Abstract
Objectives
We aim to explore whether the long inter-pregnancy interval (IPI) modifies the association between previous cesarean section (CS) and adverse maternal and neonatal outcomes in a population with a high rate of CS and a long IPI.
Methods
Adverse maternal and neonatal outcomes were compared between the previous CS and previous vaginal delivery groups. Logistic models were used to adjust for potential confounding factors and calculate the Odds Ratios (ORs) and 95 % confidence intervals (CIs). The interaction model and stratified analyses were used to evaluate the modifications of IPIs on the associations between previous CS and maternal and neonatal outcomes.
Results
Compared with previous vaginal delivery, previous CS was associated with increased risks of uterine-related complications (a OR=1.57, 95 % CI 1.25–1.98), but with decreased risks of preterm birth (a OR=0.73, 95 % CI 0.63–0.85) and severe neonatal adverse outcomes (a OR=0.59, 95 % CI 0.46–0.78). There are synergistic biological interaction effects of previous CS and a long IPI (>60 months) on the risks of placental-related complications (RERI=0.32, 95 % CI 0.05–0.58; AP=0.39, 95 % CI 0.03–0.76) but an antagonistic biological interaction effect on the risk of preterm birth (RERI=−0.35, 95 % CI -0.68 to −0.01; AP=−0.09, 95 % CI -0.68 to −0.03).
Conclusions
Previous CS was associated with increased risks of adverse maternal outcomes but decreased risks of certain adverse neonatal outcomes. Prolonged IPIs might not attenuate the adverse effects of previous CS on mothers, and might adversely exert harm on newborns.
Introduction
Cesarean section (CS), one of the most common surgeries worldwide, serves as a life-saving intervention when medically indicated [1]. However, CS is also associated with elevated risks of maternal and neonatal complications in the current and even subsequent pregnancies. China has the world’s highest CS rate, with urban women reaching a peak of 64.1 % in 2008 [2]. Notably, China has the world’s highest rate of non-indicated CS, which is a major contributor to the overall high CS rate [3], 4]. Evidences demonstrate that prior CS elevates risks of maternal complications including hysterectomy, abnormal placentation, uterine rupture, premature rupture of membranes (PROM), and postpartum hemorrhage [1], 5], 6]. Moreover, it correlates with adverse neonatal outcomes such as low birth weight, preterm birth, and neonatal intensive care unit (NICU) admission [7].
Before the implementation of the universal two-child policy in 2015, many Chinese women didn’t consider giving birth to a second child. As a result, they often preferred CS over vaginal delivery, without concerning the potential complications of CS in future pregnancies. Nevertheless, following the policy change, a substantial number of multiparous women with a history of CS decided to have a second child. The sudden transition from a one-child to two-child policy has led to at least an additional 90 million multiparous women giving birth again [8]. Notably, many of these women have a long inter-pregnancy interval (IPI), a known risk factor for adverse maternal and neonatal outcomes.
Furthermore, studies suggest prolonged IPIs may mitigate certain CS-associated maternal risks, including uterine rupture, placenta accreta spectrum, maternal perinatal mortality, and primary postpartum hemorrhage [9], [10], [11], [12]. Conversely, a recent Chinese study reports that a long IPI (≥60 months) may reduce maternal risks but elevate risks of neonatal adverse outcomes [13]. Given China’s unique public health landscape – marked by high CS rates and high second-child rate, it is important to clarify the association between previous CS and maternal-neonatal adverse outcomes. Considering both CS and IPIs as potential modifiable factors, determining their population attributable risk percent (PARP) and interactive effects on adverse maternal and neonatal outcomes is critical. Such insights are critical for informing clinical guidelines and healthcare policies to optimize perinatal care in the post-two-child policy era. The universal two-child policy in China provides us with this unique opportunity to do this analysis in a population with high rates of CS and a long IPI.
Materials and methods
Data collection
This cohort study utilized a population-based registry dataset. Clinical data on maternal-neonatal characteristics and diagnoses were extracted from the Maternity and Child Registration System (MCRS), a government-mandated platform under the Health Commission for monitoring all births and neonatal outcomes within district hospitals. The MCRS acquired the raw data from the Hospital Information System with electronic authorization. The project of this study was reviewed by the Ethics Committee of the Affiliated Hospital on November 9, 2021 (No. KY2021264) in accordance with the Measures for Ethical Review of Life Sciences and Medical Research Involving Humans in China. Informed consent for this retrospective analysis was waived due to the absence of direct patient intervention, and all data were fully anonymized.
Study subjects
The included subjects were multiparous women who delivered singleton babies≥28 weeks between December 1, 2015, and December 1, 2020. The total number of registered pregnant mothers during this period was 14,347. First, primiparous women (n=5,586) were excluded from the cohort. Multiparous women were also excluded if they had twin or multiple births (n=394) or used artificial reproductive technology (n=787). Additionally, multiparous women with important missing information were excluded (n=213), including missing birth weight information (n=84), previous delivery modes (n=52), and IPIs (n=77). Finally, a total of 7,367 multiparous women were included in the analysis, consisting of 4,711 women (63.95 %) with previous CS delivery and 2,656 women with previous vaginal delivery (Figure 1).
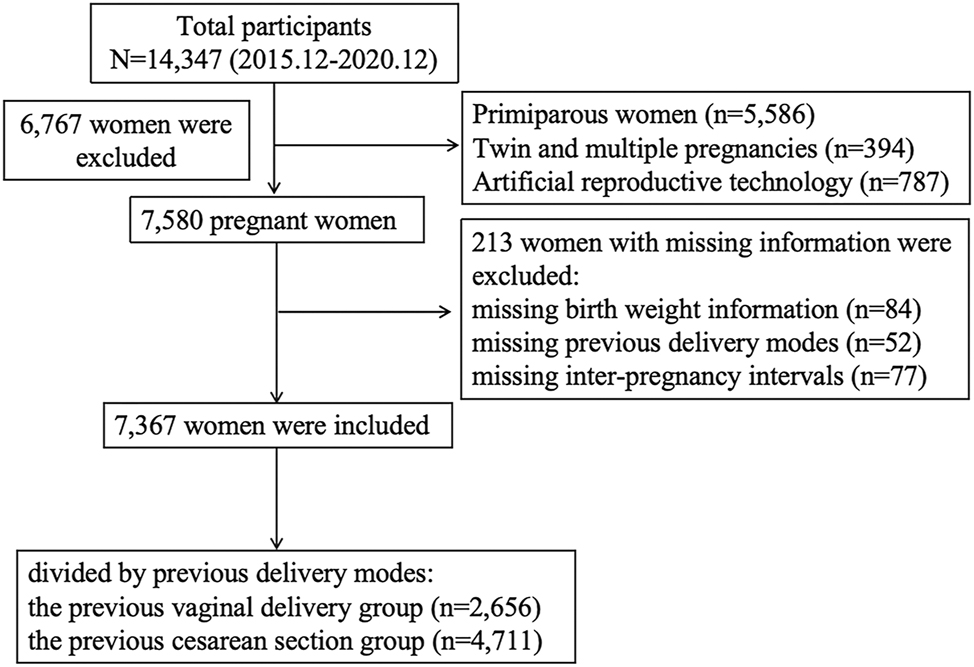
The flowchart of the whole process.
Outcomes
The outcomes examined in the study included adverse maternal and neonatal outcomes. Maternal adverse outcomes encompassed uterine-related complications (at least one of uterine rupture, partial or total hysterectomy, or postpartum hemorrhage), placental-related complications (including at least one of placenta previa or placental abruption), and PROM. Neonatal adverse outcomes included preterm birth, small for gestational age (SGA, defined as a birth weight below the 10th percentile for gestational age), and severe neonatal adverse outcomes (including at least one of stillbirth, resuscitation failure in the delivery room, or admission to NICU).
Covariates
Potential covariates were maternal age at the current delivery, gravidity (2, 3, >3), parity (2, >2), IPIs (months), body mass index (BMI, kg/m2), abortion history (yes/no), mode of the current delivery (vaginal/CS), gestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no), neonatal gestational age at birth (weeks), and birth weight (gram).
Statistical analysis
Separately by previous delivery mode (CS or vaginal delivery), baseline maternal and neonatal characteristics were summarized. Continuous variables were presented as mean±standard deviation (mean±SD) [median] and compared by t-test. Categorical variables were reported as frequencies (percentages) and compared using the chi-squared test (chi-square), while ordered categorical variables were evaluated with the Cochran-Mantel-Haenszel chi-squared test. Logistic regression models were used to estimate crude odds ratios (ORs) and adjusted odds ratios (aORs) with 95 % confidence intervals (CIs).
The modification of IPIs on the associations between previous CS and outcomes was tested by stratified analysis and additive interaction analysis. Based on a prior research, IPIs≥60 months were associated with increased risks of neonatal adverse outcomes [13]. Therefore, the stratified analysis was conducted into subgroups with different IPIs (<60, ≥60 months). The reference group of each subgroup was the corresponding previous vaginal subgroup.
Additive interaction analyses were used to evaluate the biological interactions associated between a long IPI (≥60 months) and previous CS on maternal and neonatal outcomes, with the reference group specified as those with IPIs at<60 months and previous vaginal delivery. The model was measured by two indexes: relative excess risk caused by interaction (RERI) and attributable proportion (AP), deriving 95 % CI using the delta method. RERI estimates the additional risk that is attributable to the interaction between two exposures: long IPI and previous CS [14], 15]. And it is defined as by the formula: RERI=OR11- OR10- OR01+1. The OR11 is in the group exposed to both long IPI and previous CS (1=exposed, 0=unexposed). The OR01 is in the group only exposed to long IPI and the OR10 is in the group only exposed to previous CS. AP represents the proportion of adverse outcomes that can be attributed to the interaction between long IPI and previous CS, defined as RERI/OR11 [15]. Both statistically significant RERI>0 and AP>0 indicates synergistic biological interactions (the combined risk of both exposures is higher than the sum of sum of their individual risks) and a significant RERI<0 and AP<0 indicates antagonistic biological interactions (the combined risk of both exposures is lower than the sum of sum of their individual risks) [16].
Furthermore, population attributable risk percent (PARP) was applied to calculate relative excess risk of previous CS. PARP is defined as (incidence among all participants within the population – incidence among non-exposed)/incidence among all participants within the population *100 % [17].
Multiparous women with long IPIs are frequently associated with advanced maternal age (≥35 years old), which is another risk of adverse outcomes. In our previous study, negative additive interactions (all RERIs<0) were observed between long IPI and advanced maternal age on neonatal outcomes, namely long IPI was an independent risk factor for adverse neonatal outcomes, but advanced age did not strengthen this effect [18]. To explore the association between maternal age and previous CS, the modification of maternal age was also tested by the stratified analysis (<35 years/≥35 years) and the additive interaction analysis.
To test the robustness of the results, the sensitivity analysis was performed in a 1:1 matched population using propensity score matching (PSM) between the previous vaginal delivery group and previous CS group. Covariates for matching included maternal age, gravidity, parity, IPI, BMI, and abortion history, with a matching tolerance of 0.2. Adjusted ORs for adverse outcomes were calculated after adjusting for model-specific covariates.
Statistical analyses were conducted using R software 4.2.3. All p-values were two-tailed, and a p-value <0.05 was considered significant.
Results
Maternal and neonatal characteristics
Table 1 presents the differences in maternal and neonatal characteristics between the two groups. Significant differences were observed in gravidity, parity, BMI, IPI, abortion history, mode of the current delivery, gestational diabetes mellitus, hypertensive disorders in pregnancy, and birth weight (all p<0.05).
Characteristics of the previous vaginal delivery group and the previous cesarean section delivery group.
| Characteristics N, % |
All study subjects n=7,367 |
Previous vaginal delivery n=2,656 (36.05) | Previous cesarean section delivery n=4,711 (63.95) | p-Value |
|---|---|---|---|---|
| Maternal age, year Mean±SD [median] |
31.62 ± 5.14 [31] | 31.8 ± 5.55 [31] | 31.5 ± 4.95 [31] | 0.06 |
| <30 | 2,597 (35.25) | 934 (35.17) | 1,663 (35.30) | 0.43 |
| 30–35 | 2,663 (36.15) | 940 (35.39) | 1,723 (36.57) | |
| >35 | 2,107 (28.60) | 782 (29.44) | 1,325 (28.13) | |
| Gravidity | < 0.00 | |||
| 2 | 1,824 (24.76) | 820 (30.87) | 1,004 (21.31) | |
| 3 | 2,094 (28.42) | 736 (27.71) | 1,358 (28.83) | |
| >3 | 3,449 (46.82) | 1,100 (41.42) | 2,349 (49.86) | |
| Parity | < 0.00 | |||
| 2 | 5,808 (78.84) | 2,296 (86.45) | 3,512 (74.55) | |
| >2 | 1,559 (21.16) | 360 (13.55) | 1,199 (25.45) | |
| Inter-pregnancy interval, month Mean ± SD [median] |
68.85 ± 50.72 [62] | 73.70 ± 60.60 [51] | 66.10 ± 44.00 [62] | <0.00 |
| <24 | 1,214 (16.48) | 540 (20.33) | 674 (14.31) | < 0.00 |
| 24–60 | 2,451 (33.27) | 805 (30.31) | 1,646 (34.94) | |
| >60 | 3,702 (50.25) | 1,311 (49.36) | 2,391 (50.75) | |
| Body mass index Mean ± SD [median] |
27.10 ± 3.66 [26.70] | 26.60 ± 3.47 [26.30] | 27.40 ± 3.73 [27.10] | < 0.00 |
| <25 | 1,841 (24.80, 29.10) | 760 (24.30, 28.50) | 1,081 (25.00, 29.50) | < 0.00 |
| ≥25 | 4,935 (66.99) | 1,659 (62.46) | 3,276 (69.54) | |
| Missing | 591 (8.02) | 237 (8.93) | 354 (7.51) | |
| Abortion history | < 0.00 | |||
| Yes | 5,098 (69.20) | 1,737 (65.40) | 3,361 (71.34) | |
| No | 2,269 (30.80) | 919 (34.60) | 1,350 (28.66) | |
| Mode of the current delivery | < 0.00 | |||
| Vaginal | 2,403 (32.62) | 1,984 (74.70) | 419 (8.89) | |
| Cesarean section | 4,964 (67.38) | 672 (25.30) | 4,292 (91.11) | |
| Gestational diabetes mellitus | 878 (11.91) | 289 (10.88) | 589 (12.50) | < 0.00 |
| Hypertensive disorders in pregnancy | 547 (7.42) | 218 (8.22) | 329 (6.98) | < 0.00 |
| Birth weight, gram Mean ± SD [median] |
3,113.81 ± 629.22 [3,200.00] | 3,069.00 ± 681.00 [3,220.00] | 3,139.00±597.00 [3,220.00] | <0.00 |
| Gestational age, week Mean ± SD [median] |
37.78 ± 2.35 [38] | 37.80 ± 2.71 [39] | 37.8 ± 2.12 [38] | 0.40 |
Previous CS and the risks of adverse maternal and neonatal perinatal outcomes
Compared with the previous vaginal delivery group, previous CS was associated with higher risks of uterine-related complications (aOR=1.57, 95 % CI 1.25–1.98), but with lower risks of preterm birth (aOR=0.73, 95 % CI 0.63–0.85), and severe neonatal adverse outcomes (aOR=0.59, 95 % CI 0.46–0.78) (Figure 2). No significant associations were found between previous CS and placental-related complications, PROM, and SGA.
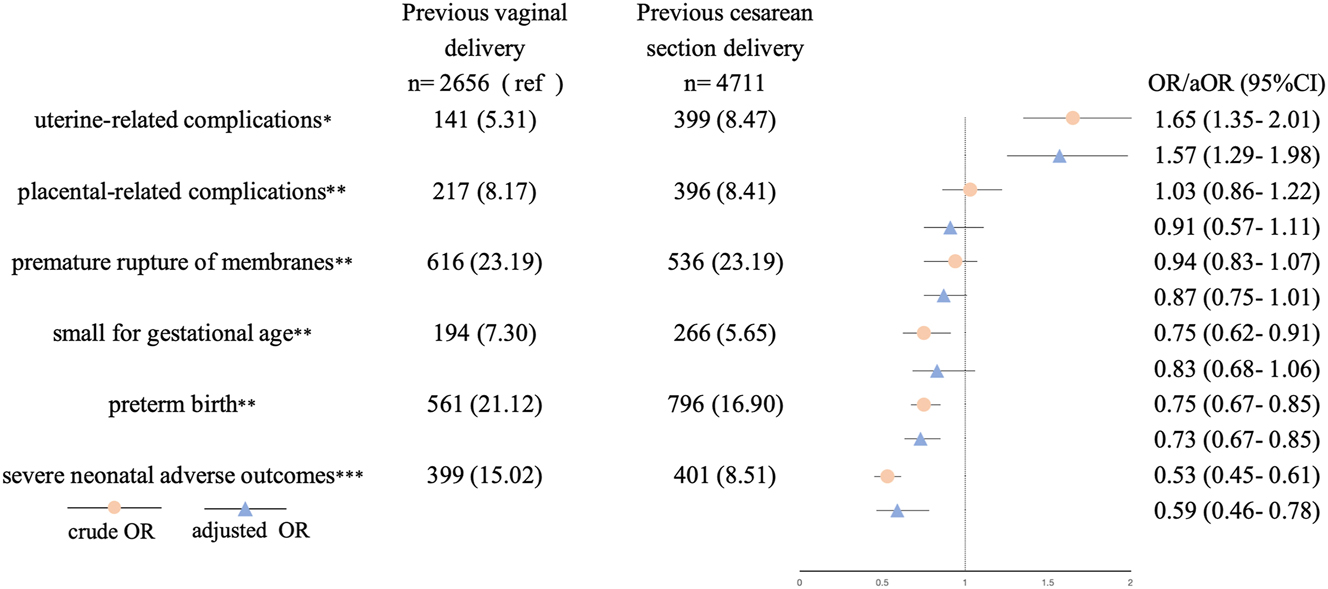
Logistic regression analysis for adverse maternal and neonatal outcomes associated with previous cesarean section. Potential covariates included maternal age of the current delivery, gravidity (2, 3, >3), parity (2, >2), body mass index, abortion history (yes/no) inter-pregnancy interval, and plus: aMode of the current delivery (vaginal/cesarean), gestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). bGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). cGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no), mode of the current delivery (vaginal/cesarean), gestational age, birth weight.
Interactions between previous CS and IPIs
In our study, the number of multiparous women with a long IPI was 3,702 (50.25 %). Stratified analyses by IPIs (<60, ≥60 months) showed consistent trends between previous CS and risks of uterine-related complications, preterm birth, and severe neonatal adverse outcomes across both subgroups (Figure 3, Supplementary Table S1). Previous CS had similar trends in higher risks of uterine-related complications (aOR=1.62, 95 % CI 1.03–2.55, aOR=1.94, 95 % CI 1.30–2.90, respectively) and in lower risks of preterm birth (aOR=0.78, 95 % CI 0.62–0.97, aOR=0.65, 95 % CI 0.54–0.79, respectively) and severe neonatal adverse outcomes (aOR=0.60, 95 % CI 0.42–0.94, aOR=0.54, 95 % CI 0.38–0.73, respectively) in subgroups with IPIs<60 and ≥ 60 months.
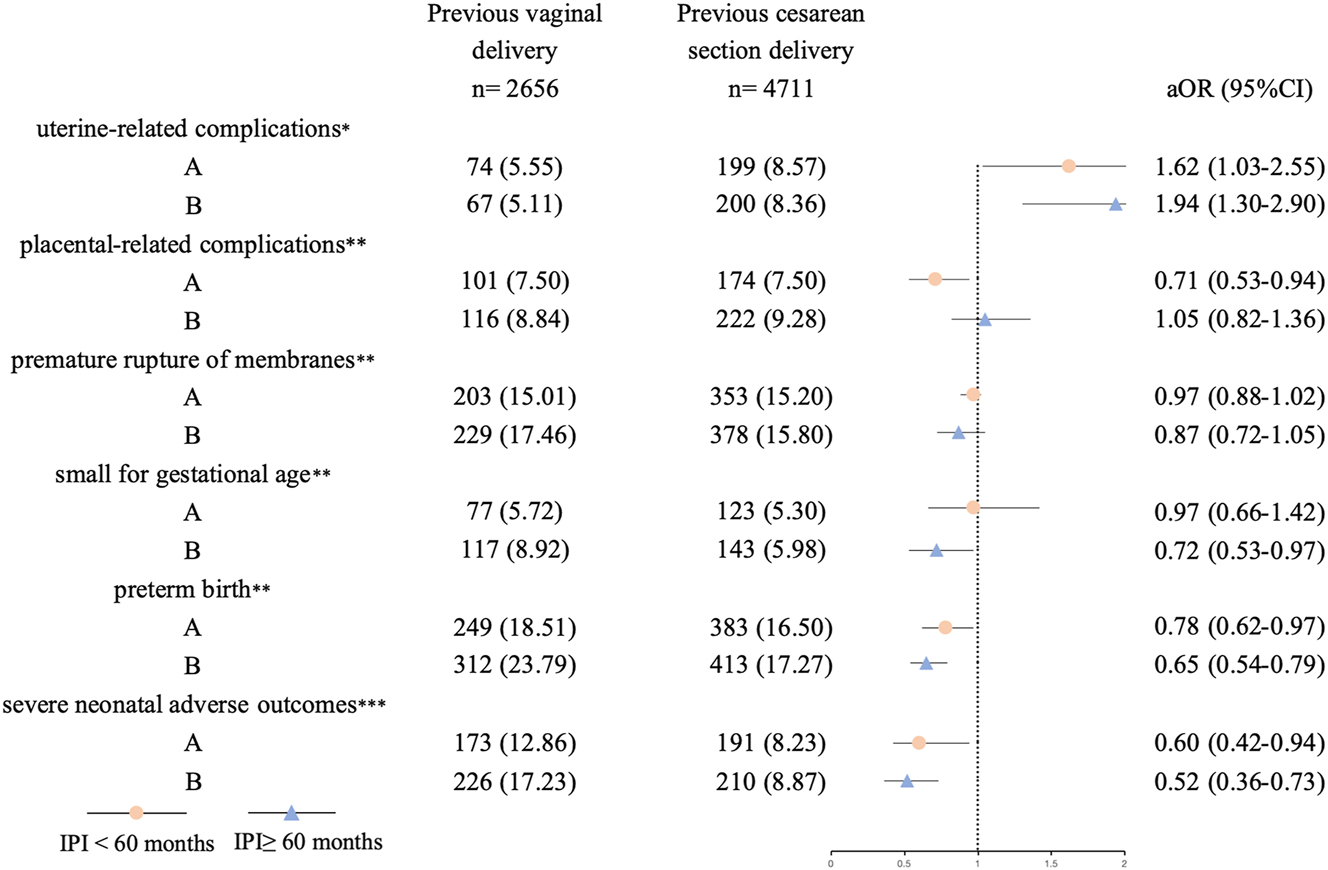
Stratified analysis to analyze for the associations between previous cesarean section and adverse maternal and neonatal outcomes in subgroups with different inter-pregnancy intervals (IPIs). Potential covariates included maternal age of the current delivery, gravidity (2, 3, >3), parity (2, >2), body mass index, abortion history (yes/no) and plus: aMode of the current delivery (vaginal/cesarean), gestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). bGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). cGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no), mode of the current delivery (vaginal/cesarean), gestational age, birth weight.
A synergistic biological interaction was observed between previous CS and a long IPI for placental-related complications (RERI=0.32, 95 % CI 0.05–0.58; AP=0.39, 95% CI 0.03–0.76). Conversely, an antagonistic interaction was found for preterm birth (RERI=−0.35, 95% CI -0.68 to −0.01; AP =-0.09, 95 % CI -0.68–0.03). No significant interactions were detected for other outcomes (Table 2).
Interactions between the long inter-pregnancy interval (≥60 months) and previous cesarean section delivery.
| Outcomes | RARI (95 % CI) | AP (95 % CI) |
|---|---|---|
| Uterine-related complicationsa | 0.01 (-0.49, 0.50) | 0.32 (-0.31, 2.47) |
| Placental-related complicationsb | 0.32 (0.05, 0.58) | 0.39 (0.03, 0.76) |
| Premature rupture of membranesb | −0.03 (−0.31, 0.25) | −1.74 (−0.32, 0.25) |
| Small for gestational ageb | −0.56 (−1.24, 0.11) | −0.46 (−0.92, 0.03) |
| Preterm birthb | −0.35 (−0.68, −0.01) | −0.09 (-0.68, −0.03) |
| Severe neonatal adverse outcomesc | −0.43 (−0.97, 0.09) | −0.42 (−0.91, 0.06) |
-
RARI, relative excess risk caused by interaction; AP, attributable proportion. The reference group is the group with both IPI<60 months and previous vaginal delivery. Potential covariates included maternal age of the current delivery, gravidity (2, 3, >3), parity (2, >2), body mass index, abortion history (yes/no) and plus: aMode of the current delivery (vaginal/cesarean), gestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). bGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no). cGestational diabetes mellitus (yes/no), hypertensive disorders in pregnancy (yes/no), mode of the current delivery (vaginal/cesarean), gestational age, birth weight.
The PARPs for previous CS were 27.57 % for uterine-related complications and 1.80 % for placental-related complications (Table S2).
Interactions between previous CS and maternal age
Similar trends of risks associated with previous CS were observed in preterm birth, uterine-related complications, and severe neonatal adverse outcomes in the analysis stratified by maternal age (Table S3). No interaction effect was observed between advanced maternal age and previous CS (Table S4).
Similarly, in the PSM model, significant associations were also observed between previous CS and the risks of uterine-related complications (aOR=1.60, 95 % CI 1.28–2.01), preterm birth (aOR=0.84, 95 % CI 0.72–0.97), and severe neonatal adverse outcomes (aOR=0.41, 95 % CI 0.32–0.53) (Table S5).
Discussion
In the present study, the findings suggest that CS is associated with a higher risk of adverse maternal outcomes in the Chinese population. However, a long IPI does not attenuate the harm of previous CS to mothers. Although previous CS appears to be associated with a lower risk of adverse neonatal outcomes, a negative impact of a long IPI is observed.
Previous CS was found to confer a higher risk of uterine complications [1], 19], 20]. Consistent with previous studies, our findings also showed that previous CS was associated with increased risks of uterine-related complications. Indeed, uterine rupture rates have been reported to be nearly 40-50-fold higher among mothers with CS history than ones without CS history [21]. This indicated that previous CS was an independent risk factor for uterine-related complications. Notably, our study estimated that 27.57 % of uterine-related complications could be prevented by avoiding previous CS in the general population.
Nonetheless, concerning IPIs after CS, the conclusions of studies are not consistent and conclusive. Sarah Cunningham et al. found that normal IPI (24–59 months) was associated with the lowest frequency of uterine rupture, while a long IPI (≥60 months) was not significantly linked to uterine rupture risk in mothers with previous CS [22]. Another study even found that a long IPI (≥60 months) was associated with a decreased risk of uterine rupture (aOR=0.83, 95 % CI 0.69–1.00) [23]. In contrast, the present study found that the prolonged IPI after previous CS did not reduce the risks of uterine-related complications. Unlike prior researches [23], we observed no antagonistic biological interaction between a long IPI and previous CS, suggesting that the adverse effects of previous CS on uterine-related complications might persist irrespective of IPI duration. Previous literature reports showed that previous CS was associated with the risk of placental-related complications, including placenta previa and placental abruption [24], 25]. Long IPI might indirectly increase placental abruption risk by elevating the likelihood of eclampsia and preeclampsia [26]. Notably, although our study didn’t detect a significant association between previous CS and placental-related complications (possibly due to sample size limitations), a positive interaction was observed between previous CS and a long IPI for these outcomes. This suggested that the combination of a long IPI and previous CS might have a synergistic effect on increasing placental complication risks.
Previous studies have indicated that certain obstetrical complications or serious maternal conditions determine the need for CS [18]. Jane Sandall et al. reported that exposure to a previous medically indicated CS delivery, including preeclampsia or maternal hyperglycemia, increased the risk of severe acute maternal morbidity in the current pregnancy, such as hemorrhage, hysterectomy, or uterine rupture [1], 27], 28]. Similarly, our findings also showed an increased risk of uterine-related complications in previous CS population with a high rate in non-medical indicated CS. These findings indicated that uterine-related complications are more associated with previous CS itself rather than with prior medical conditions. Moreover, compared with the reference group, the previous CS groups of different maternal ages and IPIs all showed higher trends of uterine-related complications. This highlighted the importance of reducing non-essential CS rates to prevent maternal complications in subsequent pregnancies.
Most prior studies reported that previous CS was associated with higher or neutral neonatal complication risks [7], 29]. In contrast, our findings indicated that previous CS appeared to be associated with lower risks of preterm birth, and severe neonatal adverse outcomes. According to previous studies, women’s socioeconomic status and the availability of healthcare services were associated with increased CS use [30]. In China, women who preferred CS perceived it to be safer for both the baby and themselves and free from pain and anxiety, despite the cost of CS being twice as high as vaginal delivery [31]. Additionally, CS delivery entailed longer maternal hospital stays and increased drug usage compared to vaginal delivery [32], 33]. Therefore, women with better financial conditions were more willing or able to afford the expense of non-medically indicated CS. After the implementation of the universal two-child policy in 2015, women with a history of non-indicated CS who wanted to give birth again usually had better social welfare benefits, health insurance, and maternity care. They had better nutrition, paid more attention to, and received more obstetric care during pregnancy to monitor fetal growth. Thus, pregnancies with previous CS seemingly appear to be particularly associated with lower risks of adverse neonatal outcomes. Similar findings have been reported in another study in the Chinese population [34]. However, it should be noted that these benefits may not be directly attributed to previous CS but rather to the effects of improved antenatal care. Therefore, better antenatal care can partly prevent neonatal complications associated with previous CS. However, our study’s reliance on data from one southwest China city might introduce bias to the results. Socioeconomic levels, healthcare access, maternal cares across China were different. So, future multi-center studies with geographically diverse data were needed to verify these associations in wider populations.
Along IPI was associated with elevated odds of preterm birth and SGA in a previous study [35]. However, the present study demonstrated that previous CS was associated with decreased risks of preterm birth and severe neonatal adverse outcomes and antagonistic effects were observed between previous CS and a long IPI for preterm birth. It should be awareness that although previous CS was associated with lower risks of adverse neonatal outcomes, prolonged IPIs might negatively modify this association. We considered that in essence, the long IPI might offset the benefits of improved antenatal care behind CS.
Several limitations existed in the present study. First, the hospital-based data were collected from a single city in southwest China, which might not represent the national general population. Second, we lacked accurate data on maternal socioeconomic status and prenatal healthcare to support our assumptions regarding the reduced risks of neonatal complications. Finally, the observational study design only allowed for association analysis rather than causal inference.
Conclusions
In a population with a high rate of previous CS, previous CS was associated with increased risks of adverse maternal outcomes but decreased risks of certain adverse neonatal outcomes. Prolonged IPIs might not diminish the adverse effects of previous CS in mothers, but might adversely harm the neonates.
Funding source: The Science and Technology Strategic Cooperation Programs of Luzhou Municipal People's Government
Award Identifier / Grant number: No. 2021LZXNYD-J21
-
Research ethics: The whole project has been examined and approved by Southwest Medical University (No. KY2021264).
-
Informed consent: Not applicable. As the data were collected in anonymization to keep the privacy of patients, informed consent was not required from patients.
-
Author contributions: X.L. conceived the study and critically revised the manuscript. Y.X. and Q.Z. collected, analyzed the data, and wrote the first draft of the manuscript. Y.L. collected the data. W.D. and Y.Z.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was partly supported by the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government, grant No. 2021LZXNYD-J21 to Xiaoping Lei. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Data availability: Not applicable.
References
1. Sandall, J, Tribe, RM, Avery, L, Mola, G, Visser, GH, Homer, CS, et al.. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392:1349–57. https://doi.org/10.1016/s0140-6736(18)31930-5.Search in Google Scholar
2. Hellerstein, S, Feldman, S, Duan, T. China’s 50% caesarean delivery rate: is it too high? BJOG 2015;122:160–4. https://doi.org/10.1111/1471-0528.12971.Search in Google Scholar PubMed
3. Song, C, Xu, Y, Ding, Y, Zhang, Y, Liu, N, Li, L, et al.. The rates and medical necessity of cesarean delivery in China, 2012-2019: an inspiration from Jiangsu. BMC Med 2021;19:14. https://doi.org/10.1186/s12916-020-01890-6.Search in Google Scholar PubMed PubMed Central
4. Ming, Y, Li, M, Dai, F, Huang, R, Zhang, J, Zhang, L, et al.. Dissecting the current caesarean section rate in Shanghai, China. Sci Rep 2019;9:2080. https://doi.org/10.1038/s41598-019-38606-7.Search in Google Scholar PubMed PubMed Central
5. Assefa, NE, Berhe, H, Girma, F, Berhe, K, Berhe, YZ, Gebreheat, G, et al.. Risk factors of premature rupture of membranes in public hospitals at Mekele city, Tigray, a case control study. BMC Pregnancy Childbirth 2018;18:386. https://doi.org/10.1186/s12884-018-2016-6.Search in Google Scholar PubMed PubMed Central
6. Calì, G, Timor-Tritsch, IE, Palacios-Jaraquemada, J, Monteaugudo, A, Buca, D, Forlani, F, et al.. Outcome of cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:169–75. https://doi.org/10.1002/uog.17568.Search in Google Scholar PubMed
7. Kietpeerakool, C, Lumbiganon, P, Laopaiboon, M, Rattanakanokchai, S, Vogel, JP, Gülmezoglu, AM. Pregnancy outcomes of women with previous caesarean sections: secondary analysis of world health organization multicountry survey on maternal and newborn health. Sci Rep 2019;9:9748. https://doi.org/10.1038/s41598-019-46153-4.Search in Google Scholar PubMed PubMed Central
8. Liu, Y, Qin, Q, Xiao, Y, Li, H, Guang, S, Tao, S, et al.. Changes of second-time mothers and their infants under the universal two-child policy in Changsha, China. Midwifery 2019;77:32–6. https://doi.org/10.1016/j.midw.2019.06.005.Search in Google Scholar PubMed
9. Nakamura, Y, Tsuda, H, Masahashi, Y, Nakamura, T, Suzuki, M, Fukuhara, N, et al.. Impact of the inter-pregnancy interval after cesarean delivery on subsequent perinatal risks: a retrospective study. Arch Gynecol Obstet 2023;308:479–85. https://doi.org/10.1007/s00404-022-06651-9.Search in Google Scholar PubMed
10. Liang, Y, Zhang, L, Huang, L, Li, Y, Chen, J, Bi, S, et al.. Association between short inter-pregnancy interval and placenta previa and Placenta accreta spectrum with respect to maternal age at first cesarean delivery. J Matern Fetal Neonatal Med 2023;36:2192853. https://doi.org/10.1080/14767058.2023.2192853.Search in Google Scholar PubMed
11. Murtaza, K, Saleem, Z, Jabeen, S, Alzahrani, AK, Kizilbash, N, Soofi, SB, et al.. Impact of inter-pregnancy intervals on perinatal and neonatal outcomes in a multiethnic Pakistani population. J Trop Pediatr 2022;68:fmac088. https://doi.org/10.1093/tropej/fmac088.Search in Google Scholar PubMed
12. Jena, BH, Biks, GA, Gete, YK, Gelaye, KA. Association of primary postpartum hemorrhage with inter-pregnancy interval in urban South Ethiopia: a matched nested case-control study. PLoS One 2022;17:e0271216. https://doi.org/10.1371/journal.pone.0271216.Search in Google Scholar PubMed PubMed Central
13. Dong, H, Chi, J, Wang, W, Liu, L. Association between inter-pregnancy interval and maternal and neonatal adverse outcomes in women with a cesarean delivery: a population-based study. BMC Pregnancy Childbirth 2023;23:284. https://doi.org/10.1186/s12884-023-05600-x.Search in Google Scholar PubMed PubMed Central
14. Loomba, R, Liu, J, Yang, HI, Lee, MH, Lu, SN, Wang, LY, et al.. Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013;11:1636–45. https://doi.org/10.1016/j.cgh.2013.04.043. e1-3.Search in Google Scholar PubMed PubMed Central
15. Tao, H, Chen, G, Wu, L, Lou, H. Synergistic impact of air pollution and artificial light at night on memory disorders: a nationwide cohort analysis. BMC Public Health 2025;25:1591. https://doi.org/10.1186/s12889-025-22863-5.Search in Google Scholar PubMed PubMed Central
16. Yang, X, So, WY, Ma, RC, Kong, AP, Lee, HM, Yu, LW, et al.. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Care 2011;34:375–80. https://doi.org/10.2337/dc10-1509.Search in Google Scholar PubMed PubMed Central
17. Hodson, DZ, Mbarga Etoundi, Y, Mbatou Nghokeng, N, Mohamadou Poulibe, R, Magne Djoko, S, Goodwin, J, et al.. Clinical characteristics of Plasmodium falciparum infection among symptomatic patients presenting to a major urban military hospital in Cameroon. Malar J 2022;21:298. https://doi.org/10.1186/s12936-022-04315-2.Search in Google Scholar PubMed PubMed Central
18. Ma, Y, Fu, H, Li, Y, Bao, ZR, Dong, WB, Lei, XP. Interactions between long inter-pregnancy interval and advanced maternal age on neonatal outcomes. World J Pediatr 2023;19:1155–61. https://doi.org/10.1007/s12519-023-00728-4.Search in Google Scholar PubMed PubMed Central
19. Boutsikou, T, Malamitsi-Puchner, A. Caesarean section: impact on mother and child. Acta Paediatr 2011;100:1518–22. https://doi.org/10.1111/j.1651-2227.2011.02477.x.Search in Google Scholar PubMed
20. Smith, GC, White, IR, Pell, JP, Dobbie, R. Predicting cesarean section and uterine rupture among women attempting vaginal birth after prior cesarean section. PLoS Med 2005;2:e252. https://doi.org/10.1371/journal.pmed.0020252.Search in Google Scholar PubMed PubMed Central
21. Matorras, R, Berreteaga, L, Laínz, L, Exposito, A, Martínez, L. Influence of caesarean section-pregnancy interval on uterine rupture risk and IVF pregnancy rates: systematic review and mathematical modelling. Reprod Biomed Online 2019;39:809–18. https://doi.org/10.1016/j.rbmo.2019.07.027.Search in Google Scholar PubMed
22. Cunningham, S, Algeo, CE, DeFranco, EA. Influence of inter-pregnancy interval on uterine rupture. J Matern Fetal Neonatal Med 2021;34:2848–53. https://doi.org/10.1080/14767058.2019.1671343.Search in Google Scholar PubMed
23. De Silva, DA, Thoma, ME. The association between inter-pregnancy interval and severe maternal morbidities using revised national birth certificate data: a probabilistic bias analysis. Paediatr Perinat Epidemiol 2020;34:469–80. https://doi.org/10.1111/ppe.12560.Search in Google Scholar PubMed
24. M, M, A, V, Gn, G, E, N, Ae, P, I, M. Association of placenta previa with a history of previous Cesarian deliveries and indications for a possible role of a genetic component. Balkan J Med Genet 2017;20:5–10. https://doi.org/10.1515/bjmg-2017-0022.Search in Google Scholar PubMed PubMed Central
25. Goldbart, A, Pariente, G, Sheiner, E, Wainstock, T. Identifying risk factors for placental abruption in subsequent pregnancy without a history of placental abruption. Int J Gynaecol Obstet 2023;161:406–11. https://doi.org/10.1002/ijgo.14446.Search in Google Scholar PubMed
26. Conde-Agudelo, A, Belizán, JM. Maternal morbidity and mortality associated with inter-pregnancy interval: cross sectional study. BMJ 2000;321:1255–9. https://doi.org/10.1136/bmj.321.7271.1255.Search in Google Scholar PubMed PubMed Central
27. Gao, Y, Xue, Q, Chen, G, Stone, P, Zhao, M, Chen, Q. An analysis of the indications for cesarean section in a teaching hospital in China. Eur J Obstet Gynecol Reprod Biol 2013;170:414–8. https://doi.org/10.1016/j.ejogrb.2013.08.009.Search in Google Scholar PubMed
28. Ye, W, Luo, C, Huang, J, Li, C, Liu, Z, Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2022;377:e067946. https://doi.org/10.1136/bmj-2021-067946.Search in Google Scholar PubMed PubMed Central
29. Ashwal, E, Lavie, A, Blecher, Y, Attali, E, Aviram, A, Hadar, E, et al.. Intrapartum cesarean delivery and the risk of perinatal complications in women with and without a single prior cesarean delivery. Int J Gynaecol Obstet 2022;157:359–65. https://doi.org/10.1002/ijgo.13798.Search in Google Scholar PubMed
30. Wyatt, S, Silitonga, PII, Febriani, E, Long, Q. Socioeconomic, geographic and health system factors associated with rising C-section rate in Indonesia: a cross-sectional study using the Indonesian demographic and health surveys from 1998 to 2017. BMJ Open 2021;11:e045592. https://doi.org/10.1136/bmjopen-2020-045592.Search in Google Scholar PubMed PubMed Central
31. Betrán, AP, Temmerman, M, Kingdon, C, Mohiddin, A, Opiyo, N, Torloni, MR, et al.. Interventions to reduce unnecessary caesarean sections in healthy women and babies. Lancet 2018;392:1358–68. https://doi.org/10.1016/s0140-6736(18)31927-5.Search in Google Scholar
32. Liu, M, Xue, M, Yang, Q, Du, W, Yan, X, Tan, J, et al.. Association between migration status and caesarean section delivery based on a modified robson classification in China. BMC Pregnancy Childbirth 2021;21:215. https://doi.org/10.1186/s12884-021-03708-6.Search in Google Scholar PubMed PubMed Central
33. Jenabi, E, Khazaei, S, Bashirian, S, Aghababaei, S, Matinnia, N. Reasons for elective cesarean section on maternal request: a systematic review. J Matern Fetal Neonatal Med 2020;33:3867–72. https://doi.org/10.1080/14767058.2019.1587407.Search in Google Scholar PubMed
34. Liu, X, Landon, MB, Cheng, W, Chen, Y. Cesarean delivery on maternal request in China: what are the risks and benefits? Am J Obstet Gynecol 2015;212:817.e1-9. https://doi.org/10.1016/j.ajog.2015.01.043.Search in Google Scholar PubMed
35. Tessema, GA, Marinovich, ML, Håberg, SE, Gissler, M, Mayo, JA, Nassar, N, et al.. Inter-pregnancy intervals and adverse birth outcomes in high-income countries: an international cohort study. PLoS One 2021;16:e0255000. https://doi.org/10.1371/journal.pone.0255000.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0280).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


