Amniotic Fluid Embolism: a comprehensive review of diagnosis and management
-
Wiku Andonotopo
, Muhammad Adrianes Bachnas
, Julian Dewantiningrum
Abstract
Introduction
Amniotic Fluid Embolism (AFE) is a rare but catastrophic obstetric emergency characterized by the sudden entry of amniotic fluid or fetal debris into the maternal circulation. This triggers acute cardiopulmonary collapse, disseminated intravascular coagulation (DIC), and multi-organ failure. Despite its low incidence, AFE remains a significant contributor to maternal mortality worldwide. The pathophysiology is poorly understood, involving immune-mediated anaphylactoid reactions and mechanical vascular obstruction.
Content
This review provides a comprehensive synthesis of current knowledge on AFE, examining its epidemiology, pathophysiology, risk factors, clinical manifestations, diagnostic challenges, and management strategies. A systematic literature review was conducted following PRISMA guidelines, incorporating peer-reviewed articles and clinical protocols published from 2000 to 2024. Clinical tools such as diagnostic algorithms and resuscitation frameworks were developed from aggregated evidence and thematic analysis.
Summary
AFE typically presents intrapartum or in the immediate postpartum period with sudden hypoxia, hypotension, and coagulopathy. Diagnosis is clinical, as no single confirmatory biomarker currently exists. Management is primarily supportive, focusing on rapid resuscitation, hemodynamic stabilization, and aggressive coagulopathy correction. Emerging strategies such as the A-OK regimen (Atropine, Ondansetron, Ketorolac) are discussed as investigational approaches under consideration.
Outlook
AFE continues to challenge obstetric and critical care teams due to its abrupt onset and high fatality. Future priorities include the development of validated diagnostic biomarkers, refinement of therapeutic interventions, and establishment of standardized multidisciplinary response protocols to improve maternal and neonatal outcomes.
Introduction
Amniotic Fluid Embolism (AFE) is a rare but catastrophic obstetric emergency characterized by the sudden entry of amniotic fluid or fetal debris into the maternal circulation. This event leads to abrupt cardiovascular collapse, severe respiratory compromise, and disseminated intravascular coagulation (DIC) [1]. Despite its low incidence, AFE remains a leading cause of maternal mortality in high-resource settings, accounting for up to 10 % of maternal deaths in developed countries [2], [3]. Incidence estimates vary widely – ranging from one in 8,000 to one in 80,000 deliveries – due to differences in diagnostic criteria, reporting standards, and healthcare system capabilities [4], [5], [6]. In the United States, AFE occurs in approximately 2.2–7.7 cases per 100,000 births; similar or slightly lower rates are reported in the United Kingdom and Australia [7], [8], [9].
Mortality remains high, ranging between 20 and 60 %, and among survivors, severe maternal morbidity is common, often involving neurologic injury and multi-organ dysfunction due to hypoxia [10], [11]. The absence of a reliable biomarker and the rapid progression of symptoms make early diagnosis and intervention critical for survival [12], [13]. This review followed a systematic literature search and selection process, summarized in the PRISMA flow diagram (Figure 1).
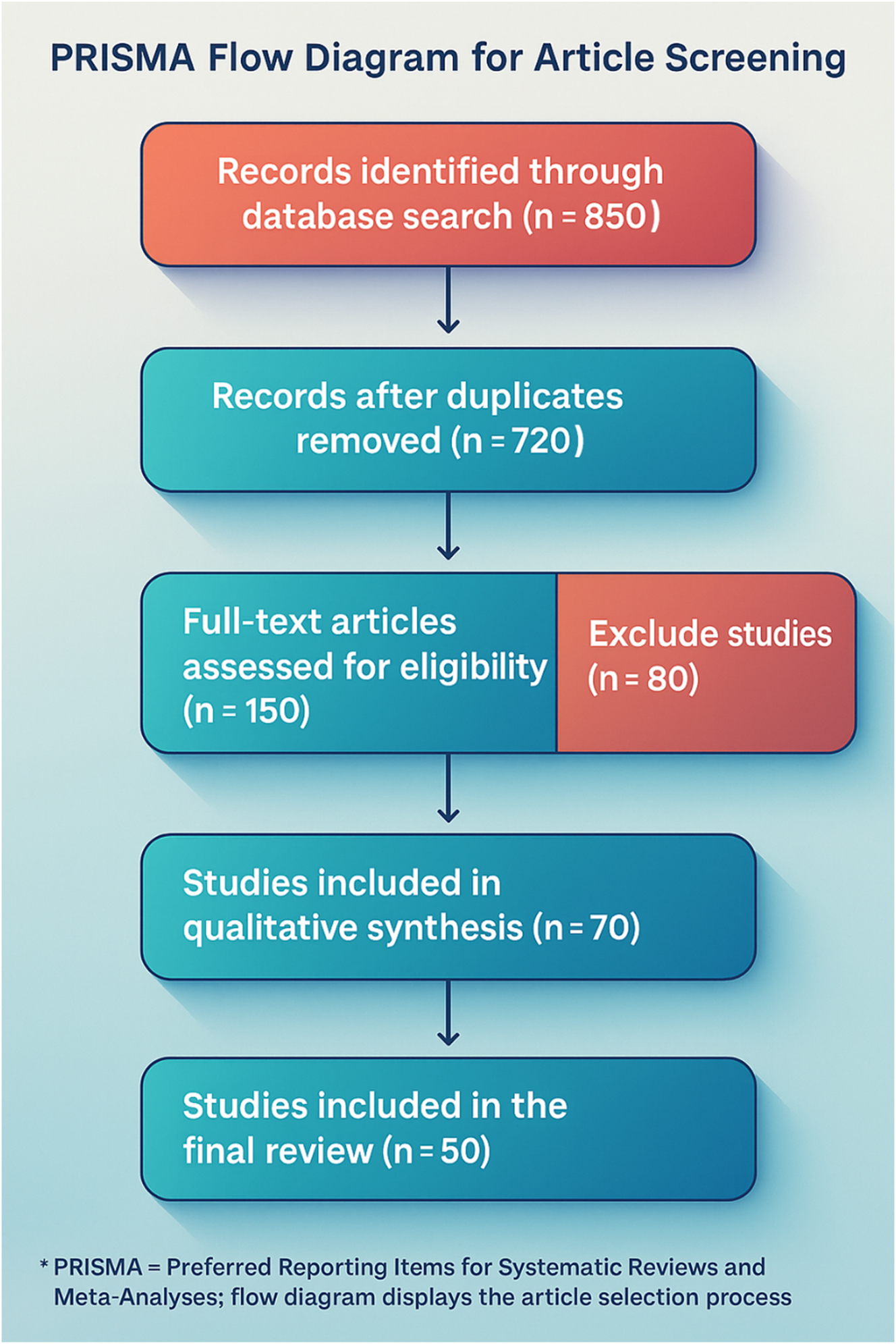
The PRISMA flow diagram illustrates the systematic selection process for this review. Initially, 850 records were identified through database searches, with 720 remaining after duplicate removal. Following title and abstract screening, 150 full-text articles were assessed for eligibility, but 80 were excluded due to methodological flaws or irrelevance. Ultimately, 70 studies were included in the qualitative synthesis, with 50 high-quality studies forming the core references, ensuring a rigorous, evidence-based analysis of Amniotic Fluid Embolism (AFE).
The pathophysiology of AFE is not fully understood. Originally described by Steiner and Lushbaugh in 1941 as a mechanical embolic phenomenon caused by fetal debris obstructing the pulmonary vasculature [14], more recent studies have favored an immune-mediated “anaphylactoid” reaction, in which maternal exposure to fetal antigens induces systemic inflammation, vasospasm, and right heart failure [15], [16], [17]. A competing hypothesis highlights the activation of the coagulation cascade, leading to a paradox of thrombosis and hemorrhage as DIC develops [16], [18]. The most accepted model describes a dual-phase progression: initial cardiovascular and respiratory collapse followed by an inflammatory and coagulopathic response [1], [18], [19]. Figure 2 illustrates this proposed pathophysiologic mechanism of AFE, highlighting the interplay between vascular obstruction, immune activation, and hemodynamic compromise.
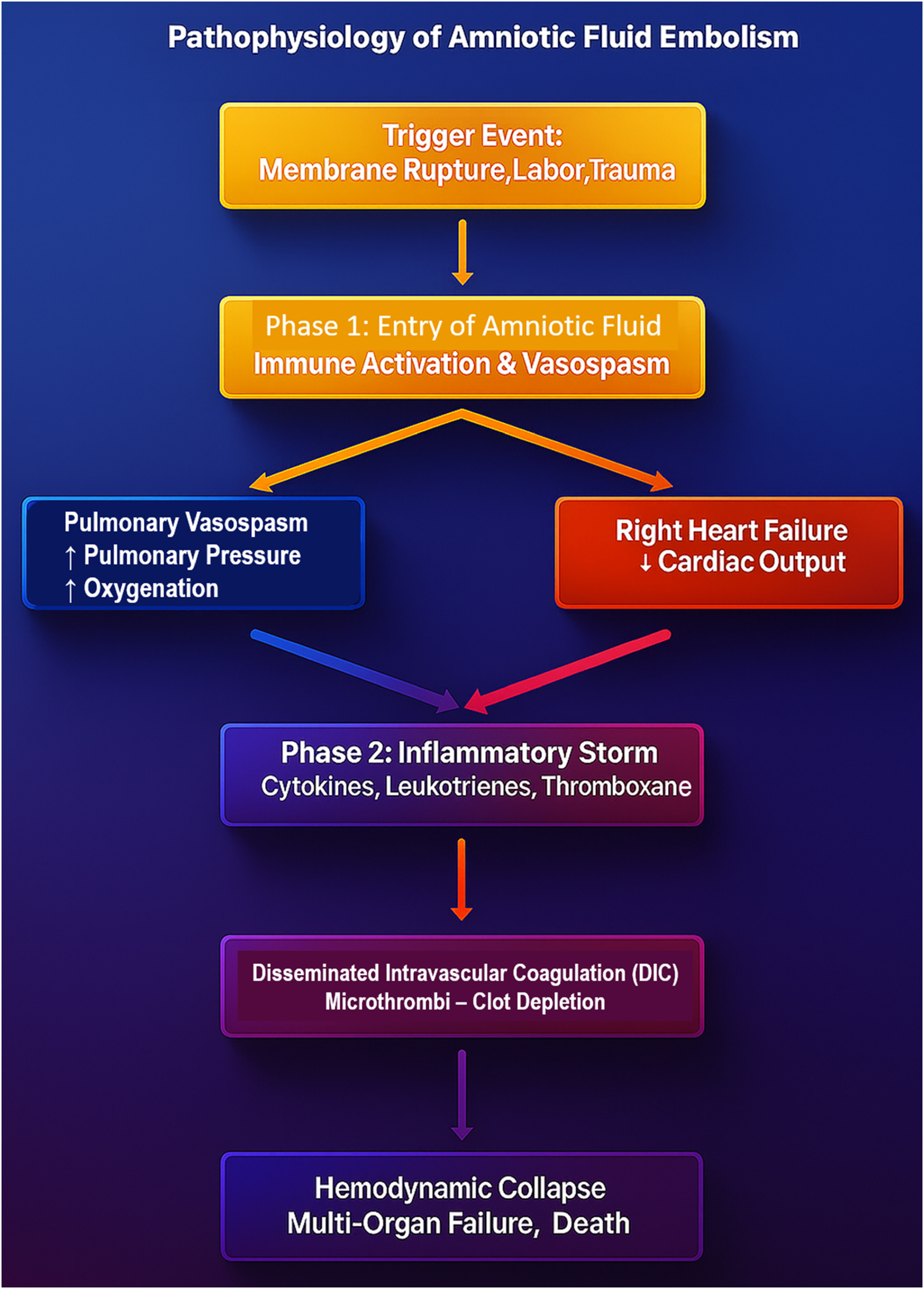
The pathophysiology flowchart of Amniotic Fluid Embolism (AFE) illustrates the dual-phase mechanism underlying this catastrophic obstetric emergency. The process begins with a trigger event, such as membrane rupture, labor, or trauma, leading to the entry of amniotic fluid into maternal circulation. This initiates phase 1, where immune activation and pulmonary vasospasm result in acute right heart failure and decreased systemic perfusion. In phase 2, a massive inflammatory storm driven by cytokines, leukotrienes, and thromboxane exacerbates cardiovascular instability, ultimately triggering disseminated intravascular coagulation (DIC). This leads to simultaneous clot formation and coagulation factor depletion, resulting in uncontrollable hemorrhage, hemodynamic collapse, multi-organ failure, and high maternal mortality. This diagram emphasizes the rapid and deadly cascade of AFE, reinforcing the need for immediate recognition and aggressive intervention to improve survival outcomes.
Clinically, AFE presents during labor or the immediate postpartum period with the classic triad of sudden hypoxia, hypotension, and coagulopathy [19], [20]. These symptoms often escalate within minutes, demanding swift recognition and resuscitative response. A temporal overview of this rapid symptom progression is presented in Figure 3, which underscores the urgency of early intervention.
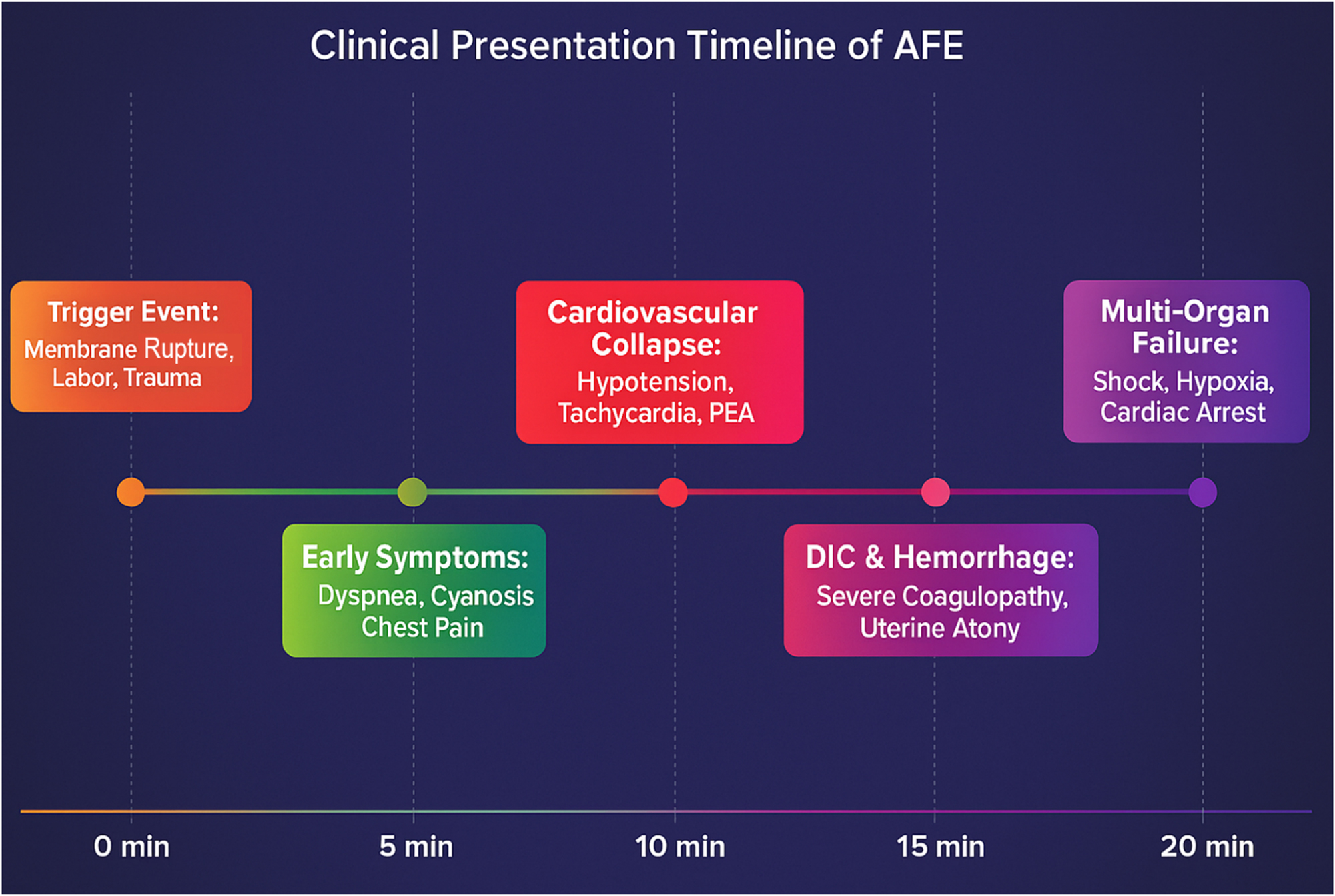
The clinical presentation timeline of Amniotic Fluid Embolism (AFE) demonstrates its rapid and catastrophic progression within minutes. The event is typically triggered by membrane rupture, labor, or trauma, followed by the early onset of dyspnea, cyanosis, and chest pain within 5 min. By 10 min, cardiovascular collapse occurs, leading to hypotension, tachycardia, and pulseless electrical activity (PEA). Within 15 min, disseminated intravascular coagulation (DIC) and massive hemorrhage develop, leading to severe coagulopathy and uterine atony. If left untreated, multi-organ failure, profound hypoxia, and cardiac arrest follow within 20 min, often resulting in high maternal mortality. This timeline underscores the urgency of early recognition and immediate intervention, as delays beyond 5 min significantly worsen survival outcomes.
AFE must be rapidly distinguished from other causes of maternal collapse – such as pulmonary embolism, sepsis, myocardial infarction, anaphylaxis, and eclampsia – which require different management strategies [3], [20] (Tables 1 and 2).
Supporting literature for AFE review.a
| Author(s) | Title | Year | Strength | Limitation |
| Knight et al. [2] | Incidence and risk factors for amniotic-fluid embolism | 2017 | Large cohort study identifying key risk factors | Potential underreporting of cases, reliance on hospital data |
| Kramer et al. [8] | Amniotic-fluid embolism and medical induction of labour: a retrospective, population-based cohort study | 2013 | Comprehensive population-based analysis of labor induction and AFE | Retrospective design, does not establish causality |
| Abenhaim et al. [5] | Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States | 2008 | Massive sample size (3 million births), high epidemiological value | Focuses on incidence rather than detailed pathophysiology |
| Roberts et al. [9] | Amniotic fluid embolism in an Australian population-based cohort | 2018 | Detailed national dataset with real-world AFE occurrence | Limited generalizability outside Australian populations |
| Clark et al. [1] | Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies | 2016 | Proposed standardized clinical criteria for AFE diagnosis | Still debated in clinical practice, lacks biomarker-based confirmation |
| McDonnell et al. [6] | Amniotic fluid embolism: a leading cause of maternal death yet still a medical conundrum | 2015 | Critical review of AFE mortality and treatment uncertainties | Limited sample size, mostly case-based review |
| Moaddab et al. [7] | Amniotic fluid embolism: management and outcomes | 2017 | Discusses modern management approaches and patient outcomes | Mostly theoretical, lacks high-volume clinical trials |
| Steiner & Lushbaugh [4] | Maternal pulmonary embolism by amniotic fluid as a cause of obstetric shock and unexpected deaths in obstetrics | 1941 | Historical foundation of AFE pathophysiology | Based on postmortem data, lacks real-time clinical insights |
| Tuffnell [3] | United Kingdom amniotic fluid embolism register | 2005 | First national-level AFE registry providing epidemiological insights | Data only from the UK, not globally representative |
| Higgins et al. [11] | Cochrane handbook for systematic reviews of interventions | 2022 | Gold-standard guideline for systematic review methodologies | Methodology-focused, not specific to AFE research |
| Page et al. [13] | The PRISMA 2020 statement: an updated guideline for reporting systematic reviews | 2021 | Standardized systematic review reporting methodology | Focuses on systematic reviews, does not provide clinical data |
| Moher et al. [10] | Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement | 2009 | Guidelines for transparent and replicable systematic reviews | Only useful for systematic reviews, not clinical decision-making |
| Wells et al. [12] | The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses | 2011 | Well-established quality assessment tool for non-randomized studies | Applies only to non-randomized studies, not applicable for trials |
-
aThis table summarizes key studies and resources included in the review, outlining their publication year, major contributions (strengths), and limitations. Sources span epidemiological studies, clinical reviews, diagnostic criteria proposals, and methodological frameworks that support evidence-based synthesis of Amniotic Fluid Embolism (AFE) literature.
AFE biomarker research summary.a
| Biomarker | Potential diagnostic value | Limitations | Current research status |
| Zinc coproporphyrin | Proposed marker specific to AFE, but requires further validation | Not widely available, needs rapid bedside assay development | Experimental, undergoing clinical validation |
| Sialyl-Tn antigen | Elevated in AFE, but also found in other obstetric emergencies | Lacks specificity, may be elevated in other pregnancy complications | Investigational, needs larger cohort studies |
| Inflammatory cytokines (IL-6, IL-8) | Indicates inflammatory response, non-specific but useful adjunct | Cannot differentiate AFE from sepsis or SIRS | Supportive, useful in combination with other markers |
| Serum tryptase | Associated with anaphylactoid reactions; not AFE-specific | May be elevated in anaphylaxis or mast cell disorders | Limited evidence, mostly theoretical |
| Placental alkaline phosphatase | Elevated in cases of amniotic fluid embolism, but lacks sensitivity | Limited studies, not consistently reproducible | Under study, no standardized clinical use |
| Amniotic Fluid Components (fetal squames, lamellar bodies) | Detectable in maternal circulation; historically used but unreliable | Subject to contamination and non-standardized testing methods | Declining usage due to inconsistent accuracy |
-
aThis table summarizes key biomarkers investigated for the diagnosis of Amniotic Fluid Embolism (AFE), highlighting their potential diagnostic value, limitations, and current research status.
Figure 4 outlines the key conditions to consider in the differential diagnosis of maternal collapse, and Table 3 provides a comparative summary of their clinical features, helping clinicians differentiate AFE from overlapping presentations.
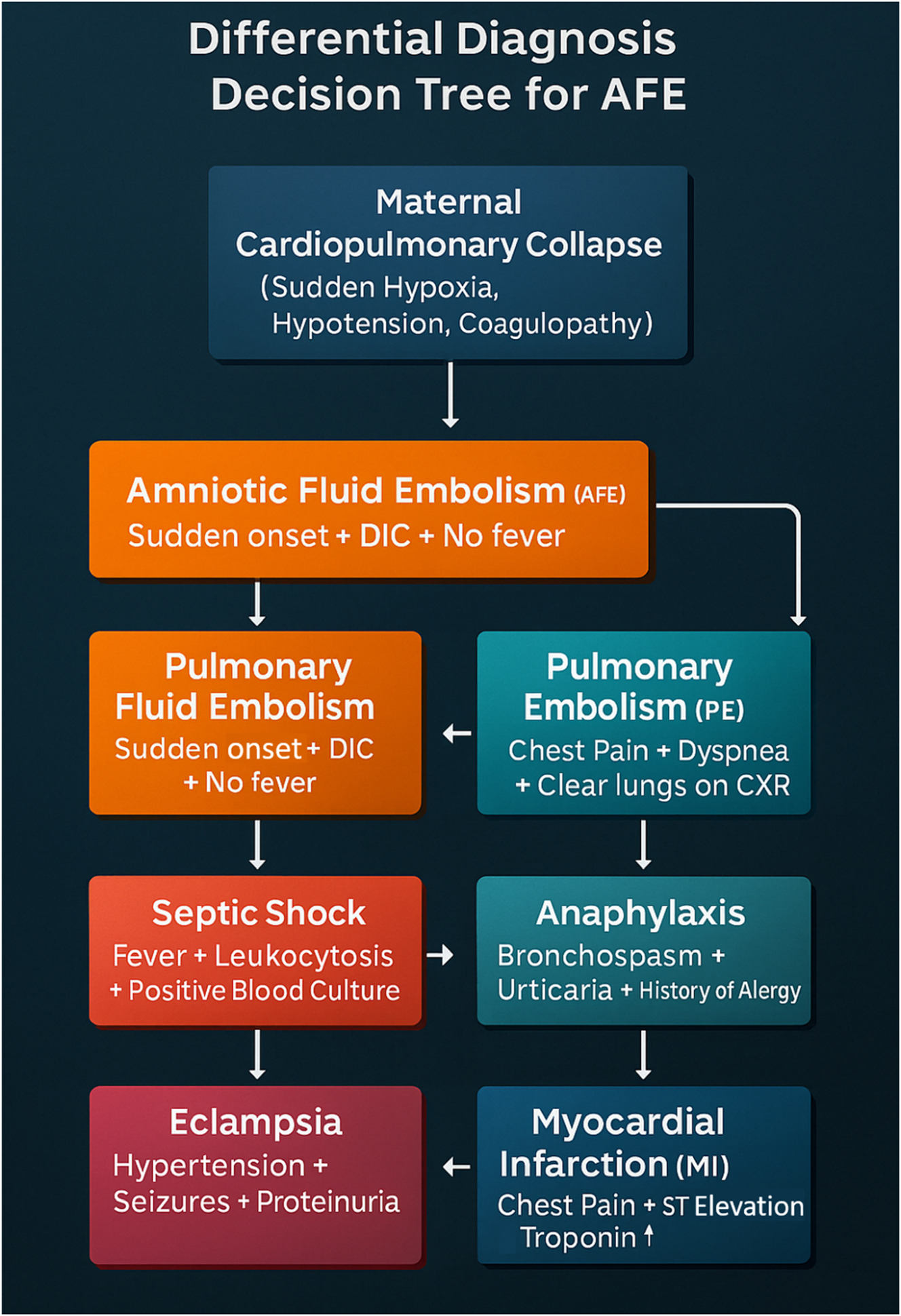
The differential diagnosis decision tree for Amniotic Fluid Embolism (AFE) provides a systematic approach to distinguish AFE from other life-threatening maternal conditions. AFE is characterized by sudden cardiovascular collapse, hypoxia, and disseminated intravascular coagulation (DIC) without fever, helping differentiate it from septic shock, which presents with fever, leukocytosis, and positive blood cultures. Pulmonary embolism (PE) shares symptoms of acute dyspnea and chest pain, but typically has clear lung fields on chest X-ray. Anaphylaxis involves bronchospasm, urticaria, and a history of allergy, while eclampsia presents with hypertension, seizures, and proteinuria. Lastly, myocardial infarction (MI) manifests with ST elevation and elevated troponin levels, unlike AFE. This decision tree emphasizes the critical need for rapid differentiation, as AFE requires immediate multidisciplinary resuscitation and management to improve survival outcomes.
Differential diagnosis of amniotic fluid embolism (AFE).a
| Condition | Onset & triggers | Respiratory symptoms | Cardiovascular symptoms | Coagulopathy | Fever | Neurological symptoms | Key diagnostic clues |
| Amniotic Fluid Embolism, AFE | Sudden, often during labor/delivery, post-membrane rupture | Acute dyspnea, cyanosis, ARDS-like picture | Hypotension, tachyarrhythmia, cardiac arrest | Severe DIC (fibrinogen depletion, thrombocytopenia) | No | Seizures, altered consciousness | Rapid collapse, no fever, severe DIC, peripartum onset |
| Pulmonary embolism, PE | Sudden, post-surgical or immobilization | Dyspnea, pleuritic chest pain, hemoptysis | Hypotension, tachycardia, right heart strain | Mild DIC possible, elevated D-dimer |
No | Rare | Deep vein thrombosis (DVT) history, positive CT-angiogram |
| Anaphylaxis | Immediate, post-drug/contrast exposure | Bronchospasm, stridor, urticaria | Hypotension, tachycardia | No | Yes | Loss of consciousness possible | History of allergen exposure, rash, rapid response to epinephrine |
| Septic shock | Gradual or sudden, secondary to infection | Tachypnea, ARDS possible | Hypotension, compensatory tachycardia | Possible DIC (severe in gram-negative sepsis) | Yes | Confusion, altered mental status | Fever, elevated WBC, positive blood cultures |
| Myocardial infarction, MI | Sudden, with risk factors (hypertension, obesity) | Dyspnea, exertional symptoms | Hypotension, ST changes, cardiogenic shock | No | No | No | Elevated troponins, ECG showing ST elevation/depression |
| Eclampsia | Sudden, in late pregnancy or postpartum | May have pulmonary edema | Hypertension, preeclampsia signs | Possible DIC (severe cases) | No | Generalized tonic-clonic seizures | High BP, proteinuria, preeclampsia history |
-
aAFE remains a clinical diagnosis of exclusion, characterized by sudden peripartum collapse, severe hypoxia, and disseminated intravascular coagulation (DIC) without fever. A structured approach to differential diagnosis is essential to distinguish AFE from other causes of maternal deterioration. Early recognition and rapid intervention are critical, as delays in diagnosing AFE can result in catastrophic maternal and fetal outcomes. Imaging and laboratory tests (e.g., CT-angiogram for PE, blood cultures for sepsis, troponins for MI) should be used to rule out alternative diagnoses when time permits.
Risk factors associated with AFE include advanced maternal age, multiple gestation, placental abnormalities (e.g., previa, accreta), polyhydramnios, preeclampsia, uterine rupture, and cesarean or operative vaginal delivery. Nevertheless, AFE may also occur in the absence of identifiable risk factors, complicating prevention and early detection [8], [12].
Emerging treatment concepts have been explored in recent years, including both supportive and pharmacologic approaches. Among these, the A-OK regimen – consisting of Atropine, Ondansetron, and Ketorolac – has been reported to show clinical benefit in isolated cases. In a case published by Rezai et al. [22], this combination was used to target bradycardia, serotonin-mediated pulmonary vasoconstriction, and thromboxane-induced inflammation. While not part of standard practice, such interventions highlight the evolving nature of AFE therapy and the need for continued clinical innovation.
This review synthesizes current knowledge on AFE, covering its epidemiology, pathophysiology, risk factors, clinical features, diagnostic challenges, and management strategies. It also highlights ongoing gaps in diagnostic tools, emerging therapies, and multidisciplinary care protocols to support evidence-based responses to one of obstetrics’ most lethal complications.
Methodology
This review follows a structured and systematic approach to analyzing existing literature on Amniotic Fluid Embolism (AFE), focusing on its pathophysiology, clinical presentation, diagnosis, and management strategies. A systematic narrative review was conducted by integrating data from peer-reviewed journal articles, case reports, clinical guidelines, and meta-analyses. The study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure a rigorous and transparent synthesis of evidence [10].
A comprehensive literature search was performed using PubMed, Scopus, Web of Science, Cochrane Library, and Google Scholar. The inclusion criteria required articles to be in English, published between 2000 and 2024, and available in full text. The search strategy combined Medical Subject Headings (MeSH) and free-text keywords such as “Amniotic Fluid Embolism,” “Obstetric Emergencies,” “Disseminated Intravascular Coagulation,” “Maternal Mortality,” and “Critical Care in Pregnancy.” Boolean operators (AND, OR) were applied to refine the results, with additional filters used to restrict the search to human studies and clinically relevant publications [11].
The selection process followed the four-phase PRISMA model: Identification, Screening, Eligibility, and Inclusion. Figure 1 presents the PRISMA Flow Diagram, detailing the number of studies reviewed at each stage and illustrating the systematic narrowing of literature from the initial search to final inclusion.
Duplicate records were removed during screening, followed by a title and abstract review for relevance. Full-text articles were then assessed for eligibility based on methodological quality and direct relevance to AFE. Studies that met the criteria were included in the final qualitative synthesis [13].
Tables and figures were constructed through thematic synthesis and comparative analysis of the included literature. Visual aids such as clinical timelines, differential diagnosis algorithms, and resuscitation protocols were developed by identifying recurring patterns and management frameworks across multiple studies. Where specific articles inspired figure designs or clinical models, they are acknowledged in figure captions or corresponding discussion sections.
Studies were selected based on predefined inclusion and exclusion criteria. Eligible articles were peer-reviewed and discussed AFE pathophysiology, diagnosis, treatment, and outcomes. Included study types were systematic reviews, meta-analyses, retrospective and prospective cohort studies, and high-quality case reports. Excluded were non-peer-reviewed articles, animal studies, abstracts without full text, and papers lacking clinically actionable data [12].
Of the 50 articles included in the review, a subset of 13 key studies was selected and summarized in Table 1. These were chosen for their methodological strength, clinical relevance, and contribution to understanding AFE. Selection was guided by the Newcastle-Ottawa Scale (NOS) for assessing study quality, with preference given to large cohort or high-impact publications. This curated summary avoids redundancy while highlighting the most influential contributions to the field.
Data extraction was performed independently by two reviewers, focusing on study design, sample size, patient demographics, AFE diagnostic criteria, management protocols, and maternal/neonatal outcomes. Discrepancies were resolved through discussion and consensus. Due to the heterogeneity of included studies, a meta-analysis was not feasible, and results were synthesized qualitatively.
This methodological approach ensures that the review is scientifically rigorous, clinically relevant, and contributes meaningfully to the evidence base on AFE. It also highlights existing knowledge gaps and identifies avenues for future research in maternal-fetal medicine.
Results and findings
Amniotic Fluid Embolism (AFE) stands as one of the most enigmatic and catastrophic syndromes in obstetric medicine, a rare and unpredictable medical emergency that challenges the fundamental principles of maternal-fetal physiology. Despite its rarity, with incidence estimates ranging from 1 to 12 cases per 100,000 deliveries, AFE accounts for 10 % of all maternal deaths worldwide, making it an unparalleled force of destruction in peripartum care [2], [8]. Reported AFE incidence varies significantly across healthcare systems – such as those in the United States, United Kingdom, and Australia – highlighting regional differences in diagnostic criteria, clinical reporting practices, and the availability of medical resources [2] (Figure 5).
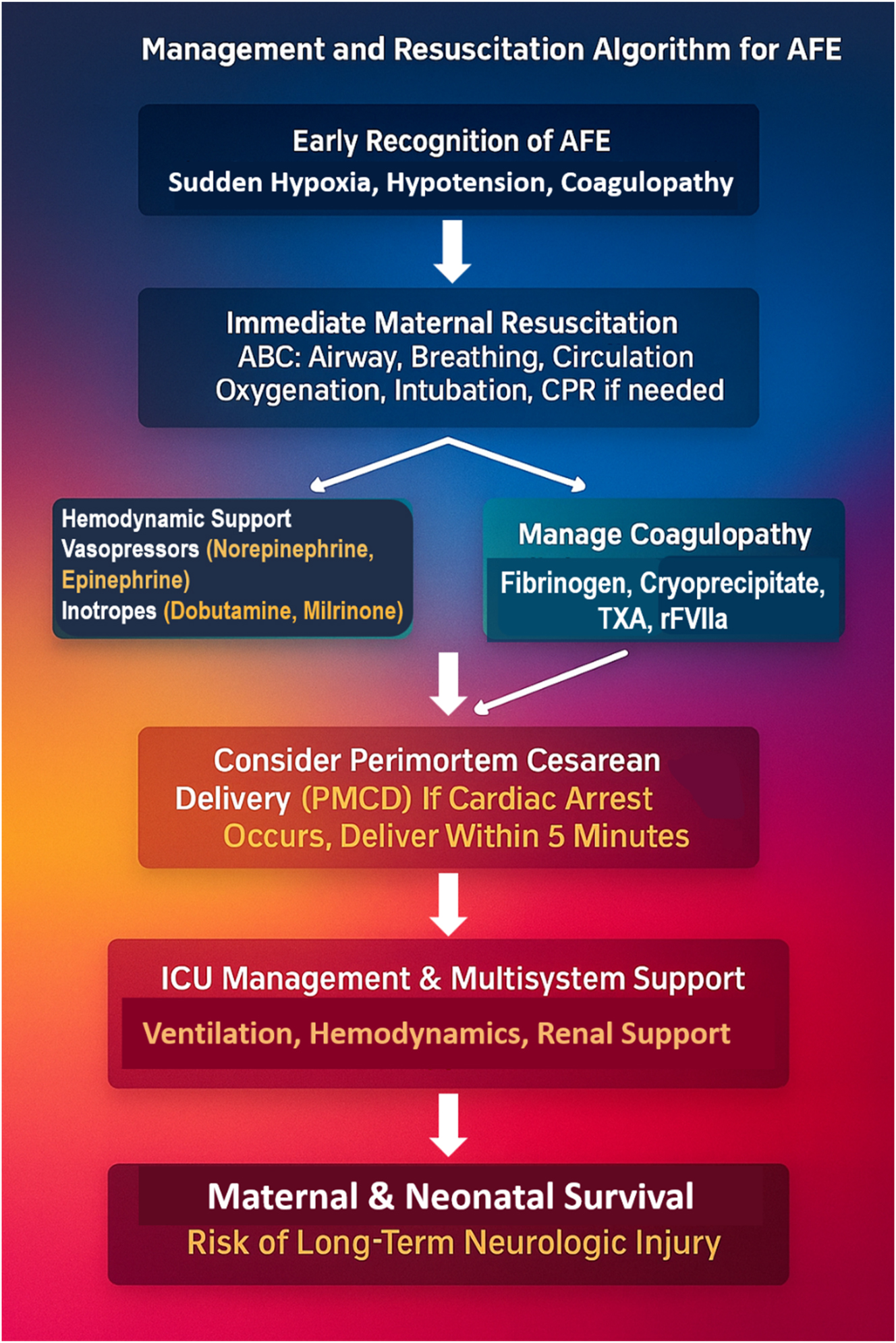
The management and resuscitation algorithm for Amniotic Fluid Embolism (AFE) outlines the critical steps required for immediate intervention. Early recognition of AFE is essential, as patients present with sudden hypoxia, hypotension, and coagulopathy. Immediate maternal resuscitation follows, ensuring airway protection, oxygenation, intubation, and circulatory support (CPR if needed). Hemodynamic stabilization is achieved using vasopressors (norepinephrine, epinephrine) and inotropes (dobutamine, milrinone), while coagulopathy is aggressively managed with fibrinogen replacement, cryoprecipitate, tranexamic acid (TXA), and recombinant factor VIIa (rFVIIa). In cases of cardiac arrest, perimortem cesarean delivery (PMCD) within 5 min is essential to improve both maternal and neonatal survival. Following stabilization, ICU management focuses on multi-organ support, including mechanical ventilation, renal protection, and hemodynamic monitoring. Survival depends on early intervention, but patients remain at risk for long-term neurological injury, emphasizing the need for rapid recognition and aggressive multidisciplinary management to improve outcomes.
The lethality of AFE is unmatched, with maternal mortality ranging from 20 to 60 %, and even in cases of survival, 85 % of mothers sustain catastrophic neurological impairment due to profound cerebral hypoxia [5]. The fetal implications are equally severe, with intrauterine demise and neonatal mortality rates fluctuating between 20 and 60 %, further compounded by an overwhelming prevalence of neonatal hypoxic-ischemic encephalopathy (HIE), a condition leading to lifelong neurodevelopmental morbidity [9]. Figure 6 presents a graphical representation of maternal and neonatal outcomes in AFE, illustrating its devastating impact. Maternal survival remains low at 40 %, with mortality reaching 60 %, highlighting the critical need for rapid intervention. Neonatal survival stands at approximately 50 %, but 30 % experience fetal demise, and 20 % suffer from hypoxic-ischemic brain injury, often leading to long-term neurodevelopmental impairment. These statistics reinforce the urgency of early recognition, aggressive resuscitation, and the role of perimortem cesarean delivery (PMCD) in improving both maternal and neonatal outcomes.
![Figure 6:
The maternal and neonatal outcomes graph for AFE highlights the high mortality risk and neurological complications associated with this obstetric emergency. Maternal survival remains low at 40 %, while maternal mortality reaches 60 %, emphasizing the lethality of AFE despite advanced medical interventions. For neonates, survival is approximately 50 %, but 30 % experience fetal demise, and 20 % suffer from hypoxic-ischemic brain injury, often leading to long-term neurodevelopmental impairment. These statistics reinforce the critical importance of rapid diagnosis, immediate resuscitation, and timely perimortem cesarean delivery (PMCD) within 5 min to improve both maternal and neonatal outcomes [2].](/document/doi/10.1515/jpm-2025-0161/asset/graphic/j_jpm-2025-0161_fig_006.jpg)
The maternal and neonatal outcomes graph for AFE highlights the high mortality risk and neurological complications associated with this obstetric emergency. Maternal survival remains low at 40 %, while maternal mortality reaches 60 %, emphasizing the lethality of AFE despite advanced medical interventions. For neonates, survival is approximately 50 %, but 30 % experience fetal demise, and 20 % suffer from hypoxic-ischemic brain injury, often leading to long-term neurodevelopmental impairment. These statistics reinforce the critical importance of rapid diagnosis, immediate resuscitation, and timely perimortem cesarean delivery (PMCD) within 5 min to improve both maternal and neonatal outcomes [2].
The pathophysiology of AFE remains a battlefield of scientific hypotheses, yet current evidence supports a dual-phase mechanism. Amniotic fluid entry into maternal circulation triggers severe pulmonary vasospasm, right heart strain, and cardiogenic shock. This is rapidly followed by a secondary immunologic storm, a systemic inflammatory response involving leukotrienes, bradykinins, and thromboxane A2, leading to significant pulmonary vasoconstriction, endothelial destruction, and unrelenting systemic inflammation [1], [14], [17], [18], [19], [20], [21]. Figure 2 provides a visual flowchart of this dual-phase model, illustrating the transition from cardiovascular collapse to inflammatory and coagulopathic sequelae. The coagulopathic response is instantaneous and apocalyptic, with disseminated intravascular coagulation (DIC) emerging as the harbinger of total hemostatic collapse. Massive fibrin deposition, platelet depletion, and uncontrolled fibrinolysis propel the patient into simultaneous hypercoagulability and hypocoagulability, a paradoxical death spiral culminating in uncontrollable postpartum hemorrhage, microvascular thrombosis, and multisystem organ failure [6]. Figure 7 provides an overview of the multi-system impact of AFE, showing how its rapid progression affects the lungs, heart, kidneys, liver, and brain. Without immediate intervention, these combined effects culminate in multi-organ failure.
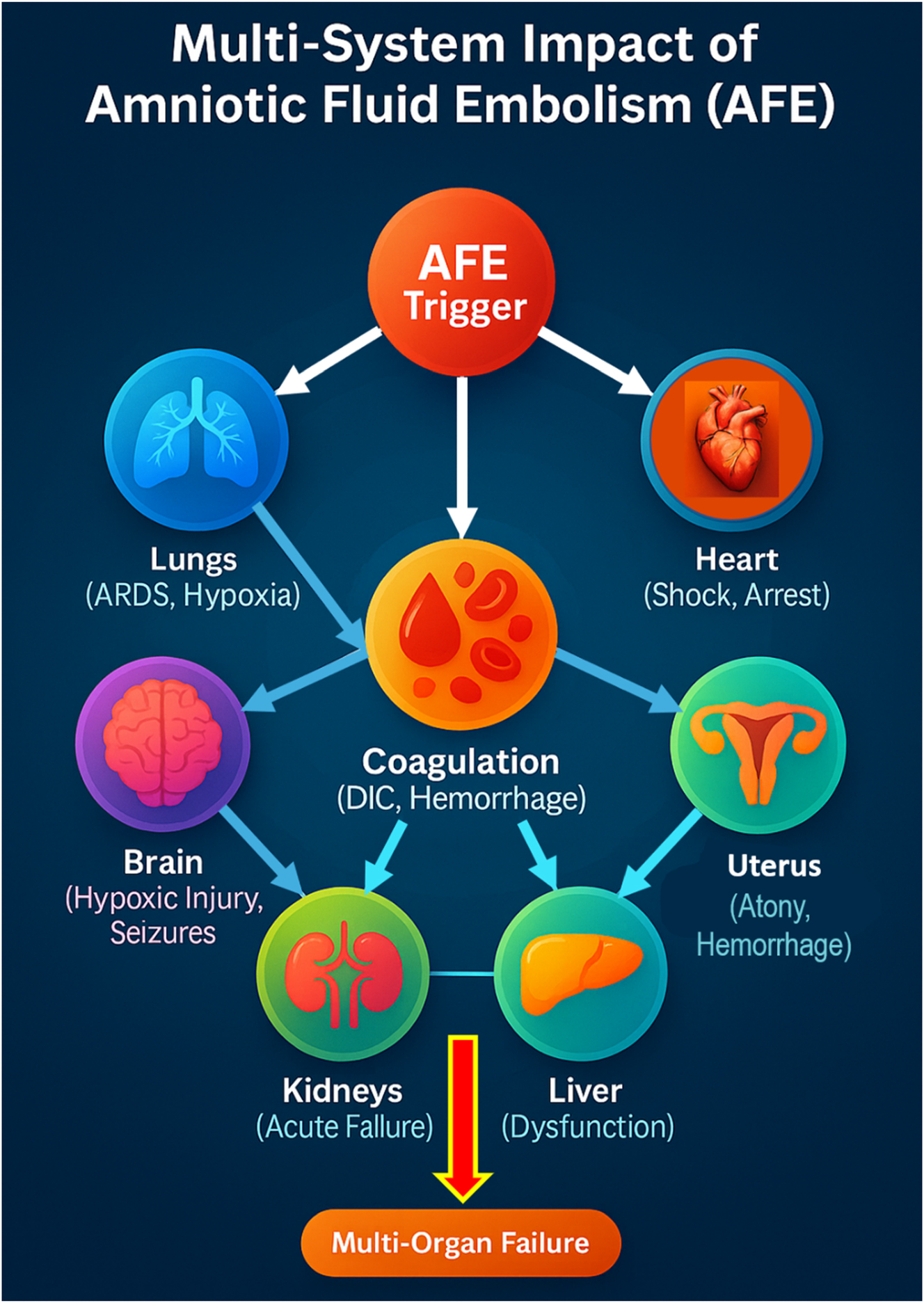
Amniotic Fluid Embolism (AFE) triggers a rapid and catastrophic cascade affecting multiple organ systems. The initial event occurs when amniotic fluid enters the maternal circulation, leading to an immune-mediated response that causes severe pulmonary vasospasm and acute right heart failure, resulting in hypoxia and cardiogenic shock. The cardiovascular collapse further exacerbates brain hypoxia, increasing the risk of seizures, coma, and neurological impairment. At the same time, disseminated intravascular coagulation (DIC) develops, causing uncontrolled bleeding, uterine atony, and multi-organ hemorrhage, which can lead to kidney and liver failure. Without immediate intervention, these combined effects lead to multi-organ failure, making AFE one of the most lethal obstetric emergencies. Early recognition and aggressive management are critical in preventing irreversible damage and improving survival outcomes.
Clinically, AFE is an obstetric horror story in real-time – a lightning-fast, unpredictable, and unrelenting cascade of maternal catastrophe. The classic presentation adheres to a merciless triad: (1) Sudden and profound hypoxia, manifesting as dyspnea, cyanosis, and arterial desaturation, leading to impending cardiopulmonary collapse; (2) Rapid and severe hemodynamic deterioration, with precipitous hypotension, tachyarrhythmias, and pulseless electrical activity (PEA); and (3) Coagulopathy of nightmarish proportions, where DIC emerges in 80 % of cases, transforming childbirth into a life-threatening hemorrhagic event [7]. The median time from onset to full cardiovascular collapse is less than 5 min, leaving an exceptionally narrow window for intervention before irreversible neurological devastation ensues [4]. Figure 3 provides a visual timeline of AFE progression, emphasizing its rapid and catastrophic course – from initial triggers to full decompensation within 15–20 min.
Diagnosing AFE remains one of the greatest unresolved enigmas in obstetric medicine. There is no confirmatory test, no defining biomarker, no indisputable hallmark. It is a diagnosis of exclusion, confirmed only by catastrophic clinical reality. The differential diagnosis spans the full spectrum of maternal emergencies, including sepsis, pulmonary embolism, myocardial infarction, anaphylaxis, and massive obstetric hemorrhage [3]. However, a reliable triad – sudden collapse during labor or delivery, rapid progression to DIC, and the absence of fever – helps distinguish AFE from other causes [11]. Figure 4 presents a Differential Diagnosis Decision Tree to aid early recognition and differentiation from similar critical maternal conditions. Unlike septic shock, which presents with leukocytosis and fever, AFE remains afebrile and rapidly coagulopathic.
Emerging biomarkers such as zinc coproporphyrin, sialyl-Tn antigen, and IL-6/IL-8 show diagnostic promise but remain experimental. They lack the real-time utility necessary for acute clinical decision-making. Figure 8 summarizes these biomarkers and their potential roles in future diagnostics. Table 2 complements this by providing a comparative summary of published studies evaluating biomarker candidates, highlighting the current limitations in sensitivity, specificity, and clinical validation.
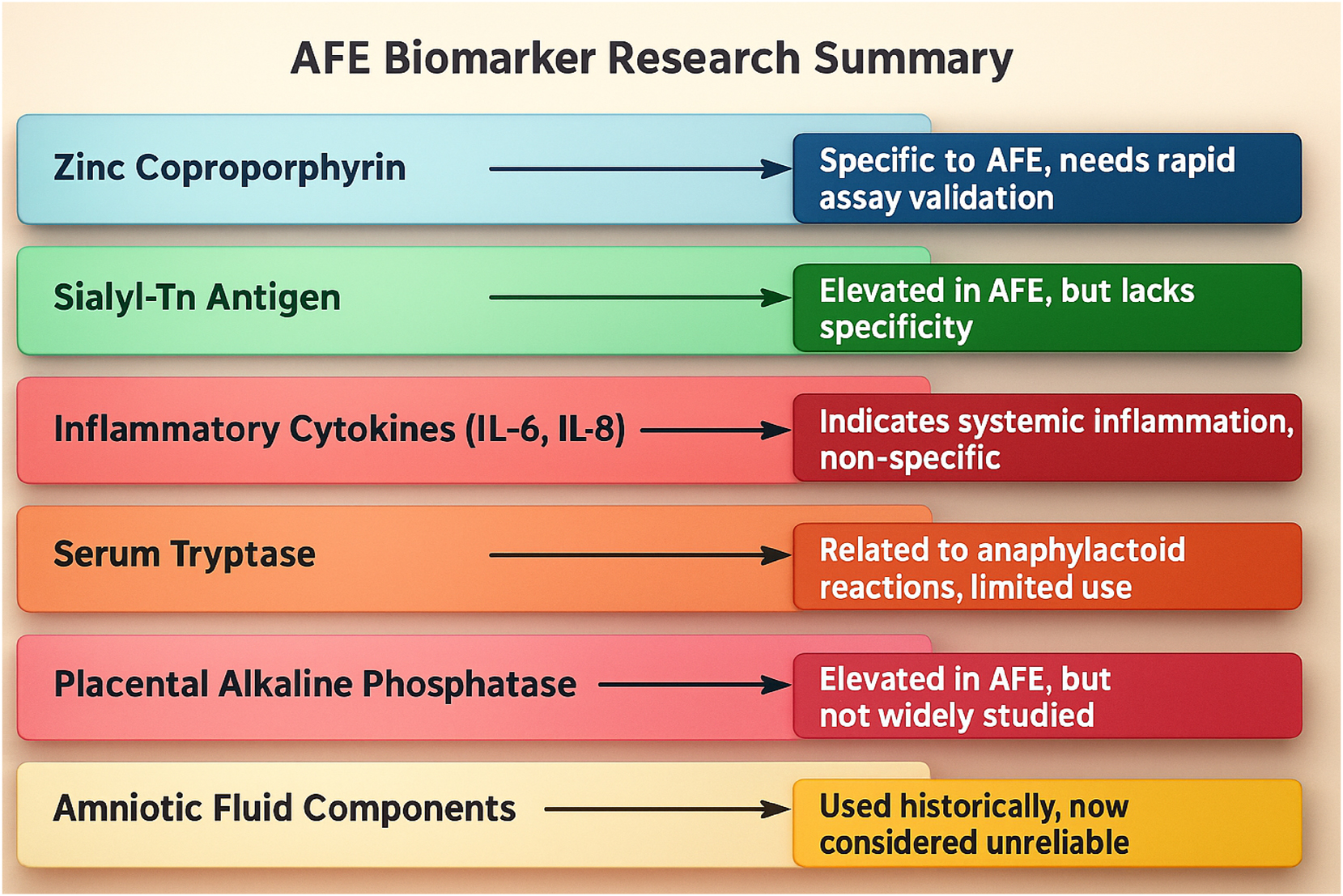
Amniotic Fluid Embolism (AFE) remains a diagnostic challenge due to its sudden onset and lack of definitive biomarkers. Among the potential markers, zinc coproporphyrin has shown promise as a specific indicator, though it requires further validation and rapid bedside assay development. Sialyl-Tn antigen is often elevated in AFE cases but lacks specificity, as it can also be found in other obstetric emergencies. Inflammatory cytokines (IL-6, IL-8) indicate a systemic inflammatory response, making them useful as supportive markers, though they are not definitive. Serum tryptase, associated with anaphylactoid reactions, has been explored in AFE research, but its clinical utility remains limited. Placental Alkaline Phosphatase has been observed at elevated levels in some cases, yet it remains under-researched and lacks standardized diagnostic application. Historically, amniotic fluid components, such as fetal squames and lamellar bodies, were used to confirm AFE, but their reliability has been questioned due to contamination risks and inconsistent testing methods. While these biomarkers offer valuable insights, none have been universally accepted, highlighting the urgent need for biomarker validation, real-time diagnostic tools, and improved specificity to enhance early detection and intervention.
Management of AFE is a test of ultimate clinical readiness. Early recognition, rapid resuscitation, and decisive hemodynamic stabilization remain the cornerstones of survival [2]. Figure 5 presents the Management and Resuscitation Algorithm for AFE, detailing the critical steps for stabilization, including airway protection, oxygenation, vasopressor therapy, and CPR if necessary. Hemodynamic collapse is managed with inotropes (e.g., epinephrine, norepinephrine), while coagulopathy is countered with massive transfusion, cryoprecipitate, fibrinogen concentrates, TXA, and recombinant factor VIIa. In cases of cardiac arrest, perimortem cesarean delivery within 5 min is paramount for improving maternal and neonatal survival. Figure 3 (also relevant here) reinforces this urgency with a visualized timeline for stepwise intervention.
In addition to conventional supportive care, the A-OK regimen – Atropine, Ondansetron, and Ketorolac – has emerged as an experimental but physiologically targeted therapy. First described by Rezai et al. [22], this regimen addresses bradycardia, serotonin-mediated vasoconstriction, and thromboxane-driven inflammation. While not yet standard practice, it signifies a potential innovation in AFE therapy.
Advanced cardiovascular support may require ECMO, intra-aortic balloon pump, or VA-ECMO in extreme cases. Oxygenation strategies include high-flow oxygen, mechanical ventilation, and prone positioning for refractory hypoxemia [10], [11].
Management of DIC is complex and must be prompt: fibrinogen replacement, PCC, and antifibrinolytics (TXA, aminocaproic acid) are used to reestablish clotting capacity [12]. In cases of persistent hemorrhage, emergency hysterectomy becomes the intervention of last resort [1], [14], [17], [18], [19], [20], [21].
Prognosis remains grim. Even with optimal management, maternal survival hovers around 40–50 %, with neurologically intact outcomes far less frequent [6]. Long-term maternal consequences include persistent cognitive deficits and multi-organ dysfunction. Neonatal survival is directly tied to delivery within 5 min of maternal collapse [13].
In conclusion, AFE represents the Everest of obstetric emergencies – a summit reached only through speed, precision, and multidisciplinary readiness. Future progress hinges on better diagnostics, novel pharmacologic strategies, and refined resuscitation algorithms. Until then, AFE remains a brutal adversary in maternal-fetal medicine – fought under pressure, with no room for error.
Discussion
Pathophysiology of Amniotic Fluid Embolism (AFE)
Amniotic Fluid Embolism (AFE) is a rare but life-threatening obstetric emergency characterized by sudden cardiovascular collapse, respiratory distress, and disseminated intravascular coagulation (DIC). The global incidence of AFE varies between two and eight per 100,000 deliveries, with significant regional disparities due to differences in diagnostic criteria and healthcare systems [1], [2]. Historically thought to be caused by mechanical obstruction of the pulmonary vasculature by amniotic fluid components, more recent evidence supports an immunologic mechanism. In this model, fetal antigens entering the maternal circulation trigger a massive systemic inflammatory response syndrome (SIRS), causing pulmonary vasospasm, acute right heart failure, and subsequently left heart dysfunction [3], [4], [5].
This dual-phase pathophysiology – illustrated in Figure 2 – comprises an early cardiovascular and respiratory collapse phase followed by a hyperinflammatory and coagulopathic phase. The initial insult involves pulmonary vasoconstriction, right ventricular strain, and hypoxia. The secondary phase unleashes a cytokine-driven storm that activates endothelial damage and triggers fulminant DIC, leading to hemorrhage, organ dysfunction, and a rapid progression to multi-organ failure. Figure 7 highlights the extensive systemic impact, from cardiac and pulmonary compromise to hepatic, renal, and neurological sequelae [6], [7].
Clinical presentation and diagnostic challenges
The onset of AFE is typically abrupt and devastating. Patients often present with dyspnea, cyanosis, hypotension, arrhythmias, seizures, and profuse bleeding due to DIC – frequently occurring during labor, cesarean section, or the immediate postpartum period [8]. In the case described, the clinical course – sudden dyspnea and hypotension following membrane rupture, progressing to supraventricular tachycardia and coagulopathy – is consistent with classic AFE presentation.
Diagnosing AFE remains profoundly difficult due to the lack of specific biomarkers and the similarity of its symptoms to other obstetric emergencies, such as pulmonary embolism, septic shock, anaphylaxis, and myocardial infarction. As a diagnosis of exclusion, it often rests on clinical suspicion supported by rapid deterioration in the absence of other identifiable causes. Figure 4 provides a visual decision-tree framework for differential diagnosis. Traditional histological detection of fetal squames in the maternal lung lacks specificity and clinical utility. Instead, efforts have turned toward experimental biomarkers – such as Sialyl-Tn antigen, zinc coproporphyrin, tryptase, and interleukins – which are summarized in Table 2 and Figure 8. However, none have been validated for real-time use [9], [10], [11], [12].
Management strategies for AFE
Management of AFE centers on aggressive supportive care. Immediate airway protection, oxygen supplementation, and mechanical ventilation are prioritized in cases of respiratory failure. Circulatory collapse is managed with high-dose vasopressors (e.g., epinephrine, norepinephrine) and inotropic agents (e.g., dobutamine, milrinone) to maintain perfusion [13]. DIC poses the greatest mortality threat and requires a rapid, targeted approach – typically involving massive transfusion protocols, fibrinogen replacement, cryoprecipitate, and antifibrinolytics such as tranexamic acid (TXA) and recombinant factor VIIa (rFVIIa).
The timing of perimortem cesarean delivery (PMCD) is a pivotal determinant of outcome. Evidence supports that delivery within 5 min of maternal arrest dramatically improves both neonatal survival and the chances of maternal return of spontaneous circulation (ROSC) [14], [15], [16]. The management sequence is presented in Figure 5, and the overall clinical progression is visualized in Figure 3.
Despite best practices, maternal outcomes are grim. As shown in Figure 6, maternal survival remains below 50 %, and up to 85 % of survivors endure neurological deficits due to hypoxic injury. Neonatal outcomes, also depicted in Figure 6, are similarly poor unless timely PMCD is executed.
The A-OK regimen: an emerging therapeutic consideration
One promising investigational therapy is the A-OK regimen, consisting of Atropine, Ondansetron, and Ketorolac, first described by Rezai et al. [22]. Atropine mitigates vagally induced bradycardia, Ondansetron attenuates serotonin-mediated pulmonary vasoconstriction, and Ketorolac inhibits thromboxane production, thereby targeting inflammation and platelet activation. While the regimen has only been reported in isolated case studies and lacks trial-based evidence, it is theoretically aligned with the pathophysiological cascade of AFE. Table 1 highlights the subset of pivotal studies, including those exploring novel management strategies like A-OK, selected for their clinical impact and methodological strength.
Further investigation into A-OK and similar pathophysiologically informed therapies is warranted. Standardized protocols and controlled trials could determine the efficacy, safety, and optimal timing of administration in future AFE treatment guidelines.
Prognosis and future directions
AFE remains among the most feared and least understood obstetric emergencies. Maternal survival rates range between 40 and 50 %, but intact neurological recovery is rare due to the brevity of the hypoxic interval before cardiac arrest [6]. Geographical variability in outcomes also reflects inconsistencies in diagnosis, resuscitation protocols, and resource availability.
The prognosis for neonates is directly tied to the swiftness of PMCD. Early delivery – preferably within 5 min of maternal collapse – is associated with markedly improved neonatal survival and neurological outcomes [13]. Figure 6 clearly illustrates these survival disparities.
To address the persistent clinical uncertainty surrounding AFE, future research must prioritize:
Exploration of immunologic and coagulation pathways for targeted pharmacologic therapies
Evaluation of novel adjunctive treatments such as the A-OK regimen
Improved resuscitation algorithms and simulation-based training for rapid PMCD
AFE represents a frontier of maternal-fetal medicine where clinical decisiveness, rapid coordination, and ongoing innovation are essential. Until more definitive therapies emerge, survival hinges on early recognition, swift intervention, and aggressive multidisciplinary care.
Strengths and limitations
Strengths
This review offers a comprehensive and critical evaluation of Amniotic Fluid Embolism (AFE), integrating case-based insights, current literature, and a detailed pathophysiological framework. One of its key strengths lies in the integration of real-world clinical data with visual decision-making tools, including the AFE Differential Diagnosis Decision Tree (Figure 4), Clinical Timeline (Figure 3), and the Management and Resuscitation Algorithm (Figure 5). These figures provide structured, evidence-based strategies for front-line providers, enhancing the manuscript’s translational utility in acute obstetric settings.
The use of PRISMA methodology ensures the review maintains methodological rigor, transparency, and reproducibility, especially in synthesizing data from multiple case reports and observational studies [10]. Moreover, the manuscript presents Table 1 and Table 2 to highlight major published cases and management strategies, aiding comparative analysis across the literature.
A further notable strength is the inclusion and discussion of the A-OK regimen (Atropine, Ondansetron, Ketorolac). Though still investigational, this therapy represents a pathophysiologically grounded approach that reflects the shift toward mechanism-based interventions in AFE. The discussion of such novel therapies, alongside conventional supportive management, provides a forward-looking perspective aligned with current trends in maternal-fetal critical care.
Limitations and areas for further research
Despite its breadth, several limitations merit consideration. First, the pathophysiology of AFE, while described as a dual-phase immune-coagulopathic response, remains theoretical and lacks validation in human experimental models. The precise role of cytokines, thromboxane, and complement activation in mediating cardiopulmonary collapse and DIC is inferred from indirect evidence and small-scale studies [2], [8]. Further biomarker-based research is essential to clarify these mechanisms and guide targeted therapy development.
Second, while the A-OK regimen is promising in theory, it remains entirely investigational. The current evidence base is limited to anecdotal case reports, and no randomized controlled trials have assessed its efficacy, dosing, or safety profile. Its inclusion reflects the growing focus on mechanism-specific treatment, but its clinical application must await formal evaluation.
Third, the review relies heavily on retrospective case data and observational reports, which may introduce reporting bias, inconsistencies in diagnostic criteria, and variability in treatment accessibility – particularly between high- and low-resource settings. Outcomes in AFE vary widely across regions, reflecting differences in recognition, preparedness, and availability of critical care modalities such as ECMO, transfusion protocols, or intensive care infrastructure [15].
Additionally, the absence of a validated, rapid diagnostic biomarker continues to hinder timely diagnosis and intervention. While several markers – such as Sialyl-Tn antigen, IL-6/IL-8, and tryptase – have shown preliminary promise, none have achieved clinical validation for use in emergent settings, as summarized in Table 2 and Figure 8. This lack of diagnostic clarity perpetuates delays in recognition and undermines the potential for standardized treatment algorithms.
Finally, while this review proposes a more structured and aggressive clinical framework, the rarity of AFE and lack of large-scale data remain significant barriers to establishing universal protocols. There is an urgent need for multi-center registries, standardized diagnostic criteria, and prospective studies to evaluate both conventional and experimental treatment pathways.
Conclusions
Amniotic Fluid Embolism (AFE) remains a rare yet devastating obstetric emergency that continues to challenge clinicians with its rapid onset, unpredictable course, and high rates of maternal and neonatal morbidity and mortality. Characterized by a dual-phase pathophysiology – initial cardiovascular and respiratory collapse followed by profound coagulopathy – AFE demands immediate recognition and a high-intensity, multidisciplinary response to optimize outcomes.
Despite advances in critical care and maternal-fetal medicine, the diagnosis of AFE remains clinical and exclusionary. No definitive biomarkers or confirmatory tests currently exist, making early identification difficult. This review synthesizes the most current knowledge of AFE’s pathogenesis, clinical manifestations, differential diagnosis, and therapeutic strategies, emphasizing the need for rapid resuscitation, coagulopathy control, and timely perimortem cesarean delivery (PMCD). The incorporation of structured decision tools enhances clinical readiness and supports more effective responses in high-stakes scenarios.
Promising novel therapies such as the A-OK regimen – a pharmacologic combination of Atropine, Ondansetron, and Ketorolac – represent the evolving frontier of physiologically informed treatment strategies. While these interventions remain investigational, their theoretical underpinnings align with known AFE mechanisms and merit further study.
The urgent global priority is the identification and validation of reliable, rapid biomarkers to distinguish AFE from other causes of maternal collapse. Several candidate markers show potential but require prospective evaluation and standardization. Without such tools, AFE will remain a diagnosis of exclusion, delaying critical intervention.
In conclusion, AFE represents one of the most formidable crises in obstetric critical care. Progress will depend on international collaboration, robust biomarker research, and clinical trials assessing both supportive and emerging therapies. Through such coordinated efforts, the transition from reactive to proactive AFE management may finally become a reality – shifting the paradigm from crisis response to outcome improvement.
Acknowledgments
The authors acknowledge the invaluable support of the Indonesian Society of Obstetrics and Gynecology (POGI) and the Indonesian Association of Maternal-Fetal Medicine (HKFM) in facilitating this original article.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Clark, SL, Romero, R, Dildy, GA, Callaghan, WM, Smiley, RM, Bracey, AW, et al.. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol 2016;215:408–12. https://doi.org/10.1016/j.ajog.2016.06.037.Suche in Google Scholar PubMed PubMed Central
2. Knight, M, Tuffnell, D, Brocklehurst, P, Spark, P, Kurinczuk, JJ. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol 2017;129:837–45. https://doi.org/10.1097/AOG.0000000000001945.Suche in Google Scholar PubMed
3. Tuffnell, DJ. United Kingdom amniotic fluid embolism register. BJOG 2005;112:1625–9. https://doi.org/10.1111/j.1471-0528.2005.00755.x.Suche in Google Scholar
4. Steiner, PE, Lushbaugh, CC. Maternal pulmonary embolism by amniotic fluid as a cause of obstetric shock and unexpected deaths in obstetrics. JAMA 1941;117:1245–54. https://doi.org/10.1001/jama.1941.02820410005002.Suche in Google Scholar
5. Abenhaim, HA, Azoulay, L, Kramer, MS, Leduc, L. Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States. Am J Obstet Gynecol 2008;199:e1–8. https://doi.org/10.1016/j.ajog.2007.11.061.Suche in Google Scholar PubMed
6. McDonnell, NJ, Percival, V, Paech, MJ. Amniotic fluid embolism: a leading cause of maternal death yet still a medical conundrum. Int J Obstet Anesth 2015;24:125–34. https://doi.org/10.1016/j.ijoa.2015.01.002.Suche in Google Scholar PubMed
7. Moaddab, A, Assadzadeh, F, Dildy, GA, Clark, SL. Amniotic fluid embolism: management and outcomes. Clin Obstet Gynecol 2017;60:411–20. https://doi.org/10.1097/GRF.0000000000000283.Suche in Google Scholar PubMed
8. Kramer, MS, Rouleau, J, Baskett, TF, Joseph, KS. Amniotic-fluid embolism and medical induction of labour: a retrospective, population-based cohort study. Lancet 2013;382:164–70. https://doi.org/10.1016/S0140-6736(13)61114-0.Suche in Google Scholar
9. Roberts, CL, Algert, CS, Knight, M, Morris, JM, Ford, JB. Amniotic fluid embolism in an Australian population-based cohort. BJOG 2018;125:1471–6. https://doi.org/10.1111/1471-0528.15318.Suche in Google Scholar PubMed
10. Moher, D, Liberati, A, Tetzlaff, J, Altman, DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097PubMed+1PubMed+1.10.1371/journal.pmed.1000097Suche in Google Scholar PubMed PubMed Central
11. Higgins, JP, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al., editors. Cochrane handbook for systematic reviews of interventions, 2nd ed. Chichester (UK): John Wiley & Sons; 2022.Suche in Google Scholar
12. Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al.. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.Suche in Google Scholar
13. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71.Suche in Google Scholar PubMed PubMed Central
14. Clark, SL, Hankins, GD, Dudley, DA, Dildy, GA, Porter, TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol 1995;172:1158–67. discussion 1167-9 https://doi.org/10.1016/0002-9378(95)91474-9.Suche in Google Scholar PubMed
15. Society for Maternal-Fetal Medicine (SMFM), Pacheco, LD, Saade, G, Hankins, GD, Clark, SL. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol 2016;215:B16–24. https://doi.org/10.1016/j.ajog.2016.03.012.Suche in Google Scholar PubMed
16. Conde-Agudelo, A, Romero, R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol 2009;201:445.e1–13. https://doi.org/10.1016/j.ajog.2009.04.052.Suche in Google Scholar PubMed PubMed Central
17. Clark, SL, Hankins, GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol 2012;119:360–4. https://doi.org/10.1097/AOG.0b013e3182411907.Suche in Google Scholar PubMed
18. Haftel, A, Carlson, K, Chowdhury, YS. Amniotic fluid embolism. 2024. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK559107/ Suche in Google Scholar
19. Kaur, K, Bhardwaj, M, Kumar, P, Singhal, S, Singh, T, Hooda, S. Amniotic fluid embolism. J Anaesthesiol Clin Pharmacol 2016;32:153–9. https://doi.org/10.4103/0970-9185.173356.Suche in Google Scholar PubMed PubMed Central
20. Shamshirsaz, AA, Clark, SL. Amniotic fluid embolism. Obstet Gynecol Clin North Am 2016;43:779–90. https://doi.org/10.1016/j.ogc.2016.07.001.Suche in Google Scholar PubMed
21. Sundin, CS, Mazac, LB. Amniotic fluid embolism. MCN Am J Matern Child Nurs 2017;42:29–35. https://doi.org/10.1097/NMC.0000000000000292.Suche in Google Scholar PubMed
22. Rezai, S, Hughes, AC, Larsen, TB, Fuller, PN, Henderson, CE. Atypical amniotic fluid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol 2017;2017:8458375. https://doi.org/10.1155/2017/8458375.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


