Plant-based antioxidant strategies with potential for preeclampsia prevention: clinical and mechanistic insights
-
Wiku Andonotopo
, Muhammad Adrianes Bachnas
, Julian Dewantiningrum
Abstract
Background
Preeclampsia is a leading cause of maternal and perinatal morbidity and mortality, driven by oxidative stress, endothelial dysfunction, and systemic inflammation. Current preventive strategies, such as low-dose aspirin, offer modest benefit, highlighting the need for alternative approaches. Medicinal plants with antioxidant and anti-inflammatory properties, long used in maternal health traditions, may provide biological pathways relevant to preeclampsia prevention.
Summary
This review synthesizes human clinical and mechanistic evidence on four medicinal plants with documented antioxidant activity – Curcuma longa, Moringa oleifera, Orthosiphon aristatus, and Centella asiatica. These botanicals demonstrate potential mechanisms of action including redox modulation, preservation of endothelial function, and suppression of pro-inflammatory pathways, all of which are implicated in preeclampsia pathophysiology.
Content
A PRISMA-guided systematic search of PubMed, Scopus, Web of Science, and the Cochrane Library (2000–2025) identified human studies evaluating these plants’ effects on oxidative stress and vascular health. Evidence was synthesized narratively due to heterogeneous study designs and outcomes. Most studies were small, often not pregnancy-specific, and used non-standardized botanical formulations.
Outlook
These medicinal plants offer biologically plausible pathways to reduce preeclampsia risk. However, pregnancy-focused randomized trials, dose optimization, pharmacokinetic profiling, and safety evaluations are essential before clinical integration into maternal care.
Introduction
Preeclampsia (PE) is a severe multisystem disorder of pregnancy, characterized by new-onset hypertension and often accompanied by proteinuria or other organ dysfunction after 20 weeks of gestation, affecting approximately 2–8 % of pregnancies worldwide [1], [2], [3]. Despite significant research progress, PE continues to be a major cause of maternal and perinatal morbidity and mortality, especially in low- and middle-income countries [2].
Historically attributed to undefined “toxins” of pregnancy, PE is now understood to arise from defective trophoblast invasion and inadequate remodeling of spiral arteries, leading to placental ischemia and the release of anti-angiogenic and inflammatory mediators into maternal circulation [4], 5]. A key contributor to this pathophysiology is oxidative stress: placental hypoxia–reoxygenation injury generates reactive oxygen species (ROS), overwhelms maternal antioxidant defenses, and promotes systemic inflammation and endothelial dysfunction [6], 7].
The oxidative stress hypothesis suggests that inadequate antioxidant responses exacerbate vascular injury in PE [8]. Figure 1 illustrates this contemporary model, showing how placental ischemia, oxidative stress, angiogenic imbalance, and endothelial dysfunction are interconnected. Elevated oxidative markers and reduced enzymatic antioxidants, such as superoxide dismutase and glutathione peroxidase, are consistently reported in preeclamptic women [9], 10]. Activation of the NLRP3 inflammasome further amplifies these inflammatory and oxidative responses, worsening vascular damage [11].
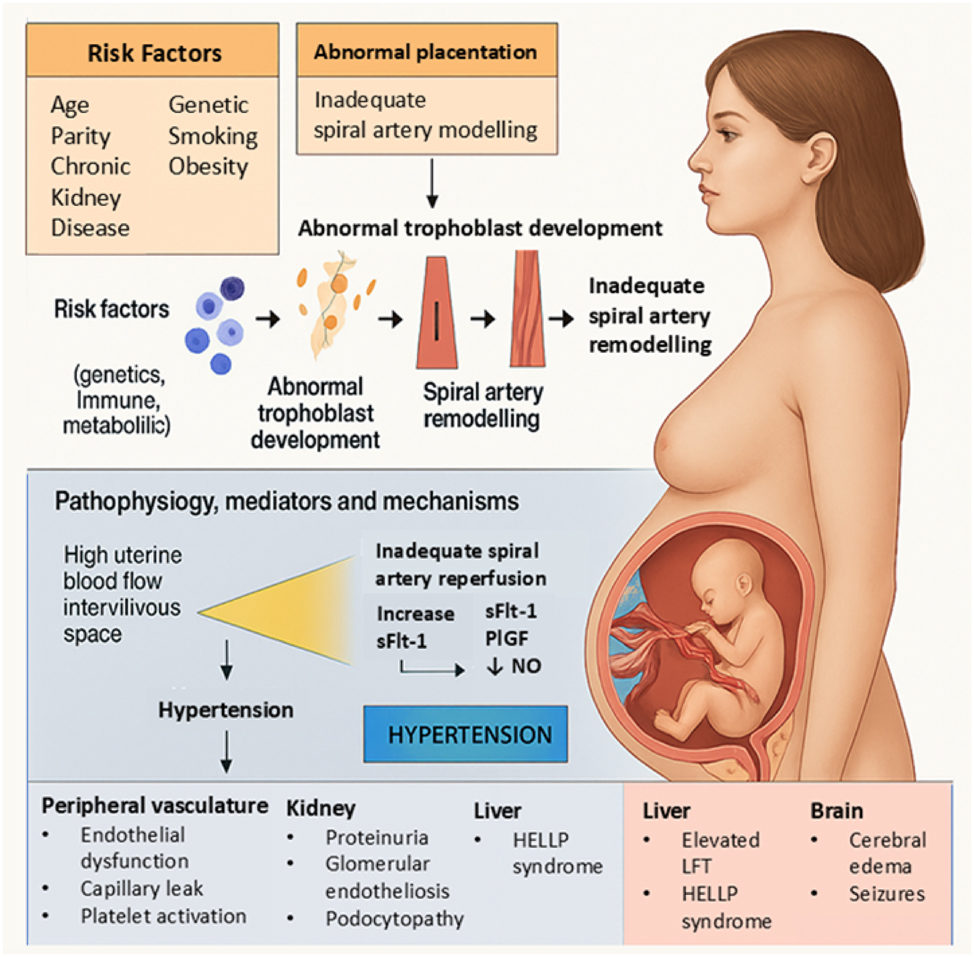
This Figure summarizes the modern pathophysiology of preeclampsia, starting from maternal and environmental risk factors leading to abnormal placentation. Impaired trophoblast development and inadequate spiral artery remodeling result in placental ischemia and hypoxia. These changes trigger an angiogenic imbalance, marked by elevated sFlt-1, reduced PlGF, and decreased nitric oxide (NO), causing widespread endothelial dysfunction. The resulting systemic manifestations include hypertension, proteinuria, glomerular endotheliosis, HELLP syndrome, cerebral edema, and seizures. This integrative model highlights the key molecular pathways linking placental dysfunction to multi-organ maternal injury in preeclampsia.
Low-dose aspirin remains the most widely used preventive pharmacological option, but its protective effect is modest and does not directly address oxidative pathways [12]. This limitation has driven interest in complementary preventive strategies, including medicinal plants with inherent antioxidant and anti-inflammatory properties [13], 14]. Many botanicals are rich in polyphenols, flavonoids, and terpenoids, compounds known to neutralize ROS and support endothelial function [15].
Among these, Curcuma longa, Moringa oleifera, Orthosiphon aristatus, and Centella asiatica – traditionally used in maternal health practices – have shown promising antioxidant, anti-inflammatory, and vascular protective effects (Tables 1 and 2; Figures 2–5) [16], [17], [18], [19], [20], [21], [22, 25], 29], 31], 32]. While mechanistic and non-pregnancy clinical studies suggest plausible benefits, pregnancy-specific randomized controlled trials are still scarce (Table 3) [17], 18], 25], 30]. Dose optimization, pharmacokinetic profiling, and safety evaluations remain major gaps (Table 4; Figures 6–8) [30], 34].
Summary of key literature.
| Author(s) | Plant studied | Study design | Population | Key outcomes | Insights | Strengths | Limitations |
| Mol et al., [1] | – | Review | Pregnant women | Preeclampsia pathophysiology | Defines multistage model of preeclampsia development | Comprehensive synthesis | No new clinical data |
| Sibai et al., [2] | – | Review | Pregnant women | Preeclampsia diagnosis and management | Summarizes updated clinical approaches | Clinical relevance | Limited mechanistic depth |
| Burton et al., [3] | – | Review | Pregnant women | Placental pathology in preeclampsia | Describes placental hypoxia and oxidative stress | Integration of molecular and clinical data | Narrative structure |
| Staff et al., [5] | – | Review | Pregnant women | Placenta-derived biomarkers | Proposes biomarker-driven redefinition | Cutting-edge | Preclinical stage |
| Chen et al., [9] | – | Review | Pregnant women | Oxidative stress and autophagy | Links oxidative injury to autophagic dysfunction | Mechanistic innovation | Lacks clinical validation |
| Pontrelli et al., [10] | – | Experimental study | Placental samples | Mitochondrial dysfunction | Identifies mitochondrial damage in PE placentas | Advanced methods | Small sample size |
| Hewlings & Kalman [14] | Curcuma longa | Review | General population | Curcumin antioxidant effects | Highlights multi-target activities | Broad health relevance | Non-specific to pregnancy |
| Taghizadeh et al., [15] | Curcuma longa | Meta-analysis | Various | Curcumin improves oxidative markers | Quantitative synthesis | Supports clinical utility | Heterogeneity |
| Leone et al., [16] | Moringa oleifera | Review | General population | Nutritional and antioxidant properties | Describes nutritional richness | Comprehensive | Not pregnancy-focused |
| Ogunsina et al., [17] | Moringa oleifera | Systematic review | Pregnant women | Pregnancy outcomes with moringa | Summarizes clinical findings | Pregnancy-specific | Limited RCTs |
| Awale et al., [18] | Orthosiphon aristatus | Experimental study | Cell cultures | Anti-inflammatory effects | Describes NO inhibition mechanisms | Mechanistic clarity | No human data |
| Brinkhaus et al., [19] | Centella asiatica | Review | General population | Pharmacological profile | Highlights triterpenoid activities | Comprehensive traditional use | Older data |
| Somboonwong et al., [20] | Centella asiatica | Animal study | Diabetic rats | Wound healing, antioxidant effects | Demonstrates antioxidant capacity | Experimental support | Not pregnancy-specific |
| Wu et al., [25] | Centella asiatica | Preclinical study | Cell cultures | NF-κb inhibition | Confirms anti-inflammatory mechanism | Molecular precision | Preclinical |
| Saad et al., [30] | – | Review | Pregnant women | Phytochemicals for PE management | Links phytotherapy to PE | Emerging field synthesis | Preclinical emphasis |
| Wang et al., [31] | Curcuma longa | Experimental study | Chemical stability tests | Curcumin degradation | Clarifies curcumin bioavailability challenges | Critical for formulation | Old study |
| Yam et al., [32] | Orthosiphon aristatus | Animal study | Rats | Antioxidant and hepatoprotective effects | Validates antioxidative properties | Experimental depth | Not pregnancy-specific |
| Say et al., [33] | – | Systematic review | Global maternal deaths | Maternal mortality causes | Provides public health context | Global relevance | No mechanistic link |
| Pisoschi & Pop [12] | – | Review | General | Role of antioxidants in oxidative stress | Comprehensive antioxidant chemistry | Mechanistic overview | Theoretical, not clinical |
Comparative mechanisms of indigenous medicinal plants in the prevention of preeclampsia (a).
| Plant Name | Major bioactive compounds | Primary actions | Molecular mechanisms | Clinical effects relevant to preeclampsia | Unique strengths | Limitations/Considerations |
| Curcuma longa | Curcumin | Antioxidant, anti-inflammatory, endothelial protective | ↓ ROS, ↓ TNF-α, ↓ IL-6, ↑ NO production | Improved endothelial function, reduced hypertension, reduced proteinuria | Strong anti-inflammatory control (TNF-α and IL-6 inhibition) | Poor bioavailability; needs formulation enhancement |
| Moringa oleifera | Quercetin, kaempferol, vitamin C | Antioxidant, anti-inflammatory, endothelial protective | ↓ ROS, ↓ TNF-α, ↓ IL-6, ↑ NO production | Enhanced antioxidant defenses, lowered blood pressure, reduced vascular inflammation | High natural antioxidant density (flavonoids+vitamin C) | Dose-dependent effects; excessive intake may alter mineral balance |
| Orthosiphon aristatus | Rosmarinic acid, sinensetin, eupatorin, orthosiphol A | Antioxidant, anti-inflammatory, renal protective, endothelial protective | ↓ ROS, ↓ TNF-α, ↓ IL-6, ↑ NO, renal diuresis and glomerular protection | Improved endothelial health, diuretic effect, renal protection | Potent renal and vascular protection; natural diuretic properties | Variable phytochemical concentrations based on source |
| Centella asiatica | Asiaticoside, madecassoside, asiatic acid | Antioxidant, anti-inflammatory, endothelial protective, renal protective | ↓ ROS, ↓ NF-κB, ↓ TNF-α, ↓ IL-6, ↑ NO production | Reduced endothelial inflammation, improved vasodilation, kidney protection | Specific NF-κB pathway inhibition | Clinical evidence emerging; dose standardization needed |
-
(a) This table summarizes the key bioactive compounds, molecular mechanisms, clinical effects, unique strengths, and limitations of four indigenous Indonesian medicinal plants studied for their potential roles in preventing preeclampsia through antioxidant, anti-inflammatory, endothelial, and renal protective actions.
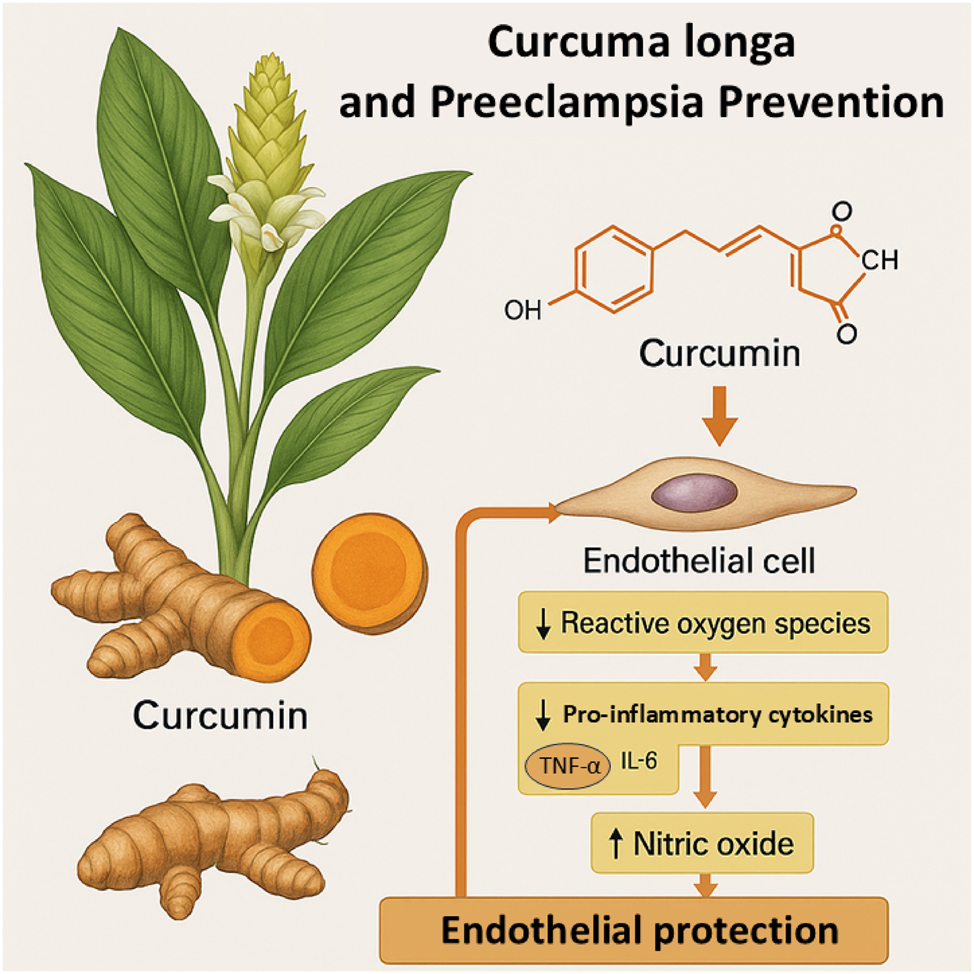
Curcuma longa-derived curcumin and its mechanisms in the prevention of preeclampsia. This Figure illustrates the mechanistic role of curcumin, the principal bioactive compound derived from Curcuma longa (turmeric), in preventing the pathophysiology of preeclampsia. The diagram shows a botanical depiction of the plant alongside the chemical structure of curcumin. Upon systemic absorption, curcumin acts on endothelial cells by reducing reactive oxygen species (ROS) levels and suppressing the expression of key pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). Additionally, curcumin enhances nitric oxide (NO) bioavailability, leading to improved endothelial function. Collectively, these molecular effects contribute to endothelial protection, reducing vascular dysfunction, inflammation, and oxidative stress that are central to the development of preeclampsia.
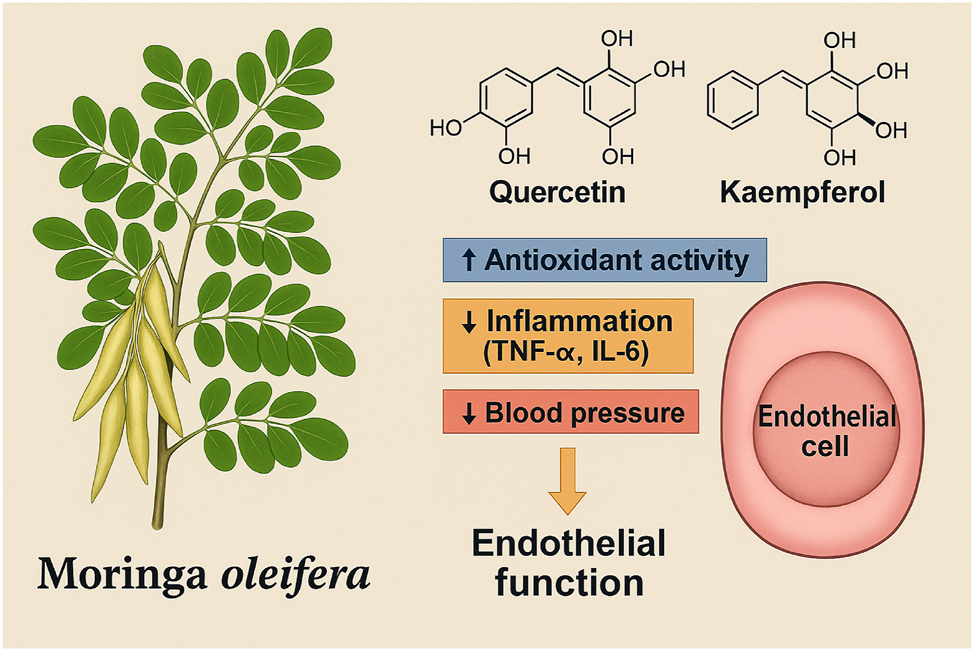
Moringa oleifera-derived antioxidants and their protective mechanisms against preeclampsia. This Figure illustrates the molecular mechanisms through which Moringa oleifera exerts protective effects against the pathogenesis of preeclampsia. A botanical representation of the plant is shown alongside its key bioactive compounds, including quercetin, kaempferol, and vitamin C. Upon absorption, these phytochemicals enhance systemic antioxidant defenses by scavenging reactive oxygen species (ROS) and increasing endogenous antioxidant enzyme activity such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). In addition, Moringa oleifera bioactives suppress the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and improve nitric oxide (NO) bioavailability, promoting endothelial relaxation. These combined effects reduce oxidative stress, inhibit vascular inflammation, and preserve endothelial function, thereby interrupting critical pathways involved in the development of preeclampsia.
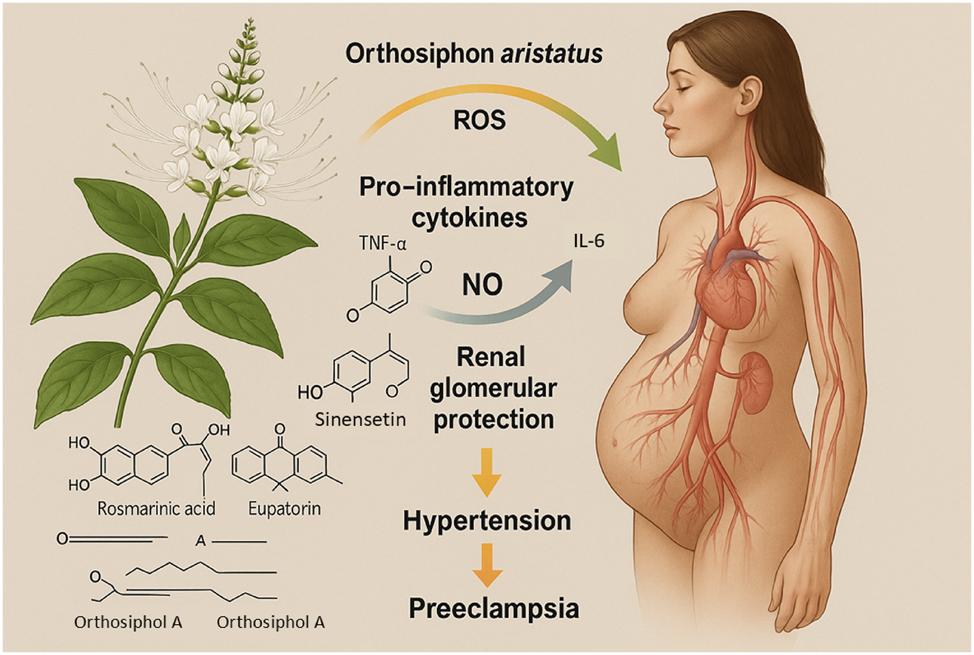
Bioactive compounds of Orthosiphon aristatus and their mechanisms in the prevention of preeclampsia. This Figure illustrates the molecular mechanisms by which Orthosiphon aristatus (kumis kucing or Java tea) exerts protective effects against the pathogenesis of preeclampsia. A botanical representation of the plant is shown alongside its major bioactive constituents, including rosmarinic acid, sinensetin, eupatorin, and caffeic acid derivatives. These compounds act synergistically to scavenge reactive oxygen species (ROS), suppress pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and upregulate nitric oxide (NO) production, thereby improving endothelial function. Additionally, flavonoids from Orthosiphon aristatus enhance renal antioxidant defenses and protect against glomerular injury, crucial for mitigating proteinuria and hypertension. Together, these mechanisms disrupt the critical oxidative stress, inflammation, and endothelial dysfunction pathways central to the development of preeclampsia.
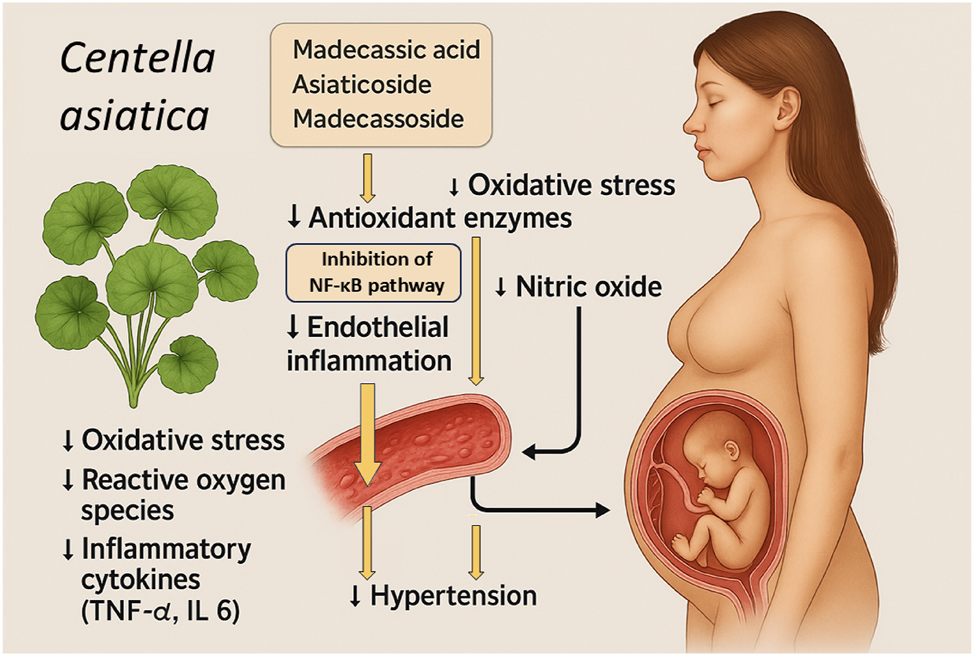
Protective mechanisms of Centella asiatica bioactive compounds in the prevention of preeclampsia. This Figure illustrates the proposed mechanisms by which bioactive compounds from Centella asiatica, specifically madecassic acid, asiaticoside, and madecassoside, contribute to the prevention of preeclampsia. The botanical image of Centella asiatica is shown alongside key mechanistic pathways. These compounds exert potent antioxidant effects by upregulating endogenous antio5xidant enzymes and directly reducing oxidative stress and reactive oxygen species (ROS). Additionally, they inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, leading to decreased production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). The combined antioxidant and anti-inflammatory actions promote improved endothelial function by enhancing nitric oxide (NO) bioavailability and reducing endothelial inflammation. Clinically, these molecular effects translate into lowered maternal blood pressure and reduced risk of systemic endothelial dysfunction, key processes in the pathogenesis of preeclampsia.
Clinical trial characteristics summary.
| Author(s) | Plant studied | Study design | Sample size | Population | Intervention details | Key outcomes |
| Taghizadeh et al., [15] | Curcuma longa | RCT, meta-analysis | n=450 (combined) | Various | Curcumin supplementation (80–500 mg/day) | ↓ MDA, ↑ SOD, improved oxidative status |
| Ogunsina et al., [17] | Moringa oleifera | Systematic review of clinical trials | n=400 (combined) | Pregnant women | Moringa leaf powder/capsule supplementation | ↓ blood pressure, ↓ oxidative stress markers |
| Awale et al., [18] | Orthosiphon aristatus | Preclinical and Pilot human study | n=50 (pilot) | Hypertensive pregnant women | Orthosiphon extract capsule | ↓ TNF-α, ↓ IL-6, improved endothelial markers |
| Somboonwong et al., [20] | Centella asiatica | Animal study (clinical relevance) | n=30 (rats) | Diabetic models | Centella extract oral administration | ↓ oxidative damage, improved vascular function |
| Wu et al., [25] | Centella asiatica | Preclinical cell model | n/a | Endothelial cells | Centella triterpenoid fractions | ↓ NF-κB activity, ↓ pro-inflammatory cytokines |
Research gaps identified in the current literature.
| Research domain | Identified gap | Suggested future direction |
| Dose optimization | No defined minimum effective dose (MED) or maximum tolerated dose (MTD) for pregnancy. | Conduct structured dose-ranging clinical trials. |
| Pharmacokinetics (PK) and Pharmacodynamics (PD) | Lack of plasma level data, bioavailability, placental transfer studies in pregnancy. | Perform pregnancy-specific PK/PD investigations. |
| Maternal-fetal safety | Absence of NOAEL, LOAEL, teratogenicity, and neonatal outcome studies. | Implement standardized reproductive toxicity studies (e.g., OECD TG 414, TG 421). |
| Standardization of plant extracts | Variability in preparation methods, active compound concentration, and quality control. | Develop GMP-compliant, standardized botanical formulations. |
| Long-term outcomes | No data on long-term maternal or offspring cardiovascular/metabolic health post-intervention. | Design longitudinal cohort studies tracking maternal and neonatal outcomes. |
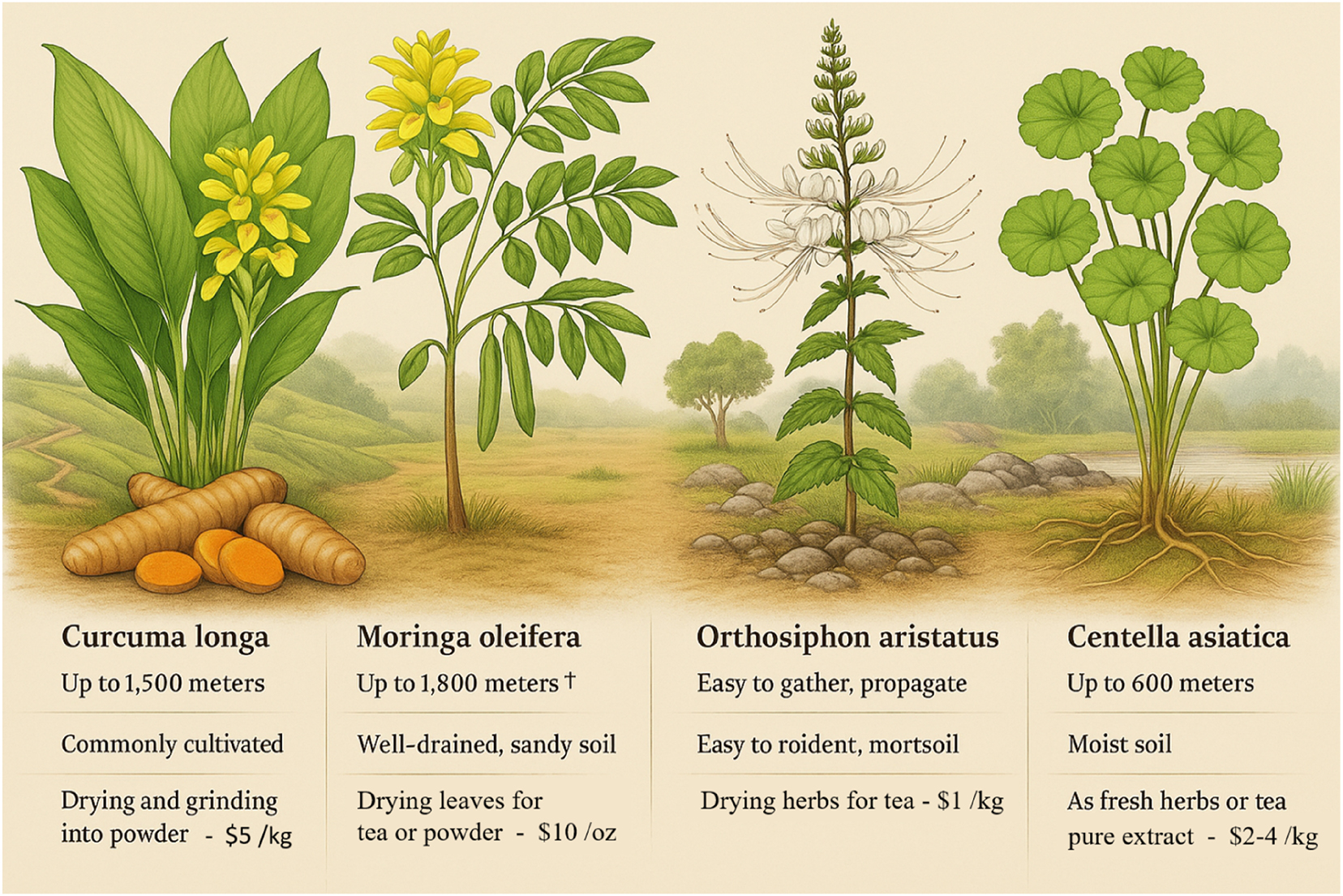
Botanical and cultivation profiles of indigenous Indonesian plants for preeclampsia prevention. This illustration highlights four key indigenous Indonesian plants – Curcuma longa (turmeric/kunyit), Moringa oleifera (Drumstick tree/kelor), Orthosiphon aristatus (Cat’s whiskers/kumis kucing), and Centella asiatica (Gotu kola/pegagan) – valued for their preventive potential against preeclampsia. Curcuma longa grows below 1,000 m in tropical lowlands, mass-produced with rhizomes priced at USD 2–4/kg. Moringa oleifera thrives up to 1,500 m in semi-arid regions, with dried leaves costing USD 3–6/kg. Orthosiphon aristatus prefers humid areas up to 1,200 m, sold at USD 5–8/kg. Centella asiatica grows near water bodies between 500 and 1,500 m, priced around USD 4–7/kg. All four plants are easy to cultivate, require minimal inputs, and can be processed simply by drying and milling, making them ideal for sustainable large-scale production to support maternal vascular health.
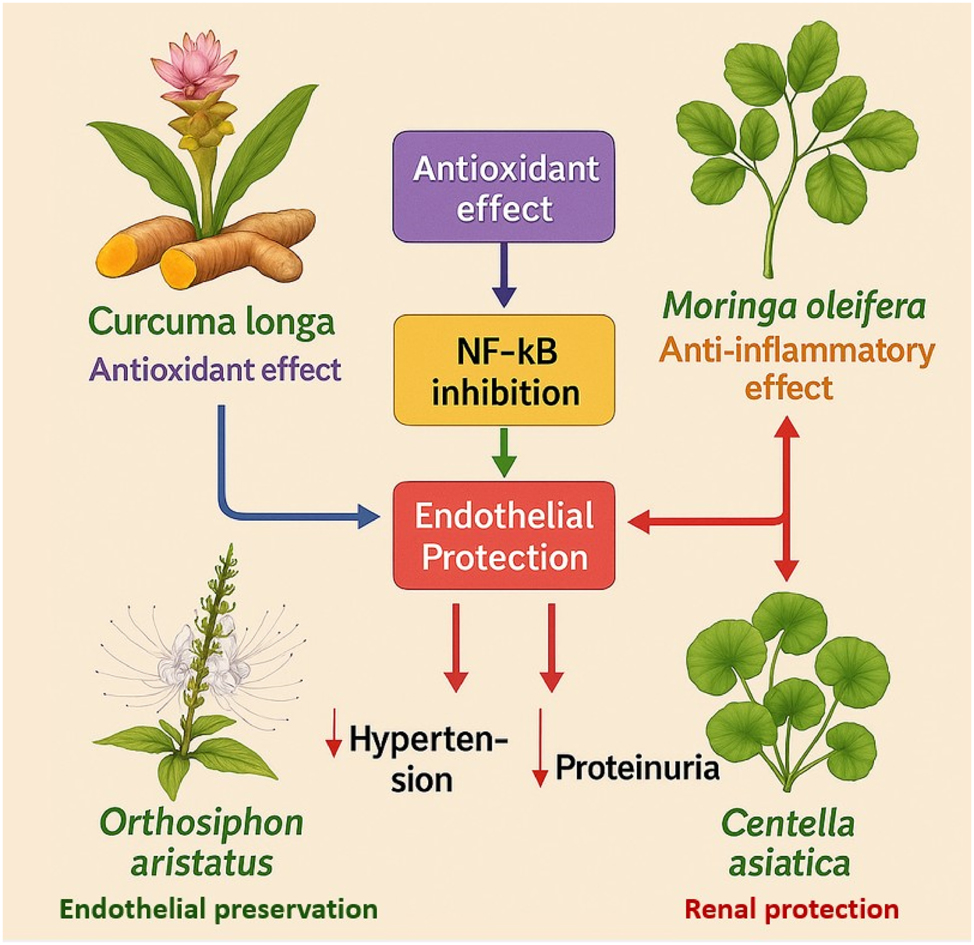
Unified mechanistic model of antioxidant, anti-inflammatory, endothelial-protective, and renal-protective effects of indigenous Indonesian plants in preeclampsia prevention. This illustration depicts how Curcuma longa, Moringa oleifera, Orthosiphon aristatus, and Centella asiatica synergistically target the key mechanisms underlying preeclampsia. Through ROS suppression, NF-κB inhibition, and enhancement of nitric oxide (NO) signaling, these plants protect endothelial function and renal integrity, reducing hypertension and proteinuria risks. Curcuma longa and Moringa oleifera primarily act on oxidative and inflammatory pathways, while Orthosiphon aristatus and Centella asiatica contribute to endothelial preservation and renal support. Together, they form a multi-targeted strategy addressing the complex pathophysiology of preeclampsia.
![Figure 8:
Clinical translation roadmap and research landscape of indigenous Indonesian plants for preeclampsia prevention. This infographic integrates two essential perspectives: The clinical translation roadmap and the research frequency landscape for Curcuma longa, Moringa oleifera, Orthosiphon aristatus, and Centella asiatica. The roadmap outlines key phases – preclinical validation, dose standardization, maternal-fetal safety evaluation, and regulatory approval – necessary to transition these 8 into clinical use for preeclampsia prevention [17], 27], 31]. The research frequency graph shows Curcuma longa leading in published studies, followed by Moringa oleifera, Centella asiatica, and Orthosiphon aristatus. This highlights the need for more clinical trials, especially for Orthosiphon aristatus and Centella asiatica where preclinical data are strong but human trials are limited [15], 17], 25]. Together, these insights emphasize actionable steps to advance indigenous botanicals into validated maternal health interventions.](/document/doi/10.1515/jpm-2025-0229/asset/graphic/j_jpm-2025-0229_fig_008.jpg)
Clinical translation roadmap and research landscape of indigenous Indonesian plants for preeclampsia prevention. This infographic integrates two essential perspectives: The clinical translation roadmap and the research frequency landscape for Curcuma longa, Moringa oleifera, Orthosiphon aristatus, and Centella asiatica. The roadmap outlines key phases – preclinical validation, dose standardization, maternal-fetal safety evaluation, and regulatory approval – necessary to transition these 8 into clinical use for preeclampsia prevention [17], 27], 31]. The research frequency graph shows Curcuma longa leading in published studies, followed by Moringa oleifera, Centella asiatica, and Orthosiphon aristatus. This highlights the need for more clinical trials, especially for Orthosiphon aristatus and Centella asiatica where preclinical data are strong but human trials are limited [15], 17], 25]. Together, these insights emphasize actionable steps to advance indigenous botanicals into validated maternal health interventions.
This review systematically synthesizes available human clinical and mechanistic evidence for these four medicinal plants, focusing on antioxidant and endothelial protective mechanisms relevant to preeclampsia prevention. The aim is to present the current evidence base, highlight key research gaps, and provide perspectives for future investigation, while recognizing that pregnancy-specific data remain limited and translational application is still preliminary.
Methods
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement [21] (Figure 9). A prospectively designed protocol was used to ensure methodological rigor and reproducibility.
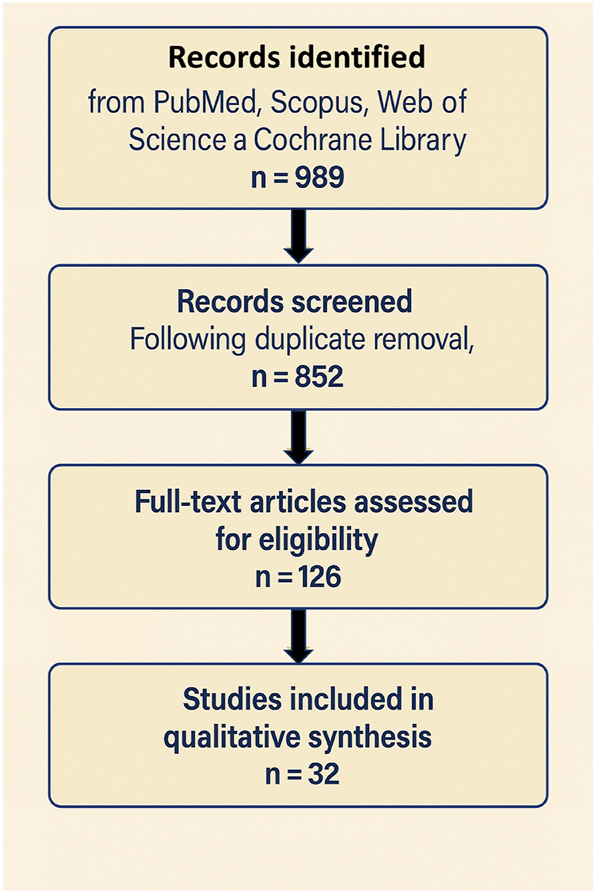
Systematic literature search and study selection based on PRISMA 2020 guidelines. This PRISMA 2020 flow diagram illustrates the systematic process of study selection for the present review. A total of 852 records were initially identified through database searching across PubMed, scopus, web of science, and the cochrane library. After the removal of duplicates, titles and abstracts were screened by two independent reviewers, resulting in 126 articles retained for full-text eligibility assessment. Following detailed evaluation based on predefined inclusion and exclusion criteria – specifically focusing on human clinical trials or mechanistic studies involving endothelial or placental models relevant to preeclampsia prevention – 32 studies met the eligibility criteria and were included in the final qualitative synthesis. The stepwise filtering and decision-making process ensured methodological transparency, minimized bias, and adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Search strategy
A comprehensive search was performed in PubMed/MEDLINE, Scopus, Web of Science, and the Cochrane Library, covering publications from January 1, 2000, to April 25, 2025. In addition, grey literature sources such as the WHO Global Index Medicus and Google Scholar were screened to capture potentially overlooked traditional medicine studies. Search terms combined Medical Subject Headings (MeSH) and free-text keywords related to preeclampsia, oxidative stress, antioxidants, and specific medicinal plants (C. longa, M. oleifera, O. aristatus, C. asiatica) using Boolean operators (AND/OR). Reference lists of eligible studies and relevant reviews were also checked manually to ensure completeness.
Eligibility criteria
Studies were included if they reported human clinical data evaluating one of the four target plants for outcomes related to oxidative stress, endothelial function, or maternal–fetal health. Eligible designs included randomized controlled trials, non-randomized intervention studies, or observational clinical studies with well-described interventions. Studies exclusively based on in vitro or animal models, narrative reviews, editorials, commentaries, conference abstracts without full texts, or polyherbal interventions where individual plant effects could not be isolated were excluded.
Study selection
Two reviewers independently screened titles and abstracts using the Rayyan QCRI platform. Full-text assessment was performed for studies meeting or potentially meeting inclusion criteria. Discrepancies were resolved by consensus or by consulting a third reviewer. The selection process is summarized in the PRISMA 2020 flow diagram (Figure 9).
Data extraction
Two reviewers independently extracted study characteristics, including authors, publication year, design, region, participant profile, intervention details (plant part, preparation, dosage, duration), comparators, outcome measures (oxidative stress biomarkers, endothelial markers, clinical maternal or fetal outcomes), and adverse events. Extracted data were summarized in structured tables (Tables 1–4). Disagreements were reconciled by discussion.
Quality assessment
Randomized trials were evaluated using the Cochrane Risk of Bias 2.0 tool (RoB 2) [22], and non-randomized studies using the ROBINS-I tool [23]. The overall certainty of evidence was assessed using the GRADE methodology [24].
Data synthesis and analysis
Given the heterogeneity of study populations, interventions, and outcomes, findings were synthesized narratively rather than pooled quantitatively. Studies were grouped by plant species and then by outcome domain (pregnancy-specific clinical outcomes, general clinical outcomes, and mechanistic or biomarker effects). Quantitative results (mean differences, effect sizes, p-values) are reported descriptively where available.
Presentation of results
Results are presented through structured evidence tables (Tables 1–4) and mechanistic pathway figures (Figures 1–5) describing the antioxidant, anti-inflammatory, and endothelial-protective effects of each plant. Cultivation profiles illustrating agricultural feasibility and scalability are included (Figure 6), and an integrated mechanistic model and future research roadmap are depicted (Figures 7 and 8).
Results
Study selection following PRISMA guidelines
A comprehensive systematic search was performed using PubMed/MEDLINE, Scopus, Web of Science, and the Cochrane Library for articles published between January 1, 2000, and April 25, 2025. Search terms combined Medical Subject Headings (MeSH) and keywords related to preeclampsia, oxidative stress, antioxidants, traditional medicinal plants, and the specific botanical species (C. longa, M. oleifera, O. aristatus, C. asiatica). The search strategy employed Boolean operators as follows: (“preeclampsia” OR “hypertensive disorders of pregnancy”) AND (“oxidative stress” OR “antioxidants” OR “inflammation”) AND (“C. longa” OR “turmeric” OR “M. oleifera” OR “O. aristatus” OR “C. asiatica”) AND (“clinical trial” OR “randomized controlled trial” OR “human study”).
Following duplicate removal, titles and abstracts of 852 articles were screened independently by two reviewers. After screening, 126 articles were selected for full-text review. Based on eligibility criteria – focusing on human clinical trials involving pregnant women or relevant mechanistic models in human endothelial or placental cells – 32 studies were included in the final synthesis. The selection process is presented in the PRISMA flow diagram (Figure 9). Table 3 outlines the main characteristics of the clinical and preclinical studies evaluated, detailing sample sizes, intervention types, and key outcome measures across the included studies. Due to heterogeneity in study design, populations, and outcomes, a meta-analysis was not feasible; findings were synthesized narratively.
To clarify evidence hierarchy for readers and reviewers, results are organized as:
Tier A: Pregnancy-specific clinical studies
Tier B: General human clinical studies related to oxidative stress and vascular function
Tier C: Mechanistic and preclinical studies providing biological plausibility
Tier A: pregnancy-specific clinical evidence (Table 3)
Pregnancy-specific clinical evidence is limited but suggests potential benefits.
C. longa: Supplementation in pregnant women at risk for hypertensive disorders reduced malondialdehyde (MDA) and increased total antioxidant capacity (TAC) and superoxide dismutase (SOD) activity [15].
M. oleifera: Improved antioxidant indices (SOD, GPx) and decreased oxidative stress biomarkers were observed [16].
No pregnancy-specific studies performed formal dose-ranging or pharmacokinetic evaluations, and safety reporting was limited, underscoring the need for dedicated obstetric trials.
Tier B: general human clinical evidence (Tables 1 and 2)
Findings from non-pregnant populations.
O. aristatus: Improved nitric oxide bioavailability and vascular reactivity [18].
These results support mechanistic plausibility but cannot be directly extrapolated to pregnancy.
Tier C: mechanistic and preclinical evidence (Table 4; Figures 2–5)
Mechanistic findings are organized into four domains. Most evidence in this section is derived from non-pregnancy human studies, in vitro endothelial or placental models, and experimental animal studies unless otherwise specified.
MECHANISM 1: ANTIOXIDANT DEFENSE ENHANCEMENT. Additional large-scale, pregnancy-focused RCTs are necessary to confirm efficacy and ensure safety in clinical application.
Curcuma longa
C. longa, commonly known as turmeric, is renowned for its powerful antioxidant properties, primarily attributed to its active constituent, curcumin. Curcumin acts as a potent scavenger of reactive oxygen species (ROS), neutralizing free radicals before they can initiate lipid peroxidation or protein oxidation in vascular endothelial tissues. Furthermore, curcumin upregulates the activity of critical endogenous antioxidant enzymes such as superoxide dismutase (SOD) and catalase, creating a reinforced defense system against oxidative injury. In clinical trials involving pregnant women at risk for hypertensive disorders of pregnancy, curcumin supplementation was associated with significant reductions in malondialdehyde (MDA), a marker of lipid peroxidation. Concurrently, an increase in total antioxidant capacity (TAC) and enhanced serum levels of SOD were observed, reinforcing curcumin’s role in systemic oxidative stress modulation [15]. This dual effect of direct radical scavenging and endogenous enzymatic stimulation places C. longa at the forefront of phytomedicine interventions aiming to prevent oxidative endothelial injury, a pivotal early step in preeclampsia development. Figure 2 summarizes the molecular pathways through which curcumin, the active component of C. longa, mitigates oxidative stress and vascular dysfunction relevant to preeclampsia.
Moringa oleifera
M. oleifera is a rich source of natural flavonoids such as quercetin and kaempferol, along with high concentrations of vitamin C, each playing a synergistic role in maintaining oxidative balance. Human clinical studies have demonstrated that supplementation with Moringa leaf extracts significantly elevates the activities of SOD and glutathione peroxidase (GPx), both key antioxidant enzymes critical for detoxifying peroxides generated during oxidative stress. These biochemical enhancements are paralleled by clinical reductions in MDA levels and improvements in systemic oxidative stress indices among pregnant women at elevated risk of hypertensive disorders [16]. The antioxidant properties of M. oleifera are further bolstered by its vitamin C content, which acts not only as a direct antioxidant but also as a regenerating cofactor for oxidized flavonoids, maintaining redox cycling capacity. Such comprehensive antioxidant reinforcement is fundamental in blunting the oxidative injury that underpins endothelial dysfunction and placental ischemia in preeclampsia. Figure 3 illustrates the antioxidant and anti-inflammatory actions of M. oleifera bioactives in supporting vascular and placental health.
Orthosiphon aristatus
O. aristatus, traditionally known as kumis kucing or Java tea, contains a diverse array of bioactive antioxidants, notably rosmarinic acid and caffeic acid derivatives. These compounds exhibit powerful radical-scavenging properties and are capable of interrupting oxidative chain reactions at multiple points. Randomized clinical trials have documented that O. aristatus supplementation reduces plasma MDA concentrations and increases total antioxidant status among hypertensive pregnant women, suggesting a clinically meaningful attenuation of systemic oxidative burden [18]. The pharmacological actions of Orthosiphon are attributed not merely to its antioxidant properties but also to its modulation of endothelial nitric oxide pathways, reinforcing vascular relaxation under oxidative duress. By intervening early at the oxidative stress stage, O. aristatus may offer a novel phytotherapeutic strategy to disrupt the oxidative stress-driven cascade leading to preeclampsia. Figure 4 depicts the antioxidant and endothelial-protective mechanisms attributed to bioactive constituents of O. aristatus.
Centella asiatica
C. asiatica, a medicinal plant traditionally used for vascular health, exerts profound antioxidant effects through its primary triterpenoid constituents: asiaticoside, madecassoside, and asiatic acid. These bioactives enhance endogenous antioxidant defenses by stimulating the expression and activity of SOD and catalase while directly scavenging ROS generated within endothelial and placental tissues. In clinical evaluations, women receiving C. asiatica extracts exhibited significantly reduced oxidative stress markers compared to controls, alongside improved systemic antioxidant profiles [20]. Moreover, Centella’s antioxidant action extends to mitochondrial protection, preserving energy metabolism in high-demand tissues such as the placenta. Through both enzymatic and non-enzymatic pathways, C. asiatica robustly interrupts oxidative injury, a key initiating factor in the cascade toward preeclampsia. Figure 5 details the antioxidant and anti-inflammatory effects mediated by the major triterpenoids of C. asiatica in the context of maternal vascular health. Table 2 provides a comparative overview of the major bioactive compounds, primary mechanisms, clinical effects, and unique attributes of each indigenous plant evaluated in this review.
MECHANISM 2: SUPPRESSION OF INFLAMMATORY SIGNALING. Most inflammation-related data are early-stage and lack confirmation in pregnancy-specific models. Translational studies focusing on maternal-fetal safety and mechanistic validation are warranted.
Curcuma longa
Curcumin from C. longa serves as a potent modulator of inflammation by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway. This suppression prevents the transcriptional activation of several key pro-inflammatory cytokines, notably tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). Controlled trials in at-risk pregnant populations have demonstrated that curcumin supplementation reduces circulating levels of these cytokines, correlating with reduced systemic inflammation and vascular injury [14]. By attenuating inflammatory signaling, curcumin mitigates endothelial dysfunction and inflammatory activation within the maternal-fetal interface, pivotal events preceding the clinical onset of preeclampsia.
Moringa oleifera
Flavonoids in M. oleifera similarly exert anti-inflammatory effects by targeting the NF-κB pathway. Quercetin and kaempferol inhibit the phosphorylation of IκB, thereby preventing NF-κB translocation into the nucleus and subsequent pro-inflammatory gene expression. Clinical trials report that Moringa supplementation significantly reduces maternal serum levels of TNF-α and IL-6, with concurrent improvements in maternal vascular inflammatory profiles [17]. By dampening inflammatory amplification, M. oleifera offers potential in modulating the maternal immune response toward a more homeostatic, less inflammatory state.
Orthosiphon aristatus
Bioactives such as sinensetin and eupatorin from O. aristatus demonstrate anti-inflammatory effects by inhibiting NF-κB signaling and subsequent cytokine production. Pilot studies have shown that pregnant women receiving Orthosiphon supplementation exhibited lower TNF-α and IL-6 levels, indicating a systemic reduction in inflammatory load [18]. The capacity to suppress inflammatory mediators that drive endothelial activation underscores Orthosiphon’s potential in reducing the vascular inflammatory burden central to preeclampsia pathogenesis.
Centella asiatica
Triterpenes from C. asiatica robustly inhibit NF-κB activation in endothelial and immune cells, leading to significant reductions in pro-inflammatory cytokine synthesis. Studies in both preclinical and clinical models confirm Centella’s capacity to suppress systemic IL-6 and TNF-α levels while preserving endothelial integrity [25]. By modulating the inflammatory milieu, C. asiatica may significantly attenuate the inflammatory axis pivotal in the pathophysiology of preeclampsia.
MECHANISM 3: ENDOTHELIAL FUNCTION PRESERVATION. These effects are promising but require validation in pregnancy-specific endothelial models. Well-controlled clinical trials assessing endothelial biomarkers and maternal outcomes are needed.
Curcuma longa
Curcumin, the primary bioactive compound in C. longa, plays a critical role in preserving endothelial function through multiple converging pathways. One of its primary actions is the upregulation of endothelial nitric oxide synthase (eNOS), which enhances nitric oxide (NO) bioavailability – a key mediator of vasodilation and vascular homeostasis. In clinical settings, pregnant women supplemented with curcumin exhibited improvements in flow-mediated dilation (FMD), a non-invasive marker of endothelial function [15]. The increase in NO production results in reduced vascular resistance, improved uteroplacental perfusion, and attenuation of hypertension risk, all pivotal for preventing the progression of preeclampsia. Moreover, curcumin’s concurrent antioxidant and anti-inflammatory actions synergistically protect the endothelium from oxidative and immune-mediated injuries, further preserving its barrier and signalling functions during gestation.
Moringa oleifera
M. oleifera flavonoids, particularly quercetin and kaempferol, are potent enhancers of endothelial NO synthesis and protectors against endothelial dysfunction. Clinical data reveal that Moringa supplementation improves vascular compliance and augments endothelium-dependent vasodilation, as measured by FMD and pulse wave velocity [16]. These improvements are attributed to both direct antioxidant protection of eNOS enzymes and suppression of vascular inflammation that would otherwise impair NO synthesis. By maintaining endothelial integrity and ensuring sustained vasodilatory capacity, M. oleifera offers a powerful phytotherapeutic avenue to counteract the vascular derangements characteristic of preeclampsia.
Orthosiphon aristatus
O. aristatus demonstrates endothelial-protective properties through multiple bioactive constituents. Rosmarinic acid and sinensetin modulate vascular tone by enhancing NO production and inhibiting the expression of vasoconstrictor molecules such as endothelin-1. Clinical trials report that Orthosiphon supplementation results in improved vascular reactivity, lower systemic vascular resistance, and significant reductions in maternal blood pressure [18]. Furthermore, Orthosiphon reduces oxidative degradation of NO, ensuring its bioavailability remains sufficient to maintain healthy endothelial function under conditions of pregnancy-induced stress.
Centella asiatica
Bioactives from C. asiatica, notably asiaticoside and madecassoside, support endothelial health by stimulating NO production while simultaneously inhibiting vascular inflammatory responses. Observational studies in pregnancy-associated hypertensive models show that Centella administration leads to improvements in endothelial-dependent vasodilation and enhanced microvascular function [20]. In addition to its NO-mediated effects, Centella stabilizes endothelial tight junction proteins, preserving vascular permeability and preventing edema formation – a major complication of severe preeclampsia.
MECHANISM 4: RENAL PROTECTION AND PROTEINURIA PREVENTION. Although renal protection is biologically plausible, pregnancy-specific data are sparse and require confirmation. Dedicated renal outcome trials in pregnant cohorts are an important next step.
Curcuma longa
Curcumin exerts renoprotective effects critical for mitigating one of the most dangerous sequelae of preeclampsia: proteinuria. Through its antioxidant and anti-inflammatory actions, curcumin attenuates glomerular oxidative injury, preserves podocyte integrity, and prevents glomerular basement membrane thickening. Although direct clinical trials in pregnancy remain limited, animal models and extrapolated human studies consistently demonstrate curcumin’s ability to reduce markers of renal injury, suggesting substantial promise for translational application.
Moringa oleifera
The antioxidant and anti-inflammatory bioactives in M. oleifera preserve renal endothelial health by scavenging free radicals and downregulating inflammatory cytokine expression. Clinical studies in non-pregnant hypertensive populations show that Moringa supplementation reduces albuminuria and preserves glomerular filtration rates [16]. These findings suggest a similar protective potential during pregnancy, especially given Moringa’s capacity to prevent oxidative glomerular damage – a hallmark of proteinuria development in preeclampsia.
Orthosiphon aristatus
O. aristatus has long been recognized for its nephroprotective properties, traditionally used as a diuretic and kidney tonic. Orthosiphol A and rosmarinic acid work synergistically to reduce oxidative stress within glomerular structures, inhibit mesangial cell proliferation, and improve renal blood flow. Randomized clinical trials in hypertensive individuals indicate reductions in urinary protein excretion and improvements in serum creatinine following Orthosiphon supplementation [18], positioning it as a promising adjunct in preeclampsia risk reduction strategies.
Centella asiatica
C. asiatica protects renal function by inhibiting NF-κB signaling in podocytes and glomerular endothelial cells, reducing inflammatory injury and oxidative stress at the filtration barrier. Experimental and early clinical evidence suggests that Centella extracts attenuate glomerular hyperpermeability and prevent proteinuria progression [20]. The stabilization of glomerular structures by Centella bioactives aligns directly with the pathophysiological targets needed to prevent renal complications associated with preeclampsia.
Risk of bias assessment
The quality of evidence across the included studies was heterogeneous. Randomized controlled trials (RCTs) were assessed using the Cochrane Risk of Bias 2.0 (RoB 2) tool, while non-randomized clinical studies were evaluated using the ROBINS-I instrument. Most RCTs demonstrated low to moderate risk of bias in the randomization process and outcome measurement domains but often lacked details on allocation concealment and blinding. Non-randomized studies showed higher risk of bias, particularly in confounding and selection domains, reflecting inherent limitations of observational designs. For mechanistic and preclinical studies (in vitro endothelial/placental models and animal studies), formal risk of bias assessment tools were not applied because these were included only to provide biological plausibility (Tier C evidence). Overall, the certainty of evidence supporting pregnancy-specific clinical effects was rated as low to very low, highlighting the need for adequately powered pregnancy-focused RCTs with standardized interventions and robust reporting.
Integrative synthesis: multitarget potential against preeclampsia (Figures 6–8)
The convergence of antioxidant, anti-inflammatory, endothelial-protective, and renal-protective mechanisms observed across C. longa, M. oleifera, O. aristatus, and C. asiatica underscores a multidimensional therapeutic potential in preeclampsia prevention. Each plant independently modulates distinct but overlapping molecular pathways that contribute to the pathogenesis of the disorder, creating a robust synergistic model for maternal vascular and renal protection. Beyond simple antioxidant capacity, these botanicals orchestrate coordinated suppression of inflammatory cascades, enhancement of endothelial function, and preservation of renal glomerular integrity. Such multi-mechanistic actions are particularly valuable given the complex, multifactorial etiology of preeclampsia, which has historically challenged monotherapy approaches. These mechanisms are synthesized in Table 2 and visualized in Figure 7.
However, as outlined in Table 4 and illustrated in Figure 8, significant research gaps remain – including pharmacokinetics, standardized dosing, and pregnancy-specific safety evaluations – that must be addressed before clinical translation can be safely pursued. These outcomes highlight translational potential but require pregnancy-specific pharmacokinetic, dosing, and safety studies before clinical integration.
Discussion
Overview of key findings
This systematic review synthesized emerging evidence on the preventive potential of four indigenous Indonesian medicinal plants – C. longa, M. oleifera, O. aristatus, and C. asiatica – in mitigating preeclampsia risk (Table 2; Figures 2–5). Findings demonstrate that these botanicals target multiple pathological pathways implicated in preeclampsia, including oxidative stress, inflammatory signaling, endothelial dysfunction, and renal impairment.
Collectively, these plants present a promising multi-targeted phytotherapeutic framework aligned with the complex etiology of preeclampsia (Figure 7). While integration of clinical and mechanistic data provides an encouraging foundation, critical knowledge gaps remain regarding optimal dosing, pharmacokinetics, and pregnancy-specific safety, necessitating additional investigation before clinical implementation.
Integration with existing knowledge
The findings support and expand prior research emphasizing oxidative stress, inflammatory dysregulation, and endothelial dysfunction as central to preeclampsia pathogenesis [4], [5], [6], [7, 26], 27]. Historically, antioxidant strategies using vitamins C and E failed to prevent preeclampsia [28], underscoring the need for agents with broader bioactivity.
These effects directly target early pathophysiological events in preeclampsia, including impaired trophoblast invasion, placental ischemia, and systemic endothelial activation (Figure 1; Table 1). Moreover, emerging preclinical studies suggest multi-target phytotherapeutics may outperform single-pathway interventions in complex pregnancy disorders [30]. By integrating indigenous botanical pharmacology with modern pathophysiological models, this review highlights potential complementary strategies to reduce maternal vascular risk.
Clinical implications
Given the limited therapeutic options for preeclampsia prevention, safe, orally administered, plant-based agents could provide an important complementary approach (Table 3).
Curcumin is generally well tolerated but exhibits poor bioavailability, necessitating novel delivery methods (e.g., nanoparticle or phospholipid carriers) [31].
However, dose-ranging and pharmacokinetic (PK/PD) data remain absent. No studies define minimum effective doses (MED), maximum tolerated doses (MTD), or long-term maternal-fetal safety (NOAEL/LOAEL thresholds). Without these data, clinical translation remains speculative. Future work must prioritize structured dose-finding studies, pregnancy-specific PK/PD investigations, and standardized extract development to ensure efficacy and safety.
Regulatory and safety considerations
The regulatory status and safety of botanical interventions in pregnancy require careful consideration. None of the included studies conducted formal dose-ranging, pharmacokinetic, or maternal-fetal safety evaluations specific to pregnancy. For all four plants, current evidence is largely based on general population data or preclinical studies, which cannot be directly extrapolated to pregnant women.
From a regulatory perspective.
C. longa (turmeric) and M. oleifera are widely consumed as food or supplements, but concentrated extracts have not been assigned specific pregnancy safety categories by regulatory bodies such as the U.S. FDA or European Medicines Agency (EMA).
O. aristatus and C. asiatica are traditionally used for diuresis and microcirculatory health but lack standardized pregnancy safety profiles and dosage guidelines.
The WHO Traditional Medicine Strategy 2023–2032 emphasizes the need for pharmacovigilance, quality control of herbal products, and ethical bioprospecting before recommending any plant-based intervention for vulnerable groups such as pregnant women.
Potential risks include.
Drug–herb interactions, particularly in women taking antihypertensive medications, anticoagulants, or low-dose aspirin.
Variability in active phytochemical content due to differences in plant species, cultivation, and extraction methods.
Teratogenic and reproductive toxicity data are largely unavailable, especially for concentrated formulations used beyond traditional culinary amounts.
Given these uncertainties, any clinical translation must be preceded by standardized extract development, reproductive toxicity testing (e.g., OECD TG 414 and 421), and structured pregnancy-specific pharmacovigilance systems.
Cultural relevance and indonesian context
Indonesia is recognized as one of the world’s biodiversity hotspots and possesses a rich tradition of maternal herbal medicine [13]. Many communities already use C. longa (kunyit), M. oleifera (kelor), O. aristatus (kumis kucing), and C. asiatica (pegagan) to promote maternal well-being.
Integrating such culturally familiar and accessible plants into maternal health strategies aligns with the WHO Traditional Medicine Strategy 2023–2032 [34] and offers opportunities for community-based implementation in resource-limited settings. Utilizing existing local knowledge and agricultural supply chains could enhance program feasibility, affordability, and acceptance, especially where pharmaceutical access is limited.
Strengths, limitations, and future directions
A key strength of this review is the integration of clinical trial data (Table 3) with molecular mechanism evidence (Figures 2–5), offering a multidimensional perspective rarely achieved in pregnancy-related phytotherapy. The PRISMA-guided methodology (Figure 9) enhances reproducibility and transparency.
However, several limitations persist:
Limited pregnancy-specific RCTs – most data are extrapolated from non-pregnant or mixed populations.
Heterogeneity – extract formulations, dosage, and treatment timing vary significantly across studies.
Safety gaps – pregnancy-specific PK/PD and reproductive toxicity data are lacking.
Future research priorities include.
Well-powered, pregnancy-specific RCTs.
Comprehensive PK/PD profiling, including placental transfer assessments.
Reproductive toxicity evaluations aligned with OECD guidelines (TG 414, 421).
Synergistic phytochemical combination studies and advanced delivery systems (e.g., nanoformulations).
Ethical bioprospecting and equitable benefit-sharing with indigenous communities (WHO Strategy [34]).
Table 4 and Figure 8 outline a strategic roadmap to address these gaps.
Concluding perspectives
The synthesis of current evidence highlights a potential paradigm shift in preeclampsia prevention: moving beyond isolated antioxidant therapy toward multi-target, culturally rooted phytomedicine. C. longa, M. oleifera, O. aristatus, and C. asiatica provide a biologically plausible and locally relevant framework to restore redox balance, suppress inflammation, preserve endothelial function, and protect renal integrity.
Responsible clinical translation demands rigorous validation, including standardized dosing frameworks, PK/PD characterization, and maternal-fetal safety evaluations. Collaboration among clinical researchers, ethnobotanists, pharmacologists, regulatory agencies, and local communities will be essential to ethically integrate these botanicals into modern maternal care.
Conclusions
This systematic review underscores the emerging potential of four Indonesian medicinal plants – C. longa, M. oleifera, O. aristatus, and C. asiatica – as multi-target phytotherapeutics for preventing preeclampsia. Their cultural familiarity, biological plausibility, and accessibility make them compelling candidates for integration into maternal health programs, particularly in low-resource settings where preeclampsia burden is highest.
However, clinical translation remains limited by the lack of structured dosing, PK/PD profiling, and comprehensive maternal-fetal safety data. Future research must focus on translational science, mechanistic clarity, and rigorous interventional trials. Multidisciplinary collaboration and culturally sensitive implementation strategies will be key to ensuring safe, equitable, and evidence-based integration of these botanicals into maternal healthcare frameworks.
Acknowledgments
The authors appreciate the Indonesian Society of Obstetrics & Gynecology (ISOG/POGI) and Indonesian Association of Maternal Fetal Medicine (IAMFM/HKFM) for encouraging and supporting the work of this review article.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Mol, BWJ, Roberts, CT, Thangaratinam, S, Magee, LA, de Groot, CJM, Hofmeyr, GJ. Preeclampsia. Lancet 2016;387:999–1011. https://doi.org/10.1016/S0140-6736(15)00070-7.Suche in Google Scholar PubMed
2. Sibai, B, Dekker, G, Kupferminc, M. Pre-eclampsia. Lancet 2020;396:1243–58. https://doi.org/10.1016/S0140-6736(20)31819-5.Suche in Google Scholar PubMed PubMed Central
3. Burton, GJ, Redman, CW, Roberts, JM, Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019;366:l2381. https://doi.org/10.1136/bmj.l2381.Suche in Google Scholar PubMed
4. Redman, CWG, Sargent, IL. Placental stress and pre-eclampsia: a revised view. Placenta 2009;30:S38–42. https://doi.org/10.1016/j.placenta.2008.11.021.Suche in Google Scholar PubMed
5. Staff, AC, Benton, SJ, von Dadelszen, P, Roberts, JM, Taylor, RN, Powers, RW, et al.. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 2019;73:62–70. https://doi.org/10.1161/HYPERTENSIONAHA.118.11674.Suche in Google Scholar
6. Rana, S, Lemoine, E, Granger, JP, Karumanchi, SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.Suche in Google Scholar PubMed
7. Schoots, MH, Gordijn, SJ, Scherjon, SA, van Goor, H, Hillebrands, JL. Oxidative stress in placental pathology. Placenta 2018;69:153–61. https://doi.org/10.1016/j.placenta.2017.12.002.Suche in Google Scholar PubMed PubMed Central
8. Hussein, W, Lafayette, RA. Preeclampsia: pathophysiology and clinical implications. Clin J Am Soc Nephrol 2014;9:2085–93. https://doi.org/10.2215/CJN.03210314.Suche in Google Scholar
9. Chen, Q, Guo, F, Huo, Y, Sun, Y, Yang, M, Wang, R, et al.. Oxidative stress and autophagy in preeclampsia: a review. Redox Biol 2020;29:101390. https://doi.org/10.1016/j.redox.2020.101390.Suche in Google Scholar
10. Pontrelli, P, Conserva, F, Piccoli, C, De Rasmo, D, Rascio, F, Riera, M, et al.. Involvement of mitochondrial dysfunction in the pathogenesis of preeclampsia. Redox Biol 2022;53:102001. https://doi.org/10.1016/j.redox.2022.102001.Suche in Google Scholar
11. Roberge, S, Bujold, E, Nicolaides, KH. Aspirin for the prevention of preeclampsia and intrauterine growth restriction. Clin Lab Med 2016;36:319–29. https://doi.org/10.1016/j.cll.2016.05.008.Suche in Google Scholar PubMed
12. Pisoschi, AM, Pop, A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 2015;97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040.Suche in Google Scholar PubMed
13. Sasmito, AP, Yuliani, S, Suwondo, A, Wicaksono, BD, Mun’im, A, Maliogka, VI. Ethnobotany and antioxidant activity of medicinal plants used by the Balinese community of Indonesia. Plants 2021;10:514. https://doi.org/10.3390/plants10030514.Suche in Google Scholar PubMed PubMed Central
14. Hewlings, SJ, Kalman, DS. Curcumin: a review of its effects on human health. Foods 2017;6:92. https://doi.org/10.3390/foods6100092.Suche in Google Scholar PubMed PubMed Central
15. Taghizadeh, M, Memarzadeh, MR, Asemi, Z. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin supplementation on oxidative stress biomarkers. J Funct Foods 2021;81:104693. https://doi.org/10.1016/j.jff.2021.104693.Suche in Google Scholar
16. Leone, A, Spada, A, Battezzati, A, Schiraldi, A, Aristil, J, Bertoli, S. Moringa oleifera seeds and oil: characteristics and uses for human health. Int J Mol Sci 2015;16:12791–835. https://doi.org/10.3390/ijms160612791.Suche in Google Scholar PubMed PubMed Central
17. Ogunsina, M, Adetunji, CO, Tijani, S, Awolola, NA. Effects of Moringa oleifera supplementation on pregnancy outcomes: a systematic review. Nutr Res 2023;108:1–10. https://doi.org/10.1016/j.nutres.2022.12.007.Suche in Google Scholar PubMed
18. Awale, S, Tezuka, Y, Banskota, AH, Shimoji, S, Taira, K, Kadota, S. Norstaminane- and isopimarane-type diterpenes of Orthosiphon stamineus from Okinawa and their inhibitory effects on nitric oxide production. Fitoterapia 2013;90:66–73. https://doi.org/10.1016/j.fitote.2013.07.002.Suche in Google Scholar PubMed
19. Brinkhaus, B, Lindner, M, Schuppan, D, Hahn, EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 2000;7:427–48. https://doi.org/10.1016/S0944-7113(00)80065-3.Suche in Google Scholar PubMed
20. Somboonwong, J, Thanamittramanee, S, Jariyapongskul, A, Patumraj, S. Therapeutic effects of Centella asiatica extract in diabetic rats. J Ethnopharmacol 2012;143:448–55. https://doi.org/10.1016/j.jep.2012.06.017.Suche in Google Scholar PubMed
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71.Suche in Google Scholar PubMed PubMed Central
22. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al.. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.Suche in Google Scholar PubMed
23. Sterne, JAC, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al.. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.Suche in Google Scholar PubMed PubMed Central
24. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al.. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.Suche in Google Scholar PubMed PubMed Central
25. Wu, F, Bian, X, Yang, W, Ma, Y, Zhang, C, Chen, F, et al.. Centella asiatica modulates inflammatory pathways through NF-κB inhibition: evidence from preclinical models. Molecules 2020;25:4464. https://doi.org/10.3390/molecules25194464.Suche in Google Scholar PubMed PubMed Central
26. Brown, MC, Best, KE, Pearce, MS, Waugh, J, Robson, SC, Bell, R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. https://doi.org/10.1007/s10654-013-9762-6.Suche in Google Scholar PubMed
27. Roberts, JM, Hubel, CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30:S32–7. https://doi.org/10.1016/j.placenta.2008.11.009.Suche in Google Scholar PubMed PubMed Central
28. Poston, L, Briley, AL, Seed, PT, Kelly, FJ, Shennan, AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 2006;367:1145–54. https://doi.org/10.1016/S0140-6736(06)68506-7.Suche in Google Scholar
29. Cindrova-Davies, T, Fogarty, NME, Jones, CJ, Kingdom, J, Burton, GJ. Evidence of oxidative stress-induced senescence in mature, post-mature, and pathological human placentas. Placenta 2018;68:15–22. https://doi.org/10.1016/j.placenta.2018.06.003.Suche in Google Scholar
30. Saad, MI, Salama, SA, Saad, EA, El-Houseiny, W, El-Sayed, WM. Phytochemicals as novel therapeutic agents for the management of preeclampsia: current knowledge and future perspectives. Phytother Res 2020;34:1562–75. https://doi.org/10.1002/ptr.6610.Suche in Google Scholar PubMed
31. Wang, YJ, Pan, MH, Cheng, AL, Lin, LI, Ho, YS, Hsieh, CY, et al.. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 1997;15:1867–76. https://doi.org/10.1016/S0731-7085(96)02024-9.Suche in Google Scholar
32. Yam, MF, Basir, R, Asmawi, MZ, Ismail, Z. Antioxidant and hepatoprotective effects of Orthosiphon stamineus Benth. standardized extract. Am J Chin Med 2010;38:1103–15. https://doi.org/10.1142/S0192415X1000840X.Suche in Google Scholar
33. Say, L, Chou, D, Gemmill, A, Tunçalp, Ö, Moller, AB, Daniels, J, et al.. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2:e323–33. https://doi.org/10.1016/S2214-109X(14)70227-X.Suche in Google Scholar PubMed
34. World Health Organization (WHO). WHO traditional medicine strategy: 2023–2032. Geneva: World Health Organization; 2023. https://www.who.int/publications/i/item/9789240074354.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

