Measurements of the fetal heart and prenatal biomarkers in children born with an isolated ventricular septum defect – a large, prospective, multicenter cohort study
-
Cathrine Vedel
, Christian Pihl
Abstract
Objectives
To compare 2nd-trimester cardiac and fetal biometries, 1st-trimester biomarkers, and obstetric outcome between children with an isolated ventricular septum defect (iVSD) and healthy children in a large cohort of children with neonatal echocardiography.
Methods
The Copenhagen Baby Heart Study offered all neonates born in Copenhagen an echocardiography within the 60 days of life between 2016 and 2018. The mothers were recruited at the 2nd-trimester scan (18+0 to 22+6). The documentation from the 2nd-trimester anomaly scan was supplemented with the four-chamber view with Doppler flow, the sagittal view of the aortic arch, the mid-umbilical artery flow, and the ductus venosus flow. All prenatal images were evaluated and cardiac biometries measured by two blinded investigators. VSDs were classified as muscular or as “other type” (perimembranous, inlet, and outlet VSDs). We compared cardiac biometries, biomarkers, and pregnancy outcome between fetuses with a neonatally diagnosed iVSD and controls.
Results
We included 25,556 neonates for an echocardiography, of which 9,155 fetuses were scanned in the period with extended cardiac imaging prenatally. We identified 294 fetuses with a postnatal VSD. Fetal measurements were similar between those with an iVSD compared to those without, and obstetric outcome were similar.
Conclusions
Our results indicate that children diagnosed with an iVSD through neonatal echocardiography in an unselected population have an otherwise structurally normal heart prenatally and a normal pregnancy outcome.
Introduction
Ventricular septum defect (VSD) is a common congenital malformation with an incidence of about 3 % [1]. VSD is a defect in the septum between the ventricles of the heart and is categorized according to location [2]. Postnatally, the defect allows oxygenated blood from the left ventricle to flow across the septum. In large VSDs, the increased flow in the pulmonary circulation can over time result in pulmonary arterial hypertension and ventricular dysfunction [3], 4]. Whether there is an effect on the cardiac structures in fetuses with an isolated VSD is to the best of our knowledge yet unknown.
Previously, fewer, and only larger VSDs were detected prenatally, but the detection of VSDs has increased over time due to technological advancements now including smaller and often isolated defects [5], 6]. Prenatal counselling is based on literature including primarily fetuses with larger VSDs, associated malformations, and chromosomal aberrations. Hence, the prenatal counselling is difficult as there may be a difference in both etiology and consequences between small muscular VSDs and larger VSDs with associated malformations, where small muscular VSDs may be a normal variation [7].
The Copenhagen Baby Heart Study (CBHS), a multicenter, population-based cohort study with neonatal echocardiography on more than 25,000 newborns, found a prevalence of VSDs of 3.3 % [1]. We therefore anticipate that the number of prenatally detected isolated small VSDs will increase in the future as the ultrasound equipment and techniques rapidly improve. Consequently, a greater knowledge on possible associated changes in and around the heart, as well as pregnancy outcome is important, when a VSD is present.
The aim of this study was to compare 2nd-trimester cardiac and fetal biometries, 1st-trimester biomarkers, and obstetric outcome between children with an isolated VSD and children without heart malformations (controls) in a unique, large cohort of children with a subsequent neonatal echocardiography.
Methods
The study is part of the CBHS; a multicenter cohort study including newborns for neonatal echocardiography [8]. The mothers were included at the 2nd-trimester anomaly scan at three hospitals in greater Copenhagen – Herlev Hospital, Hvidovre Hospital, and Rigshospitalet – from April 2016 to October 2018. All parents gave written informed consent to participate in the CBHS including sub-studies. The study was approved by the Regional Ethics Committee of the Capital City Region of Denmark (H-16001518), and the Danish Data Protection Agency (I-Suite no.: 04546, ID-no. HGH-2016-53). The CBHS is registered with Clinical Trial registration: clinicaltrials.gov identifier NCT02753348.
All pregnant women in Denmark are offered a malformation scan in the 2nd trimester (around 20 weeks’ gestation), and approximately 95 % of pregnant women attend this screening [9]. During the inclusion period of the CBHS, the prenatal image documentation of the heart at the 2nd-trimester anomaly scan was extended compared to what the national guidelines required [10]. We added the four-chamber view with color Doppler flow across the atrioventricular valves, the sagittal view of the aortic arch, the mid-umbilical artery pulsatility index (PI), and the ductus venosus PI, to the standard views already part of the national guideline for the 2nd-trimester anomaly scan [11]; end-diastolic four-chamber view, left ventricular outflow tract including the aortic valve, right ventricular outflow tract, and the three-vessel trachea view.
The prenatal scans were performed by experienced sonographers, certified by the Fetal Medicine Foundation, London. We used Voluson E8 scanners, GE Medical Systems, Zipf, Austria. All prenatal cardiac images were subsequently evaluated by two blinded investigators. We measured the diameters of the aortic valve, pulmonary valve, the ascending aorta (3-vessel view), the aortic isthmus (sagittal view), and the end-diastolic width and length of the ventricles heart, and the axis of the heart in thorax (the angle between the heart’s interventricular septum and the chest wall’s anteroposterior axis). If the quality of the image was poor with not clearly defined structures in and around the heart, we did not measure the structure.
Gestational age was determined by the 1st-trimester crown-rump length [12]. Maternal characteristics were obtained from the local obstetric databases, the patient file system (Epic Systems, Wisconsin, USA) and the ultrasound database Astraia (Astraia GmBH, Munich, Germany). The 1st-trimester parameters e.g., crown rump length, nuchal translucency thickness in mm (NT), ductus venosus PI, free β-human Chorion Gonadotropin (β-hCG, multiple of the median, MoM), and Pregnancy-Associated Plasma Protein A (PAPP-A, MoM) were measured and collected by sonographers at the routine 1st-trimester screening exam. We used the biparietal diameter, the abdominal circumference, and the femur length at the 2nd-trimester scan as proxies for fetal growth. Pregnancy outcome data included gestational age at delivery and birthweight. Whether the newborn was growth restricted (FGR) was calculated using reference charts from Marsal et al.’s [13]; a birthweight below 2 standard deviations was considered FGR.
All neonates in the CBHS who underwent a transthoracic echocardiography were scanned by a sonographer or physician preferably within the first four weeks of life using Vivid E9 ultrasound equipment (General Electric, Horten, Norway). All the examiners were trained by specialists in pediatric echocardiography. Any echocardiographic finding suspected to be abnormal was reviewed by a cardiologist specialized in pediatric echocardiography. A VSD was defined as flow across the interventricular septum identified on color Doppler. All children with a VSD were evaluated and followed up to ensure the defect would close spontaneously, and in the case of larger defects, they were referred to the department of Pediatric Cardiology. The VSDs were grouped as being either muscular or other type – a compound variable consisting of inlet, outlet, and perimembranous VSDs due to few cases. The VSD diameter was measured from inner-to-inner edge, diagonally to the flow in all available views, and sub-analyses were performed regarding size of the VSD irrespective of the type or location of VSD and were classified as large when ≥3 mm (90th-percentile), and small when <3 mm. We did not include neonates with VSDs detected prenatally that had spontaneously closed before the neonatal echocardiography. The controls consisted of all other singleton fetuses without cardiac anomalies.
The data were analyzed with Stata, version 13.1 (StataCorp, College Station, TX, USA). For the analyses we only included scans performed between 18+0 and 22+6 weeks’ gestation, and we excluded twins and children with any cardiac abnormalities other than VSD. We summarized baseline characteristics using frequencies or medians where relevant and compared the two groups using the Student’s t-test or the Mann-Whitney U test where appropriate. Differences in biometries between fetuses with VSD and heart healthy fetuses were examined using linear regression analysis.
Results
Of the 25,556 neonates who were enrolled in the CBHS for a systematic echocardiography, 9,155 fetuses underwent extended cardiac imaging prenatally. As described, we only measured on optimal prenatal images, hence the numbers of measurements vary. For this study we excluded 319 twins (3.5 %), and 347 children with other cardiac abnormalities (3.8 %) including hypertrophic left ventricle, bicuspid aortic valve, atrium septum defect, pulmonary stenosis, coarctation of the aorta, and transposition of the great arteries, Figure 1. We also excluded 17 fetuses that had the 2nd-trimester scan performed later than 22+6 weeks’ gestation. Consequently, 8,472 fetuses were eligible for analysis.
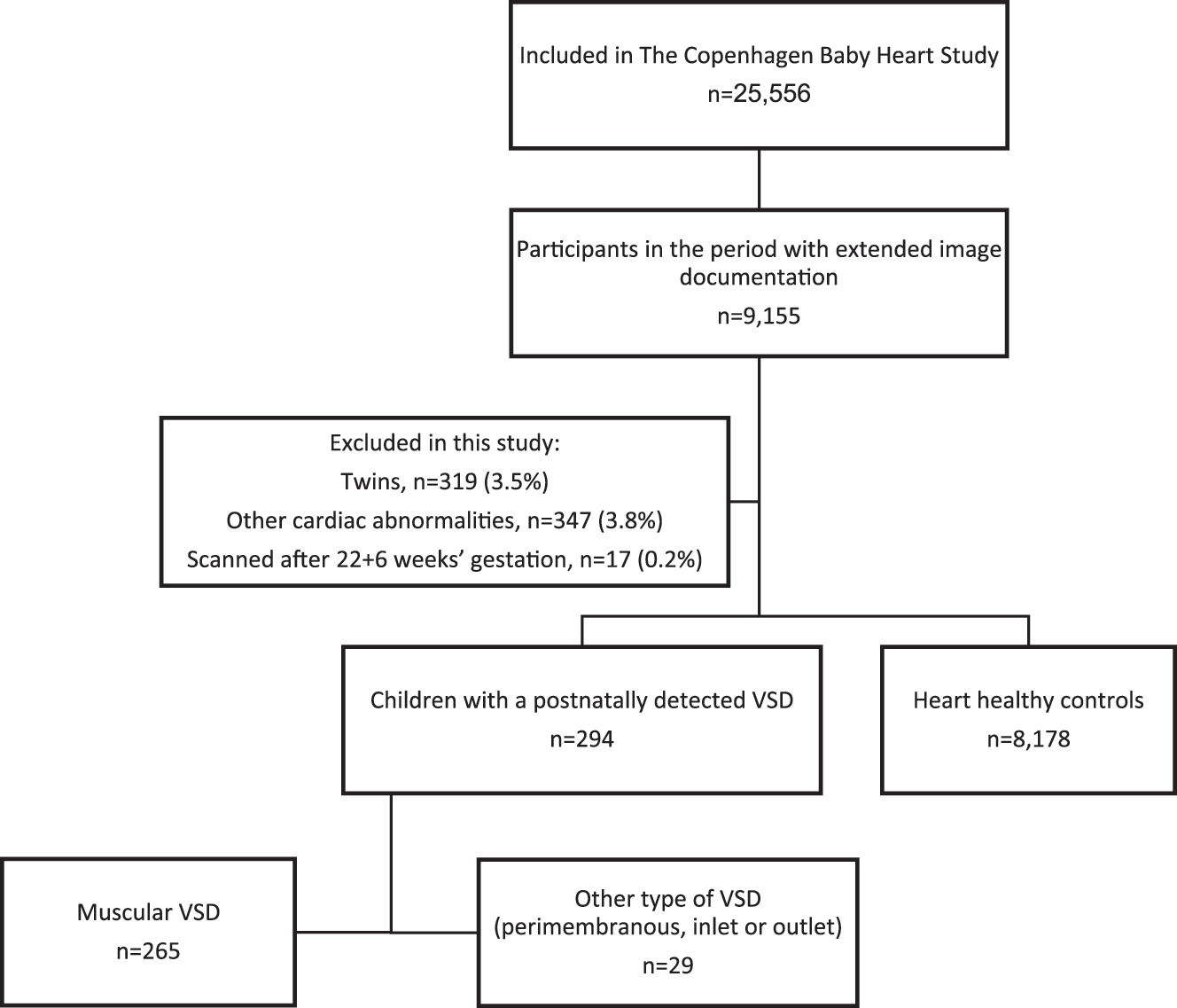
Flowchart of the study population.
A VSD was detected in 294 newborns – 265 with a muscular VSD and 29 with another type of VSD (perimembranous=16, inlet=7, outlet=5, sub-arterial=1). Of the 294 VSDs identified neonatally, 15 had also been detected prenatally (5.1 %), of which 14 were muscular VSDs. Table 1 shows the maternal characteristics of the women in the VSD groups and in the control group. There were no differences between the muscular VSD group and controls nor the other types of VSDs and controls.
Characteristics of mothers to children with a ventricular septum defect compared to mothers of children without a heart malformation.
| Muscular VSD (n=265) |
Perimembranous, inlet and outlet VSDs (n=29) |
Controls (n=8,178) |
Differences between muscular VSD and controls p-Value | Differences between perimembranous, inlet and outlet VSDs and controls p-Value | |
|---|---|---|---|---|---|
| Age, years (mean, SD) | 30.8 (4.4) | 32.2 (4.3) | 31.4 (4.5) | 0.05 | 0.3 |
| BMI, kg/m2 (median, IQR) Missing n (%) |
22.8 (20.5–25.6) 14 (5.3) |
21.7 (19.6–25.6) 2 (6.9) |
22.5 (20.6–25.2) 363 (4.4) |
0.6 | 0.6 |
| Smoking (yes), pre-gestational n (%) Missing n (%) |
5 (2.1) 24 (9.1) |
0 (0.0) 3 (10.3) |
216 (2.9) 768 (9.4) |
0.4 | 0.4 |
-
VSD, ventricular septum defect; SD, standard deviation; BMI, body mass index; IQR, interquartile range.
Table 2 shows 1st-trimester biomarkers, 2nd-trimester growth biometries and obstetric outcomes. The results were generally similar between the groups, however, with tendencies towards lower levels of 1st-trimester biochemical markers and a larger proportion born with FGR in the group with other types of VSDs (perimembranous, inlet, and outlet), though not significant.
1st trimester biomarkers and 2nd trimester growth biometries in fetuses with VSD compared to fetuses without a heart malformation.
| Muscular VSD (n=265) |
Perimembranous, inlet and outlet VSDs (n=29) |
Control group (n=8,178) |
Difference between fetuses with muscular VSD and controls (95 % confidence interval) |
Difference between fetuses with other types of VSDs and controls (95 % confidence interval) | |
|---|---|---|---|---|---|
| 1st trimester | |||||
|
|
|||||
| CRL in mm, mean, SD Data available, n (%) |
65.9 (7.4) 252 (95.1) |
64.0 (6.9) 27 (93.1) |
65.8 (8.2) 7,593 (92.8) |
−0.09 (−0.24; 0.05)a | 0.04 (−0.40; 0.49)a |
| NT thickness ≥3.5 mm, n (%) Data available, n (%) |
2 (0.8) 252 (95.1) |
0 (0.0) 27 (93.1) |
33 (0.4) 7,562 (92.5) |
p=0.4 | p=0.7 |
| Ductus venosus pulsatility index, mean, SD Data available, n (%) |
1.0 (0.1) 70 (26.4) |
1.0 (0.1) 9 (31.0) |
1.0 (0.2) 2,231 (27.3) |
0.03 (−0.01; 0.06)a | −0.03 (−0.13; 0.07)a |
| β-hCG MoM, median (IQR) Data available, n (%) |
1.08 (0.7–1.5) 252 (95.1) |
0.97 (0.7–1.3) 27 (93.1) |
1.02 (0.7–1.5) 7,540 (92.2) |
0.02 (−0.08;0.12)a | −0.16 (−0.45;0.14)a |
| PAPP-A MoM, median (IQR) Data available, n (%) |
1.03 (0.7–1.6) 252 (95.1) |
0.92 (0.6–1.3) 27 (93.1) |
1.03 (0.7–1.5) 7,540 (92.2) |
0.01 (−0.07; 0.09)a | −0.25 (−0.50; 0.00)a |
|
|
|||||
| 2nd trimester | |||||
|
|
|||||
| Biparietal diameter in mm, SD Data available, n (%) |
48.0 (2.5) 259 (97.7) |
47.9 (2.7) 29 (100.0) |
48.2 (2.4) 8,007 (97.9) |
−0.11 (−0.38; 0.15)a | −0.41 (−1.19; 0.38)a |
| Abdominal circumference in mm, SD Data available, n (%) |
152.2 (7.9) 256 (96.6) |
153.1 (9.7) 28 (96.6) |
152.1 (8.3) 7,899 (96.6) |
0.44 (−0.43; 1.32)a | 0.72 (−1.89; 3.34)a |
| Femur length in mm, SD Data available, n (%) |
32.0 (1.7) 255 (96.2) |
32.0 (1.6) 29 (100.0) |
32.0 (1.8) 7,900 (96.6) |
0.08 (−0.10; 0.26)a | −0.04 (−0.56; 0.48)a |
|
|
|||||
| Obstetric outcome | |||||
|
|
|||||
| Delivery before 37 weeks’ gestation, n (%) Data available, n (%) |
12 (4.5) 265 (100) |
2 (6.9) 29 (100) |
338 (4.1) 8,178 (100) |
p=0.8 | p=0.5 |
| Birthweight (median, IQR) Data available, n (%) |
3,555 (3,204–3,800) 265 (100) |
3,412 (2,980–3,675) 29 (100) |
3,500 (3,190–3,826) 8,153 (99.7) |
29 (23; 80)a | −136 (−290; 18)a |
| Fetal growth restriction, n (%) Data available, n (%) |
5 (1.9) 265 (100) |
2 (6.9) 29 (100) |
184 (2.3) 8,153 (99.7) |
p=0.7 | p=0.09 |
-
aadjusted for gestational age. VSD, ventricular septum defect; CRL, crown rump length; SD, standard deviation; NT, nuchal translucency; β-hCG, free β-human chorion gonadotropin; IQR, interquartile range; PAPP-A, pregnancy associated plasma protein A.
The cardiac biometries and flows are listed in Table 3, and there were no differences between the measurements in fetuses neither with muscular VSD and controls nor between the other types of VSDs and controls. As listed, there were big differences in the number of obtained images ranging from 15 % (sagittal view of the aortic arch) to 78 % (ascending aorta in the three-vessel trachea view).
The total numbers of obtained measurements and the mean sizes of the cardiac structures in fetuses with ventricular septal defect compared to heart healthy controls.
| Muscular VSD (n=265) |
Perimembranous, inlet and outlet VSDs (n=29) |
Control group (n=8,178) |
Mean difference between muscular VSD group and control group adjusted for gestational age (95 % confidence interval) |
Mean difference between perimembranous, inlet and outlet VSD group and control group adjusted for gestational age (95 % confidence interval) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | mean (SD) | n (%) | mean (SD) | n (%) | mean (SD) | |||
| Aortic valve diameter, mm | 161 (60.8) | 3.0 (0.3) | 22 (75.9) | 3.0 (0.3) | 5,257 (64.3) | 3.0 (0.3) | −0.02 (−0.07; 0.02) | −0.02 (−0.14; 0.10) |
| Ascending aorta in 3-vessel view, mm | 206 (77.7) | 3.5 (0.4) | 21 (72.4) | 3.5 (0.3) | 6,079 (74.3) | 3.5 (0.4) | 0.01 (−0.05; 0.06) | 0.02 (−0.15; 0.19) |
| Aortic isthmus in sagittal view, mm | 42 (15.8) | 3.4 (0.5) | 2 (6.9) | 3.4 (0.4) | 1,234 (15.1) | 3.3 (0.4) | 0.09 (−0.04; 0.22) | 0.04 (−0.55; 0.63) |
| Pulmonary valve diameter, mm | 201 (75.8) | 3.9 (0.4) | 20 (69.0) | 4.0 (0.3) | 5,867 (71.7) | 3.9 (0.4) | 0.02 (−0.04; 0.07) | 0.05 (−0.13; 0.22) |
| Left ventricular length, mm | 128 (48.3) | 11.2 (1.5) | 10 (34.5) | 10.9 (1.3) | 4,123 (50.4) | 11.3 (1.4) | −0.15 (−0.39; 0.10) | −0.31 (−1.18; 0.55) |
| Right ventricular length, mm | 128 (48.3) | 5.7 (0.7) | 10 (34.5) | 5.8 (1.1) | 4,123 (50.4) | 5.8 (0.9) | −0.08 (−0.23; 0.07) | 0.06 (−0.47; 0.60) |
| Left ventricular width, mm | 128 (48.3) | 6.3 (1.0) | 10 (34.5) | 6.1 (0.8) | 4,123 (50.4) | 6.3 (0.8) | −0.01 (−0.16; 0.13) | −0.11 (−0.61; 0.39) |
| Right ventricular width, mm | 128 (48.3) | 5.8 (0.8) | 10 (34.5) | 5.9 (0.9) | 4,123 (50.4) | 5.9 (0.8) | 0.00 (−0.14; 0.14) | 0.17 (−0.33; 0.67) |
| Heart length, mm | 128 (48.3) | 19.0 (1.7) | 10 (34.5) | 18.3 (1.7) | 4,123 (50.4) | 19.0 (1.7) | −0.08 (−0.37; 0.21) | −0.45 (−1.47; 0.58) |
| Heart width, mm | 128 (48.3) | 13.6 (1.4) | 10 (34.5) | 13.2 (1.6) | 4,123 (50.4) | 13.6 (1.4) | −0.07 (−0.32; 0.17) | −0.17 (−1.03; 0.70) |
| Axis of heart in the thorax, degrees | 57 (21.5) | 45.9 (6.6) | 4 (13.8) | 45.2 (6.6) | 1,568 (19.2) | 44.7 (7.2) | 1.28 (−0.62; 3.18) | 0.50 (−6.56; 7.56) |
| Ductus venosus pulsatility index | 57 (21.5) | 0.71 (0.1) | 11 (37.9) | 0.72 (0.2) | 2,302 (28.1) | 0.72 (0.2) | −0.01 (−0.06; 0.04) | 0.00 (−0.10; 0.11) |
| Umbilical artery pulsatility index | 89 (33.6) | 1.20 (0.2) | 13 (44.8) | 1.19 (0.2) | 2,852 (34.9) | 1.22 (0.2) | −0.02 (−0.06; 0.02) | −0.02 (−0.12; 0.08) |
-
VSD, ventricular septum defect; SD, standard deviation.
Sub-analyses of the prenatal scans were performed regarding size of the VSD (Supplementary Table 1). The cohort consisted of 218 small VSDs <3 mm and 45 larger VSDs ≥3 mm defined postnatally, and 31 VSDs without prenatal measurements, thus not included in this analysis. The length of the fetal heart was marginally smaller in the group with large VSDs compared to the control group (mean difference −0.75 mm (95 % CI -1.47; -0.02)). The abdominal circumference was also marginally larger when the fetus had a small VSD compared to the controls (mean difference 1.04 mm (95 % CI 0.07; 2.00)). For all other biomarkers and cardiac measurement variables, no differences were found between fetuses with small VSD and controls nor fetuses with large VSDs and controls.
Sub-analyses were performed on the prenatally detected VSDs (n=15) compared to the non-detected. There were no differences in cardiac biometries between the prenatally detected and non-detected VSDs. Moreover, there were no differences in 1st nor 2nd-trimester biomarkers, gestational age at delivery, birthweight, or numbers born FGR.
Discussion
In this large, prospective, multicenter cohort study with neonatal echocardiography, we compared cardiac and fetal biometries, 1st-trimester biomarkers, and pregnancy outcome between fetuses with a neonatally diagnosed isolated VSD and children without a heart malformation. This is the first study to examine the prenatal cardiac biometries in fetuses neonatally diagnosed with an isolated VSD. The fetuses with a postnatally identified isolated VSD did not differ in any of the evaluated measurements and birth outcomes from the controls. The study was performed as the number of prenatally detected isolated small, muscular VSDs will probably increase in the future. Consequently, a greater knowledge on possible associated changes in and around the heart, as well as pregnancy outcome, is important, when a small VSD is present, to expand our knowledge and to provide optimal prenatal counseling.
The present results indicate that the cardiac structures in fetuses with isolated VSDs are not affected. Due to the size of the cohort, even small differences could be demonstrated; however, that was not the case, except from a marginally smaller length of the fetal heart in the group with postnatally identified larger VSDs. A study by Radhakrishna et al. found an overrepresentation of genes linked to the development of other cardiac structures such as the ventricles from pregnancies where the fetus had a VSD compared to normal pregnancies [14]. In this study, however, we did not find any direct effect on other cardiac structures. This could be due to difference in penetrance, thus not consequently resulting in different altered structures. On the other hand, one could hypothesize that the hemodynamic changes seen postnatally, could also be seen prenatally. The flow across the ventricular septum could potentially change the hemodynamics, hence sizes, of the ventricles and ventricular outflow tracts. However, we did not find any evidence of this independent of the size of the VSD. This supports our hypothesis that small VSDs as a single finding may not be of great significance or severity. It is also supported by a recent study, which showed that fetuses with isolated VSDs were not at increased risk of chromosomal aberrations compared to the non-selected background population [15], 16].
Embryologically, the fetal heart and placenta develop at the same time and are probably directed by some of the same mechanisms, which is called the placenta-heart axis [17]. This phenomenon is thought to play a significant role in non-syndromic congenital heart disease. We did not find a significant difference in placental biomarkers PAPP-A and free β-hCG between the groups, although PAPP-A was marginally lower in the group with other VSDs compared to the controls, though not significant (0.92 vs. 1.03 (median difference −0.25 (95 % CI -0.50; 0.00))). There was no difference in PAPP-A between muscular VSDs and controls. This finding is interesting, as it differentiates between subtypes of VSDs. Muscular VSDs appear to be either a milder lesion or not correlated to lower PAPP-A levels, which is a marker of placental function, whereas other types of VSDs might be correlated to lower PAPP-A levels.
Several studies have found a lower birthweight in children with CHD, also specifically for VSDs [18], 19]. The study by Matthiesen et al. showed differences in birthweight between children with large VSDs and the controls, but not when the VSD was small [19]. We did not find any significant differences in birthweight or proportion of FGR; however, we found an insignificantly lower birthweight and a larger proportion born FGR when the child had perimembranous, inlet or outlet VSD.
The two significant findings on heart length and abdominal circumference in the analyzes of small and large VSDs show very small differences. It should be noticed that we performed multiple comparisons, and a Type 1 error, especially when the differences are small, is not impossible. However, it is also possible that the heart is smaller, if the fetus has a large VSD.
There are some limitations in this study. Although our cohort size is large, more cases with compound outcome “other types” of VSDs would have been beneficial as these subtypes may represent a less benign variation. The extended image documentation was carried out by sonographers at the 2nd trimester anomaly scan and was not mandatory to finish the exam. This had a bigger impact than we expected, as some of the images were only documented in 15–20 % of the cases. However, the number of missing images or measurements was comparable in the two groups and our results would most likely not change with a larger sample size to analyze. The study also has several strengths. It is the first study to examine the prenatal cardiac biometries in fetuses neonatally diagnosed with an isolated VSD. Moreover, it is a large prospective study including 9,155 fetuses for prenatal image evaluation who also underwent neonatal echocardiography. As a result of the prospective study design, the investigators reviewing the images did not know whether the child was diagnosed with a VSD which decreases the risk of bias.
In conclusion, our results indicate that children diagnosed with an isolated VSD through neonatal echocardiography in an unselected population have an otherwise structurally normal heart prenatally and a normal pregnancy outcome.
Funding source: The Danish Heart Association
Funding source: Candys Foundation
Funding source: The Danish Children’s Heart Foundation
Funding source: Rigshospitalet’s Research Foundation
Funding source: Herlev-Gentofte Hospital Research Foundation
Funding source: Toyota Foundation
-
Research ethics: The study was approved by the Regional Ethics Committee of the Capital City Region of Denmark (H-16001518), and the Danish Data Protection Agency (I-Suite no.: 04546, ID-no. HGH-2016-53).
-
Informed consent: Informed consent was obtained from all individuals included in this study or their legal guardians.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: CV was funded by Rigshospitalet’s Research Foundation. The Copenhagen Baby Heart Study was supported by the Danish Heart Association; the Danish Children’s Heart Foundation; Candy’s Foundation; the Toyota Foundation; and the Herlev-Gentofte Hospital Research Foundation. The funder had no role in the design, data collection, data analysis, and reporting of this study. OBP held a professorship funded by the Novo Nordic Foundation grant NNFSA170030576.
-
Data availability: Data available on request from the authors.
References
1. Pihl, C, Sillesen, AS, Norsk, JB, Vøgg, ROB, Vedel, C, Boyd, HA, et al.. The prevalence and spontaneous closure of ventricular septal defects the first year of life. Neonatology 2024:1–10. https://doi.org/10.1159/000538810.Search in Google Scholar PubMed
2. Lopez, L, Houyel, L, Colan, SD, Anderson, RH, Beland, MJ, Aiello, VD, et al.. Classification of ventricular septal defects for the eleventh iteration of the international classification of diseases-striving for consensus: a report from the international society for nomenclature of paediatric and congenital heart disease. Ann Thorac Surg 2018;106:1578–89. https://doi.org/10.1016/j.athoracsur.2018.06.020.Search in Google Scholar PubMed
3. Duffels, MG, Engelfriet, PM, Berger, RM, van Loon, RL, Hoendermis, E, Vriend, JW, et al.. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 2007;120:198–204. https://doi.org/10.1016/j.ijcard.2006.09.017.Search in Google Scholar PubMed
4. Penny, DJ, Vick, GW3rd. Ventricular septal defect. Lancet 2011;377:1103–12. https://doi.org/10.1016/s0140-6736-10-61339-6.Search in Google Scholar
5. Oyen, N, Poulsen, G, Boyd, HA, Wohlfahrt, J, Jensen, PK, Melbye, M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J 2009;157:467–73.e1. https://doi.org/10.1016/j.ahj.2008.10.017.Search in Google Scholar PubMed
6. Liu, Y, Chen, S, Zuhlke, L, Black, GC, Choy, MK, Li, N, et al.. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. https://doi.org/10.1093/ije/dyz009.Search in Google Scholar PubMed PubMed Central
7. Chau, AC, Jones, A, Sutherland, M, Lilje, C, Sernich, S, Hagan, J, et al.. Characteristics of isolated ventricular septal defects less likely to close in utero. J Ultrasound Med 2018;37:1891–8. https://doi.org/10.1002/jum.14535.Search in Google Scholar PubMed
8. Sillesen, AS, Raja, AA, Pihl, C, Vogg, ROB, Hedegaard, M, Emmersen, P, et al.. Copenhagen baby heart study: a population study of newborns with prenatal inclusion. Eur J Epidemiol 2019;34:79–90. https://doi.org/10.1007/s10654-018-0448-y.Search in Google Scholar PubMed
9. FØTOdatabasen. Årsrapport 2018 2020 Available from: https://www.sundhed.dk/content/cms/47/61247_2020-03-18-aarsrapport_foeto_2018_officiel.pdf.Search in Google Scholar
10. DFMS. National guideline on the 2nd-trimester anomaly scan: Danish fetal medicine society 2017. Available from: https://static1.squarespace.com/static/5d8120d60fe9717b4299a867/t/5dd57d655828035255a60bf3/1574272361493/gennemscanningaffostret.pdf.Search in Google Scholar
11. http://www.dfms.dk/images/Guidelines/Guideline_Revideret_19.10.18_VG_IK_docx.pdf.Search in Google Scholar
12. Robinson, HP, Fleming, JE. A critical evaluation of sonar “crown-rump length” measurements. Br J Obstet Gynaecol 1975;82:702–10. https://doi.org/10.1111/j.1471-0528.1975.tb00710.x.Search in Google Scholar PubMed
13. Marsal, K, Persson, PH, Larsen, T, Lilja, H, Selbing, A, Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–8. https://doi.org/10.1111/j.1651-2227.1996.tb14164.x.Search in Google Scholar PubMed
14. Radhakrishna, U, Albayrak, S, Zafra, R, Baraa, A, Vishweswaraiah, S, Veerappa, AM, et al.. Placental epigenetics for evaluation of fetal congenital heart defects: Ventricular septal defect (VSD). PLoS One 2019;14:e0200229. https://doi.org/10.1371/journal.pone.0200229.Search in Google Scholar PubMed PubMed Central
15. Maya, I, Singer, A, Yonath, H, Reches, A, Rienstein, S, Zeligson, S, et al.. What have we learned from 691 prenatal chromosomal microarrays for ventricular septal defects? Acta Obstet Gynecol Scand 2019. https://doi.org/10.1111/aogs.13708.Search in Google Scholar PubMed
16. Vedel, C, Rode, L, Jørgensen, FS, Petersen, OB, Sundberg, K, Tabor, A, et al.. Prenatally detected isolated ventricular septum defects and the association with chromosomal aberrations-A nationwide register-based study from Denmark. Prenat Diagn 2020. https://doi.org/10.1002/pd.5853.Search in Google Scholar PubMed
17. Maslen, CL. Recent advances in placenta-heart interactions. Front Physiol 2018;9:735. https://doi.org/10.3389/fphys.2018.00735.Search in Google Scholar PubMed PubMed Central
18. Courtney, JA, Cnota, JF, Jones, HN. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol 2018;9:1045. https://doi.org/10.3389/fphys.2018.01045.Search in Google Scholar PubMed PubMed Central
19. Matthiesen, NB, Henriksen, TB, Gaynor, JW, Agergaard, P, Bach, CC, Hjortdal, VE, et al.. Congenital heart defects and indices of fetal cerebral growth in a nationwide cohort of 924 422 liveborn infants. Circulation 2016;133:566–75. https://doi.org/10.1161/circulationaha.115.019089.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0167).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

