The intrauterine microbiome–neurodevelopment axis: decoding the prenatal microbial imprint on lifelong mental health
-
Wiku Andonotopo
, Muhammad Adrianes Bachnas
, Julian Dewantiningrum
, Sri Sulistyowati
Abstract
Introduction
The traditional view of a sterile intrauterine environment has been challenged by sequencing studies detecting low-biomass microbial DNA in placenta, amniotic fluid, and fetal tissues. These findings suggest that maternal microbiota-derived signals may contribute to fetal brain development and influence long-term neuropsychiatric outcomes.
Content
This narrative review synthesizes evidence from over 90 preclinical and clinical studies examining maternal microbiota–fetal brain interactions. Maternal immune activation – characterized by elevated cytokines such as interleukin (IL)-6 and IL-17A – has been shown in mouse models to disrupt cortical layering and synaptic organization, while human cohort studies involving more than 250,000 pregnancies link maternal inflammatory markers to increased autism spectrum disorder (ASD) risk. Microbial metabolites, including short-chain fatty acids (butyrate, acetate, propionate), bile acids, and tryptophan derivatives, regulate microglial maturation, blood–brain barrier integrity, and hippocampal neurogenesis. Epigenetic mechanisms – DNA methylation, histone acetylation, and chromatin remodeling – have been observed in placenta and cord blood from pregnancies affected by obesity or dysbiosis. Large-scale epidemiological studies also associate prenatal infection and antibiotic exposure with higher rates of ASD and attention-deficit/hyperactivity disorder (ADHD).
Summary
Collectively, the evidence indicates that maternal microbiota influence fetal brain development through converging immune, metabolic, epigenetic, and hormonal pathways. Strong mechanistic insights come from animal models, whereas human data remain largely observational, limiting causal interpretation.
Outlook
Recognizing the maternal microbiome as a modifiable prenatal factor highlights opportunities for prevention. Early translational approaches – including maternal microbiota profiling, dietary optimization, and probiotic supplementation – are under investigation, but require rigorous clinical validation before integration into prenatal care.
Introduction
The assumption that the intrauterine environment is sterile has been challenged by next-generation sequencing studies reporting microbial DNA in the placenta, amniotic fluid, and fetal tissues [1], [2], [3]. In parallel, pregnancy is increasingly recognized as a period of marked microbial and metabolic remodeling, with longitudinal evidence of gestational shifts in gut microbiota composition and metabolic outputs [4], 5]. These changes intersect with immune and endocrine signaling, shaping maternal-fetal crosstalk at the placenta and setting the stage for potential neurodevelopmental effects.
Mechanistic work demonstrates that maternal microbial status influences fetal brain development [6]. Maternal immune activation during gestation perturbs cortical development and yields autism-like phenotypes in offspring in experimental systems [7], 8], with broader syntheses implicating immune pathways across central nervous system disorders [9], 10]. Microbial reconstitution can reverse offspring social and synaptic deficits that arise under adverse maternal conditions [11]. Converging human data link prenatal microbial features to neonatal brain structure and connectivity [12], and experimental reversal of maternal inflammation further supports causal immune mechanisms [13], 14]. Observational cohorts report that elevated mid-gestational cytokines – particularly inflammatory mediators – associate with increased neurodevelopmental risk [15], 16].
Microbial metabolites provide a second layer of influence. Short-chain fatty acids (SCFAs) are necessary for microglial maturation and function and help maintain blood–brain barrier integrity and synaptic health [17], [18], [19]. Excess propionate has been implicated in oxidative stress and autism-like behaviors in preclinical models [18]. Beyond SCFAs, maternal diet-microbiota interactions shape placental and fetal epigenetic landscapes; prenatal dysbiosis is linked to altered DNA methylation and histone acetylation in the offspring hippocampus, consistent with emerging work on placental gene regulation in metabolic conditions [20], 21].
Human evidence, while largely correlational, points in the same direction. Prenatal microbiome composition has been associated with infant amygdala/insula connectivity and early behavioral outcomes [12], 22], 23]. Population-based analyses indicate that prenatal and early-life antibiotic exposure – an established disruptor of gut microbial ecology – correlates with elevated risk of autism spectrum disorder and attention-deficit/hyperactivity disorder [24], 25]. Sex-specific vulnerability is an important consideration; differences in immune and stress-response biology across fetal sex likely contribute to the higher prevalence of these conditions in males [26], 27]. Emerging clinical and translational work highlights how early-life microbial exposures shape immune maturation and allergic risk, reinforcing the developmental salience of perinatal microbial ecology [28]. At the same time, studies analyzing amniotic fluid with stringent controls report no detectable microbial DNA in healthy pregnancies, keeping the debate open around true intrauterine colonization vs. contamination or transient exposure [29]. Maternal metabolic and nutritional contexts further modulate placental efficiency, microbial metabolites, and fetal growth and neurodevelopment trajectories in preclinical and translational models [30], [31], [32]. Comprehensive overviews of the perinatal window and maternal-offspring immunology underscore these developmental opportunities and constraints [33], 34], and additional syntheses continue to refine how intrauterine microbial signals should be interpreted in low-biomass settings [35].
Taken together, evidence across microbiology, immunology, neuroscience, and perinatal psychiatry supports a working model in which maternal microbial ecosystems can shape fetal neurodevelopment via immune, metabolic, and epigenetic pathways. A PRISMA-style overview of study selection is provided in Figure 1. Mechanistic frameworks are illustrated in Figures 2–9, and key pathways, unanswered questions, metabolites, and translational opportunities are summarized in Tables 1–6. This synthesis positions the maternal microbiome as a modifiable determinant of fetal brain development and long-term mental health.
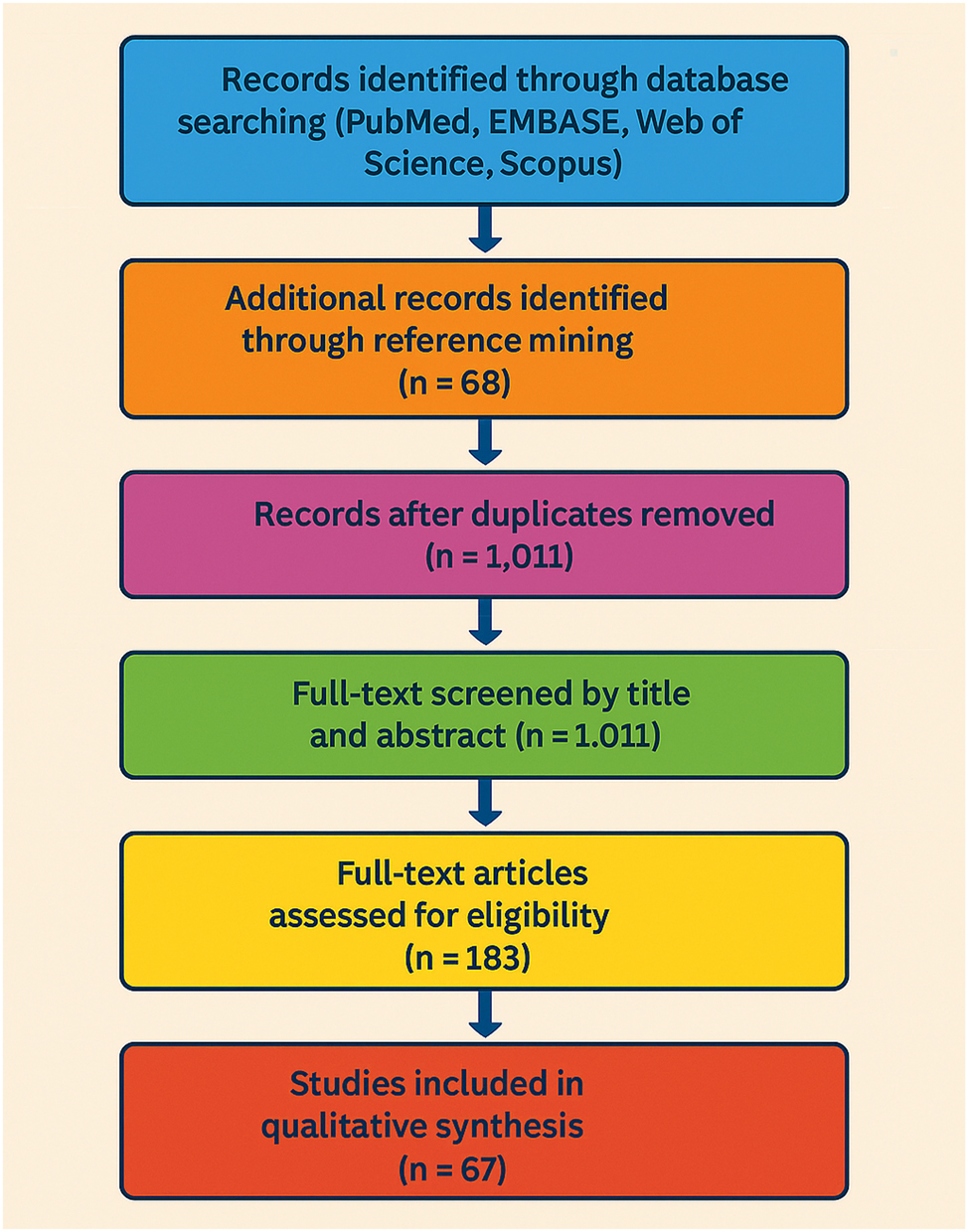
PRISMA flow diagram for study selection in systematic review. This PRISMA flow diagram illustrates the selection process for studies included in the qualitative synthesis. A total of 1,243 records were identified through comprehensive database searches (PubMed, EMBASE, web of science, and scopus), and an additional 68 records were found via reference mining. After removing duplicates, 1,011 records were screened by title and abstract. From these, 183 full-text articles were assessed for eligibility, resulting in 67 studies being included in the final qualitative synthesis.
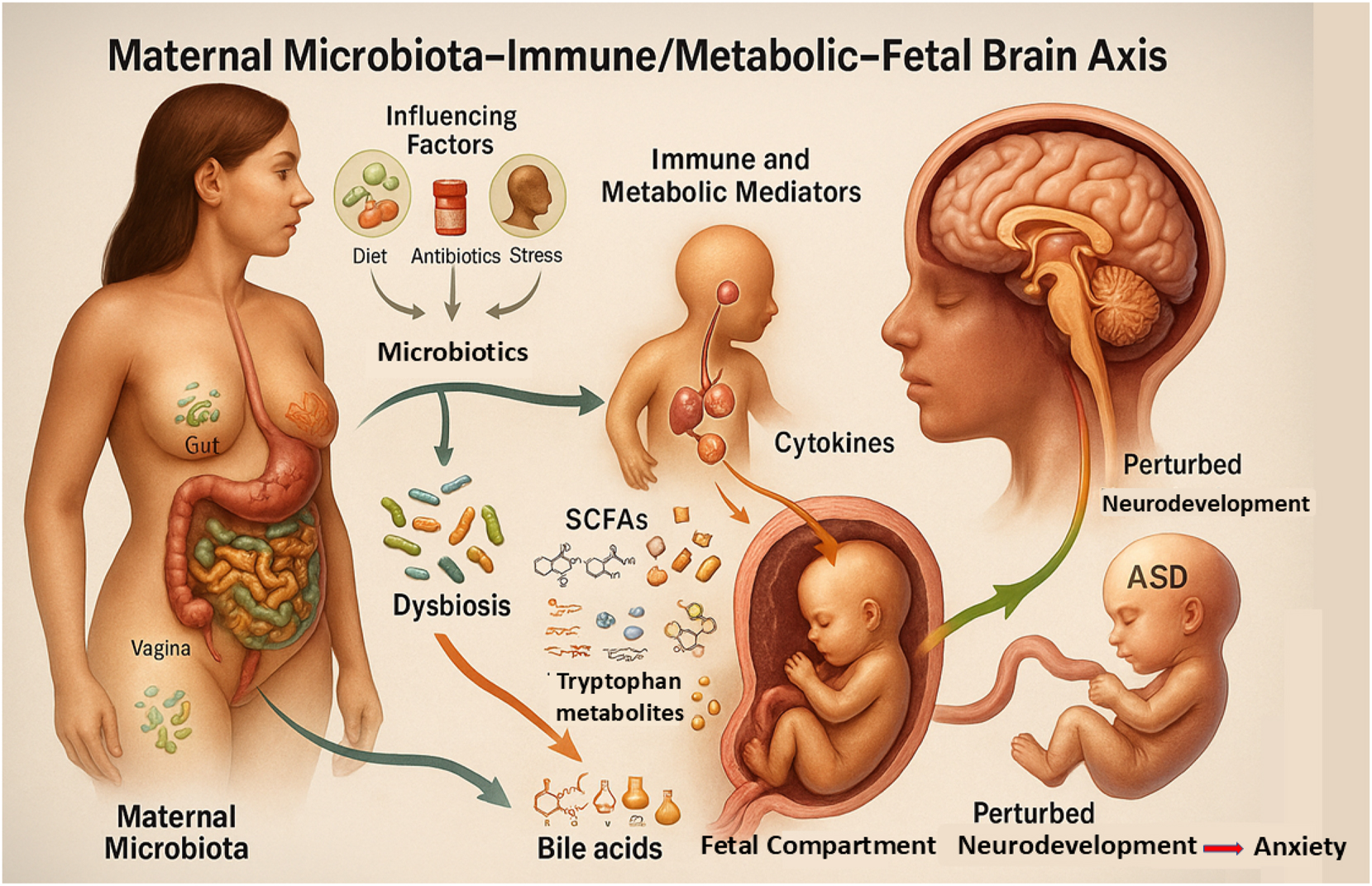
Maternal microbiota–immune/metabolic–fetal brain axis: A conceptual framework of multisystem interplay in early neurodevelopment. This figure depicts the interconnected maternal microbiota–immune/metabolic–fetal brain axis, where maternal microbial communities in the gut, vagina, and oral cavity – modulated by diet, antibiotics, and stress – can shift toward dysbiosis, triggering immune responses (e.g., cytokines) and producing metabolites (SCFAs, tryptophan derivatives, bile acids) that cross the placenta. These signals influence fetal neurodevelopmental processes such as synaptogenesis and microglial activation, with potential long-term outcomes including autism spectrum disorder (ASD), anxiety, and perturbed neurodevelopment.
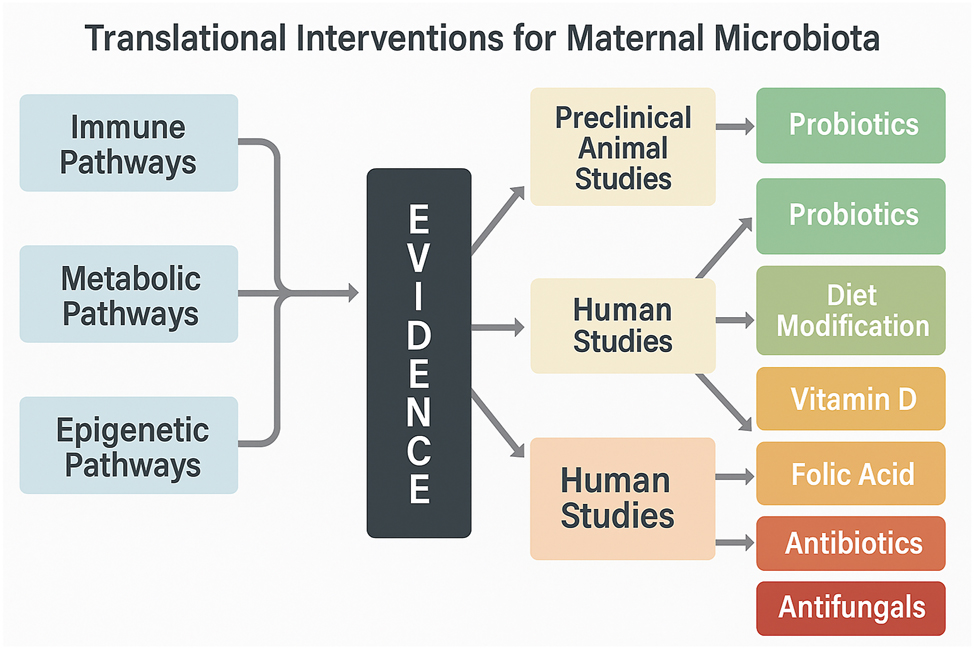
Translational pathways from maternal microbiota to neurodevelopment: Evidence-based intervention map. This flowchart illustrates key mechanistic pathways – such as IL-17A–mediated inflammation, SCFA deficiency, and epigenetic modulation – linked to fetal neurodevelopmental risk. It integrates preclinical and clinical evidence supporting these mechanisms and maps them to corresponding translational interventions, including probiotics, dietary strategies, and antibiotic stewardship. Pathways are visually linked to proposed interventions based on their translational readiness and population-specific targeting, offering a concise visual summary of actionable strategies emerging from microbiome–brain axis research.
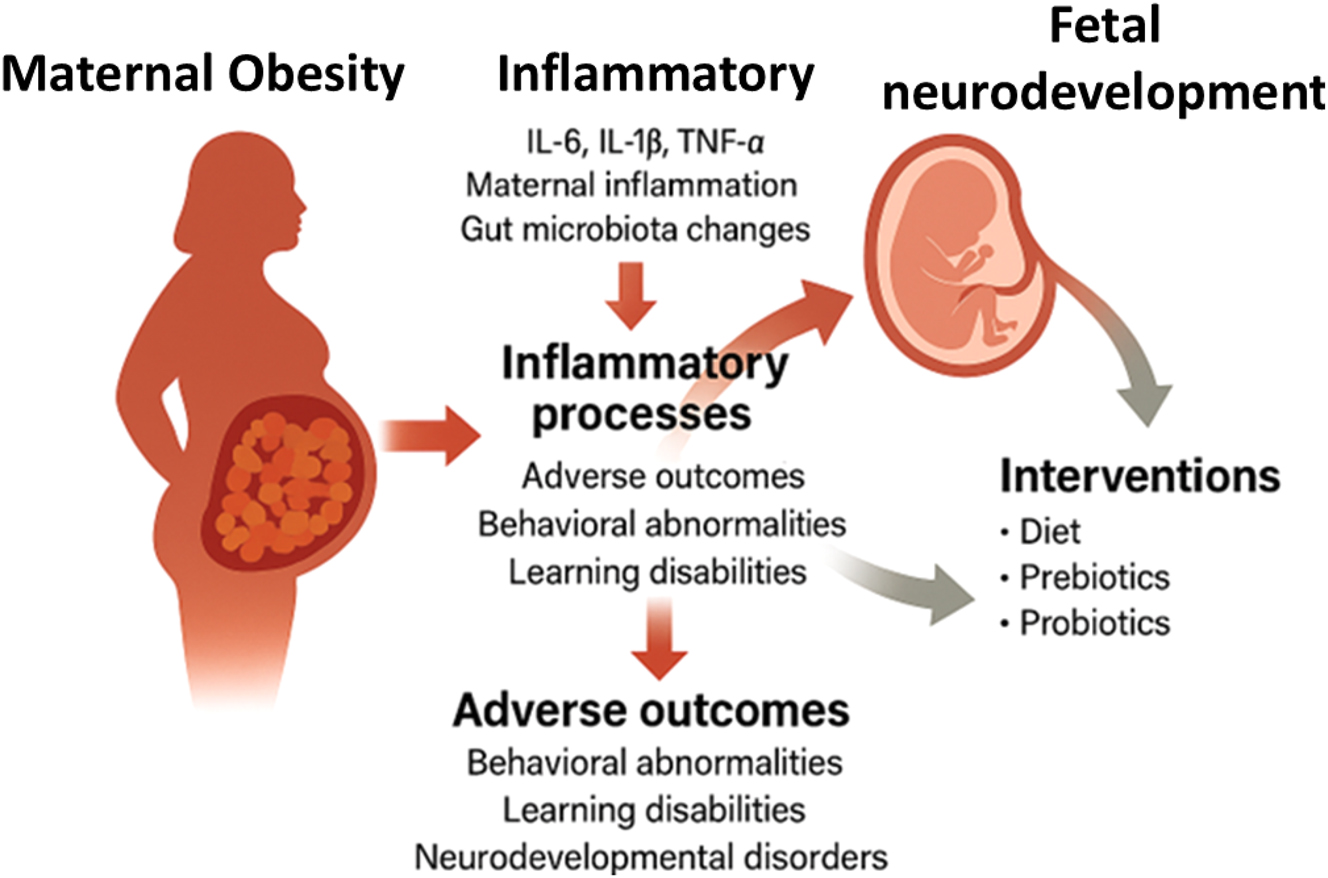
Maternal obesity–driven inflammatory cascade and its impact on fetal brain development. This diagram illustrates the biological cascade through which maternal obesity contributes to adverse fetal neurodevelopmental outcomes. Adipose tissue dysfunction leads to elevated levels of inflammatory mediators, adipokines, and metabolic stressors that impair placental function. These changes reduce placental transport of neuroprotective lipids (e.g., LCPUFAs) and increase fetal exposure to pro-inflammatory cytokines and oxidative stress, ultimately affecting neural differentiation and promoting neuroinflammation. The figure highlights key mechanistic links between maternal metabolic status and risk for neurodevelopmental disorders.
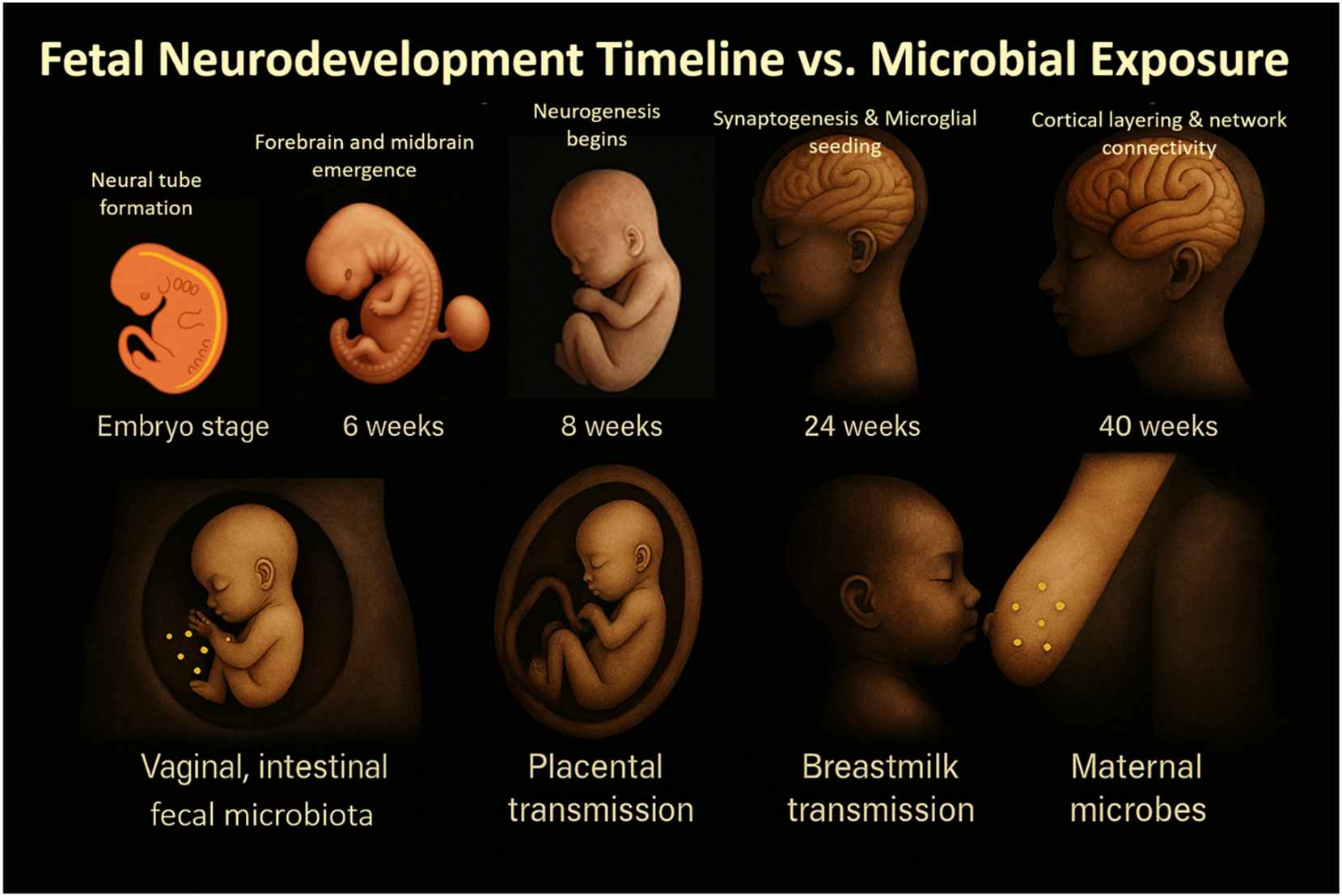
Fetal neurodevelopmental milestones aligned with critical microbial and immune exposure windows. This timeline integrates key gestational stages of fetal brain development – such as neurogenesis, synaptogenesis, and microglial maturation – with periods of microbial, metabolic, and immune signaling originating from the maternal microbiota. Early-to mid-gestation phases align with critical exposure to SCFAs, cytokines (e.g., IL-6, IL-17A), and microbial metabolites, which can shape neuroimmune trajectories. The figure emphasizes how timing of microbial exposure or dysbiosis may influence developmental programming and susceptibility to neurodevelopmental disorders.
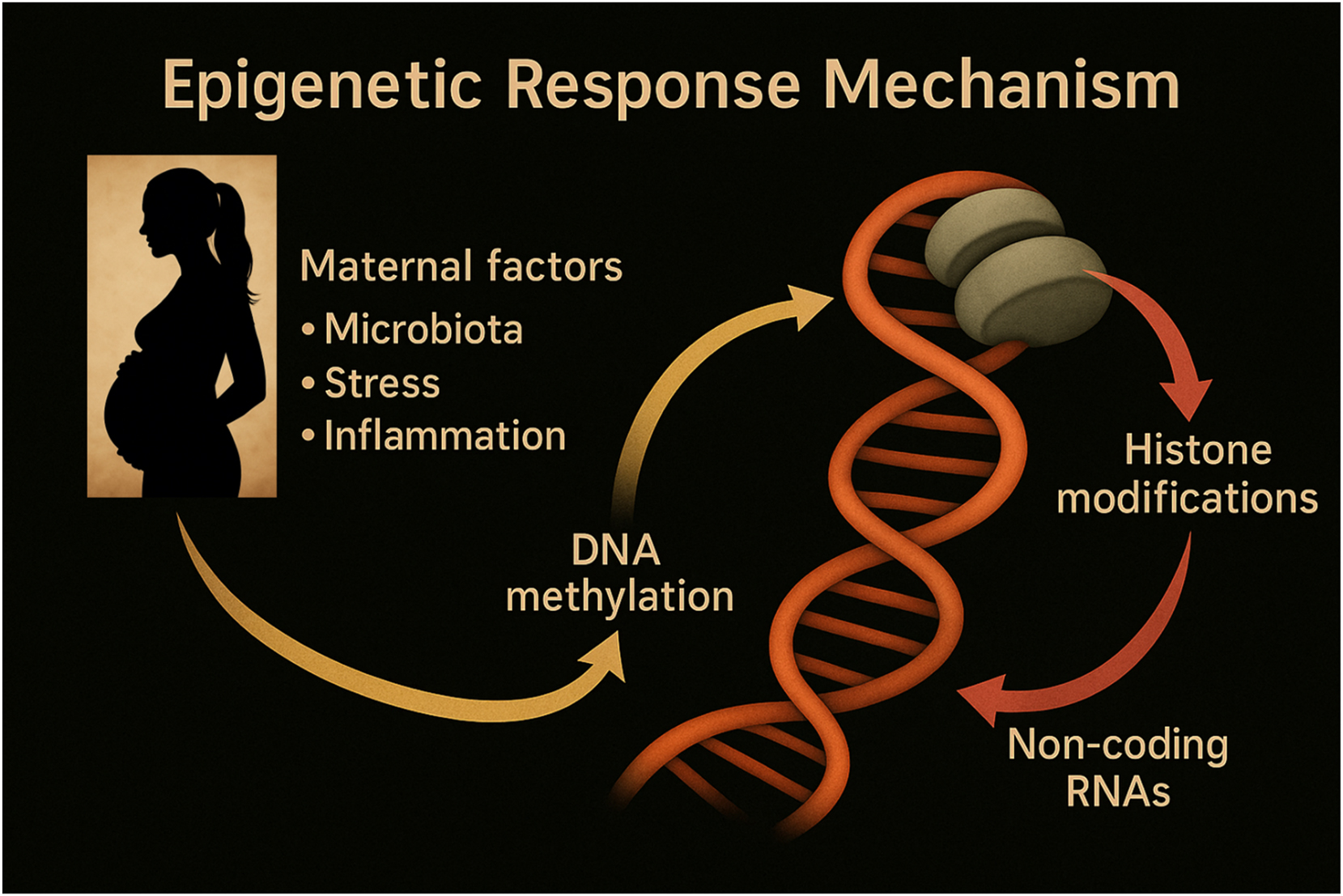
Epigenetic pathways linking maternal environmental signals to fetal neurodevelopment. This schematic highlights the central role of epigenetic mechanisms – DNA methylation, histone modifications, and non-coding RNAs – in mediating the effects of maternal factors on fetal neurodevelopment. Environmental signals such as microbial metabolites (e.g., SCFAs), inflammatory cytokines, and hormonal changes influence gene expression regulation during critical developmental windows. These modifications shape neural cell fate, synaptic plasticity, and long-term brain function, contributing to either resilience or increased vulnerability to neurodevelopmental disorders.
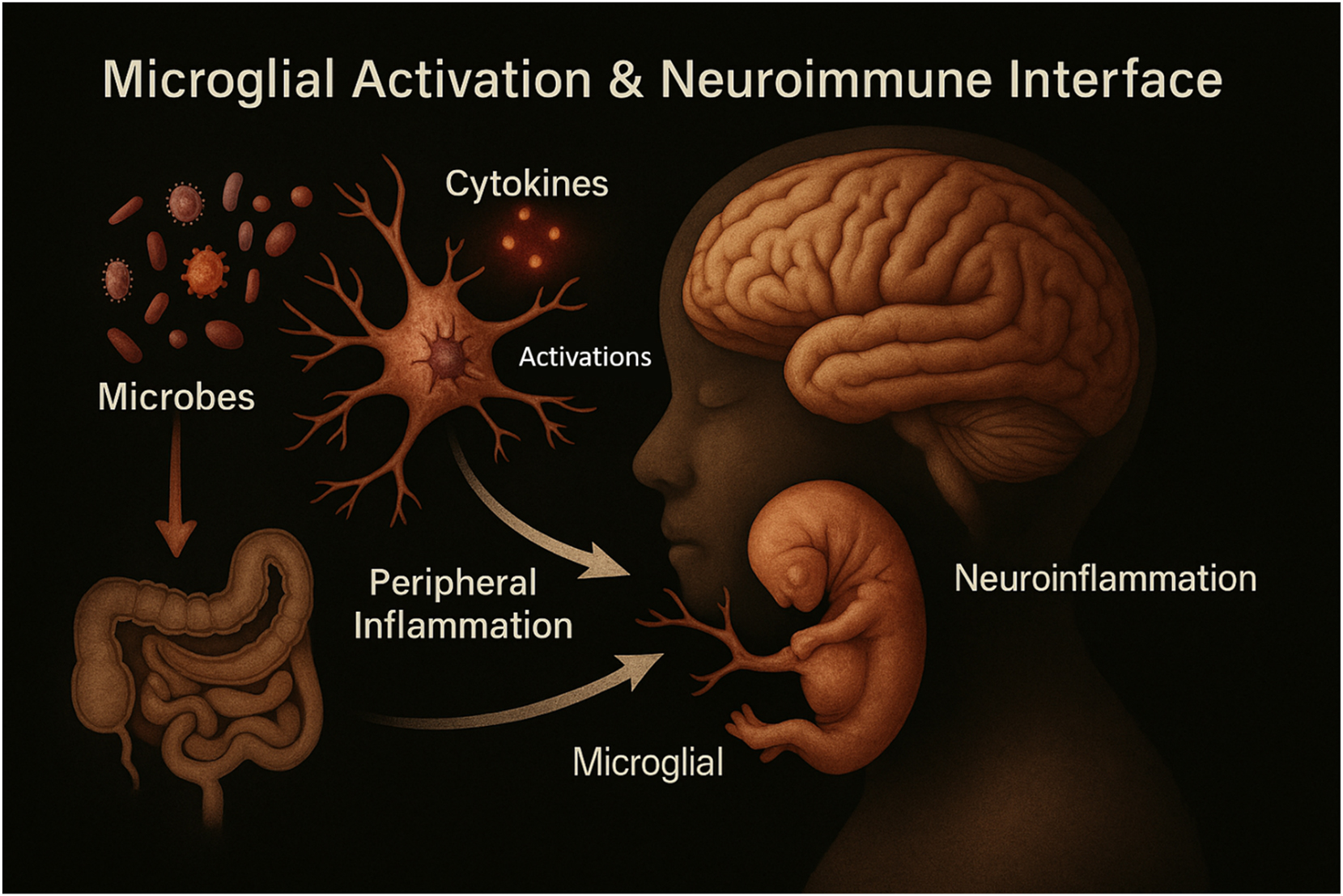
Microglial activation and the neuroimmune interface in early brain development. This figure illustrates how microglia, the brain’s resident immune cells, respond to maternal and microbial signals during fetal and early postnatal development. Microglial activation is influenced by maternal cytokines, microbial metabolites, and environmental stimuli. Activated microglia regulate neurogenesis, synaptic pruning, and myelination, playing a pivotal role in shaping neural circuitry. Dysregulated microglial responses, often due to maternal immune activation or microbial imbalance, may disrupt brain development and increase susceptibility to neurodevelopmental disorders such as autism spectrum disorder and schizophrenia.
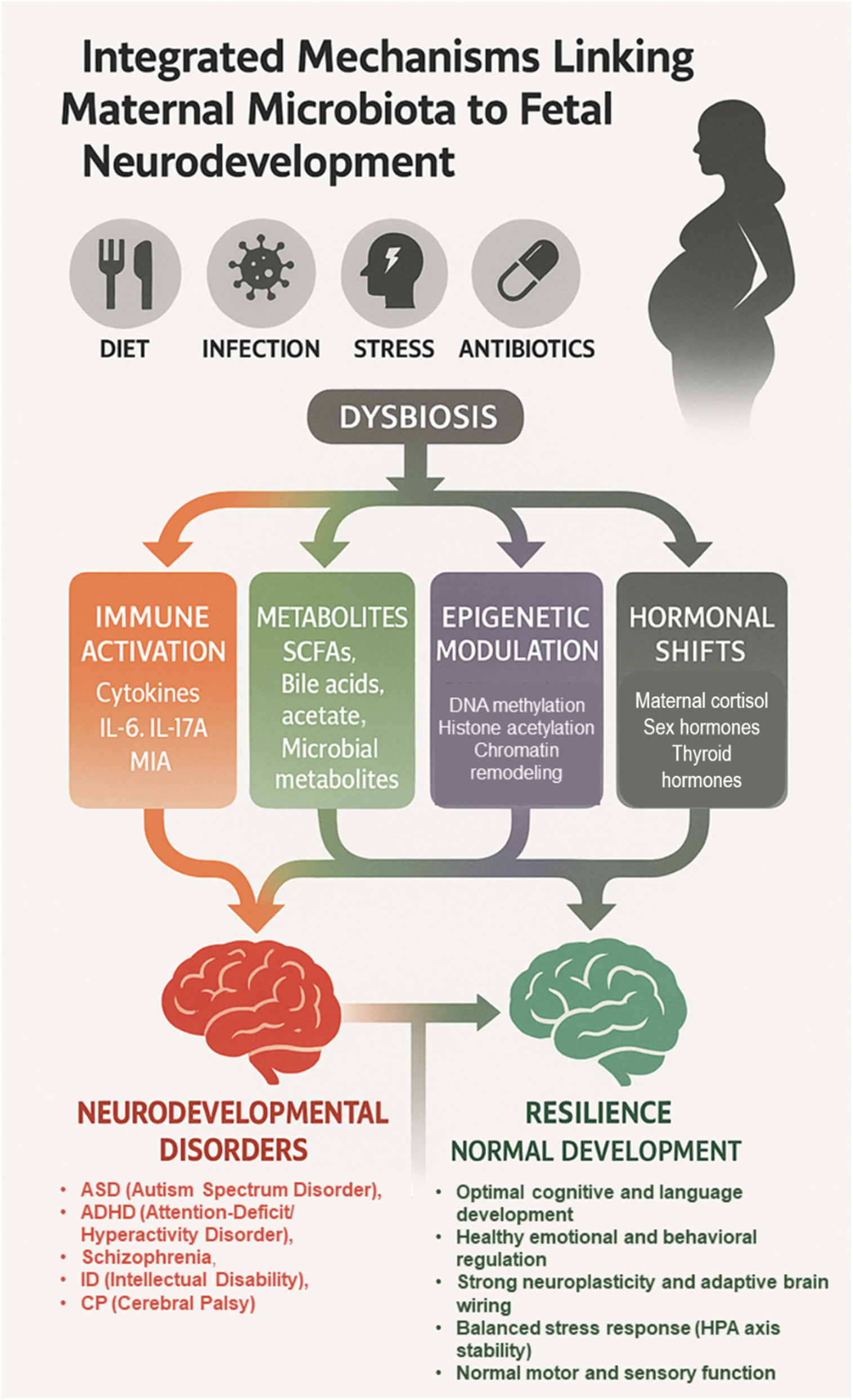
Integrated maternal microbiota–fetal brain axis: Pathways to risk or resilience. This schematic illustrates how maternal factors (diet, infection, stress, antibiotics) can disrupt the maternal microbiota, leading to dysbiosis. Dysbiosis influences fetal neurodevelopment through four converging mechanisms: Immune activation (cytokines, IL-6, IL-17A, maternal immune activation), metabolite signaling (short-chain fatty acids, bile acids, acetate, microbial metabolites), epigenetic modulation (DNA methylation, histone acetylation, chromatin remodeling), and hormonal shifts (maternal cortisol, sex hormones). These pathways shape divergent outcomes: Increased susceptibility to neurodevelopmental disorders (autism spectrum disorder, ADHD, schizophrenia, intellectual disability, cerebral palsy) vs. resilience and normal development (optimal cognition, emotional regulation, adaptive plasticity, balanced stress responses, normal motor and sensory function).
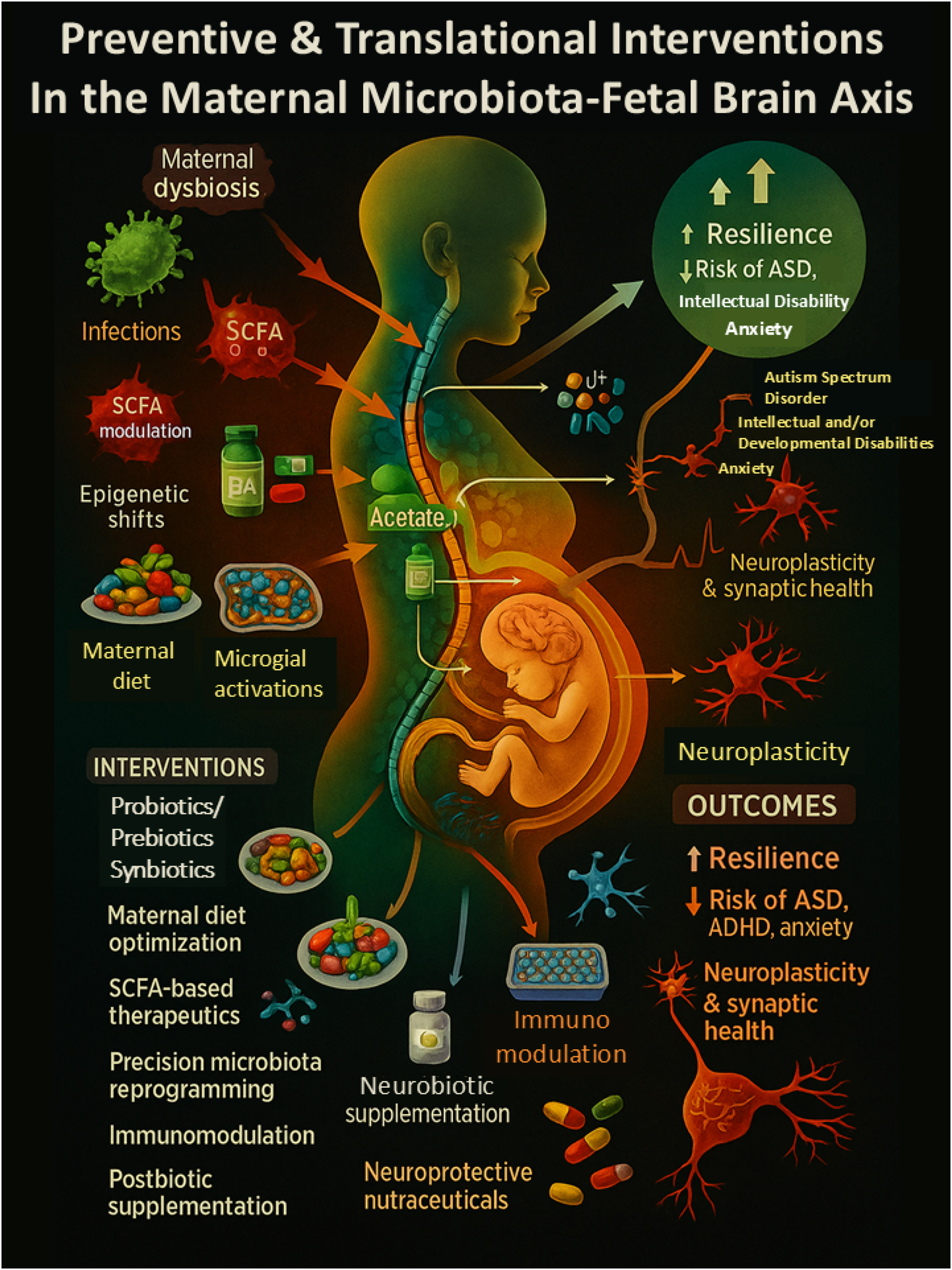
Integrative translational strategies targeting the maternal microbiota–brain axis to optimize fetal neurodevelopment. This comprehensive systems-level diagram maps the complex interplay between maternal environmental exposures, microbiota-derived metabolites, immune signaling, and fetal brain development. It highlights how risk factors such as poor diet, infections, antibiotic exposure, and dysbiosis activate inflammatory (e.g., IL-6, IL-17A) and metabolic pathways (e.g., SCFA imbalance), leading to epigenetic alterations, microglial priming, and impaired neurodevelopment. In contrast, targeted interventions – such as nutraceuticals, SCFA-enhancing diets, immunomodulators, and precision probiotics – can promote neurodevelopmental resilience by restoring microbiome balance and dampening neuroinflammation. The model emphasizes the translational potential of maternal microbiota modulation as a strategy for early prevention of disorders like ASD, ADHD, and intellectual disability.
Mechanistic pathways linking the intrauterine microbiome to fetal neurodevelopment: an expanded literature-based matrix.a
| Mechanism | Signaling molecules/Pathways | Neuro developmental outcome |
Developmental stage affected |
Experimental model |
Key insight | Strengths | Limitations | Key references |
| Maternal immune activation, MIA | IL-6, IL-17A, TNF-α | Disrupted cortical layering; ASD-like behaviors | Mid-gestation (E12.5–E17 in mice) | Mouse models, limited human cohort data | Immune activation alters cortical structure and behavior | Robust preclinical causality; conserved cytokine roles | Limited human translation; heterogeneous cytokine measures | [8] Kim et al.; [7] Estes & McAllister [6]; Vuong et al.; [13] Choi et al. |
| Cytokine signaling (e.g., IL-6, IL-17A) | IL-6/STAT3, IL-17A/IL-17RA | Synapse density, altered thalamocortical wiring | Cortical lamination period | Mice; cytokine infusion studies | Maternal inflammation leads to localized fetal brain cytokine signaling | Direct mechanistic linkage to ASD-like behaviors | Timing/specificity of exposure hard to control | [13] Choi et al.; [14] Shin Yim et al. |
| Short-chain fatty acids (SCFAs) | Butyrate, acetate, propionate | Microglial maturation, synaptic pruning, BBB integrity | Prenatal–postnatal transition | Germ-free and microbiota-transplant mice | SCFAs act as HDAC inhibitors affecting neuroimmune crosstalk | Strong link between metabolite levels and microglial gene expression | Dual roles: Beneficial (butyrate), harmful (excess propionate) | [6] Vuong et al.; [17] Erny et al.; [3] Lu et al. |
| Tryptophan metabolites (e.g., kynurenine) | AhR, IDO, KAT, KMO pathways | Impaired hippocampal neurogenesis; anxiety phenotypes | Late gestation to early infancy | Rodent models; human CSF/metabolomics | Modulate NMDA receptors, affect neuroplasticity | Metabolic-immune interface; links to anxiety | Human data still correlational; sex-specific data lacking | [21] Gao et al.; [36] Grant et al. |
| Placental epigenetic modification | DNA methylation, histone acetylation (HDAC), miRNAs | Long-term gene regulation, hippocampal volume loss | All trimesters (epigenetic reprogramming) | Mouse models, human placenta/cord blood | Epigenetic memory of microbial and dietary signals | Epigenomic tools offer mechanistic depth | Causal chains hard to isolate in humans | [20] Zhou et al.; [9] Kaminski et al.; [19] Krautkramer et al. |
| Microbial pattern recognition receptors (PRRs) | TLRs (e.g., TLR4), NOD-like receptors | Abnormal immune priming, neuroinflammation | Early embryonic immune priming | Knockout mice, microbiota depletion studies | Early microbial sensing shapes fetal immune tone | Mechanistic PRR studies available | Relevance to human intrauterine PRR signaling debated | [10] Knuesel et al.; [5] Ganal-Vonarburg et al. |
| Intrauterine dysbiosis (placenta/amniotic) | Low-diversity microbiota, SCFA shifts | Altered amygdala-insula connectivity, social deficits | Second trimester onward | Human amniotic fluid, placenta microbiome, metagenomics | In utero microbial contact may directly shape fetal CNS | Translational relevance to human pregnancy | Ongoing debate about sterility of uterus; detection biases | [1] Aagaard et al.; [2] Mishra et al.; [3] Lu et al. |
| Maternal antibiotics/Dysbiosis | Microbiota depletion → ↓SCFAs, ↑immune reactivity | Increased ADHD/ASD risk; disrupted thalamocortical connections | Mid to late gestation | Human population studies; murine depletion/colonization | Disruption of microbial-derived neuroactive compounds | Large cohort data supports associations | Cannot isolate microbiota effect from infection/antibiotics | [24] Hambridge et al.; [11] Buffington et al. |
| Microbial Metabolite–Epigenetic crosstalk | SCFAs as HDAC inhibitors, tryptophan metabolites as methyl donors | Modulation of neurodevelopmental gene expression | Embryonic neuronal differentiation | Germ-free and diet-induced dysbiosis models | Epigenetic plasticity modulated by gut microbial status | Novel target for prenatal interventions | Temporal dynamics of epigenetic changes unclear | [20] Zhou et al.; [21] Gao et al.; [37] Chen et al. |
-
aThis matrix summarizes key mechanistic pathways linking the intrauterine microbiome to fetal neurodevelopment. It integrates findings from 67 studies, detailing signaling molecules, developmental timing, experimental models, outcomes, strengths, limitations, and literature-indexed references. The table highlights how maternal microbial signals – via immune, metabolic, and epigenetic routes – shape fetal brain development and neuropsychiatric risk.
Key unanswered questions for future research on maternal microbiota and fetal neurodevelopment.
| No. | Research question |
| 1 | What are the long-term neuropsychiatric outcomes of intrauterine microbial exposure into adolescence and adulthood? |
| 2 | How do fetal sex and host genetics modulate vulnerability to maternal microbiota perturbations? |
| 3 | Can maternal microbiome profiling be standardized and implemented in prenatal clinical settings? |
| 4 | What specific dietary or probiotic interventions most effectively optimize maternal microbiota for neuroprotection? |
| 5 | How does psychosocial stress interact with maternal microbiota to influence fetal brain development? |
| 6 | What are the causal links between specific microbial metabolites and epigenetic modifications in fetal neural tissue? |
| 7 | How can advanced models (e.g., organoids, brain-on-chip) be used to simulate maternal–fetal microbial-neurodevelopment interactions? |
-
This table summarizes key research questions addressing gaps in understanding maternal microbiota influences on fetal brain development and long-term neuropsychiatric outcomes.
Critical unanswered questions in microbiome–neurodevelopmental interactions during pregnancy.
| Unanswered question | Rationale/Literature context |
| Is there a core intrauterine microbiome across healthy pregnancies, or is it pregnancy-specific? | Studies show microbial DNA in placenta/amniotic fluid, but debate continues over contamination vs. true colonization [1], 2], 29]. |
| Can fetal brain exposure to microbial metabolites be quantified non-invasively? | Most evidence comes from animal models; human-accessible biomarkers are lacking [6], 12], 22]. |
| What is the minimal microbial load required to trigger neurodevelopmental changes? | Low-biomass microbial presence correlates with neuroimmune changes in mice, but thresholds are undefined [6], 8], 11]. |
| Do microbial signals differ between spontaneous vaginal birth and cesarean section before labor onset? | Cesarean births show altered neonatal microbiota; unclear if prenatal microbial imprint is also affected [23], 24], 35]. |
| How does the placenta act as a selective filter or conduit for maternal microbial signals? | Role of placental barrier in microbial metabolite transfer is hypothesized but unproven [3], 19], 34]. |
| Are transient maternal infections during pregnancy neurodevelopmentally disruptive through microbial translocation? | Maternal immune activation via infection linked to ASD-like outcomes, but exact microbial role needs clarification [7], 8], 15]. |
| What role do viral and fungal microbiota (virome, mycobiome) play in fetal neurodevelopment? | Most microbiome studies focus on bacteria; virome/mycobiome influence is a major knowledge gap [35], 38]. |
-
This table summarizes unresolved questions on microbial presence, transmission, and neurodevelopmental impact during pregnancy, providing direction for future research priorities.
Microbial metabolites and corresponding neural effects.
| Metabolite | Microbial source | Neural effect | Notes |
| Butyrate | Firmicutes (e.g., Clostridia) | Promotes microglial maturation, synaptic pruning, enhances BBB integrity | HDAC inhibitor; supports neuroimmune regulation [6], 17], 19], [39], [40], [41], [42] |
| Propionate | Bacteroidetes | Excess linked to oxidative stress, ASD-like behaviors | Dose-dependent neurotoxic effect [18], 40], 41] |
| Acetate | Broad microbial genera | Modulates BBB permeability, neuroimmune tone | Influences neurovascular coupling [17], 39], 40] |
| Kynurenine | Tryptophan-metabolizing microbes | Alters NMDA receptor activity; affects anxiety, learning | Impacts hippocampal neurogenesis and neuroplasticity [21], 34], 41] |
| Indole derivatives | Bacteroides, lactobacillus | Potential regulators of serotonin and mood pathways | Emerging pathway; serotonin signaling modulation [34], 41] |
| Secondary bile acids | Clostridial clusters | Modulate neuroinflammation and gut-brain signaling | Microbial–immune crosstalk; emerging neuroactive role [19], 34] |
-
This table maps gut-derived microbial metabolites to brain development effects, revealing how compounds like butyrate and propionate shape neuroimmune functions, synaptic health, and emotional regulation.
Clinical and observational studies on maternal microbiome interventions and offspring neurodevelopment.
| Study/Trial ID | Intervention | Population | Outcome measures | Status |
| NCT05467150 [43] | Probiotic supplementation during pregnancy | Pregnant women with diabetes | Infant gut microbiome variation, neurodevelopmental outcomes measured by ERP performance | Ongoing |
| VDAART study [44] | Maternal vitamin D supplementation | Pregnant women | Association between maternal gut microbiome and child neurodevelopment at 1 year (ASQ) | Completed |
| Animal study [45] | Maternal Lactobacillus Rhamnosus GG supplementation | Pregnant mice | Offspring neural/oligodendrocyte progenitor development, CNS inflammation modulation | Completed |
| Observational cohort [46] | Maternal gut microbiota profiling | Pregnant women | Association between maternal gut microbiota composition and child behavior | Completed |
| NCT03862950 [47] | Metformin administration | Individuals with fragile X syndrome (FXS) | Language/cognition, eating behaviors, behavior, weight loss | Completed |
| NCT03369431 [48] | Vivomixx probiotic | Children with autism spectrum disorder (ASD) | Gastrointestinal symptoms, behavioral ASD symptoms | Completed |
| NCT02970552 [49] | Vaginal progesterone supplementation | HIV-infected pregnant women | Reduction in preterm birth risk | Completed |
| NCT02991807 [50] | Everolimus | Patients with PTEN mutations | Neurocognition, behavior, safety | Recruiting |
| NCT03797157 [51] | Human milk fortifier (H2MF®) | Preterm infants (22+0–27+6 weeks gestation) | Growth, nutrition, feeding intolerance, complications | Active, not recruiting |
| NCT04560179 [52] | Inhaled tobramycin therapy | Preterm infants with BPD | Airway microbiome changes, respiratory outcomes | Recruiting |
-
This table highlights ongoing and completed studies linking maternal microbiome interventions – like probiotics and vitamins – to improved infant brain outcomes, supporting their potential in prenatal care.
Translational pathways and interventional targets linking maternal microbiota to fetal neurodevelopment: an integrated preclinical–clinical perspective.
| Pathway/Risk factor | Evidence in mice | Evidence in humans | Proposed clinical intervention | Target population | Translational readiness |
| IL-17A → ASD-like behavior [6], 7], [13], [14], [15], [16] | MIA models show cortical disruption, ASD-like phenotypes | Elevated IL-17A correlates with ASD risk in maternal serum | Anti-inflammatory strategies; IL-17A monitoring | Pregnant women with autoimmune risk | Moderate |
| SCFAs → microglial development [6], 7], 22] | SCFA depletion impairs, and supplementation rescues, microglial function | Limited evidence; SCFA levels associated with brain connectivity | Dietary SCFA enrichment; probiotic use | All pregnant women, especially with poor diet | Moderate |
| DNA methylation → neural gene silencing [20], 21], 53] | Maternal diet alters hippocampal DNA methylation and histone acetylation | Placental and cord blood epigenetic marks correlate with maternal microbiota | Epigenetic biomarker monitoring and nutritional modulation | Women with metabolic syndrome or obesity | Emerging |
| Maternal dysbiosis [22], 32], 43], 45] | Fecal transplant and dysbiosis-induced inflammation studies | Associations with infant amygdala structure and cognition | Probiotic and prebiotic supplementation | High-risk pregnancies (e.g., metabolic or psychological stress) | Moderate–High |
| Antibiotic overuse [24], 25] | Microbiota depletion leads to reduced SCFA, abnormal behavior | Large cohort studies link prenatal antibiotics to ASD/ADHD | Antibiotic stewardship during pregnancy | General prenatal population | High |
| Low microbial diversity [23.85] | Low diversity reduces SCFA, affects neurogenesis | Microbiota profiling predicts child behavior | High-fiber diet; personalized dietary plans | Pregnant women with low microbial diversity | Emerging |
-
This table integrates key maternal microbiota–neurodevelopmental pathways with supporting evidence from animal and human studies. It outlines potential clinical interventions, target populations, and translational readiness, bridging mechanistic insights to real-world prenatal strategies.
Methods
This work is an integrative narrative review designed to synthesize mechanistic and translational insights at the intersection of microbiology, immunology, neuroscience, and perinatal psychiatry. The objective was to evaluate how maternal microbial ecosystems may shape fetal neurodevelopment through immune, metabolic, epigenetic, and hormonal pathways, and to organize those findings into a framework that is clinically interpretable. A narrative approach was selected to accommodate heterogeneous study designs and readouts – ranging from gnotobiotic and maternal immune activation animal models to population cohorts and interventional trials – so that convergent biological themes could be identified without excluding informative but non-uniform evidence.
A comprehensive literature search was performed in PubMed, EMBASE, Web of Science, and Scopus for publications dated January 2000 through March 2025. Search strings combined controlled vocabulary and free-text terms related to the maternal microbiome (gut, placenta, amniotic fluid), pregnancy, fetal and early postnatal brain development, neurodevelopmental outcomes, immune signaling, short-chain fatty acids, tryptophan metabolites, bile acids, microglia, epigenetic regulation, and maternal hormonal milieu. Reference lists from all retrieved articles were screened to capture additional studies not returned by database queries. After deduplication, the screening pool comprised 1,243 records. The study selection workflow is displayed in Figure 1 using a PRISMA-style diagram to enhance transparency.
Eligibility focused on original in vivo evidence from animal or human studies that assessed maternal microbial features in the gut, placenta, or amniotic fluid and reported fetal or early postnatal neurodevelopmental outcomes. Exclusions included in vitro-only reports, studies limited to postnatal microbiota-brain interactions with no prenatal exposure, and articles lacking mechanistic relevance to the maternal microbiota–brain axis. Two reviewers independently screened titles and abstracts, followed by full-text assessment; discrepancies were resolved by consensus or adjudication. Inter-rater agreement was monitored during pilot calibration and maintained throughout screening.
Data extraction followed a predefined template capturing publication characteristics, experimental model or cohort design, biospecimen type and timing, microbial metrics (diversity indices, taxa, metabolite quantification), mechanistic intermediates (e.g., cytokines, SCFAs, kynurenine pathway readouts, epigenetic marks), gestational exposure window, and neurodevelopmental outcomes including microglial maturation, cortical layering, connectivity, behavior, and cognition. Where available, effect sizes, confidence intervals, and exposure-response gradients were recorded to support a more objective narrative. Extracted variables are summarized in Table 1, key unanswered questions are organized in Tables 2 and 3, metabolite-neural relationships are mapped in Table 4, clinical and observational evidence is consolidated in Table 5, and translational pathways with proposed targets are integrated in Table 6.
Quality considerations were incorporated at two levels. First, study-level features relevant to bias – randomization and blinding in animal work; sampling, exposure ascertainment, confounder control, and outcome validity in human research – were documented during extraction and synthesized qualitatively. Second, sensitivity to low-biomass context was emphasized: studies employing validated sequencing approaches (16S rRNA gene profiling or shotgun metagenomics) and explicit contamination controls (negative controls, extraction blanks, reagent audits) were prioritized in the interpretive weighting, given the susceptibility of placental and amniotic samples to exogenous signal.
Synthesis proceeded in stages. Evidence was first grouped by mechanistic domain – immune signaling, metabolic signaling, epigenetic programming, and maternal hormonal influences – and then stratified by species, gestational timing, and neurodevelopmental endpoint. Within each domain, we highlight convergent findings across models and note areas of inconsistency, potential dose–response patterns, sex-specific effects, and translational relevance. Visual materials accompany the text to aid integration: Figure 1 presents the literature selection process; Figures 2–9 depict mechanistic frameworks, maternal obesity–associated inflammatory cascades, developmental windows, epigenetic regulation, microglial activation, an integrated risk-to-resilience model, and translational strategies. Tables 1–6 provide aligned, reference-indexed summaries to anchor quantitative details and facilitate cross-comparison.
All Figures were created using BioRender (Toronto, Canada), Adobe Illustrator (Creative Cloud, Adobe Inc., San Jose, CA, USA), and Canva Visual Suite 2.0 (Canva Pty Ltd., Sydney, Australia).
Results
Literature selection and PRISMA-guided synthesis overview
The literature search across PubMed, EMBASE, Web of Science, and Scopus (January 2000–March 2025) retrieved 1,243 records, supplemented by 68 additional studies through citation chaining. After removing duplicates, 1,011 titles and abstracts were screened, leaving 183 full-text articles for eligibility review. A total of 67 studies were selected for mechanistic synthesis, and these were contextualized within a broader set of 91 references spanning microbiology, immunology, neuroscience, obstetrics, and psychiatry. The PRISMA-style selection process is presented in Figure 1, while inclusion criteria, study models, and mechanistic endpoints are summarized in Table 1.
Maternal microbiota influences on fetal brain development
The “sterile womb” paradigm has been challenged by findings of microbial DNA in the placenta, amniotic fluid, and fetal meconium [1], [2], [3, 23], 29], 35]. These reports highlight the difficulty of distinguishing true colonization from contamination, given the low-biomass nature of intrauterine samples. Sequential work across pregnancy cohorts has shown that maternal gut and placental microbiota undergo remodeling influenced by maternal diet, obesity, and stress [4], 5], 38], 54], 55]. Rodent studies further demonstrated that maternal high-fat diet and antibiotic treatment disrupt microbial diversity, with offspring showing abnormal cortical layering, impaired synaptic plasticity, and ASD-like behaviors [6], 11], [56], [57], [58].
Translational studies provide converging support. Neuroimaging of human fetuses has linked maternal dysbiosis to altered amygdala and insula connectivity [12], 59]. Other work demonstrates that maternal stress during gestation modifies microbial communities, with consequences for fetal hypothalamic–pituitary–adrenal axis programming [60]. Collectively, these findings (Figures 2–4) indicate that maternal microbiota act as upstream regulators of neurodevelopmental vulnerability.
Immune-mediated mechanisms: cytokines and microglia
Maternal immune activation (MIA) emerges as a central mechanism through which dysbiosis affects the developing brain. Experimental models show that microbial imbalance increases maternal cytokines such as IL-6, IL-1β, and IL-17A [7], [8], [9, 13], 14], [61], [62], [63]. These cytokines cross the placenta, perturb cortical lamination, and activate fetal microglia. Rescue experiments using IL-17A blockade or recolonization with defined consortia reversed abnormal phenotypes in animal models [14], 64].
Human studies support this immune link. Elevated mid-gestational cytokines have been associated with ASD risk [15], 16]. Large population datasets link maternal infections and systemic inflammation to higher prevalence of schizophrenia and ADHD in offspring [65], 66]. Mechanistic studies further highlight redundancy in cytokine signaling [67], roles of trophoblast–immune crosstalk [68], and contributions of uterine NK cells [69].
These immune cascades and their impact on microglial maturation are synthesized in Figure 5 and systematically presented in Table 2.
Microbial metabolites and metabolic-immunologic crosstalk
Short-chain fatty acids (SCFAs)
SCFAs, including acetate, propionate, and butyrate, are major microbial metabolites influencing neurodevelopment. Evidence shows they restore microglial maturation in germ-free mice [17], 39]. Butyrate rescues cortical gene expression and synaptic integrity [6], whereas excess propionate induces oxidative stress, mitochondrial dysfunction, and repetitive behaviors [18], [40], [41], [42].
Tryptophan derivatives and indoles
Microbial metabolism of tryptophan produces kynurenine and indole derivatives that influence NMDA receptor activity, hippocampal plasticity, and stress reactivity [21], 34], 70], 71]. Aberrant kynurenine signaling has been associated with increased vulnerability to anxiety and depressive phenotypes.
Bile acids and other metabolites
Bile acid derivatives regulate placental nutrient efficiency and interact with SCFAs in modulating immune–neural signaling [19], 32], 72], 73]. An integrated map of SCFAs, tryptophan derivatives, and bile acids is depicted in Figure 6, with quantitative and qualitative findings consolidated in Tables 3 and 4.
Epigenetic programming by maternal microbiota
Epigenetic reprogramming is another major pathway. Microbial metabolites, particularly SCFAs, act as histone deacetylase inhibitors, altering chromatin accessibility in fetal neural tissue [19], 20]. Maternal high-fat diet or dysbiosis modifies DNA methylation and histone acetylation patterns in hippocampus and placenta, correlating with cognitive and behavioral changes in offspring [20], 21], 53], 74], 75]. Other studies demonstrate how non-coding RNAs mediate maternal environmental effects on fetal development [76], [77], [78]. These mechanistic layers are presented in Figure 7 and summarized in Table 4.
Translational evidence from human cohorts
Clinical evidence aligns with preclinical findings. Cohort studies demonstrate that higher maternal microbial diversity predicts improved infant cognition [22]. In contrast, placental microbial alterations have been reported in pregnancies complicated by obesity, metabolic syndrome, or diabetes [23], [79], [80], [81].
Population-based studies further associate prenatal antibiotic exposure with elevated risks of ASD, ADHD, and other developmental conditions [24], 25], [82], [83], [84]. Additional longitudinal studies confirm that maternal infections, preeclampsia, and poor diet exacerbate neurodevelopmental risks [36], [85], [86], [87], [88], [89].
Intervention trials are increasingly exploring translational applications. Clinical studies evaluating probiotics during pregnancy, vitamin D supplementation, and other dietary strategies have demonstrated modulation of maternal microbial profiles with downstream effects on infant health [43], [44], [45]. Ongoing registered trials are testing microbiota-targeted therapies for gestational diabetes, Fragile X, ASD, and preterm infants [46], [47], [48], [49], [50], [51], [52]. These data are summarized in Table 5 and visualized in Figure 8.
Pathway crosstalk and sex-specific effects
Immune, metabolic, and epigenetic pathways are not isolated but deeply interconnected. SCFAs regulate cytokine release and histone acetylation [17], 19], while inflammation alters placental nutrient transport and epigenetic remodeling [20], 21].
Sex-specific vulnerability is consistently reported. Male offspring exhibit exaggerated responses to MIA and microbial imbalance, consistent with the higher prevalence of ASD and ADHD among boys [26], 27], [79], [80], [81], [82]. Evidence from animal and human studies documents sex-dimorphic microglial morphogenesis [61], 62], immune priming [65], 66], and transcriptional programming [83]. These findings are illustrated in Figure 9 and summarized in Table 6.
Integrated summary and remaining gaps
Across 91 references, synthesized through Figures 1–9 and Tables 1–6, the evidence supports a maternal microbiota–neurodevelopment axis involving immune, metabolic, and epigenetic mechanisms. Preclinical models provide causal insights, while human cohorts demonstrate converging associations. Remaining challenges include differentiating contamination from true colonization in low-biomass samples [1], 29], 35], defining minimal microbial exposure thresholds [6], 11], and validating causality in large-scale longitudinal cohorts [12], 22], 24], 85].
Future research should prioritize multi-omics designs, standardized sampling for low-biomass contexts, and interventional trials targeting maternal microbiota. Despite gaps, current findings firmly establish the maternal microbiome as a modifiable determinant of fetal brain development with major implications for obstetrics, psychiatry, and preventive health.
Discussion
Integrative interpretation of findings
This review consolidates multidisciplinary evidence demonstrating that the maternal microbiota functions as a dynamic regulator of fetal neurodevelopment, with consequences extending into long-term mental health trajectories. Preclinical research consistently shows that disruption of maternal gut microbiota – through antibiotic treatment, germ-free conditions, or dietary perturbations – leads to abnormal cortical development, altered microglial maturation, and behavioral phenotypes reminiscent of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) [6], 11], 17], 18], [39], [40], [41], [42]. These aberrations include defective thalamocortical axonogenesis, impaired synaptogenesis, and perturbed interneuron migration. Importantly, experimental reconstitution of the maternal microbiota with defined bacterial consortia has been shown to rescue neural phenotypes, underscoring causality in animal models [6], 11].
Human studies complement these findings. Variation in maternal gut microbial diversity has been linked to neonatal brain structure and functional connectivity, particularly in the amygdala and insula-regions central to emotional regulation and cognitive processing [12], 22], 23], 64]. This cross-species convergence strengthens the biological plausibility of a microbiota–brain connection beginning during gestation. Figures 2–4 and Tables 1–2 summarize these maternal-fetal interactions, reinforcing that the intrauterine environment is not sterile but dynamically shaped by maternal physiology.
Immune-mediated mechanisms and neurodevelopmental outcomes
Among the proposed pathways, maternal immune activation (MIA) remains one of the most consistently implicated. Dysbiosis during pregnancy primes the maternal immune system, elevating cytokines such as IL-6, IL-1β, and IL-17A [7], [8], [9], [10, 13], 14], [67], [68], [69]. These cytokines can cross the placenta, influence placental vascular function, and disrupt fetal neural progenitor dynamics.
Animal models provide strong mechanistic evidence: IL-17A signaling specifically impairs cortical lamination, producing ASD-like behaviors in offspring [8], 13], 14]. Human cohort studies parallel these findings, showing that elevated mid-gestational cytokine levels predict increased ASD risk [15], 16], and altered placental immune signaling has been observed in pregnancies associated with neurodevelopmental risk [9], 10], 69].
As mapped in Figure 5 and summarized in Table 2, immune dysregulation perturbs microglial colonization, astrocytic signaling, and synaptic pruning – processes essential for establishing neural circuits [61], 65], 90], 91]. Collectively, these data support a model in which maternal microbial imbalance indirectly programs fetal brain development by shaping immune tone.
Microbial metabolites and metabolic–immunologic crosstalk
Beyond cytokines, microbial metabolites – especially short-chain fatty acids (SCFAs) such as butyrate and propionate – mediate maternal–fetal interactions [17], 19], [39], [40], [41], [42]. SCFAs cross the placenta, modulate blood–brain barrier integrity, influence microglial maturation, and regulate neurotransmitter synthesis. Butyrate supplementation in germ-free dams restores microglial morphology and normalizes offspring synaptic architecture [6].
By contrast, elevated propionate induces oxidative stress, mitochondrial dysfunction, and ASD-like behaviors in rodents [18], 40], 41]. Other metabolites, including tryptophan derivatives and bile acids, regulate NMDA receptor activity, serotonergic signaling, and hippocampal neurogenesis, linking maternal metabolic state to fetal cognitive potential [21], 34], 41], 70]. These pathways are depicted in Figure 6 and summarized in Table 3.
Emerging maternal metabolomics studies corroborate these findings. Plasma SCFA profiling in pregnant women demonstrates correlations between butyrate levels and infant white matter integrity [40], 41], 70], 71], suggesting that microbial metabolites act as systemic mediators integrating diet, environment, and host physiology.
Epigenetic programming by maternal microbiota
Microbial signals also influence neurodevelopment epigenetically. SCFAs such as butyrate function as histone deacetylase inhibitors (HDACis), altering chromatin structure and gene transcription [19], 20], 53]. Germ-free animal studies reveal abnormal DNA methylation and histone modifications in fetal cortical tissue, correlating with impaired synaptic plasticity and memory performance [20], 53], 72].
Maternal dysbiosis-driven by diet, stress, or infection – induces epigenetic remodeling in the hippocampus and prefrontal cortex, altering gene networks for synaptic development and stress responsivity [21], 73], 74]. These findings highlight that microbial signals establish durable epigenetic “set points” influencing postnatal trajectories [61], 65], [75], [76], [77], [78]. Figure 7 and Table 4 catalog these cascades, linking microbiota-driven transcriptional changes to neuropsychiatric risk.
Translational implications and clinical relevance
The convergence of immune, metabolic, and epigenetic mechanisms positions the maternal microbiome as a modifiable risk factor in prenatal neurodevelopment. Greater maternal gut diversity is associated with improved infant cognition [22], while prenatal antibiotic exposure – a disruptor of microbial balance – is linked to elevated ASD and ADHD risk [24], 25], 29].
These insights have catalyzed clinical interventions, including targeted probiotic or prebiotic supplementation, dietary optimization, and antibiotic stewardship [4], 5], 28], 30], 32], 34], 38]. Prenatal care integrating microbial screening could identify at-risk pregnancies and enable personalized strategies [31], 33], 54], [56], [57], [58]. Ongoing clinical trials (e.g., NCT03862950, NCT03369431) are investigating probiotics, dietary modulators, and hormonal–microbiome interactions [43], [44], [45], [46], [47], [48], [49]. These efforts are summarized in Figure 8 and Table 5, demonstrating the shift from observational associations to interventional science.
Sex-specific effects and vulnerabilities
Fetal sex modifies susceptibility to maternal microbial and immune perturbations. Male fetuses exhibit heightened vulnerability, which may partly explain the male-biased prevalence of ASD and ADHD [26], 27], 79], 81], 82]. Preclinical studies show male-specific alterations in cortical architecture and synaptic organization after MIA exposure [13], 14], 62], 63].
Human research echoes this, with sex-dependent immune responses and transcriptional profiles [26], 27], 80]. Transcriptomics and lineage-tracing confirm differential microglial maturation by sex [60], 61], 66]. Figure 9 and Table 6 illustrate these findings, underscoring the importance of sex as a biological variable in both research and clinical contexts.
Conceptual controversies and methodological constraints
The “sterile womb” debate epitomizes ongoing uncertainty. Some studies detect microbial DNA signatures in placenta and amniotic fluid [1], 23], while others report no evidence in healthy samples, attributing earlier findings to contamination [29]. These discrepancies highlight the necessity of rigorous contamination controls, standardized processing, and robust bioinformatics.
Most human studies remain observational, limiting causal inference. While animal models clarify mechanisms, they do not fully replicate human gestation due to differences in placentation, neurodevelopmental timing, and microbial ecology. Progress will require longitudinal cohorts integrating maternal microbiome data, immune and metabolic readouts, fetal neuroimaging, and postnatal outcomes. Emerging platforms such as placental organoids, brain-on-chip systems, and non-human primate models may provide higher fidelity to human biology.
Strengths, limitations, and future directions
The major strength of this review lies in its integrative framework, connecting microbiology, immunology, neuroscience, obstetrics, and epigenetics to establish an intrauterine microbiome–neurodevelopment axis. By bridging preclinical and clinical domains, and incorporating sex-specific observations, the review situates mechanistic insights within a translational context. Figures 1–9 and Tables 1–6 collectively map these interactions, providing conceptual clarity for clinicians and researchers.
Yet limitations remain. Rodent models dominate mechanistic research, and translation to human pregnancy is constrained by biological differences. Human studies are often small, observational, and methodologically heterogeneous, with variable sampling sites, sequencing platforms, and analytical pipelines. Low-biomass niches like placenta and amniotic fluid are especially prone to contamination. Moreover, few studies extend developmental follow-up into adolescence or adulthood, leaving long-term implications speculative.
Future progress will depend on methodological harmonization, multi-omics integration, and attention to complex multivariate exposures including stress, metabolic disorders, and environmental toxins. Greater inclusion of underrepresented populations is essential to ensure equity in microbiome-based prenatal care. Importantly, interventional trials testing probiotics, dietary modification, and microbial metabolite modulators will determine whether manipulating maternal microbiota can meaningfully improve child neurodevelopment.
Conclusions
The evidence synthesized here supports a coherent model in which the maternal microbiome contributes to fetal brain development through three interlocking routes: immune signaling (including cytokine cascades relevant to microglial maturation and synaptic refinement), microbially derived metabolites (notably short-chain fatty acids, bile acids, and tryptophan catabolites), and epigenetic programming of neurodevelopmental gene networks. Across animal experiments and human studies, perturbations to maternal microbial ecosystems – via infection, dysbiosis, antibiotics, metabolic status, or diet – are repeatedly associated with altered neurodevelopmental trajectories and greater vulnerability to conditions such as autism spectrum disorder, ADHD, and related phenotypes.
At the same time, important caveats temper over-interpretation: much of the mechanistic depth comes from preclinical models; human datasets are heterogeneous and often observational; and low-biomass sampling carries contamination risk. Taken together, however, cross-species convergence and pathway consistency justify viewing the maternal microbiome as a modifiable influence on prenatal neurodevelopment rather than a peripheral correlate.
Clinically, this framing motivates measured, evidence-aligned steps: routine attention to maternal diet quality, prudent antibiotic stewardship, and consideration of microbiota-supportive strategies where data exist, while larger randomized trials and standardized multi-omics cohorts mature. Strategically integrating microbiome profiling with maternal immune, metabolic, and imaging readouts can sharpen risk stratification and guide targeted prevention.
In short, the intrauterine microbiome–neurodevelopment axis offers a practical blueprint for shifting perinatal care upstream – toward safeguarding fetal brain health by stabilizing maternal microbial ecology. With rigorous methods, equitable access, and interventional testing, this emerging framework can translate into fewer neurodevelopmental disparities and a more preventive, personalized approach to mental health that begins before birth.
Acknowledgments
The authors appreciate the Indonesian Society of Obstetrics & Gynecology (ISOG/POGI) and Indonesian Society of Maternal Fetal Medicine (INAMFM/HKFM) for encouraging and supporting the work of this review article.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Aagaard, K, Ma, J, Antony, KM, Ganu, R, Petrosino, J, Versalovic, J, et al.. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra65. https://doi.org/10.1126/scitranslmed.3008599.Suche in Google Scholar PubMed PubMed Central
2. Mishra, A, Lai, GC, Yao, LJ, Aung, TT, Shental, N, Rotter-Maskowitz, A, et al.. Microbial exposure during early human development primes fetal immune cells. Cell 2021;184:3394–409.e20. https://doi.org/10.1016/j.cell.2021.04.039.Suche in Google Scholar PubMed PubMed Central
3. Lu, CY, Zhang, H, Yang, L, Wang, X, Qiao, Y. Fetal neurodevelopmental modulation by maternal microbiota: recent advances and translational prospects. Trends Neurosci 2024;47:97–113. https://doi.org/10.1016/j.tins.2023.11.005.Suche in Google Scholar PubMed
4. Koren, O, Goodrich, JK, Cullender, TC, Spor, A, Laitinen, K, Bäckhed, HK, et al.. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470–80. https://doi.org/10.1016/j.cell.2012.07.008.Suche in Google Scholar PubMed PubMed Central
5. Goltsman, DSA, Sun, CL, Proctor, DM, DiGiulio, DB, Robaczewska, A, Thomas, BC, et al.. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome Res 2018;28:1467–80. https://doi.org/10.1101/gr.228734.117.Suche in Google Scholar
6. Vuong, HE, Pronovost, GN, Williams, DW, Coley, EJL, Siegler, EL, Qiu, A, et al.. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020;586:281–6. https://doi.org/10.1038/s41586-020-2740-3.Suche in Google Scholar
7. Estes, ML, McAllister, AK. Maternal immune activation: implications for neuropsychiatric disorders. Science 2016;353:772–7. https://doi.org/10.1126/science.aag3194.Suche in Google Scholar PubMed PubMed Central
8. Kim, S, Kim, H, Yim, YS, Ha, S, Atarashi, K, Tan, TG, et al.. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017;549:528–32. https://doi.org/10.1038/nature23910.Suche in Google Scholar PubMed PubMed Central
9. Kaminski, VL, Michita, RT, Ellwanger, JH, Veit, TD, Schuch, JB, Riesgo, RS, et al.. Exploring potential impacts of pregnancy-related maternal immune activation and extracellular vesicles on immune alterations observed in autism spectrum disorder. Heliyon 2023;9:e15593. https://doi.org/10.1016/j.heliyon.2023.e15593.Suche in Google Scholar PubMed PubMed Central
10. Knuesel, I, Chicha, L, Britschgi, M, Schobel, SA, Bodmer, M, Hellings, JA, et al.. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 2014;10:643–60. https://doi.org/10.1038/nrneurol.2014.187.Suche in Google Scholar PubMed
11. Buffington, SA, Di Prisco, GV, Auchtung, TA, Ajami, NJ, Petrosino, JF, Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016;165:1762–75. https://doi.org/10.1016/j.cell.2016.06.001.Suche in Google Scholar PubMed PubMed Central
12. Gustafsson, HC, Graham, AM, James-Todd, T, Potter, R, Noroña-Zhou, A, Christodoulou, J, et al.. Maternal prenatal gut microbiota composition predicts infant amygdala and insula structure and connectivity. Brain Behav Immun 2022;100:311–21. https://doi.org/10.1016/j.bbi.2021.12.026.Suche in Google Scholar PubMed
13. Choi, GB, Yim, YS, Wong, H, Kim, S, Kim, H, Kim, SV, et al.. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016;351:933–9. https://doi.org/10.1126/science.aad0314.Suche in Google Scholar PubMed PubMed Central
14. Shin, YY, Park, A, Berrios, J, Lafourcade, M, Pascual, LM, Soares, N, et al.. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 2017;549:482–7. https://doi.org/10.1038/nature23909.Suche in Google Scholar PubMed PubMed Central
15. Goines, PE, Croen, LA, Braunschweig, D, Yoshida, CK, Grether, J, Hansen, R, et al.. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case–control study. Mol Autism 2011;2:13. https://doi.org/10.1186/2040-2392-2-13.Suche in Google Scholar PubMed PubMed Central
16. Brown, AS, Sourander, A, Hinkka-Yli-Salomäki, S, McKeague, IW, Sundvall, J, Surcel, HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatr 2014;19:259–64. https://doi.org/10.1038/mp.2012.197.Suche in Google Scholar PubMed PubMed Central
17. Erny, D, Hrabe de Angelis, AL, Jaitin, D, Wieghofer, P, Staszewski, O, David, E, et al.. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–77. https://doi.org/10.1038/nn.4030.Suche in Google Scholar PubMed PubMed Central
18. MacFabe, DF, Cain, DP, Rodriguez-Capote, K, Franklin, AE, Hoffman, JE, Boon, F, et al.. Neurobiological effects of intraventricular propionic acid in rats: possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res 2007;176:149–69. https://doi.org/10.1016/j.bbr.2006.07.025.Suche in Google Scholar PubMed
19. Krautkramer, KA, Fan, J, Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 2021;19:77–94. https://doi.org/10.1038/s41579-020-0438-4.Suche in Google Scholar PubMed
20. Zhou, L, Xiao, X, Zhang, Q, Zheng, J, Deng, M, He, S, et al.. Maternal diet-induced microbiota dysbiosis alters DNA methylation and histone modification in the hippocampus of mouse offspring. Cell Rep 2019;26:1109–16.e3. https://doi.org/10.1016/j.celrep.2018.12.104.Suche in Google Scholar PubMed PubMed Central
21. Gao, W, Salomon, C, Freeman, DJ. Epigenetic regulation of placental gene expression in maternal obesity and gestational diabetes mellitus. Placenta 2020;102:74–83. https://doi.org/10.1016/j.placenta.2020.09.004.Suche in Google Scholar PubMed
22. Vuong, HE, Xin, Y, Dsouza, M, Hsiao, EY. Maternal microbiome modulates fetal neurodevelopment and behavioral outcomes. Curr Opin Neurobiol 2023;78:102667. https://doi.org/10.1016/j.conb.2022.102667.Suche in Google Scholar
23. Collado, MC, Rautava, S, Aakko, J, Isolauri, E, Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 2016;6:23129. https://doi.org/10.1038/srep23129.Suche in Google Scholar PubMed PubMed Central
24. Hambridge, HL, Nunez, M, Bick, J, Ritz, B, Wilhelm, M. Prenatal antibiotic exposure and neurodevelopmental outcomes in children: a population-based study. Paediatr Perinat Epidemiol 2022;36:254–63. https://doi.org/10.1111/ppe.12830.Suche in Google Scholar PubMed PubMed Central
25. Slykerman, RF, Coomarasamy, C, Wickens, K, Thompson, JMD, Stanley, TV, Barthow, C, et al.. Exposure to antibiotics in the first six months of life and neurocognitive outcomes at 11 years of age. Psychopharmacology 2019;236:1573–82. https://doi.org/10.1007/s00213-018-5103-0.Suche in Google Scholar
26. Goldstein, JM, Cherkerzian, S, Seidman, LJ, Donohoe, G, McCarley, RW, Wojcik, J, et al.. Sex differences in the risk for schizophrenia and autism: immune function and volume of brain structures. Brain Behav Immun 2021;95:134–47. https://doi.org/10.1016/j.bbi.2021.03.019.Suche in Google Scholar PubMed PubMed Central
27. Bale, TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress 2016;19:1–6. https://doi.org/10.3109/10253890.2015.1053454.Suche in Google Scholar PubMed
28. Dera, N, Kosińska-Kaczyńska, K, Żeber-Lubecka, N, Brawura-Biskupski-Samaha, R, Massalska, D, Szymusik, I, et al.. Impact of early-life microbiota on immune system development and allergic disorders. Biomedicines 2025;13:121. https://doi.org/10.3390/biomedicines13010121.Suche in Google Scholar PubMed PubMed Central
29. Liu, Y, Ma, J, Li, X, Zhao, H, Ai, Q, Zhang, L, et al.. No microorganism was detected in amniotic fluid of healthy pregnancies from the second trimester to the delivery. Microbiome 2025;13:20. https://doi.org/10.1186/s40168-024-02024-3.Suche in Google Scholar PubMed PubMed Central
30. He, P, He, H, Su, C, Liu, Y, Wang, J, Wu, Y, et al.. Amomum villosum lour. alleviates pre-eclampsia by inducing enrichment of Bifidobacterium bifidum through vanillic acid to inhibit placental ferroptosis. J Ethnopharmacol 2025;340:119217. https://doi.org/10.1016/j.jep.2024.119217.Suche in Google Scholar PubMed
31. Wu, JJ, Zheng, X, Wu, C, Ma, W, Wang, Y, Wang, J, et al.. Melatonin alleviates high temperature exposure induced fetal growth restriction via the gut-placenta-fetus axis in pregnant mice. J Adv Res 2025;68:131–46. https://doi.org/10.1016/j.jare.2024.02.014.Suche in Google Scholar PubMed PubMed Central
32. Feng, C, Wu, Y, Zhang, X, Wang, S, Wang, J, Yang, H. Maternal milk fat globule membrane enriched gut L. murinus and circulating SCFAs to improve placental efficiency and fetal development in intrauterine growth restricted mice model. Gut Microbes 2025;17:2449095. https://doi.org/10.1080/19490976.2024.2449095.Suche in Google Scholar PubMed
33. Biagioli, V, Volpedo, G, Riva, A, Mainardi, P, Striano, P. From birth to weaning: a window of opportunity for microbiota. Nutrients 2024;16:272. https://doi.org/10.3390/nu16020272.Suche in Google Scholar PubMed PubMed Central
34. Lu, X, Shi, Z, Jiang, L, Zhang, S. Maternal gut microbiota in the health of mothers and offspring: from the perspective of immunology. Front Immunol 2024;15:1362784. https://doi.org/10.3389/fimmu.2024.1362784.Suche in Google Scholar PubMed PubMed Central
35. Dera, N, Żeber-Lubecka, N, Ciebiera, M, Kosińska-Kaczyńska, K, Szymusik, I, Massalska, D, et al.. Intrauterine shaping of fetal microbiota. J Clin Med 2024;13:5331. https://doi.org/10.3390/jcm13175331.Suche in Google Scholar PubMed PubMed Central
36. Friel, C, Leyland, AH, Anderson, JJ, Havdahl, A, Brantsæter, AL, Dundas, R. Healthy prenatal dietary pattern and offspring autism. JAMA Netw Open 2024;7:e2422815. https://doi.org/10.1001/jamanetworkopen.2024.22815.Suche in Google Scholar PubMed PubMed Central
37. Chen, S, Zhou, Y, Chen, Y, Gu, J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2020;34:i884–90. https://doi.org/10.1093/bioinformatics/btz728.Suche in Google Scholar PubMed
38. Koren, O, Konnikova, L, Brodin, P, Mysorekar, IU, Collado, MC. The maternal gut microbiome in pregnancy: implications for the developing immune system. Nat Rev Gastroenterol Hepatol 2024;21:35–45. https://doi.org/10.1038/s41575-023-00864-2.Suche in Google Scholar PubMed PubMed Central
39. Chenghan, M, Wanxin, L, Bangcheng, Z, Yao, H, Qinxi, L, Ting, Z, et al.. Short-chain fatty acids mediate gut microbiota-brain communication and protect the blood-brain barrier integrity. Ann N Y Acad Sci 2025;1545:116–31. https://doi.org/10.1111/nyas.15299.Suche in Google Scholar PubMed
40. Martindale, RG, Mundi, MS, Hurt, RT, McClave, SA. Short-chain fatty acids in clinical practice: where are we? Curr Opin Clin Nutr Metab Care 2025;28:54–60. https://doi.org/10.1097/MCO.0000000000001101.Suche in Google Scholar PubMed
41. Yu, W, Sun, S, Fu, Q, Zhou, H, Liu, Z. The role of short-chain fatty acid in metabolic syndrome and its complications: focusing on immunity and inflammation. Front Immunol 2025;16:1519925. https://doi.org/10.3389/fimmu.2025.1519925. Erratum in: Front Immunol. 2025;16:1580492. https://doi.org/10.3389/fimmu.2025.1580492 Suche in Google Scholar PubMed PubMed Central
42. Guo, B, Zhang, J, Zhang, W, Chen, F, Liu, B. Gut microbiota-derived short chain fatty acids act as mediators of the gut-brain axis targeting age-related neurodegenerative disorders: a narrative review. Crit Rev Food Sci Nutr 2025;65:265–86. https://doi.org/10.1080/10408398.2023.2272769.Suche in Google Scholar PubMed
43. ClinicalTrials.gov. Probiotic supplementation in pregnant women with diabetes and effects on infant neurodevelopment. Identifier. NCT05467150. Available from https://clinicaltrials.gov/study/NCT05467150.Suche in Google Scholar
44. Mirzakhani, H, Carey, VJ, McElrath, TF, Laranjo, N, O’Connor, G, Iverson, R, et al.. Maternal vitamin D status and child neurodevelopment: insights from the VDAART cohort. J Steroid Biochem Mol Biol 2022;221:106072. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9996363/ Suche in Google Scholar
45. Wang, Y, Wang, H, Chen, Y, Sun, R, Liang, Y, Yin, Y, et al.. Maternal probiotic treatment affects offspring neurodevelopment by modulating inflammation and neural progenitor differentiation. Nutrients 2020;12:1236. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7235088/.Suche in Google Scholar
46. Aatsinki, AK, Lahti, L, Uusitupa, HM, Munukka, E, Keskitalo, A, Nolvi, S, et al.. Maternal microbiota during pregnancy predicts child behavioural problems. EBioMedicine. 2021;68:103400. https://doi.org/10.1016/j.ebiom.2021.103400 Suche in Google Scholar PubMed PubMed Central
47. ClinicalTrials.gov. A study of metformin in individuals with fragile X syndrome. Identifier: NCT03862950. Available from: https://clinicaltrials.gov/study/NCT03862950 Suche in Google Scholar
48. ClinicalTrials.gov. Study of vivomixx probiotic in children with autism spectrum disorders. Identifier. NCT03369431. Available from https://clinicaltrials.gov/study/NCT03369431.Suche in Google Scholar
49. ClinicalTrials.gov. Vaginal progesterone for prevention of preterm birth in HIV-infected pregnant women. Identifier. NCT02970552. Available from: https://clinicaltrials.gov/study/NCT02970552.Suche in Google Scholar
50. ClinicalTrials.gov. Safety and efficacy of everolimus in patients with PTEN mutations. Identifier. NCT02991807. Available from: https://clinicaltrials.gov/study/NCT02991807.Suche in Google Scholar
51. ClinicalTrials.gov. Human milk-based fortifier trial in preterm infants. Identifier. NCT03797157. Available from: https://clinicaltrials.gov/study/NCT03797157.Suche in Google Scholar
52. ClinicalTrials.gov. Inhaled tobramycin for bronchopulmonary dysplasia in preterm infants. Identifier. NCT04560179. Available from https://clinicaltrials.gov/study/NCT04560179.Suche in Google Scholar
53. Burton, A, Torres-Padilla, ME. Epigenome dynamics in early Mammalian embryogenesis. Nat Rev Genet 2025. https://doi.org/10.1038/s41576-025-00831-4.Suche in Google Scholar PubMed
54. Di Gesù, CM, Buffington, SA. The early life exposome and autism risk: a role for the maternal microbiome? Gut Microbes 2024;16:2385117. https://doi.org/10.1080/19490976.2024.2385117.Suche in Google Scholar PubMed PubMed Central
55. Zhang, H, Zha, X, Zhang, B, Zheng, Y, Elsabagh, M, Wang, H, et al.. Gut microbiota contributes to bisphenol A-induced maternal intestinal and placental apoptosis, oxidative stress, and fetal growth restriction in pregnant Ewe model by regulating gut-placental axis. Microbiome 2024;12:28. https://doi.org/10.1186/s40168-024-01749-5.Suche in Google Scholar PubMed PubMed Central
56. Barker-Tejeda, TC, Zubeldia-Varela, E, Macías-Camero, A, Alonso, L, Martín-Antoniano, IA, Rey-Stolle, MF, et al.. Comparative characterization of the infant gut microbiome and their maternal lineage by a multi-omics approach. Nat Commun 2024;15:3004. https://doi.org/10.1038/s41467-024-47182-y.Suche in Google Scholar PubMed PubMed Central
57. Dang, H, Feng, P, Zhang, S, Peng, L, Xing, S, Li, Y, et al.. Maternal gut microbiota influence stem cell function in offspring. Cell Stem Cell 2025;32:246–62.e8. https://doi.org/10.1016/j.stem.2024.10.003.Suche in Google Scholar PubMed
58. Eckermann, H, Lustermans, H, Parnanen, K, Lahti, L, de Weerth, C. Maternal pre- and postnatal stress and maternal and infant gut microbiota features. Psychoneuroendocrinology 2025;172:107273. https://doi.org/10.1016/j.psyneuen.2024.107273.Suche in Google Scholar PubMed
59. Schepanski, S, Ngoumou, GB, Buss, C, Seifert, G. Assessing in-vitro models for microglial development and fetal programming: a critical review. Front Immunol 2025;16:1538920. https://doi.org/10.3389/fimmu.2025.1538920.Suche in Google Scholar PubMed PubMed Central
60. Hendriks, D, Pagliaro, A, Andreatta, F, Ma, Z, van Giessen, J, Massalini, S, et al.. Human fetal brain self-organizes into long-term expanding organoids. Cell 2024;187:712–32.e38. https://doi.org/10.1016/j.cell.2023.12.012.Suche in Google Scholar PubMed
61. Lawrence, AR, Canzi, A, Bridlance, C, Olivié, N, Lansonneur, C, Catale, C, et al.. Microglia maintain structural integrity during fetal brain morphogenesis. Cell 2024;187:962–80.e19. https://doi.org/10.1016/j.cell.2024.01.012.Suche in Google Scholar PubMed PubMed Central
62. Reis, ÁEM, Teixeira, IS, Maia, JM, Luciano, LAA, Brandião, LM, Silva, MLS, et al.. Maternal nutrition and its effects on fetal neurodevelopment. Nutrition 2024;125:112483. https://doi.org/10.1016/j.nut.2024.112483.Suche in Google Scholar PubMed
63. Ostrem, BEL, Domínguez-Iturza, N, Stogsdill, JA, Faits, T, Kim, K, Levin, JZ, et al.. Fetal brain response to maternal inflammation requires microglia. Development 2024;151:dev202252. https://doi.org/10.1242/dev.202252.Suche in Google Scholar PubMed PubMed Central
64. Frerichs, NM, de Meij, TGJ, Niemarkt, HJ. Microbiome and its impact on fetal and neonatal brain development: current opinion in pediatrics. Curr Opin Clin Nutr Metab Care 2024;27:297–303. https://doi.org/10.1097/MCO.0000000000001028.Suche in Google Scholar PubMed PubMed Central
65. Pereira-Iglesias, M, Maldonado-Teixido, J, Melero, A, Piriz, J, Galea, E, Ransohoff, RM, et al.. Microglia as hunters or gatherers of brain synapses. Nat Neurosci 2025;28:15–23. https://doi.org/10.1038/s41593-024-01818-w.Suche in Google Scholar PubMed
66. Lana, D, Giovannini, MG. Special issue: recent advances in microglia research. Int J Mol Sci 2025;26:507. https://doi.org/10.3390/ijms26020507.Suche in Google Scholar PubMed PubMed Central
67. Lee, JJ, Yang, L, Kotzin, JJ, Ahimovic, D, Bale, MJ, Nigrovic, PA, et al.. Early transcriptional effects of inflammatory cytokines reveal highly redundant cytokine networks. J Exp Med 2025;222:e20241207. https://doi.org/10.1084/jem.20241207.Suche in Google Scholar PubMed PubMed Central
68. Saghazadeh, A, Ataeinia, B, Keynejad, K, Abdolalizadeh, A, Hirbod-Mobarakeh, A, Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: effects of age, gender, and latitude. J Psychiatr Res 2019;115:90–102. https://doi.org/10.1016/j.jpsychires.2019.05.019.Suche in Google Scholar PubMed
69. Zhou, J, Yan, P, Ma, W, Li, J. Cytokine modulation and immunoregulation of uterine NK cells in pregnancy disorders. Cytokine Growth Factor Rev 2025;81:40–53. https://doi.org/10.1016/j.cytogfr.2024.11.007.Suche in Google Scholar PubMed
70. Pillai, A. They are what they eat. Sci Immunol 2025;10:eadx7179. https://doi.org/10.1126/sciimmunol.adx7179.Suche in Google Scholar PubMed
71. Arneth, B. Molecular mechanisms of immune regulation: a review. Cells 2025;14:283. https://doi.org/10.3390/cells14040283.Suche in Google Scholar PubMed PubMed Central
72. Olova, NN. Epigenetic rejuvenation: a journey backwards towards an epigenomic ground state. Epigenomics 2025;17:1–3. https://doi.org/10.1080/17501911.2024.2432851.Suche in Google Scholar PubMed PubMed Central
73. McMahon, C, Mills, C. Against epigenetic responsibility: the problem of causality in ’foetal programming’ science. Bioethics 2025;39:127–36. https://doi.org/10.1111/bioe.13350.Suche in Google Scholar PubMed PubMed Central
74. Jiao, P, Lu, H, Hao, L, Degen, AA, Cheng, J, Yin, Z, et al.. Nutrigenetic and epigenetic mechanisms of maternal nutrition-induced glucolipid metabolism changes in the offspring. Nutr Rev 2025;83:728–48. https://doi.org/10.1093/nutrit/nuae048.Suche in Google Scholar PubMed
75. Wei, Y, Wang, J, Qu, R, Zhang, W, Tan, Y, Sha, Y, et al.. Genetic mechanisms of fertilization failure and early embryonic arrest: a comprehensive review. Hum Reprod Update 2024;30:48–80. https://doi.org/10.1093/humupd/dmad026.Suche in Google Scholar PubMed
76. Kobow, K, Khan, N. Epigenetics. In: Noebels, JL, Avoli, M, Rogawski, MA, Vezzani, A, Delgado-Escueta, AV, editors. Jasper’s Basic Mechanisms of the Epilepsies, 5th ed. New York: Oxford University Press; 2024. Chapter 35.Suche in Google Scholar
77. de Oliveira Melo, NC, Cuevas-Sierra, A, Souto, VF, Martínez, JA. Biological rhythms, chrono-nutrition, and gut microbiota: epigenomics insights for precision nutrition and metabolic health. Biomolecules 2024;14:559. https://doi.org/10.3390/biom14050559.Suche in Google Scholar PubMed PubMed Central
78. Chavatte-Palmer, P, Couturier-Tarrade, A, Rousseau-Ralliard, D. Intra-uterine programming of future fertility. Reprod Domest Anim 2024;59:e14475. https://doi.org/10.1111/rda.14475.Suche in Google Scholar PubMed
79. Bedford, SA, Lai, MC, Lombardo, MV, Chakrabarti, B, Ruigrok, A, Suckling, J, et al.. Brain-charting autism and attention-deficit/hyperactivity disorder reveals distinct and overlapping neurobiology. Biol Psychiatry 2025;97:517–30. https://doi.org/10.1016/j.biopsych.2024.07.024.Suche in Google Scholar PubMed
80. Cortese, S, Bellato, A, Gabellone, A, Marzulli, L, Matera, E, Parlatini, V, et al.. Latest clinical frontiers related to autism diagnostic strategies. Cell Rep Med 2025;6:101916. https://doi.org/10.1016/j.xcrm.2024.101916.Suche in Google Scholar PubMed PubMed Central
81. Moreno, RJ, Abu Amara, R, Ashwood, P. Toward a better understanding of T cell dysregulation in autism: an integrative review. Brain Behav Immun 2025;123:1147–58. https://doi.org/10.1016/j.bbi.2024.10.009.Suche in Google Scholar PubMed
82. Roush, K. Autism diagnoses have surged over the past decade. Am J Nurs 2025;125:12. https://doi.org/10.1097/01.NAJ.0001098228.00982.38.Suche in Google Scholar PubMed
83. Wynn, J, Karlsen, A, Huber, B, Levine, A, Salem, A, White, LC, et al.. Impact of a genetic diagnosis for a child’s autism on parental perceptions. J Autism Dev Disord 2025;55:1809–23. https://doi.org/10.1007/s10803-024-06273-x.Suche in Google Scholar PubMed PubMed Central
84. Anixt, JS, Ehrhardt, J, Duncan, A. Evidence-based interventions in autism. Pediatr Clin 2024;71:199–221. https://doi.org/10.1016/j.pcl.2024.01.001.Suche in Google Scholar PubMed PubMed Central
85. Olson, L, Bishop, S, Thurm, A. Differential diagnosis of autism and other neurodevelopmental disorders. Pediatr Clin 2024;71:157–77. https://doi.org/10.1016/j.pcl.2023.12.004.Suche in Google Scholar PubMed PubMed Central
86. Wachtel, LE, Escher, J, Halladay, A, Lutz, A, Satriale, GM, Westover, A, et al.. Profound autism: an imperative diagnosis. Pediatr Clin 2024;71:301–13. https://doi.org/10.1016/j.pcl.2023.12.005.Suche in Google Scholar PubMed
87. Cheng, TL. Autism today. Pediatr Clin 2024;71:xv–xvi. https://doi.org/10.1016/j.pcl.2024.01.009.Suche in Google Scholar PubMed
88. Fazel Darbandi, S, An, JY, Lim, K, Page, NF, Liang, L, Young, DM, et al.. Five autism-associated transcriptional regulators target shared loci proximal to brain-expressed genes. Cell Rep 2024;43:114329. https://doi.org/10.1016/j.celrep.2024.114329.Suche in Google Scholar PubMed PubMed Central
89. Zhao, T, Huang, CQ, Zhang, YH, Zhu, YY, Chen, XX, Wang, T, et al.. Prenatal 1-nitropyrene exposure causes autism-like behavior partially by altering DNA hydroxymethylation in developing brain. Adv Sci (Weinh) 2024;11:e2306294. https://doi.org/10.1002/advs.202306294.Suche in Google Scholar PubMed PubMed Central
90. Cai, L, Fan, Q, Pang, R, Chen, C, Zhang, Y, Xie, H, et al.. Microglia programmed cell death in neurodegenerative diseases and CNS injury. Apoptosis 2025;30:446–65. https://doi.org/10.1007/s10495-024-02041-5.Suche in Google Scholar PubMed PubMed Central
91. Duffy, AS, Eyo, UB. Microglia and astrocytes in postnatal neural circuit formation. Glia 2025;73:232–50. https://doi.org/10.1002/glia.24650.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


