quality dashboards. Byatt et al. [13] argued that without measurable outcomes—such as anxiety remission rates or referral-to-treatment intervals—mental-health initiatives risk becoming symbolic rather than transformative. Integrating these indicators within electronic health records enables longitudinal tracking and accountability across obstetric networks [38], [39], [40], [41]. As demonstrated by Unützer’s collaborative-care trials [65] and extended by Solberg et al. [63], continuous measurement is not administrative but therapeutic: it reinforces responsiveness, ensuring that care adjusts dynamically as maternal needs evolve.
Emerging global perspectives underscore the importance of cultural humility and local adaptation. Studies from South Africa [1], Pakistan [23], and Latin-American populations [38] converge on a shared insight: interventions succeed when they reflect the social fabric of the women they serve. Training midwives and community health workers to deliver first-line psychosocial support, as advocated by Tandon et al. [64] and Singla et al. [61], extends reach in resource-constrained environments. Such approaches redefine expertise as a continuum rather than a hierarchy, fostering a “whole-team literacy” in mental health that aligns with WHO’s stepped-care philosophy [48], 49].
Building upon these strands, an emerging consensus envisions an integrated system map for perinatal-anxiety care. Figure 6 depicts this synthesis: from policy enablers and funding streams at the macro level to screening, triage, and stepped interventions within clinical practice, and community supports operating in parallel. It emphasizes that true integration requires interoperability—not merely coexistence—among obstetric, psychiatric, and social systems. The model positions perinatal anxiety not as a specialized niche but as a litmus test for the maturity of maternal-health systems.
Future research directions identified in Table 6 reinforce this systems perspective. Economic analyses remain sparse [6], 31], 65]; long-term outcomes beyond twelve months postpartum are rarely tracked [22], 52]; and biomarker-informed approaches linking psychological and physiological recovery are only beginning to emerge [23], 46], 58]. Incorporating these dimensions will help quantify the full return on investment for integrated care. As O’Hara and McCabe [52] observed, the next generation of perinatal research must balance mechanistic precision with pragmatic reach, ensuring that discoveries translate into scalable policy.
Ultimately, the strategic pathway forward rests on three interlocking principles. First, perinatal anxiety screening and management must become a universal expectation embedded in routine obstetric encounters. Second, every obstetric system should maintain a minimum operational standard—defined by access to validated tools, trained providers, and bidirectional referral mechanisms. Third, implementation must be ethically equitable, addressing disparities across geography, culture, and socioeconomic status. These principles, visualized collectively in Figure 5, are not aspirational but actionable. They represent the next maturity stage of perinatal medicine, where mental-health integration defines quality as much as safety or survival.
The convergence of evidence, innovation, and ethics points toward a singular conclusion: integrating mental health into obstetric practice is no longer a theoretical ambition but a moral and professional imperative. As Austin [5] once argued, “The health of the mother is the health of the child.” Extending that sentiment into the modern era, the health of the mind is inseparable from the health of the pregnancy. Collaborative care, informed by decades of research [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67] and embodied in the evolving models summarized here, offers not just a framework for treatment but a philosophy of obstetric care—one that recognizes emotional wellbeing as the most fundamental indicator of maternal resilience.
Proposed framework and conceptual model for integrated perinatal anxiety care
The preceding synthesis demonstrates that collaborative care has matured from a promising intervention to an established principle of perinatal practice. Yet despite the breadth of supporting evidence, its real-world implementation remains inconsistent, fragmented by unequal resources and varying clinical priorities. As Unützer et al. [65] and Grote et al. [31] underscored, effective integration requires more than evidence—it demands a structural and conceptual framework capable of guiding obstetric systems across contexts. The proposed model here offers such a scaffold: a coherent, ethically grounded, and adaptable design for embedding mental-health care into obstetric practice. It is not a prescriptive formula, but a living architecture that translates existing science into sustained clinical function.
The conceptual framework arises from several converging insights. First, as Austin [5] and Howard et al. [32] have long argued, screening alone cannot achieve meaningful change without defined response pathways. Second, studies by Byatt et al. [14], 15] and Avalos et al. [6] confirmed that collaborative success depends on care coordination, feedback loops, and continuous measurement of progress. Third, qualitative work by Abrahams et al. [1] and Ganann et al. [27] revealed that cultural context determines both help-seeking behavior and therapeutic engagement. Synthesizing these perspectives, the proposed model envisions integration as a dynamic interplay between clinical workflow, digital infrastructure, and policy support, illustrated conceptually in Figure 5.
At its core, the model defines three interconnected domains: clinical integration, digital augmentation, and systemic reinforcement. Clinical integration situates mental-health screening and follow-up within every obstetric encounter. This involves validated tools such as the GAD-7 [41], PASS [33], or culturally adapted local equivalents [37], 45]. Digital augmentation ensures that these data flow seamlessly through electronic records, enabling communication between obstetricians, care managers, and mental-health professionals as demonstrated in Lewkowitz et al. [40] and Schwartz et al. [57]. Systemic reinforcement encompasses policy alignment and workforce development, ensuring that reimbursement, training, and supervision sustain clinical practice rather than rely on short-term grants [6], 36], 61]. Together these domains form a triadic structure—each dependent on the others for stability and scalability.
Figure 6 situates this triad within an integrated system map linking individual, clinical, and policy layers. At the individual level, routine screening leads to tailored interventions within a stepped-care pathway [41], 48]. At the clinical level, interdisciplinary collaboration—supported by care coordinators as in the PRISM model [14]—facilitates real-time communication between obstetric and mental-health teams. At the system level, feedback loops capture quality metrics such as treatment adherence and symptom remission, which inform ongoing funding and training cycles [13], 63], 66]. This recursive design ensures that clinical outcomes drive policy refinement, creating a self-correcting mechanism of care improvement.
The framework’s ethical rationale is equally significant. As Kozhimannil et al. [36] and Lara-Cinisomo et al. [37] documented, inequities in mental-health access persist even within ostensibly universal systems. Therefore, the model prioritizes equity as a design principle, not a secondary outcome. It advocates for bilingual and culturally competent providers, peer support networks, and flexible modes of engagement—digital, in-person, or hybrid—to accommodate diverse populations [1], 27], 38], 49]. In this way, perinatal-anxiety care becomes an instrument of justice as well as of clinical efficacy.
Sustainability also demands economic logic. Avalos et al. [6] demonstrated that universal obstetric screening coupled with collaborative management yields cost savings through reduced emergency visits and improved birth outcomes. Yet such benefits often remain invisible to policymakers without structured evaluation. The proposed model therefore embeds cost-effectiveness monitoring into its feedback loops, echoing the call by O’Hara and McCabe [52] and Vigod et al. [66] for evidence-based funding mechanisms. When financial incentives reward continuity rather than crisis response, integration becomes economically self-sustaining rather than dependent on advocacy alone.
Research implications flow naturally from this design. Table 6 outlines persistent gaps: limited long-term follow-up, underrepresentation of low- and middle-income countries, and insufficient integration of biological markers [23], 46], 58]. The proposed framework incorporates these needs by encouraging mixed-method evaluation—linking psychological outcomes with physiological indicators such as cortisol or inflammatory profiles—to capture the full biopsychosocial spectrum of maternal health [7], [8], [9, 23], 46]. Such multidimensional monitoring will allow future studies to map not only symptom change but also biological recovery, bridging obstetric and psychiatric research traditions.
While digitally enabled systems promise scalability, they also raise questions of privacy and autonomy. As Papapetrou et al. [53] cautioned, ethical implementation requires explicit consent and respect for maternal agency. Data platforms must therefore adhere to transparent governance standards, limiting algorithmic decision-making to supportive—not directive—roles. The human relationship remains central: digital infrastructure should extend empathy, not mechanize it. The model thus positions technology as a connective tissue linking clinicians and patients rather than as a substitute for professional judgment or relational care.
The integration of this framework within obstetric systems necessitates a shift in professional identity. Obstetricians, midwives, psychologists, and community workers must function as a continuum of care, unified by shared literacy in perinatal mental health. Singla et al. [61] and Tandon et al. [64] demonstrated that non-specialists can deliver effective first-line interventions when trained and supervised under collaborative structures. Incorporating such distributed competency within the proposed model enhances resilience, ensuring that perinatal mental-health support is not confined to specialized centers but available wherever pregnancy is managed.
Policy alignment remains the keystone of implementation. As highlighted in Table 5, integrated screening protocols, insurance coverage, and peer-supported initiatives represent complementary policy mechanisms that translate conceptual ideals into actionable practice [4], 6], 14], 31], 63], 65]. The framework adopts these mechanisms as operational levers. In well-resourced systems, this means embedding mental-health metrics into obstetric quality indicators; in resource-limited settings, it means leveraging task-sharing and mobile-health technology to maintain continuity of care. In both cases, the framework’s modularity ensures that principles of integration remain constant even as operational strategies adapt to local capacity.
The broader philosophical contribution of this model lies in its redefinition of perinatal anxiety as an index of system maturity. When mental health becomes a built-in expectation—measured, resourced, and respected alongside blood pressure or fetal growth—the obstetric field achieves a new ethical standard. Collaborative care thus transcends its clinical origins to become a moral architecture for maternal wellbeing. This conceptualization echoes the argument of Howard et al. [32] and Wisner et al. [67], who viewed mental-health integration as the ultimate measure of compassionate obstetric care.
In sum, the proposed framework synthesizes two decades of global evidence [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67] into a coherent vision for twenty-first-century perinatal medicine. It defines integration not as a discrete innovation but as a continuous process linking evidence, ethics, and implementation. The diagrams in Figures 5 and 6, together with the operational priorities in Tables 5 and 6, illustrate how such a model can evolve within real systems—iterative, equitable, and self-sustaining. The next section will turn to the horizon beyond this framework, examining the ethical imperatives and future directions required to transform integration from a professional aspiration into a universal right for every mother and child.
Future directions and ethical imperatives in perinatal mental health integration
The integration of mental health into obstetric care is no longer constrained by a lack of evidence but by the pace of ethical and systemic adaptation. As Austin et al. [5] and Howard et al. [32] have noted, the remaining questions are not whether collaborative care works, but how swiftly and equitably it can be delivered to every pregnant and postpartum woman. This future depends on the willingness of obstetric systems to embed emotional health as a universal quality indicator rather than a specialized adjunct.
Research horizons and clinical evolution
The next decade will likely witness a shift from efficacy to scalability. Studies by Byatt et al. [14], 15] and Grote et al. [31] demonstrated that integrated models can sustain engagement when supported by structured supervision, but replication outside academic settings remains limited. Pragmatic trials comparing diverse health-system models—urban vs. rural, public vs. private—are essential to identify the core operational elements of success. As emphasized by O’Hara and McCabe [52], long-term follow-up is the missing cornerstone of perinatal research; outcomes should extend beyond 12 months to evaluate maternal resilience, child development, and family adaptation. Table 6 outlines these priorities, emphasizing the need for interdisciplinary designs that capture the biological, psychological, and social dimensions of recovery.
The incorporation of biomarker and neuroendocrine data also represents an important frontier. Meltzer-Brody et al. [46] and Sherer et al. [58] highlighted cortisol dysregulation as a possible mediator linking anxiety to obstetric complications. Integrating such markers within clinical trials could illuminate how psychological and physiological healing align. These data would not replace self-reported outcomes but would strengthen mechanistic understanding, advancing the conceptual unity of perinatal medicine.
Digital transformation and artificial intelligence
Technological acceleration continues to reshape the boundaries of care. As Lewkowitz et al. [40] and Schwartz et al. [57] showed, telemedicine and digital cognitive-behavioral platforms have become essential extensions of clinical services. Artificial intelligence now introduces the possibility of predictive screening, adaptive triage, and personalized treatment monitoring. Yet, as Papapetrou et al. [53] cautioned, algorithms trained on biased data risk perpetuating inequities rather than correcting them. The ethical imperative is therefore dual: to harness innovation while safeguarding autonomy, privacy, and justice.
The responsible use of digital tools must preserve the centrality of human judgment. Automated alerts and dashboards can enhance clinical vigilance but should never dictate decisions. The lessons from collaborative care are clear—empathy, continuity, and cultural sensitivity cannot be mechanized. Figure 5 situates these technological functions within the human framework of integration, reminding practitioners that data serve compassion, not the reverse.
Global equity and policy accountability
Equity will define the moral credibility of future perinatal systems. Kozhimannil et al. [36] and Lara-Cinisomo et al. [37], 38] demonstrated that minority and low-income women remain disproportionately excluded from specialized mental-health services. In low- and middle-income countries, task-sharing initiatives described by Singla et al. [61] and Tandon et al. [64] have shown that trained midwives and community workers can deliver first-line psychosocial interventions effectively. Scaling these programs requires sustained investment in supervision, remuneration, and digital connectivity. As reflected in Figure 6, global progress will depend on developing interoperable systems capable of linking local initiatives to national maternal-health agendas.
Policy accountability must evolve in parallel. Avalos et al. [6] demonstrated that reimbursement reform increases screening and treatment rates, but without performance measurement such gains may fade. National health systems should adopt integrated quality indicators—time to referral, remission rates, and follow-up continuity—to ensure that mental-health integration remains measurable and transparent. Byatt et al. [13] argued that inclusion of mental-health metrics within obstetric dashboards transforms policy from aspiration into enforceable practice. When funding and evaluation are explicitly tied to psychological outcomes, integration becomes a structural expectation rather than a discretionary ideal.
Ethical foundations for the next era
The ethical imperatives underlying perinatal mental-health integration extend beyond access. They encompass respect for maternal agency, recognition of cultural plurality, and protection from digital exploitation. As Abrahams et al. [1] and Ganann et al. [27] observed, women’s decisions about disclosure and help-seeking are shaped by trust; coercive or data-driven models risk undermining that trust. Transparent consent, shared decision-making, and narrative-based care should therefore guide every technological or policy innovation.
Environmental and intergenerational ethics also emerge as new considerations. Evidence linking maternal anxiety to epigenetic and developmental outcomes [7], [8], [9, 23], 46] implies that neglecting mental health may transmit vulnerability across generations. Addressing perinatal anxiety is thus an act of preventive public health, with implications extending to the wellbeing of children and communities. The framework proposed earlier situates this ethical obligation at the heart of modern perinatology: to protect not only survival, but the capacity for emotional flourishing.
The vision forward
Looking ahead, the convergence of collaborative care, digital innovation, and social equity forms a new triad of progress. Research must move from isolated efficacy studies to learning health systems capable of continuous refinement. Policymakers must treat mental health as a fundamental obstetric outcome. Clinicians must cultivate the competencies that bridge obstetric skill with psychological insight. When these elements coalesce, the integration of mental health into obstetric practice becomes not a reform, but a redefinition of care itself.
The ethical horizon is clear. As Howard et al. [32] wrote, “The most advanced obstetric systems will be those that make psychological wellbeing a measure of safety.” Integrating mental health is therefore not a supplement to obstetric medicine—it is its culmination. The science summarized in Figures 5–6 and the priorities outlined in Table 6 together illuminate a singular direction: toward an obstetric paradigm where the mind and body of the mother are treated as one. In that unity lies the true measure of progress for the next century of perinatal medicine.
Conclusions
Integrating mental health into obstetric practice represents a necessary evolution in modern maternity care. The evidence and reflections presented throughout this paper affirm that perinatal anxiety is not a peripheral issue but a vital dimension of maternal wellbeing. Addressing it requires a fundamental cultural shift—one that recognizes emotional health as inseparable from physical safety during pregnancy and childbirth. This integration is not only a clinical reform but an ethical redefinition of care. When mental health is embedded within obstetric systems, the approach to pregnancy becomes more humane, preventive, and continuous. Women are no longer treated as patients defined by biological risk but as whole individuals whose emotional experiences shape both recovery and resilience. Such transformation demands commitment at every level—clinicians trained to listen and respond empathetically, institutions that prioritize psychological outcomes, and policies that sustain access to comprehensive perinatal support.
The future of obstetric medicine will be measured not only by survival rates but by the quality of emotional flourishing that accompanies birth. Integrative models of care offer a path toward this balance, blending scientific precision with compassion and dignity. The challenge ahead lies in ensuring that such care becomes standard rather than exceptional. Ultimately, the integration of mental health within obstetric practice restores the most essential promise of medicine: to care for the person as a whole. It calls on healthcare systems to honor the mind and body as a unified experience and to safeguard the wellbeing of every mother and child within that unity. In doing so, perinatal medicine moves beyond treatment toward transformation—where empathy, ethics, and evidence coexist as the foundation of truly comprehensive care.
Acknowledgments
The authors acknowledge the invalu- able support of the Indonesian Society of Obstetrics & Gyncology (ISOG/POGI) and Indonesian Society of Maternal-Fetal Medicine (INAMFM/HKFM) in facilitating this article.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Abrahams, Z, Boisits, S, Schneider, M, Honikman, S, Lund, C. Facilitators and barriers to detection and treatment of depression, anxiety and experiences of domestic violence in pregnant women. Sci Rep 2023;13:12457. https://doi.org/10.1038/s41598-023-36150-z.Suche in Google Scholar PubMed PubMed Central
2. Alhusen, JL, Frohman, N, Purcell, G. Intimate partner violence and suicidal ideation in pregnant women. Arch Womens Ment Health 2015;18:573–8. https://doi.org/10.1007/s00737-015-0515-2.Suche in Google Scholar PubMed PubMed Central
3. Athinaidou, AM, Vounatsou, E, Pappa, I, Harizopoulou, VC, Sarantaki, A. Influence of antenatal education on birth outcomes: a systematic review focusing on primiparous women. Cureus 2024;16:e64508. https://doi.org/10.7759/cureus.64508.Suche in Google Scholar PubMed PubMed Central
4. Austin, MP, Middleton, P, Reilly, NM, Highet, NJ. Detection and management of mood disorders in the maternity setting: the Australian clinical practice guidelines. Women Birth 2013;26:2-9. https://doi.org/10.1016/j.wombi.2011.12.001Suche in Google Scholar PubMed
5. Austin, MP. Antenatal screening and early intervention for “perinatal” distress, depression and anxiety: where to from here? Arch Womens Ment Health 2004;7:1–6. https://doi.org/10.1007/s00737-003-0034-4.Suche in Google Scholar PubMed
6. Avalos, LA, Raine-Bennett, T, Chen, H, Adams, AS, Flanagan, T. Improved perinatal depression screening, treatment, and outcomes with a universal obstetric program. Obstet Gynecol 2016;127:917–25. https://doi.org/10.1097/AOG.0000000000001403.Suche in Google Scholar PubMed PubMed Central
7. Ayers, S, Sinesi, A, Coates, R, Cheyne, H, Maxwell, M, Best, C, et al.. When is the best time to screen for perinatal anxiety? A longitudinal cohort study. J Anxiety Disord 2024;103:102841. https://doi.org/10.1016/j.janxdis.2024.102841.Suche in Google Scholar PubMed
8. Best, C, Ayers, S, Sinesi, A, Meades, R, Cheyne, H, Maxwell, M, et al.. Socioeconomic deprivation and perinatal anxiety: an observational cohort study. BMC Public Health 2024;24:3183. https://doi.org/10.1186/s12889-024-20608-4.Suche in Google Scholar PubMed PubMed Central
9. Biaggi, A, Conroy, S, Pawlby, S, Pariante, CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord 2016;191:62–77. https://doi.org/10.1016/j.jad.2015.11.014.Suche in Google Scholar PubMed PubMed Central
10. Bjertrup, AJ, Rasmussen, KHE, Miskowiak, KW. Psychological prevention of postpartum depression for expectant parents. J Affect Disord 2025;378:58–73. https://doi.org/10.1016/j.jad.2025.02.077.Suche in Google Scholar PubMed
11. Brand, SR, Brennan, PA. Impact of antenatal and postpartum maternal mental illness: how are the children? Clin Obstet Gynecol 2009;52:441–55. https://doi.org/10.1097/GRF.0b013e3181b52930.Suche in Google Scholar PubMed
12. Byatt, N, Biebel, K, Friedman, L, Debordes-Jackson, G, Ziedonis, D, Pbert, L. Patient’s views on depression care in obstetric settings: how do they compare to the views of perinatal health care professionals? Gen Hosp Psychiatry 2013;35:598–604. https://doi.org/10.1016/j.genhosppsych.2013.07.011.Suche in Google Scholar PubMed PubMed Central
13. Byatt, N, Levin, LL, Ziedonis, D, Moore Simas, TA, Allison, J. Enhancing participation in depression care in outpatient perinatal care settings: a systematic review. Obstet Gynecol 2015;126:1048–58. https://doi.org/10.1097/AOG.0000000000001067.Suche in Google Scholar PubMed PubMed Central
14. Byatt, N, Pbert, L, Hosein, S, Swartz, HA, Weinreb, L, Allison, J, et al.. Program in support of moms (PRISM): development and beta testing. Psychiatr Serv 2016;67:824–6. https://doi.org/10.1176/appi.ps.201600049.Suche in Google Scholar PubMed PubMed Central
15. Byatt, N, Simas, TA, Lundquist, RS, Johnson, JV, Ziedonis, DM. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol 2012;33:143–61. https://doi.org/10.3109/0167482X.2012.728649.Suche in Google Scholar PubMed
16. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71.Suche in Google Scholar PubMed PubMed Central
17. Cheng, ER, Rifas-Shiman, SL, Perkins, ME, Rich-Edwards, JW, Gillman, MW, Wright, R, et al.. The influence of antenatal partner support on pregnancy outcomes. J Womens Health (Larchmt) 2016;25:672–9. https://doi.org/10.1089/jwh.2015.5462.Suche in Google Scholar PubMed PubMed Central
18. Clinkscales, N, Golds, L, Berlouis, K, MacBeth, A. The effectiveness of psychological interventions for anxiety in the perinatal period: a systematic review and meta-analysis. Psychol Psychother 2023;96:296–327. https://doi.org/10.1111/papt.12441.Suche in Google Scholar PubMed
19. Cluxton-Keller, F, Olson, A. A family-based collaborative care model for treatment of depressive and anxiety symptoms in perinatal women: results from a pilot study. JMIR Pediatr Parent 2023;6:e45616. https://doi.org/10.2196/45616.Suche in Google Scholar PubMed PubMed Central
20. Coll, CVN, Domingues, MR, Stein, A, da Silva, BGC, Bassani, DG, Hartwig, FP, et al.. Efficacy of regular exercise during pregnancy on the prevention of postpartum depression: the PAMELA randomized clinical trial. JAMA Netw Open 2019;2:e186861. https://doi.org/10.1001/jamanetworkopen.2018.6861.Suche in Google Scholar PubMed PubMed Central
21. Dennis, CL, Falah-Hassani, K, Shiri, R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry 2017;210:315–23. https://doi.org/10.1192/bjp.bp.116.187179.Suche in Google Scholar PubMed
22. Dominiak, M, Antosik-Wojcinska, AZ, Baron, M, Mierzejewski, P, Swiecicki, L. Recommendations for the prevention and treatment of postpartum depression. Ginekol Pol 2021;92:153–64. https://doi.org/10.5603/GP.a2020.0141.Suche in Google Scholar PubMed
23. Etyemez, S, Mehta, K, Tutino, E, Zaidi, A, Atif, N, Rahman, A, et al.. The immune phenotype of perinatal anxiety in an anxiety-focused behavioral intervention program in Pakistan. Brain Behav Immun 2024;120:141–50. https://doi.org/10.1016/j.bbi.2024.05.028.Suche in Google Scholar PubMed
24. Fairbrother, N, Young, AH, Janssen, P, Antony, MM, Tucker, E. Depression and anxiety during the perinatal period. BMC Psychiatry 2015;15:206. https://doi.org/10.1186/s12888-015-0526-6.Suche in Google Scholar PubMed PubMed Central
25. Falah-Hassani, K, Shiri, R, Dennis, CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med 2017;47:2041–53. https://doi.org/10.1017/S0033291717000617.Suche in Google Scholar PubMed
26. Figueiredo, B, Dias, CC, Brandão, S, Canário, C, Nunes-Costa, R. Breastfeeding and postpartum depression: state of the art review. J Pediatr 2013;89:332–8. https://doi.org/10.1016/j.jped.2012.12.002.Suche in Google Scholar PubMed
27. Ganann, R, Sword, W, Newbold, KB, Thabane, L, Armour, L, Kint, B. Influences on mental health and health services accessibility in immigrant women with post-partum depression: an interpretive descriptive study. J Psychiatr Ment Health Nurs 2020;27:87–96. https://doi.org/10.1111/jpm.12557.Suche in Google Scholar PubMed
28. Gedzyk-Nieman, SA. Postpartum and paternal postnatal depression: identification, risks, and resources. Nurs Clin North Am 2021;56:325–43. https://doi.org/10.1016/j.cnur.2021.04.001.Suche in Google Scholar PubMed
29. Gopalan, P, Spada, ML, Shenai, N, Brockman, I, Keil, M, Livingston, S. Postpartum depression-identifying risk and access to intervention. Curr Psychiatry Rep 2022;24:889–96. https://doi.org/10.1007/s11920-022-01392-7.Suche in Google Scholar PubMed PubMed Central
30. Grigoriadis, S, VonderPorten, EH, Mamisashvili, L, Tomlinson, G, Dennis, CL, Koren, G, et al.. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 2013;74:e321-41. https://doi.org/10.4088/JCP.12r07968.Suche in Google Scholar PubMed
31. Grote, NK, Katon, WJ, Russo, JE, Lohr, MJ, Curran, M, Carson, K. Collaborative care for perinatal depression in socioeconomically disadvantaged women. Psychiatr Serv 2014;65:434–42. https://doi.org/10.1176/appi.ps.201300099.Suche in Google Scholar
32. Howard, LM, Molyneaux, E, Dennis, CL, Rochat, T, Stein, A, Milgrom, J. Non-psychotic mental disorders in the perinatal period. Lancet 2014;384:1775–88. https://doi.org/10.1016/S0140-6736(14)61276-9.Suche in Google Scholar PubMed
33. Jradi, H, Alfarhan, T, Alsuraimi, A. Validation of the Arabic version of the perinatal anxiety screening scale (PASS) among antenatal and postnatal women. BMC Pregnancy Childbirth 2020;20:758. https://doi.org/10.1186/s12884-020-03451-4.Suche in Google Scholar PubMed PubMed Central
34. Katon, WJ, Lin, EH, Von Korff, M, Ciechanowski, P, Ludman, EJ, Young, B, et al.. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–20. https://doi.org/10.1056/NEJMoa1003955.Suche in Google Scholar PubMed PubMed Central
35. Ko, JY, Farr, SL, Dietz, PM, Robbins, CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. J Womens Health 2012;21:830–6. https://doi.org/10.1089/jwh.2011.3466.Suche in Google Scholar PubMed PubMed Central
36. Kozhimannil, KB, Trinacty, CM, Busch, AB, Huskamp, HA, Adams, AS. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv 2011;62:619–25. https://doi.org/10.1176/ps.62.6.pss6206_0619.Suche in Google Scholar PubMed PubMed Central
37. Lara-Cinisomo, S, Clark, CT, Wood, J. Increasing diagnosis and treatment of perinatal depression in Latinas and African American women: addressing stigma is not enough. Womens Health Issues 2018;28:201–4. https://doi.org/10.1016/j.whi.2018.01.003.Suche in Google Scholar PubMed PubMed Central
38. Lara-Cinisomo, S, Ramirez Olarte, A, Rosales, M, Barrera, AZ. A systematic review of technology-based prevention and treatment interventions for perinatal depression and anxiety in Latina and African American women. Matern Child Health J 2021;25:268–81. https://doi.org/10.1007/s10995-020-03028-9.Suche in Google Scholar PubMed
39. Lewis, BA, Schuver, K, Dunsiger, S, Samson, L, Frayeh, AL, Terrell, CA, et al.. Randomized trial examining the effect of exercise and wellness interventions on preventing postpartum depression and perceived stress. BMC Pregnancy Childbirth 2021;21:785. https://doi.org/10.1186/s12884-021-04257-8.Suche in Google Scholar PubMed PubMed Central
40. Lewkowitz, AK, Whelan, AR, Ayala, NK, Hardi, A, Stoll, C, Battle, CL, et al.. The effect of digital health interventions on postpartum depression or anxiety: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol 2024;230:12–43. https://doi.org/10.1016/j.ajog.2023.06.028.Suche in Google Scholar PubMed PubMed Central
41. Loughnan, SA, Wallace, M, Joubert, AE, Haskelberg, H, Andrews, G, Newby, JM. A systematic review of psychological treatments for clinical anxiety during the perinatal period. Arch Womens Ment Health 2018;21:481–90. https://doi.org/10.1007/s00737-018-0812-7.Suche in Google Scholar PubMed
42. McCarthy, M, Houghton, C, Matvienko-Sikar, K. Women’s experiences and perceptions of anxiety and stress during the perinatal period: a systematic review and qualitative evidence synthesis. BMC Pregnancy Childbirth 2021;21:811. https://doi.org/10.1186/s12884-021-04271-w.Suche in Google Scholar PubMed PubMed Central
43. McMahon, CA, Boivin, J, Gibson, FL, Hammarberg, K, Wynter, K, Saunders, D. Age at first birth, mode of conception and psychological wellbeing in pregnancy: findings from the parental age and transition to parenthood Australia (PATPA) study. Hum Reprod 2011;26:1389–98. https://doi.org/10.1093/humrep/der076.Suche in Google Scholar PubMed
44. Meades, R, Ayers, S. Anxiety measures validated in perinatal populations: a systematic review. J Affect Disord 2011;133:1–15. https://doi.org/10.1016/j.jad.2010.10.009.Suche in Google Scholar PubMed
45. Meades, R, Sinesi, A, Williams, LR, Delicate, A, Cheyne, H, Maxwell, M, et al.. Evaluation of perinatal anxiety assessment measures: a cognitive interview study. BMC Pregnancy Childbirth 2024;24:507. https://doi.org/10.1186/s12884-024-06641-6.Suche in Google Scholar PubMed PubMed Central
46. Meltzer-Brody, S, Stuebe, A, Dole, N, Savitz, D, Rubinow, D, Thorp, J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). J Clin Endocrinol Metab 2011;96:E40-7. https://doi.org/10.1210/jc.2010-0978.Suche in Google Scholar PubMed PubMed Central
47. Moulds, ML, Bisby, MA, Black, MJ, Jones, K, Harrison, V, Hirsch, CR, et al.. Repetitive negative thinking in the perinatal period and its relationship with anxiety and depression. J Affect Disord 2022;311:446–62. https://doi.org/10.1016/j.jad.2022.05.070.Suche in Google Scholar PubMed
48. Mwita, M, Dewey, D, Konje, ET, Patten, S. Non-pharmacological interventions for perinatal depression and anxiety among adolescent mothers: a systematic review and meta-analysis. J Affect Disord 2025;379:168–75. https://doi.org/10.1016/j.jad.2025.03.056.Suche in Google Scholar PubMed
49. Nielsen-Scott, M, Fellmeth, G, Opondo, C, Alderdice, F. Prevalence of perinatal anxiety in low- and middle-income countries: a systematic review and meta-analysis. J Affect Disord 2022;306:71–9. https://doi.org/10.1016/j.jad.2022.03.032.Suche in Google Scholar PubMed
50. Nomura, Y, Finik, J, Salzbank, J, Ly, J, Huynh, N, Davey, T, et al.. The effects of preeclampsia on perinatal risks and infant temperaments among mothers with antenatal depression. Psychol Res 2014;4:451–61. https://doi.org/10.17265/2159-5542/2014.06.005.Suche in Google Scholar PubMed PubMed Central
51. O’Dea, GA, Youssef, GJ, Hagg, LJ, Francis, LM, Spry, EA, Rossen, L, et al.. Associations between maternal psychological distress and mother-infant bonding: a systematic review and meta-analysis. Arch Womens Ment Health 2023;26:441–52. https://doi.org/10.1007/s00737-023-01332-1.Suche in Google Scholar PubMed PubMed Central
52. O’Hara, MW, McCabe, JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 2013;9:379–407. https://doi.org/10.1146/annurev-clinpsy-050212-185612.Suche in Google Scholar PubMed
53. Papapetrou, C, Panoulis, K, Mourouzis, I, Kouzoupis, A. Pregnancy and the perinatal period: the impact of attachment theory. Psychiatriki 2020;31:257–70. https://doi.org/10.22365/jpsych.2020.313.257.Suche in Google Scholar PubMed
54. Ponti, L, Ghinassi, S, Tani, F. Spontaneous and induced labor: association with maternal well-being three months after childbirth. Psychol Health Med 2022;27:896–901. https://doi.org/10.1080/13548506.2021.1956554.Suche in Google Scholar PubMed
55. Rogers, AM, Youssef, GJ, Teague, S, Sunderland, M, Le Bas, G, Macdonald, JA, et al.. Association of maternal and paternal perinatal depression and anxiety with infant development: a longitudinal study. J Affect Disord 2023;338:278–88. https://doi.org/10.1016/j.jad.2023.06.020.Suche in Google Scholar PubMed
56. Rondung, E, Massoudi, P, Nieminen, K, Wickberg, B, Peira, N, Silverstein, R, et al.. Identification of depression and anxiety during pregnancy: a systematic review and meta-analysis of test accuracy. Acta Obstet Gynecol Scand 2024;103:423–36. https://doi.org/10.1111/aogs.14734.Suche in Google Scholar PubMed PubMed Central
57. Schwartz, H, McCusker, J, Da Costa, D, Singh, S, Baskaran, S, Belzile, E, et al.. A pilot randomized controlled trial of a lay telephone coaching and web-based intervention for postpartum depression and anxiety: the MPOWER study. Internet Interv 2022;31:100597. https://doi.org/10.1016/j.invent.2022.100597.Suche in Google Scholar PubMed PubMed Central
58. Sherer, ML, Voegtline, KM, Park, HS, Miller, KN, Shuffrey, LC, Klein, SL, et al.. The immune phenotype of perinatal anxiety. Brain Behav Immun 2022;106:280–8. https://doi.org/10.1016/j.bbi.2022.09.005.Suche in Google Scholar PubMed
59. Shorey, S, Chee, CYI, Ng, ED, Chan, YH, Tam, WWS, Chong, YS. Prevalence and incidence of postpartum depression among healthy mothers: a systematic review and meta-analysis. J Psychiatr Res 2018;104:235–48. https://doi.org/10.1016/j.jpsychires.2018.08.001.Suche in Google Scholar PubMed
60. Shorey, SY, Ng, ED, Chee, CYI. Anxiety and depressive symptoms of women in the perinatal period during the COVID-19 pandemic: a systematic review and meta-analysis. Scand J Public Health 2021;49:730–40. https://doi.org/10.1177/14034948211011793.Suche in Google Scholar PubMed
61. Singla, DR, Savel, KA, Magidson, JF, Vigod, SN, Dennis, CL. The role of peer providers to scale up psychological treatments for perinatal populations worldwide. Curr Psychiatry Rep 2023;25:735–40. https://doi.org/10.1007/s11920-023-01459-z.Suche in Google Scholar PubMed
62. Sockol, LE, Epperson, CN, Barber, JP. Preventing postpartum depression: a meta-analytic review. Clin Psychol Rev 2013;33:1205–17. https://doi.org/10.1016/j.cpr.2013.10.004.Suche in Google Scholar PubMed PubMed Central
63. Solberg, JJ, Deyo-Svendsen, ME, Nylander, KR, Bruhl, EJ, Heredia, D, Angstman, KB. Collaborative care management associated with improved depression outcomes in patients with personality disorders, compared to usual primary care. J Prim Care Community Health 2018;9:2150132718773266. https://doi.org/10.1177/2150132718773266.Suche in Google Scholar PubMed PubMed Central
64. Tandon, SD, Leis, JA, Mendelson, T, Perry, DF, Kemp, K. Six-month outcomes from a randomized controlled trial to prevent perinatal depression in low-income home visiting clients. Matern Child Health J 2014;18:873–81. https://doi.org/10.1007/s10995-013-1313-y.Suche in Google Scholar PubMed PubMed Central
65. Unützer, J, Katon, W, Callahan, CM, Williams, JWJr, Hunkeler, E, Harpole, L, et al.. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836–45. https://doi.org/10.1001/jama.288.22.2836.Suche in Google Scholar PubMed
66. Vigod, SN, Frey, BN, Clark, CT, Grigoriadis, S, Barker, LC, Brown, HK, et al.. Canadian network for mood and anxiety treatments 2024 clinical practice guideline for the management of perinatal mood, anxiety, and related disorders: guide de pratique 2024 du Canadian network for mood and anxiety treatments pour le traitement des troubles de l’humeur, des troubles anxieux et des troubles connexes périnatals. Can J Psychiatr 2025;70:429–89. https://doi.org/10.1177/07067437241303031.Suche in Google Scholar PubMed PubMed Central
67. Wisner, KL, Sit, DK, McShea, MC, Rizzo, DM, Zoretich, RA, Hughes, CL, et al.. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 2013;70:490–8. https://doi.org/10.1001/jamapsychiatry.2013.87.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


