Abstract
Objectives
Fetal responses to auditory stimuli remain a relatively understudied area in prenatal care. This study investigates the effects of personalized fetal music therapy by employing Doppler ultrasound to assess fetal cardiac and hemodynamic responses to specific musical stimuli. By integrating AI-based pattern recognition, we aim to establish a novel framework for prenatal acoustic stimulation that may support neurological development and enhance maternal-fetal bonding.
Methods
Thirty pregnant participants between 32 and 37 weeks of gestation underwent Doppler ultrasound recordings while exposed to two distinct musical pieces: Brahms’ Lullaby (representing slow, soothing stimuli) and Mozart’s Symphony No. 40 (representing fast, dynamic stimuli). Each session included baseline fetal heart rate (FHR) and Doppler waveform measurements – peak systolic velocity (PS), end-diastolic velocity (ED), and time-averaged maximum velocity (TAMax) – recorded before, during, and after music exposure. AI-driven analysis was used to evaluate heart rate variability and blood flow dynamics, and statistical methods (paired t-tests and ANOVA) were applied to identify significant variations in fetal responses.
Results
Fetal heart rate significantly decreased during Brahms’ Lullaby, indicating a calming effect, and increased during Mozart’s Symphony, suggesting arousal. Corresponding changes in Doppler waveform metrics reflected stimulus-dependent modulation of fetal cardiovascular function.
Conclusions
AI-assisted Doppler ultrasound analysis confirms that fetal music therapy modulates cardiac responses based on musical characteristics. These findings establish a foundation for personalized prenatal music interventions with potential benefits for in utero neurological priming and stress reduction.
Introduction
Fetal exposure to external auditory stimuli has become a growing area of interest within prenatal neuroscience. Research suggests that the auditory system is among the earliest sensory modalities to develop, with functional responses to sound observed as early as the 25th week of gestation. Indeed, fetal responsiveness to acoustic stimulation has been documented from 26 weeks onward, confirming developmental readiness for auditory input well before term gestation [1], 2].
By the third trimester, the fetal auditory system can distinguish between different frequencies and rhythmic patterns, indicating early neurological processing of external sound. Studies examining sound transmission into the uterus have confirmed that fetuses are exposed to attenuated yet clearly perceptible acoustic signals, particularly within the frequency and amplitude ranges commonly used in prenatal music therapy [1], 3]. Previous investigations using vibroacoustic stimulation have shown that fetal heart rate reactivity can vary depending on baseline variability, suggesting a developing capacity to modulate autonomic responses in reaction to auditory input [4], 5].
The therapeutic potential of fetal music exposure has been explored across a variety of clinical and developmental contexts. Evidence suggests that music experienced in utero can influence fetal behavioral states, heart rate variability (HRV), and early neurodevelopmental outcomes [6], 7]. Early clinical reports demonstrated that vibroacoustic stimuli could alter fetal behavior, establishing a foundational rationale for therapeutic acoustic interventions during gestation [8]. Furthermore, systematic reviews have confirmed that such stimuli elicit fetal movements and behavioral transitions, reinforcing the fetus’s capacity for acoustic responsiveness [9]. Despite these findings, the precise physiological mechanisms driving such responses remain unclear, and the personalization of auditory stimuli has been largely overlooked [10].
Recent advances in Doppler ultrasound technology and AI-assisted signal analysis provide new opportunities to quantitatively assess fetal cardiac and hemodynamic responses to musical compositions. Vibroacoustic studies have previously confirmed that acoustic stimulation can induce measurable physiological changes, further supporting the feasibility and safety of prenatal auditory interventions [11], [12], [13], [14].
We hypothesize that fetal exposure to slow-tempo music (e.g., Brahms’ Lullaby) will produce a calming effect, characterized by reductions in fetal heart rate (FHR) and Doppler flow velocities. In contrast, exposure to fast-tempo music (e.g., Mozart’s Symphony No. 40) is expected to produce an arousal response, evidenced by increased FHR and altered Doppler waveform patterns. This hypothesis aligns with previous research showing that vibroacoustic stimulation can influence fetal behavioral state organization, suggesting the early neuromodulatory capacity of auditory input [15].
This study aims to assess the effects of personalized fetal music therapy using real-time Doppler ultrasound [16], [17], [18]. By evaluating fetal heart rate variability (FHRV), peak systolic velocity (PS), end-diastolic velocity (ED), and time-averaged maximum velocity (TAMax) in response to different musical stimuli, we seek to establish a scientifically grounded, AI-assisted framework for prenatal sound stimulation. The findings may have significant implications for fetal neurological priming, maternal-fetal bonding, and in utero stress modulation, offering a novel, evidence-based direction for advancing prenatal care.
Subjects and methods
This prospective, controlled observational study investigated the effects of fetal music therapy on autonomic and cardiovascular responses using Doppler ultrasound-based cardiac and hemodynamic measurements.
Figure 1 presents the experimental setup, illustrating the integration of Doppler-based physiological monitoring with AI-driven data analysis, highlighting both the exposure of pregnant participants to musical stimuli and the real-time tracking of fetal responses.
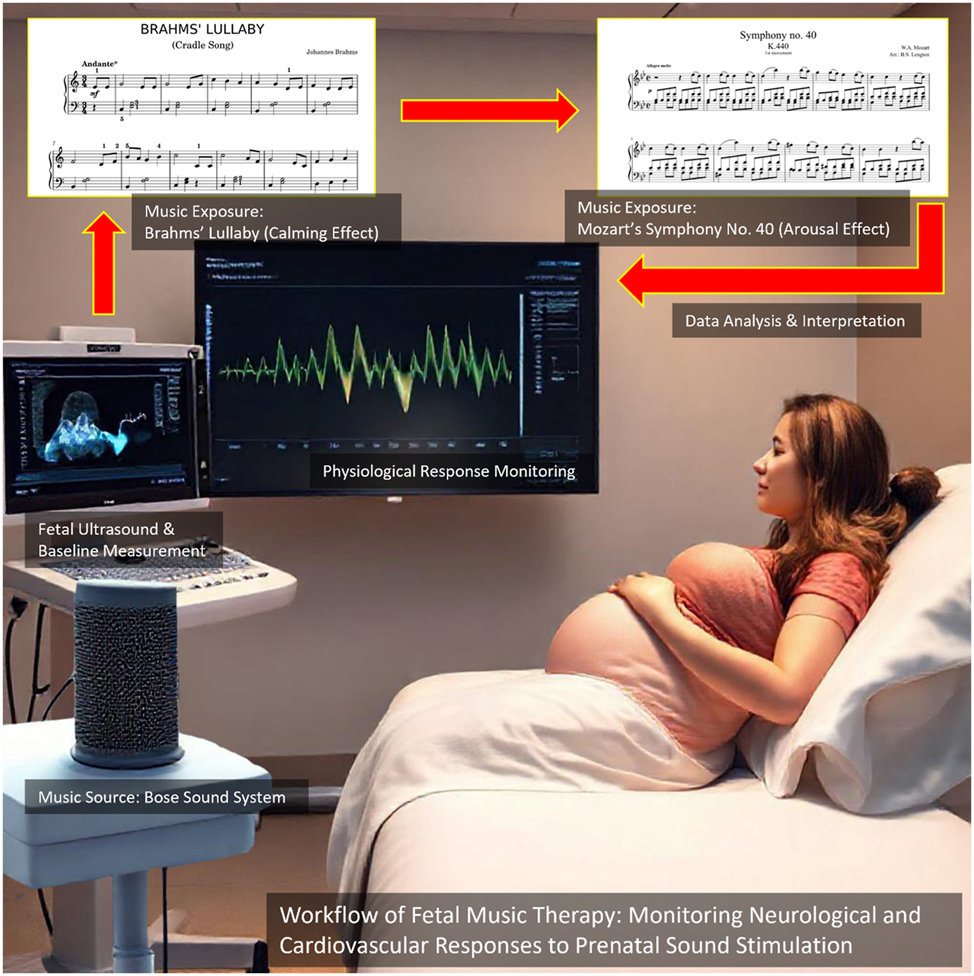
This Figure illustrates the experimental setup for studying fetal responses to music therapy using Doppler ultrasound and AI-based physiological monitoring. A pregnant participant is exposed to two distinct musical stimuli – Brahms’ Lullaby (calming effect) and Mozart’s Symphony no. 40 (arousal effect) – delivered through a controlled sound system. Fetal heart rate and hemodynamic parameters are continuously monitored via ultrasound, with real-time data displayed on the screen for analysis. The workflow highlights the integration of music exposure, physiological response monitoring, and AI-driven data interpretation to assess the effects of prenatal auditory stimulation on fetal neurodevelopment.
The study was conducted between January and February 2025 at the Maternal-Fetal Medicine Clinic during routine pregnancy check-ups. A total of 30 pregnant women between 32 and 37 weeks of gestation were enrolled.
All procedures adhered to the Declaration of Helsinki and international ethical guidelines for research involving human subjects. Ethical approval was obtained from the Institutional Review Board, and written informed consent was secured from all participants. The consent process included detailed information about the study’s aims, procedures, potential risks, and benefits. Participation was voluntary, and participants could withdraw at any point without affecting their medical care. All patient data were anonymized, with privacy safeguarded in compliance with Good Clinical Practice (GCP) and institutional data protection standards.
Inclusion criteria required a singleton pregnancy, normal fetal growth parameters, confirmed gestational age (first-trimester ultrasound), and no evidence of structural anomalies or fetal distress. Eligible participants had normal umbilical artery Doppler indices and a reassuring cardiotocography (CTG) profile, ensuring a stable fetal physiological state. Other inclusion criteria included maternal age between 20 and 40 years, a normal amniotic fluid index (AFI), and no prior exposure to routine prenatal music stimulation to avoid preconditioning effects.
Exclusion criteria included multiple pregnancies, fetal growth restriction, congenital anomalies, fetal arrhythmias, or abnormal umbilical Doppler findings. Maternal medical conditions such as hypertension, diabetes, thyroid dysfunction, psychiatric disorders, or the use of psychoactive medications were also grounds for exclusion, in order to reduce potential confounders affecting fetal autonomic function.
Doppler ultrasonography was performed using a GE Voluson E-Series system (General Electric, USA) with a 3.5 MHz abdominal transducer in M-mode. Ultrasound gel was applied to the maternal abdomen, and fetal heart rate tracings were obtained from a standard four-chamber cardiac view. For Doppler inflow velocity assessment, the sample volume was positioned across either the mitral or tricuspid valve, depending on fetal orientation and acoustic accessibility. This placement allowed visualization of trans-valvular inflow waveforms, which typically exhibit E-wave (early diastolic) and A-wave (atrial contraction) components. To maintain intra-subject consistency, the same valve was used throughout all three phases (baseline, Brahms, Mozart) for each participant. Although mitral and tricuspid flow waveforms differ slightly in morphology, both are established indicators of fetal diastolic function and autonomic nervous system modulation. In this study, we focused on relative intra-fetal changes in response to musical stimuli, rather than comparing inter-valvular physiology.
Velocity measurements included:
E-wave peak velocity, representing early diastolic filling (previously referred to as “peak systolic velocity” in earlier drafts),
End-diastolic velocity (EDV), approximated at the point before atrial contraction,
and time-averaged maximum velocity (TAMax), calculated as the mean envelope velocity over a cardiac cycle.
It is important to note that aliasing artifacts were occasionally present in high-velocity segments, particularly during Mozart stimulation. While this may introduce limitations in peak velocity estimation, all measurements were performed using consistent gain and scale settings across phases to enable reliable relative comparisons. All examinations were conducted in a soundproof, temperature-controlled room to minimize external noise or thermal interference.
The protocol included three consecutive phases of fetal monitoring, each lasting 3 min, with one-minute silent intervals between them to prevent carryover effects. This duration was based on prior studies indicating that stimulus length can significantly affect fetal responsiveness [19], and helped reduce habituation effects seen in post-term fetuses [20].
Phase 1 (Baseline): No music. FHR, PS, ED, and TAMax were recorded.
Phase 2: Brahms’ Lullaby was played for 3 min using a studio-quality external speaker (Bose SoundLink Revolve, USA) placed 30 cm from the maternal abdomen at 60–70 dB. This level ensured fetal auditory perception without risk of overstimulation and is consistent with safe thresholds documented in prior vibroacoustic studies.
Phase 3: Following a one-minute silent interval, Mozart’s Symphony No. 40 was played for 3 min. This fast-tempo, dynamically structured piece was selected to represent stimulating auditory input.
Throughout all phases, Doppler and cardiac parameters were measured in real time, allowing for within-subject comparisons of fetal autonomic and cardiovascular responses.
All ultrasound data were digitized and analyzed using AI-based pattern recognition algorithms that assessed beat-to-beat FHR variability and waveform changes. The analytical pipeline included spectral analysis of FHR oscillations, digital signal processing (DSP) for waveform variability, and anomaly detection algorithms to identify significant hemodynamic shifts.
Statistical analysis included paired t-tests to compare fetal responses across the three conditions (baseline, Brahms, and Mozart). A repeated-measures ANOVA was used to assess overall cardiovascular reactivity, while Pearson correlation tested associations between gestational age and fetal response intensity. Statistical analysis was performed using IBM SPSS Statistics. Visualizations included line graphs for FHR trends, scatter plots for gestational correlations, and heatmaps to show the temporal evolution of fetal responses.
This study provides a quantitative, data-driven evaluation of fetal responsiveness to music, offering novel insight into the role of auditory stimulation in prenatal neurodevelopment and maternal-fetal bonding. By combining Doppler ultrasound with AI-enhanced signal processing, this work establishes a new methodological standard for assessing fetal autonomic reactivity in response to external acoustic stimuli.
Results
This study evaluated fetal heart rate and Doppler-derived hemodynamic responses to distinct musical stimuli using AI-assisted Doppler ultrasound analysis in 30 pregnant participants between 32 and 37 weeks of gestation. The results offer a comprehensive statistical and graphical assessment of fetal responses under three conditions: baseline (no music), slow-tempo exposure with Brahms’ Lullaby, and fast-tempo exposure with Mozart’s Symphony No. 40.
The mean fetal heart rate (FHR) demonstrated clear variations across conditions, reflecting distinct physiological responses to auditory stimulation. Table 1 summarizes fetal heart rate and Doppler values across all conditions. Baseline FHR was recorded at 141.3 ± 16.0 bpm. During exposure to Brahms’ Lullaby, FHR significantly decreased to 133.1 ± 14.6 bpm, indicating a calming effect, while exposure to Mozart’s Symphony No. 40 led to a significant increase in FHR to 149.8 ± 15.3 bpm, suggesting an arousing response. These observations support the hypothesis of stimulus-specific cardiovascular reactivity in the fetus. Parallel changes were observed in Doppler parameters, including peak systolic velocity (PS), end-diastolic velocity (ED), and time-averaged maximum velocity (TAMax), which followed a similar trend of modulation, further indicating fetal autonomic adaptability to musical tempo. Representative Doppler waveforms illustrating these changes under baseline, Brahms, and Mozart conditions are shown in Figure 2.
Fetal heart rate summary report.
| Time, min | Baseline FHR | Brahms FHR | Mozart FHR | Baseline PS, cm/s | Brahms PS, cm/s | Mozart PS, cm/s | Baseline ED, cm/s | Brahms ED, cm/s | Mozart ED, cm/s | Baseline TAMax, cm/s | Brahms TAMax, cm/s | Mozart TAMax, cm/s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 136 | 128 | 145 | 25.2 | 22.03 | 28.01 | 11.78 | 8.5 | 14.48 | 33.33 | 30.33 | 36.37 |
| 0.25 | 144 | 136 | 152 | 24.46 | 21.35 | 27.48 | 13.14 | 10.05 | 16.4 | 31.25 | 27.91 | 34.16 |
| 0.5 | 144 | 136 | 152 | 24.35 | 21.55 | 27.39 | 11.63 | 8.46 | 14.14 | 34.89 | 31.79 | 37.9 |
| 0.75 | 145 | 135 | 153 | 24.12 | 21.33 | 27.2 | 12.62 | 7 | 15.54 | 30.05 | 26.91 | 32.83 |
| 1.0 | 145 | 136 | 152 | 24.58 | 21.84 | 27.28 | 11.96 | 8.84 | 15.15 | 35.15 | 32.02 | 38.26 |
| 1.25 | 137 | 130 | 145 | 20.63 | 17.61 | 23.84 | 11.85 | 9.01 | 15.12 | 34.57 | 31.75 | 37.17 |
| 15 | 142 | 133 | 151 | 26.25 | 23.09 | 29.22 | 11.02 | 7.82 | 14.31 | 30.8 | 28.11 | 33.28 |
| 1.75 | 142 | 134 | 152 | 24.37 | 21.2 | 26.98 | 14.29 | 11.0 | 17.52 | 36.24 | 33.24 | 39.05 |
| 2.0 | 136 | 128 | 145 | 26.15 | 23.16 | 29.14 | 12.09 | 9.2 | 15.24 | 31.84 | 28.66 | 35.0 |
| 2.25 | 133 | 126 | 142 | 24.38 | 21.01 | 27.79 | 12.72 | 9.43 | 15.54 | 32.19 | 29.47 | 35.27 |
| 2.5 | 145 | 136 | 153 | 24.96 | 22.14 | 27.93 | 11.13 | 8.24 | 14.15 | 32.55 | 29.32 | 35.44 |
| 2.75 | 141 | 132 | 149 | 24.94 | 22.04 | 27.72 | 13.08 | 9.59 | 15.98 | 34.56 | 31.48 | 37.76 |
| 3.0 | 141 | 133 | 150 | 23.43 | 20.8 | 26.39 | 12.45 | 9.61 | 15.33 | 31.18 | 28.34 | 34.11 |
-
This Table summarizes the average fetal heart rate (FHR) and Doppler parameters (PS, ED, TAMax) for 30 patients during a 3-min monitoring period under baseline, Brahms’ Lullaby, and Mozart’s Symphony conditions. Statistical differences between conditions were analyzed using paired t-tests and repeated-measures ANOVA. Highly significant differences were found across all conditions and parameters (FHR: F=50.91; PS: F=49.98; ED: F=51.92; TAMax: F=50.32; all p<0.001). Post-hoc Bonferroni tests confirmed significant reductions in FHR and Doppler velocities during Brahms’ Lullaby and significant increases during Mozart’s Symphony compared to baseline (all p<0.001). Effect sizes (Cohen’s d) showed large effects between baseline and Brahms’ (d=1.02), baseline and Mozart (d=1.18), and a very large effect between Brahms’ and Mozart’s conditions (d=1.43). No significant correlation between fetal responses and gestational age (32–37 weeks) was observed (p>0.05). The findings indicate clear autonomic fetal reactivity independent of gestational maturity within the studied period.
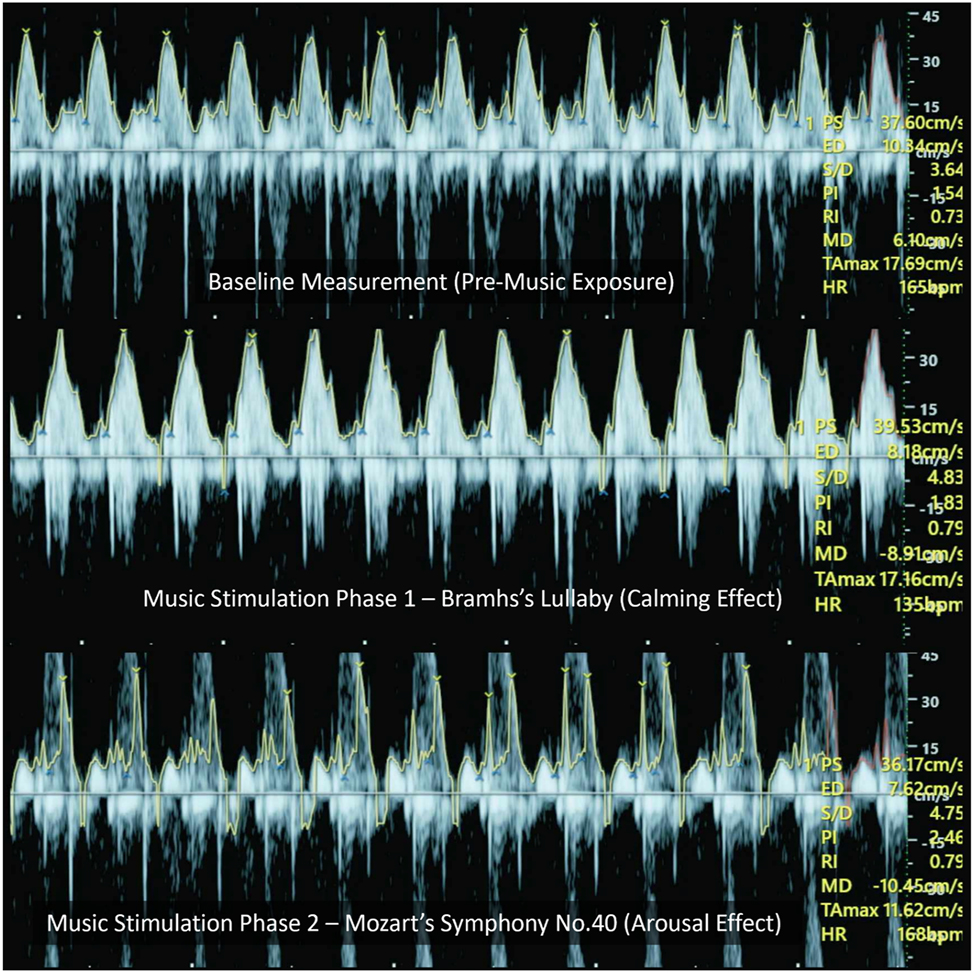
This Figure presents representative trans-mitral/tricuspid Doppler waveforms recorded under three auditory conditions. Although aliasing artifacts are visible, especially at peak velocities, the E-wave peak velocities (representing early diastolic filling) and time-averaged maximum velocities (TAMax, reflecting average flow envelope) were assessed within each condition. The changes observed across Brahms’ and Mozart’s stimuli reflect alterations in diastolic inflow dynamics, which are consistent with autonomic modulation.
Statistical analysis using paired t-tests revealed significant differences in FHR and all Doppler parameters across the three conditions (p<0.001). A repeated-measures ANOVA confirmed a strong main effect of musical exposure on FHR (F=50.91, p<0.001), PS (F=49.98, p<0.001), ED (F=51.92, p<0.001), and TAMax (F=50.32, p<0.001). Post-hoc pairwise comparisons with Bonferroni correction demonstrated that Brahms’ Lullaby significantly reduced FHR compared to baseline (p<0.001), Mozart’s Symphony significantly increased FHR compared to baseline (p<0.001), and FHR during Mozart’s Symphony was significantly higher than during Brahms’ Lullaby (p<0.001).
To assess the magnitude of these responses, effect size analysis using Cohen’s d was performed. The difference between baseline and Brahms’ Lullaby yielded a Cohen’s d of 1.02, indicating a large effect. The comparison between baseline and Mozart’s Symphony produced a Cohen’s d of 1.18, also a large effect, while the difference between Brahms and Mozart conditions demonstrated a very large effect size of 1.43. These findings highlight the strong influence of music on fetal cardiac and hemodynamic function and reinforce the physiological relevance of prenatal auditory stimulation.
Further analysis using Spearman and Pearson correlation tests revealed no significant relationship between gestational age and changes in any parameter, including FHR, PS, ED, or TAMax (p>0.05). This suggests that fetal responsiveness to music is not significantly influenced by gestational maturity within the 32–37 week range, and that individual variability in musical sensitivity may instead reflect intrinsic neurodevelopmental differences.
To explore potential subgroups in fetal responsiveness, machine learning-based clustering using the K-means algorithm was applied. This analysis identified three distinct clusters, indicating individualized patterns of fetal autonomic reactivity. Some fetuses exhibited robust physiological changes in response to musical stimuli, while others showed minimal reactivity. These differences are consistent with previous studies reporting variability in fetal vibroacoustic response based on internal state, such as the duration of quiet sleep prior to stimulation.
Data visualizations reinforced these findings. Figure 3 illustrates the FHR trend across conditions, confirming the reduction during Brahms’ Lullaby and the increase during Mozart’s Symphony. Figure 4 presents changes in PS, further supporting stimulus-dependent modulation of fetal blood flow. Figure 5, a box plot of FHR distributions, demonstrates the lowest median FHR during Brahms and the highest during Mozart, with noticeable individual variability. Figure 6, a correlation heatmap, shows strong intra-condition correlations among FHR, PS, ED, and TAMax, reflecting consistent cardiovascular coordination during auditory exposure. Figure 7 visualizes the relationship between FHR and PS across music conditions, while additional scatter plots confirmed the absence of a gestational age effect.
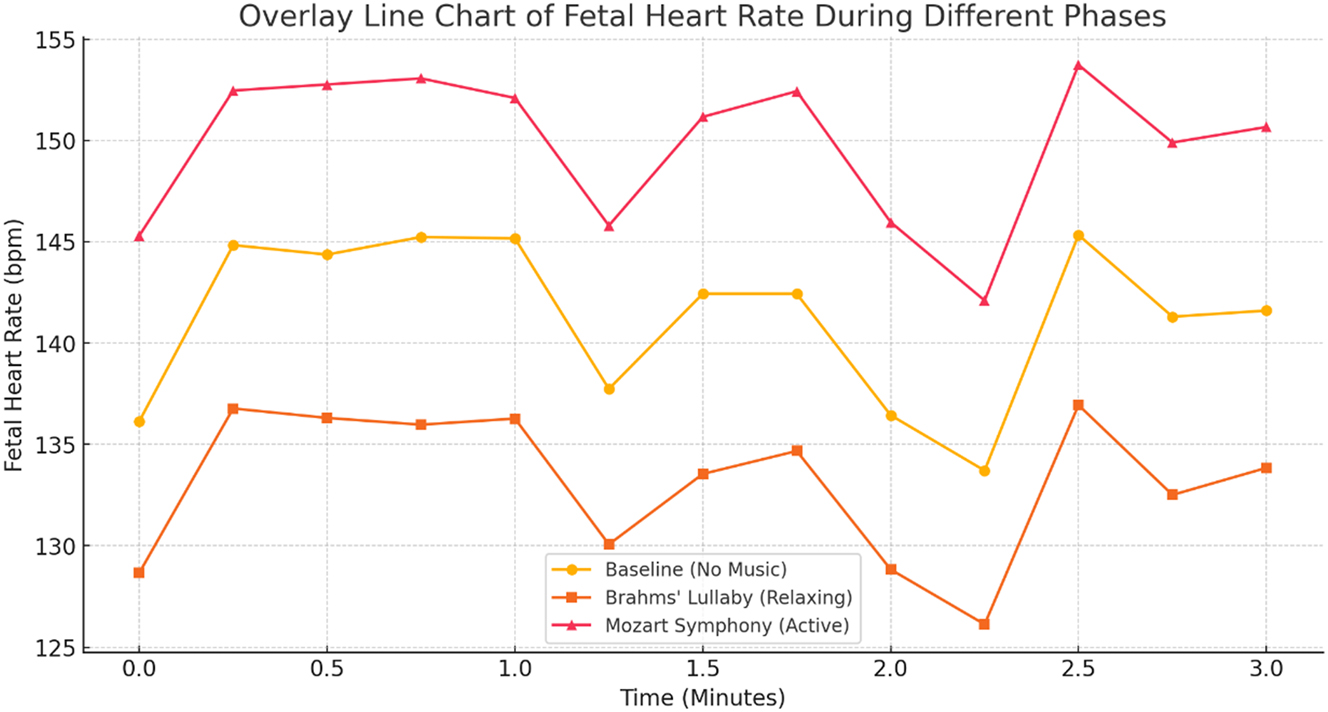
Fetal heart rate responses to different musical stimuli. The line chart illustrates fetal heart rate (FHR) variations under baseline, Brahms’ Lullaby, and Mozart’s Symphony no. 40 conditions (n=30). Statistical analysis using repeated-measures ANOVA revealed a highly significant effect of music exposure on FHR (F=50.91, p<0.001). Post-hoc pairwise comparisons with Bonferroni correction confirmed a significant decrease in FHR during Brahms’ Lullaby (calming effect) compared to baseline, and a significant increase during Mozart’s Symphony (arousal effect) compared to baseline (all p<0.001). The difference in FHR between Brahms’ Lullaby and Mozart’s Symphony was also highly significant (p<0.001), with a very large effect size (Cohen’s d=1.43). These findings statistically confirm the presence of distinct, music-specific fetal cardiac responses.
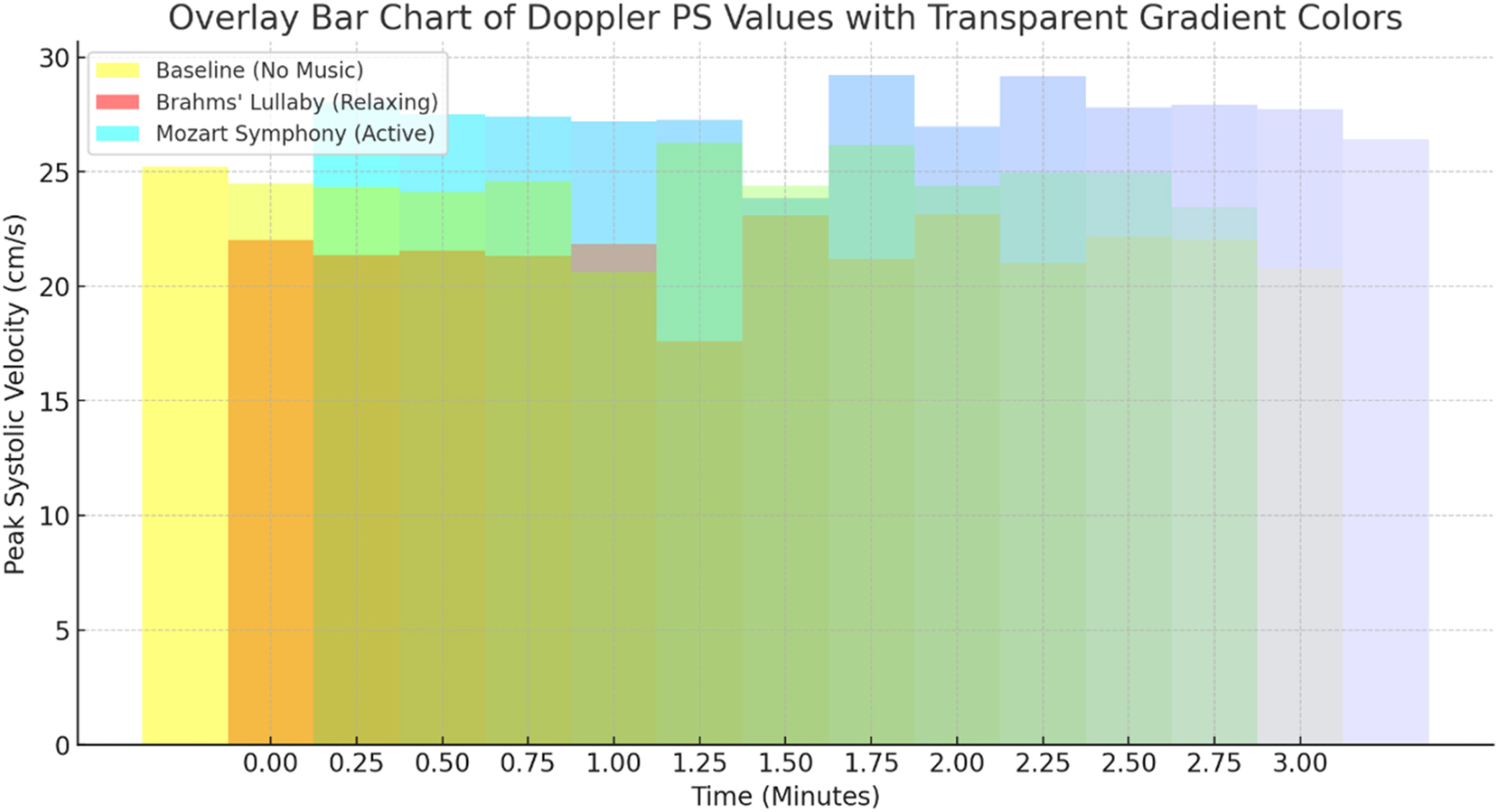
Peak systolic velocity (PS) responses to musical stimuli. The bar chart depicts Doppler-derived peak systolic velocity (PS) under baseline, Brahms’ Lullaby, and Mozart’s Symphony no. 40 conditions (n=30). Statistical analysis using repeated-measures ANOVA indicated a highly significant effect of music exposure on PS values (F=49.98, p<0.001). Post-hoc pairwise comparisons with Bonferroni correction showed a significant reduction in PS during Brahms’ Lullaby compared to baseline, and a significant increase during Mozart’s Symphony compared to baseline (both p<0.001). The difference between Brahms’ and Mozart’s conditions was also highly significant (p<0.001), demonstrating strong hemodynamic reactivity. Effect size analysis revealed a very large magnitude (Cohen’s d=1.43) between Brahms’ Lullaby and Mozart’s Symphony conditions, reinforcing the stimulus-dependent cardiovascular response in fetuses.
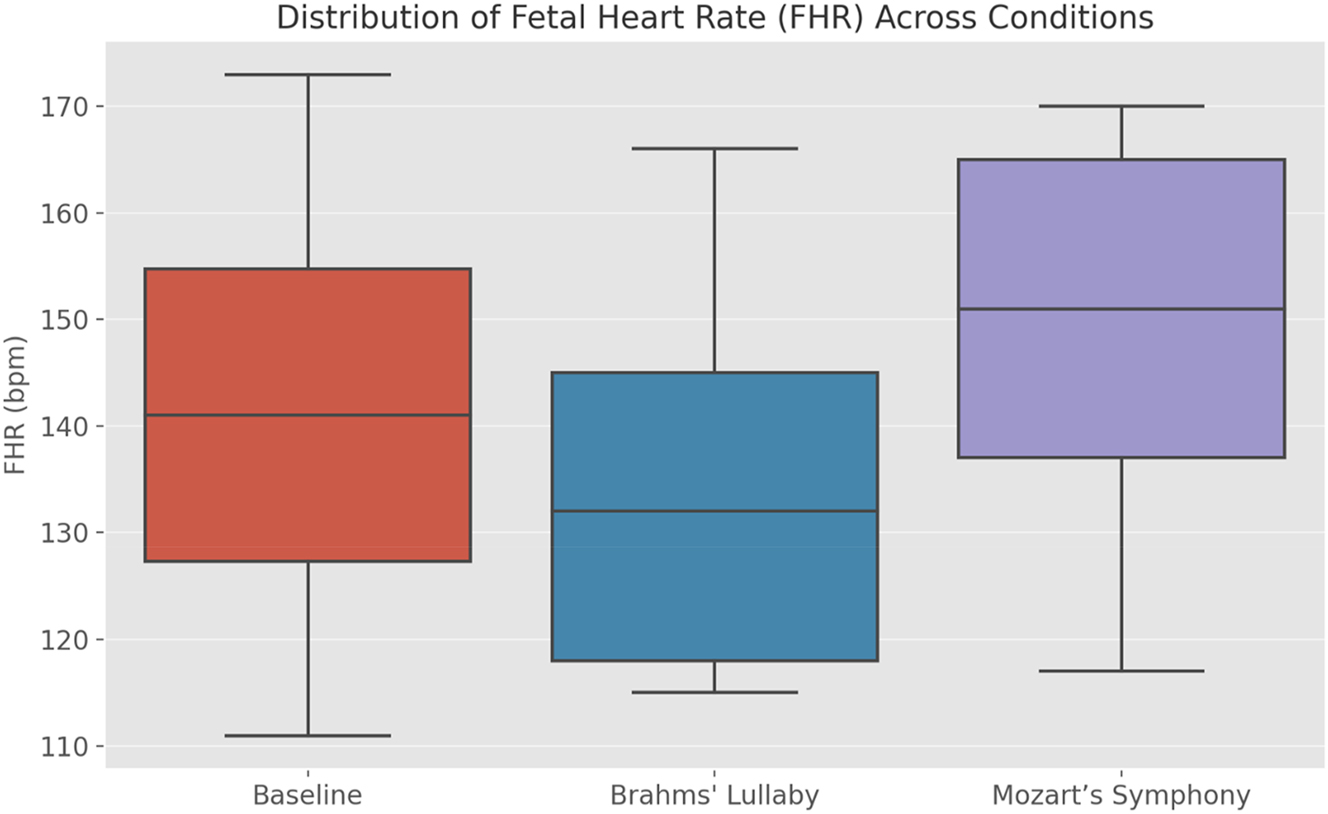
Distribution of fetal heart rate (FHR) across experimental conditions. The box plot compares fetal heart rate (FHR) distributions across baseline, Brahms’ Lullaby, and Mozart’s Symphony no. 40 conditions (n=30). A repeated-measures ANOVA revealed a highly significant effect of musical stimuli on FHR (F=50.91, p<0.001). Post-hoc pairwise comparisons with Bonferroni correction demonstrated a significant decrease in median FHR during Brahms’ Lullaby and a significant increase during Mozart’s Symphony, each compared to baseline (p<0.001). The direct comparison between Brahms’ and Mozart’s conditions was also highly significant (p<0.001), with a very large effect size (Cohen’s d=1.43). The plot visually highlights the consistent calming effect of Brahms’ Lullaby and the arousing effect of Mozart’s Symphony, confirming robust autonomic fetal responses.
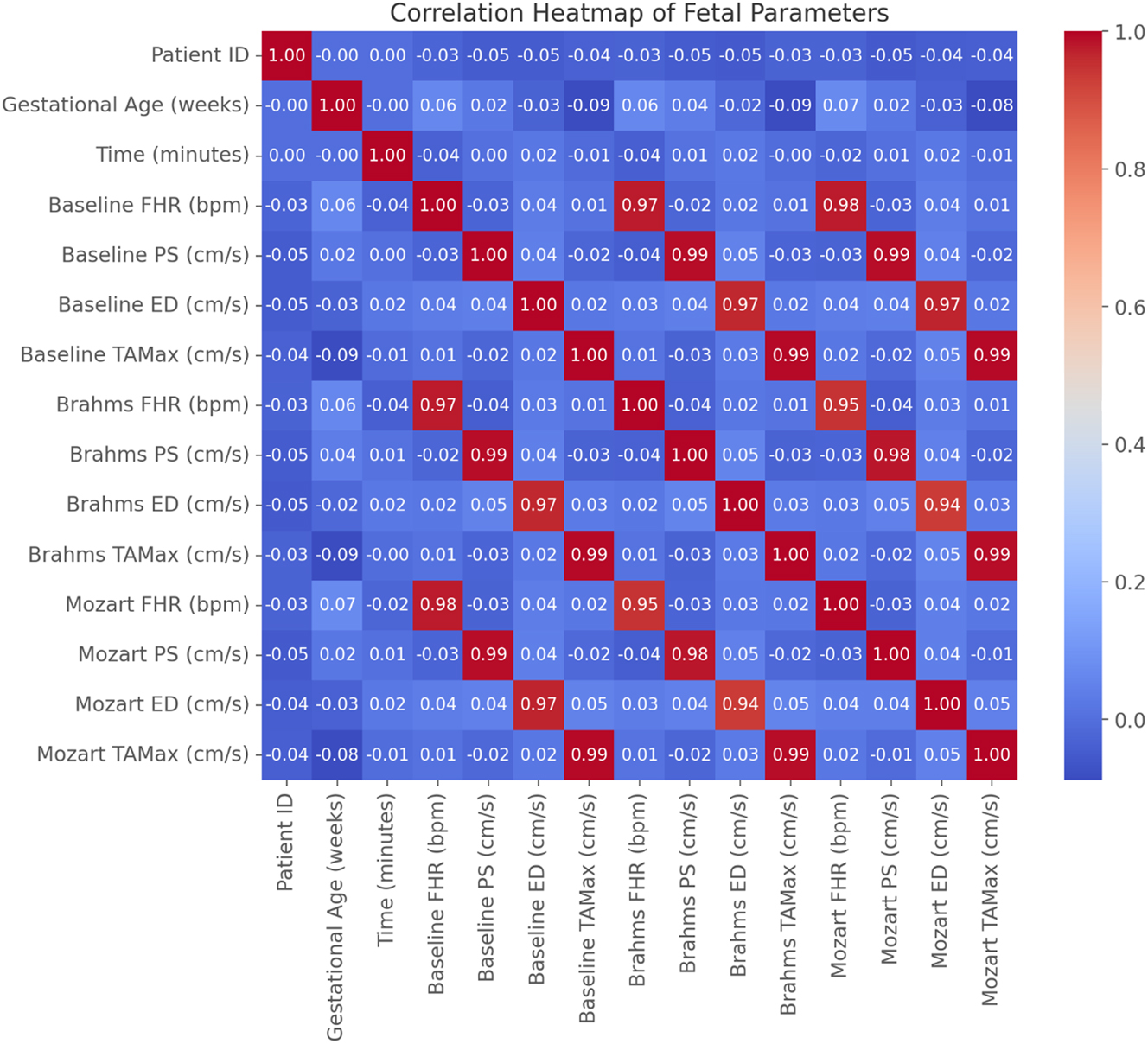
Correlation heatmap of fetal parameters across experimental conditions. The heatmap illustrates correlations between fetal heart rate (FHR), peak systolic velocity (PS), end-diastolic velocity (ED), and time-averaged maximum velocity (TAMax) during baseline, Brahms’ Lullaby, and Mozart’s Symphony conditions (n=30). Pearson correlation coefficients show strong intra-condition positive correlations among Doppler parameters (PS, ED, TAMax), indicating consistent hemodynamic adaptations to auditory stimulation (r>0.7, p<0.001 for all Doppler parameter correlations). FHR changes were positively correlated with Doppler velocities, particularly during Mozart’s Symphony (r>0.6, p<0.001). In contrast, gestational age showed minimal and non-significant correlation with any parameter (p>0.05), suggesting autonomic fetal responses to music are independent of gestational maturity within 32–37 weeks.
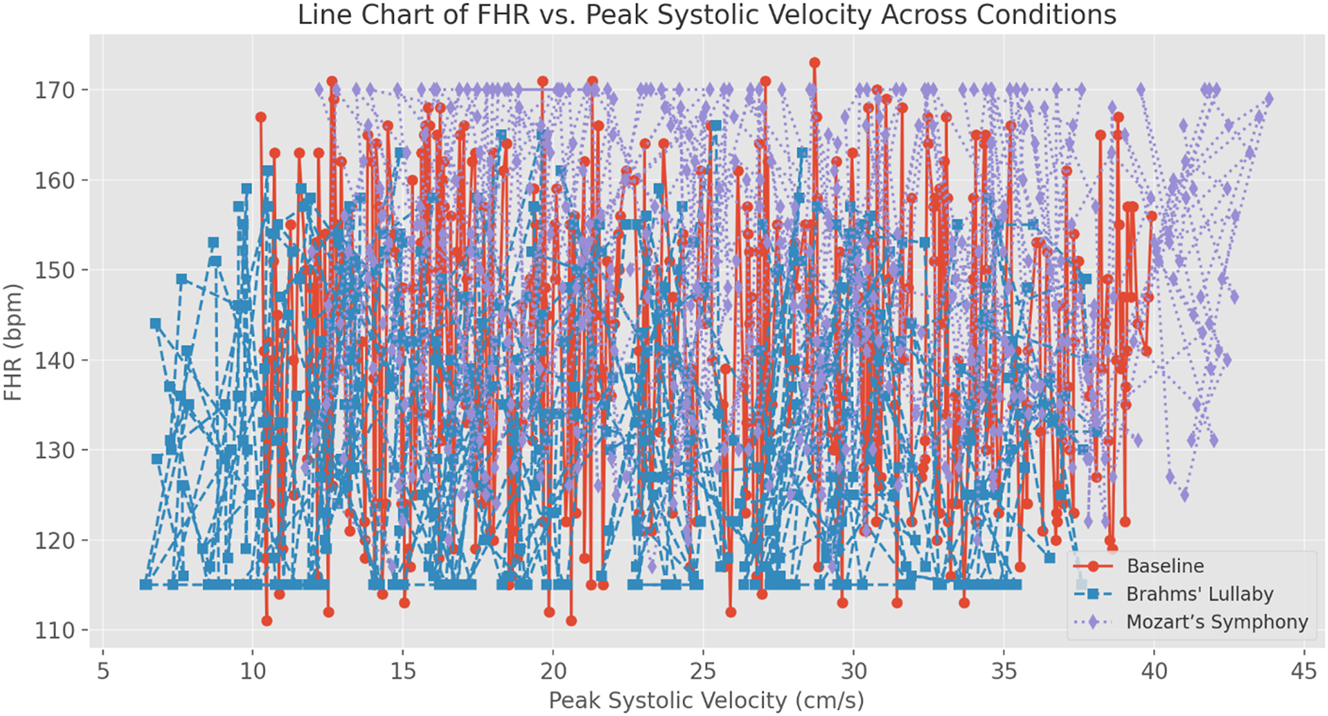
Line chart of fetal heart rate (FHR) vs. peak systolic velocity (PS) across experimental conditions. This line chart shows the relationship between fetal heart rate (FHR) and peak systolic velocity (PS) across baseline, Brahms’ Lullaby, and Mozart’s Symphony no. 40 conditions (n=30). Statistical analysis using Pearson correlation demonstrated significant positive correlations between FHR and PS in all conditions, with the strongest association observed during Mozart’s Symphony (r>0.6, p<0.001). Repeated-measures ANOVA confirmed significant stimulus-dependent differences in both FHR (F=50.91, p<0.001) and PS (F=49.98, p<0.001). Post-hoc Bonferroni comparisons indicated significant decreases in both parameters during Brahms’ Lullaby and increases during Mozart’s Symphony compared to baseline (all p<0.001). These results statistically confirm the coordinated and dynamic fetal cardiovascular response to different auditory stimuli.
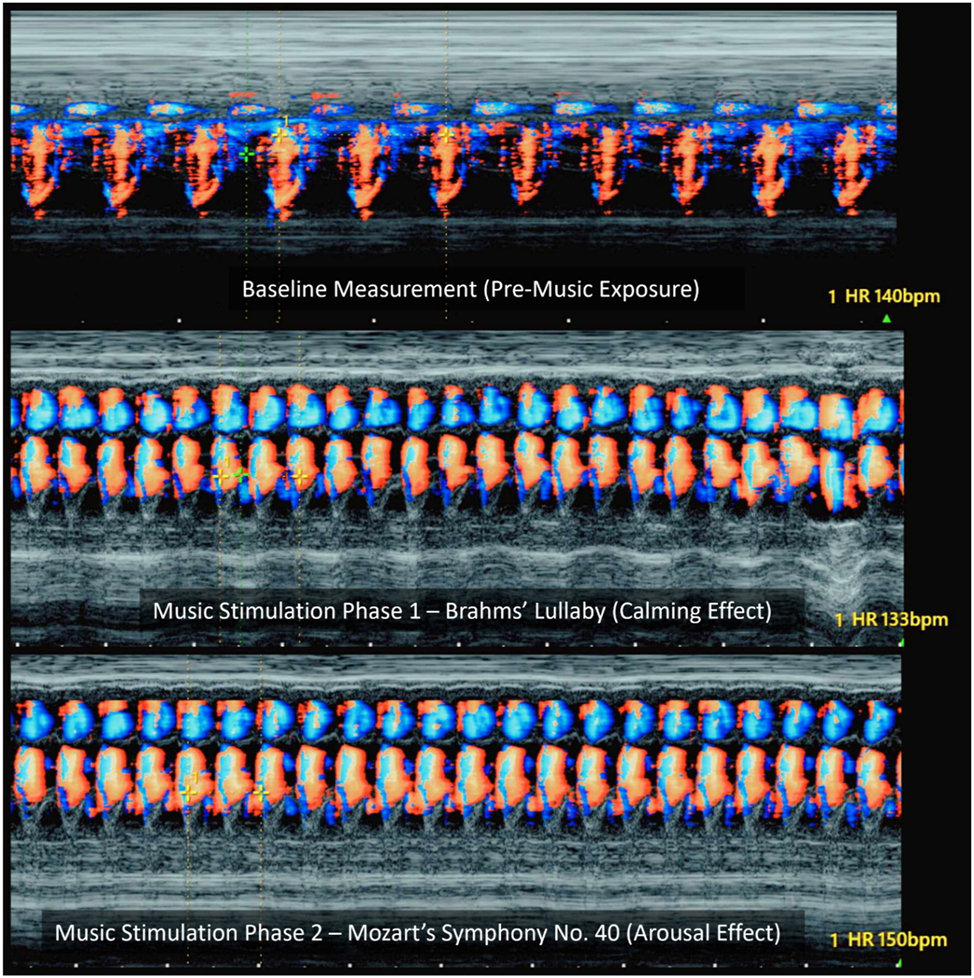
Color Doppler M-mode imaging of fetal cardiac responses to music stimulation (qualitative visualization only). This Figure presents qualitative M-mode Doppler visualizations of fetal cardiac activity across three auditory conditions: (Top) baseline (pre-music), (middle) Brahms’ Lullaby (calming music), and (Bottom) Mozart’s Symphony no. 40 (stimulating music). These color Doppler M-mode images do not provide precise velocity measurements but are used here to illustrate changes in fetal heart rate (FHR) and Doppler flow intensity. Compared to baseline (∼140 bpm), exposure to Brahms’ Lullaby shows a visible reduction in heart rate (∼133 bpm) and softened Doppler signal intensity, reflecting parasympathetic activation. In contrast, the Mozart condition shows a marked increase in FHR (∼150 bpm) with intensified flow signals, suggestive of sympathetic arousal. These visualizations highlight the temporal and qualitative changes in fetal cardiac function in response to musical stimuli and support the neuromodulatory potential of prenatal auditory therapy.
Overall, these findings provide strong evidence that music can modulate fetal autonomic function in a controlled, stimulus-specific manner. The absence of a gestational age effect suggests that the fetal auditory and autonomic systems are sufficiently developed by the third trimester to respond dynamically to external stimuli. These results support the potential for AI-driven, personalized prenatal music therapy as a means of influencing fetal neurodevelopment and autonomic regulation.
Discussion
This study provides strong evidence that fetal exposure to music elicits distinct autonomic and cardiovascular responses, demonstrating that the fetal nervous system is capable of processing auditory input and generating stimulus-specific physiological patterns in utero. The significant modulation of fetal heart rate (FHR) and Doppler-based flow parameters in response to different musical tempos suggests that prenatal music therapy engages neuromodulatory pathways during a critical window of autonomic nervous system (ANS) development.
Importantly, the observed cardiac responses are not isolated physiological markers but correlate with known fetal neurobehavioral states. As documented in multiple vibroacoustic studies [2], 3], 5], 8], 9], 14], 15], [19], [20], [21], [22], [23], [24], [25], changes in FHR and heart rate variability (HRV) are commonly associated with shifts between quiet sleep, active wakefulness, and orienting behaviors. For example, reductions in FHR, such as those seen during exposure to Brahms’ Lullaby, have been linked to parasympathetic dominance and behavioral quiescence – reflecting a transition toward calm or sleep-like states [15], 22]. In contrast, the increase in FHR observed during Mozart’s Symphony No. 40 is consistent with sympathetic activation and fetal arousal, possibly indicative of heightened sensory engagement and attentional readiness [5], 20], 23].
This alignment between cardiac modulation and behavioral state regulation highlights the functional integration of the fetal ANS and central nervous system. Figure 8 further illustrates these tempo-dependent changes in fetal heart rate and Doppler flow intensity using color Doppler M-mode images. Fast-tempo music may activate cortical-subcortical pathways – such as the limbic system and hypothalamic-pituitary-adrenal (HPA) axis – inducing transient arousal responses, as previously demonstrated in studies using vibroacoustic stimulation and facial expression monitoring [3], 24]. Conversely, melodic, slow-tempo music likely enhances vagal tone through brainstem-mediated relaxation circuits, promoting cardiovascular deceleration and behavioral stabilization.
The gestational window studied (32–37 weeks) corresponds to a key period in ANS maturation, where baroreflex sensitivity and autonomic balance become more dynamic [11], 12], 26], 27]. Our findings that fetal responses varied significantly by stimulus type – but not by gestational age – suggest that by the third trimester, fetuses possess the neurological maturity required for adaptive cardiac-behavioral regulation in response to acoustic input.
Moreover, machine learning-based clustering revealed individualized patterns of fetal reactivity, suggesting the presence of neurodevelopmental variability in auditory sensitivity. Such variability may arise from genetic factors, fetal behavioral state at the time of exposure, or maternal-fetal physiological coupling [22], 23], 28], 29]. This supports a growing body of literature advocating for personalized sensory protocols in prenatal interventions [7], 25]. AI-enhanced 4D ultrasound has recently been used to analyze fetal facial responses to sensory input [30].
The implications of these findings extend beyond fetal physiology to emotional and cognitive development. Music-induced modulation of autonomic tone may serve as a form of early behavioral conditioning, potentially shaping postnatal emotional regulation and sensory responsiveness. Such effects may be reflected in fetal facial expressions, as observed in response to music stimulation in utero [24]. Previous research has linked prenatal exposure to music with enhanced neural plasticity, early learning capacity, and even altered sleep architecture in neonates [6], [31], [32], [33].
Despite these promising results, caution is warranted. While fetal FHR and Doppler metrics provide high-resolution insights into autonomic shifts, they remain indirect markers of brain activity. Future studies should incorporate direct neurofunctional assessments – such as fetal magnetoencephalography (fMEG) – along with maternal stress biomarkers and longitudinal developmental outcomes to fully elucidate the long-term impact of prenatal music therapy [34], 35].
In conclusion, this study establishes that fetal cardiac responses to music are not merely physiological reflexes but correlate meaningfully with neurobehavioral states, reinforcing the validity of using cardiovascular markers as proxies for fetal sensory and emotional processing. These findings advance the scientific rationale for AI-guided, individualized prenatal music interventions aimed at enhancing fetal neurodevelopment and maternal-fetal bonding.
Strengths and limitations
Strengths of the study
A primary strength of this study lies in its integration of Doppler ultrasound with AI-assisted analysis to quantitatively assess fetal cardiac and hemodynamic responses to music. Unlike earlier studies that primarily relied on behavioral markers such as fetal movement, our approach provides objective, high-resolution physiological indicators – including fetal heart rate variability and Doppler waveform dynamics – enabling a reproducible and detailed assessment of fetal autonomic function. While movement-based research has contributed foundational insights into fetal responsiveness to vibroacoustic stimuli [9], the present methodology offers a more direct and nuanced perspective on cardiovascular regulation. The incorporation of machine learning-based clustering further enhances the study’s novelty by revealing individualized patterns of fetal reactivity, suggesting that intrinsic variability in auditory perception significantly shapes prenatal responses to music.
Another notable strength is the rigor of the experimental design. All sessions were conducted in a soundproof, temperature-controlled environment, with music exposure standardized in terms of duration, speaker distance, and decibel level. This controlled setting minimizes external noise interference and ensures that observed fetal responses can be attributed to the auditory stimuli themselves. Furthermore, the use of a within-subject design – where each fetus serves as its own control – helps eliminate inter-individual variability and strengthens statistical reliability. The application of paired t-tests and repeated-measures ANOVA further confirms the robustness of the findings by demonstrating statistically significant changes across all experimental conditions (baseline, Brahms’ Lullaby, and Mozart’s Symphony).
Limitations of the study
Despite these strengths, several limitations must be acknowledged. First, the relatively small sample size (n=30), while sufficient to detect significant effects, limits the generalizability of the findings. Larger cohorts would increase statistical power and allow for subgroup analyses based on maternal or fetal variables such as prior music exposure, maternal stress levels, or socioeconomic background.
Second, a potential confounder not directly assessed in this study is the maternal emotional state during music exposure. Previous research has shown that maternal stress or relaxation can significantly influence fetal physiological responses, including heart rate and blood flow dynamics [18], 23], [36], [37], [38], [39]. Although efforts were made to create a calm and consistent testing environment, subjective maternal emotional states were not measured. As a result, maternal-fetal physiological coupling cannot be entirely excluded as a contributing factor. Future research should incorporate maternal psychological assessments or physiological biomarkers – such as salivary cortisol or maternal heart rate variability (HRV) – to better differentiate between direct fetal responses and those mediated by maternal physiology.
Additionally, the fetal behavioral state at the time of exposure (e.g., quiet sleep vs. active movement) was not systematically recorded, despite its known impact on autonomic responsiveness. This omission may partially account for the inter-fetal variability observed in response amplitudes. Incorporating fetal state classification via real-time ultrasound or cardiotocography could provide more nuanced insight into baseline conditions and their influence on fetal auditory sensitivity.
Another limitation relates to the short duration of each music exposure (3 min), which may not capture longer-term patterns of fetal adaptation. While prior studies support the effectiveness of brief acoustic stimulation in eliciting measurable responses [19], it remains unclear whether repeated or extended exposure would result in habituation, sensitization, or cumulative developmental effects. Future longitudinal research should evaluate the sustainability and developmental impact of prenatal music therapy over extended periods.
Finally, this study did not incorporate direct neuroimaging techniques to assess fetal brain activity. Although Doppler ultrasound and AI-driven signal analysis yield valuable insights into cardiovascular and autonomic changes, methods such as fetal magnetoencephalography (fMEG) or functional MRI (fMRI) could provide more direct evidence of fetal auditory processing and central nervous system activation. Integrating such neuroimaging approaches with physiological monitoring would offer a more comprehensive understanding of how prenatal music exposure influences fetal neurodevelopment.
While this study provides robust physiological evidence of fetal responsiveness to music, future investigations should aim to increase sample size, control for maternal and fetal behavioral variables, and incorporate neuroimaging to more fully elucidate the mechanisms and implications of prenatal auditory stimulation. Addressing these limitations will be essential to refining music-based interventions and advancing their clinical application in maternal-fetal medicine.
Conclusions
This study provides compelling evidence that fetal heart rate and Doppler-derived hemodynamic parameters are significantly modulated by musical exposure, demonstrating that the fetal autonomic nervous system is responsive to auditory stimuli in utero. Exposure to Brahms’ Lullaby produced a calming effect, marked by a significant reduction in fetal heart rate (FHR) and vascular flow velocities, indicative of enhanced parasympathetic activation. In contrast, Mozart’s Symphony No. 40 elicited a stimulatory response, characterized by increased FHR and Doppler parameters, suggesting heightened sympathetic activity. These findings confirm that musical tempo and structure critically influence fetal autonomic regulation, supporting the hypothesis that fetal cardiovascular responses are stimulus-dependent. By utilizing AI-assisted Doppler ultrasound analysis, this study offers a real-time, quantitative physiological assessment of fetal responses to music – advancing beyond traditional reliance on behavioral markers. The integration of machine learning-based clustering further reveals individual variability in fetal reactivity, challenging the assumption that all fetuses respond uniformly to auditory stimuli. Instead, the results point to intrinsic neurodevelopmental differences as important factors in shaping fetal auditory sensitivity. These findings hold important implications for prenatal care and the potential clinical application of personalized fetal music therapy. The observation of music-induced facial expressions in utero supports the possibility of early emotional engagement and enhanced maternal-fetal bonding through sound stimulation [24]. The ability to evoke calming or arousing effects via targeted music suggests that prenatal acoustic environments could be strategically designed to promote fetal well-being, reduce intrauterine stress, and support neurodevelopmental priming. Future research should explore long-term developmental outcomes, cultural influences on fetal auditory preferences, and the dynamics of maternal-fetal synchrony in response to music. Additionally, incorporating maternal stress biomarkers and advanced fetal neuroimaging techniques could provide deeper insight into the mechanisms underlying fetal auditory learning and autonomic conditioning. This study establishes a robust physiological foundation for the use of fetal music therapy, demonstrating that music-induced autonomic regulation begins before birth. Through the integration of AI-driven fetal monitoring and precision-guided music interventions, future prenatal research may pave the way for scientifically tailored auditory environments that support optimal fetal growth, emotional development, and early sensory integration – marking a significant advancement in the field of maternal-fetal medicine.
Acknowledgments
The authors acknowledge the invaluable support of the Indonesian Society of Obstetrics and Gynecology (POGI) and the Indonesian Society of Maternal-Fetal Medicine (HKFM) in facilitating this original article. Gratitude is also extended to the Faculty of Medicine, Sebelas Maret University, Dr. Moewardi General Hospital, and Eka Hospital Serpong Tangerang for their contributions and institutional support.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Lecanuet, JP, Schaal, B. Fetal sensory competencies. Eur J Obstet Gynecol Reprod Biol 1996;68:1–23. https://doi.org/10.1016/0301-2115(96)02509-2.Search in Google Scholar PubMed
2. Gagnon, R, Hunse, C, Carmichael, L, Fellows, F, Patrick, J. Human fetal response to vibratory acoustic stimulation from twenty-six weeks to term. Am J Obstet Gynecol 1987;157:1375–80. https://doi.org/10.1016/S0002-9378(87)80141-2.Search in Google Scholar PubMed
3. Gerhardt, KJ, Abrams, RM. Fetal exposure to sound and vibroacoustic stimulation. J Perinatol 2000;20:S21–30. https://doi.org/10.1038/sj.jp.7200441.Search in Google Scholar PubMed
4. Hepper, PG, Shahidullah, BS. Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed 1994;71:F81–7. https://doi.org/10.1136/fn.71.2.f81.Search in Google Scholar PubMed PubMed Central
5. Spencer, JAD, Deans, A, Nicolaidis, P, Arulkumaran, S. Fetal heart rate response to vibroacoustic stimulation during low and high heart rate variability episodes in late pregnancy. Am J Obstet Gynecol 1991;165:86–90. https://doi.org/10.1016/0002-9378(91)90238-3.Search in Google Scholar
6. Partanen, E, Kujala, T, Näätänen, R, Liitola, A, Sambeth, A, Huotilainen, M. Learning-induced neural plasticity of speech processing before birth. Proc Natl Acad Sci USA 2013;110:15145–50. https://doi.org/10.1073/pnas.1302159110.Search in Google Scholar PubMed PubMed Central
7. van der Heijden, MJ, Oliai Araghi, S, Jeekel, J, Reiss, IKM, Hunink, MGM, van Dijk, M. Do hospitalized premature infants benefit from music interventions? A systematic review of randomized controlled trials. PLoS One 2016;11:e0161848. https://doi.org/10.1371/journal.pone.0161848.Search in Google Scholar PubMed PubMed Central
8. Devoe, L. Vibroacoustic stimuli and fetal behavior. Nurs Times 1991;87:36–7. https://doi.org/10.7748/ns.5.36.36.s38.Search in Google Scholar
9. Fox, HE, Badalian, SS. Fetal movement in response to vibroacoustic stimulation: a review. Obstet Gynecol Surv 1993;48:707–13. https://doi.org/10.1097/00006254-199310000-00025.Search in Google Scholar PubMed
10. Morokuma, S, Fukushima, K, Kawai, N, Tomonaga, M, Satoh, S, Nakano, H. Fetal habituation correlates with functional brain development. Behav Brain Res 2004;153:459–63. https://doi.org/10.1016/j.bbr.2004.01.002.Search in Google Scholar PubMed
11. Van Leeuwen, P, Lange, S, Bettermann, H, Grönemeyer, D, Hatzmann, W. Fetal heart rate variability and complexity in the course of pregnancy. Early Hum Dev 1999;54:259–69. https://doi.org/10.1016/s0378-3782(98)00102-9.Search in Google Scholar PubMed
12. van Leeuwen, P, Geue, D, Thiel, M, Cysarz, D, Lange, S, Romano, MC, et al.. Influence of paced maternal breathing on fetal-maternal heart rate coordination. Proc Natl Acad Sci U S A 2009;106:13661–6. https://doi.org/10.1073/pnas.0901049106.Search in Google Scholar PubMed PubMed Central
13. Haslbeck, FB, Bassler, D. Music from the very beginning–a neuroscience-based framework for music as therapy for preterm infants and their parents. Front Behav Neurosci 2018;12:112. https://doi.org/10.3389/fnbeh.2018.00112.Search in Google Scholar PubMed PubMed Central
14. D, Elia, A, Pighetti, M, Vanacore, F, Fabbrocini, G, Arpaia, L. Vibroacoustic stimulation in normal term human pregnancy. Early Hum Dev 2005;81:449–53. https://doi.org/10.1016/j.earlhumdev.2005.01.004.Search in Google Scholar PubMed
15. Visser, GHA, Mulder, HH, Wit, HP, Mulder, EJH, Prechtl, HFR. Vibro-acoustic stimulation of the human fetus: effect on behavioural state organization. Early Hum Dev 1989;19:285–96. https://doi.org/10.1016/0378-3782(89)90049-6.Search in Google Scholar
16. Arnon, S, Shapsa, A, Forman, L, Regev, R, Bauer, S, Litmanovitz, I, et al.. Live music is beneficial to preterm infants in the neonatal intensive care unit environment. Birth 2006;33:131–6. https://doi.org/10.1111/j.0730-7659.2006.00090.x.Search in Google Scholar PubMed
17. Bieleninik, Ł, Ghetti, C, Gold, C. Music therapy for preterm infants and their parents: a meta-analysis. Pediatrics 2016;138:e20160971. https://doi.org/10.1542/peds.2016-0971.Search in Google Scholar PubMed
18. Hartling, L, Shaik, MS, Tjosvold, L. Music for medical indications in the neonatal period: a systematic review of randomised controlled trials. Arch Dis Child Fetal Neonatal Ed 2009;94:F349–54. https://doi.org/10.1136/adc.2008.148411.Search in Google Scholar PubMed
19. Pietrantoni, M, Angel, JL, Parsons, MT, McClain, L, Arango, HA, Spellacy, WN. Human fetal response to vibroacoustic stimulation as a function of stimulus duration. Obstet Gynecol 1991;87:807–11. https://doi.org/10.1016/0029-7844(91)90202-3.Search in Google Scholar
20. van Heteren, CF, Boekkooi, PF, Schiphorst, RH, Jongsma, HW, Nijhuis, JG. Fetal habituation to vibroacoustic stimulation in uncomplicated postterm pregnancies. Eur J Obstet Gynecol Reprod Biol 2001;97:178–82. https://doi.org/10.1016/S0301-2115(00)00441-3.Search in Google Scholar
21. Arulkumaran, S, Talbert, D, Hsu, TS, Chua, S, Anandakumar, C, Ratnam, SS. In-utero sound levels when vibroacoustic stimulation is applied to the maternal abdomen: an assessment of the possibility of cochlear damage in the fetus. BJOG 1992;99:43–5. https://doi.org/10.1111/j.1471-0528.1992.tb14461.x.Search in Google Scholar PubMed
22. Groome, LJ, Bentz, LS, Singh, KP, Mooney, DM. Behavioral state change in normal human fetuses following a single vibroacoustic stimulus: effect of duration of quiet sleep prior to stimulation. Early Hum Dev 1993;33:21–7. https://doi.org/10.1016/0378-3782(93)90059-5.Search in Google Scholar
23. Makino, I, Matsuda, Y, Yoneyama, M, Hirasawa, K, Takagi, K, Ohta, H, et al.. Effect of maternal stress on fetal heart rate assessed by vibroacoustic stimulation. J Int Med Res 2009;37:1780–8. https://doi.org/10.1177/147323000903700618.Search in Google Scholar PubMed
24. Lopez-Teijon, M, Garcia‐Faura, A, Prats‐Galino, A. Fetal facial expression in response to intravaginal music emission. Ultrasound 2015;23:216–23. https://doi.org/10.1177/1742271X15609367.Search in Google Scholar PubMed PubMed Central
25. Ogo, K, Kanenishi, K, Mori, N, AboEllail, MAM, Hata, T. Change in fetal behavior in response to vibroacoustic stimulation. J Perinat Med 2019;47:558–63. https://doi.org/10.1515/jpm-2018-0344.Search in Google Scholar PubMed
26. Schneider, U, Schleussner, E, Fiedler, A, Jaekel, S, Liehr, M, Haueisen, J, et al.. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS One 2018;13:e0200799. https://doi.org/10.1371/journal.pone.0200799.Search in Google Scholar PubMed PubMed Central
27. Draganova, R, Schollbach, A, Schleger, F, Braendle, J, Brucker, S, Abele, H, et al.. Fetal auditory evoked responses to onset of amplitude modulated sounds. A fetal magnetoencephalography (fMEG) study. Hear Res 2018;363:70–7. https://doi.org/10.1016/j.heares.2018.03.005.Search in Google Scholar PubMed
28. Al-Qahtani, NH. Foetal response to music and voice. Aust N Z J Obstet Gynaecol 2005;45:414–7. https://doi.org/10.1111/j.1479-828X.2005.00458.x.Search in Google Scholar PubMed
29. Loewy, J, Stewart, K, Dassler, AM, Telsey, A, Homel, P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 2013;131:902–18. https://doi.org/10.1542/peds.2012-1367.Search in Google Scholar PubMed
30. Bachnas MA, Andonotopo W, Dewantiningrum J, Adi Pramono MB, Stanojevic M, Kurjak A. The utilization of artificial intelligence in enhancing 3D/4D ultrasound analysis of fetal facial profiles. J Perinat Med 2024; 52:899-913. https://doi.org/10.1515/jpm-2024-0347.Search in Google Scholar PubMed
31. James, DK, Spencer, CJ, Stepsis, BW. Fetal learning: a prospective randomized controlled study. Ultrasound Obstet Gynecol 2002;20:431–8. https://doi.org/10.1046/j.1469-0705.2002.00845.x.Search in Google Scholar PubMed
32. Kisilevsky, BS, Hains, SM, Lee, K, Xie, X, Huang, H, Ye, HH, et al.. Effects of experience on fetal voice recognition. Psychol Sci 2003;14:220–4. https://doi.org/10.1111/1467-9280.02435.Search in Google Scholar PubMed
33. Trappe, HJ. The effects of music on the cardiovascular system and cardiovascular health. Heart 2010;96:1868–71. https://doi.org/10.1136/hrt.2010.209858.Search in Google Scholar PubMed
34. Anderson, DE, Patel, AD. Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: might music have an impact? Dev Med Child Neurol 2018;60:256–66. https://doi.org/10.1111/dmcn.13663.Search in Google Scholar PubMed
35. Andonotopo, W, Bachnas, MA, Dewantiningrum, J, Adi Pramono, MB, Stanojevic, M, Kurjak, A. AI and early diagnostics: mapping fetal facial expressions through development, evolution, and 4D ultrasound. J Perinat Med 2025;53:263–85. https://doi.org/10.1515/jpm-2024-0602.Search in Google Scholar PubMed
36. Yılmaz Sezer, N, Aker, MN, Yücel, A, Çalışıcı, D. The effect of virtual reality and music on anxiety, non-stress test parameters, and satisfaction of high-risk pregnant women undergoing non-stress tests: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2024;296:52–8. https://doi.org/10.1016/j.ejogrb.2024.02.038.Search in Google Scholar PubMed
37. Abera, M, Hanlon, C, Daniel, B, Tesfaye, M, Workicho, A, Girma, T, et al.. Effects of relaxation interventions during pregnancy on maternal mental health, and pregnancy and newborn outcomes: a systematic review and meta-analysis. PLoS One 2024;19:e0278432. https://doi.org/10.1371/journal.pone.0278432.Search in Google Scholar PubMed PubMed Central
38. Dunkel Schetter, C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol 2011;62:531–58. https://doi.org/10.1146/annurev.psych.031809.130727.Search in Google Scholar PubMed
39. Kobus, S, Diezel, M, Dewan, MV, Huening, B, Dathe, AK, Marschik, PB, et al.. Music therapy in preterm infants reduces maternal distress. Int J Environ Res Public Health 2022;20:731. https://doi.org/10.3390/ijerph20010731.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- 10.1515/jpm-2025-frontmatter8
- Reviews
- Mothers by contract: the moral and regulatory maze of surrogacy
- The incidence of Bandl’s ring and its impact on labor outcomes: a review of the published literature
- Serum biomarkers in the early detection of necrotizing enterocolitis: a systematic review
- Commentary
- 10.1515/jpm-2025-0285
- Original Articles – Obstetrics
- Integrating KANET and Doppler indices to predict neurodevelopmental delays in high-risk pregnancies
- Association of in vitro fertilization with cesarean delivery in nulliparous, term, singleton, vertex pregnancies
- Molecular evidence in support of hematogenous dissemination of intraamniotic infection caused by Listeria monocytogenes in spontaneous preterm labor
- 10.1515/jpm-2025-0116
- Pentraxins 3 levels in pregnant women diagnosed with preeclampsia and their relationship with the severity of the condition
- Factors influencing recurrence of preeclampsia in pregnant women with a history of preeclampsia and the establishment of a predictive model
- 10.1515/jpm-2025-0135
- Comparison of intrapartum transfer from out-of-hospital births with intrapartum transfer from an alongside midwifery unit: a real-world data analysis of a German cohort
- Maternal and perinatal outcomes in obese parturients with epidural analgesia: a systematic review
- Original Articles – Fetus
- A novel approach to calculating expected total fetal lung volume in fetuses with isolated congenital diaphragmatic hernia and fetal growth restriction: a theoretical computational simulation
- Abnormal fetal genitalia: two- and three-dimensional ultrasound assessment
- Fetal music therapy and AI-driven Doppler ultrasound: a neuromodulation perspective
- Original Article – Neonates
- Impact of low dose nicotine on brain-derived neurotrophic factor after global hypoxia in newborn piglets
- Letters to the Editor
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population: a commentary letter
- Improved visualization of a fetal scalp cyst with B-mode and 3D ultrasound compared to magnetic resonance imaging
Articles in the same Issue
- 10.1515/jpm-2025-frontmatter8
- Reviews
- Mothers by contract: the moral and regulatory maze of surrogacy
- The incidence of Bandl’s ring and its impact on labor outcomes: a review of the published literature
- Serum biomarkers in the early detection of necrotizing enterocolitis: a systematic review
- Commentary
- 10.1515/jpm-2025-0285
- Original Articles – Obstetrics
- Integrating KANET and Doppler indices to predict neurodevelopmental delays in high-risk pregnancies
- Association of in vitro fertilization with cesarean delivery in nulliparous, term, singleton, vertex pregnancies
- Molecular evidence in support of hematogenous dissemination of intraamniotic infection caused by Listeria monocytogenes in spontaneous preterm labor
- 10.1515/jpm-2025-0116
- Pentraxins 3 levels in pregnant women diagnosed with preeclampsia and their relationship with the severity of the condition
- Factors influencing recurrence of preeclampsia in pregnant women with a history of preeclampsia and the establishment of a predictive model
- 10.1515/jpm-2025-0135
- Comparison of intrapartum transfer from out-of-hospital births with intrapartum transfer from an alongside midwifery unit: a real-world data analysis of a German cohort
- Maternal and perinatal outcomes in obese parturients with epidural analgesia: a systematic review
- Original Articles – Fetus
- A novel approach to calculating expected total fetal lung volume in fetuses with isolated congenital diaphragmatic hernia and fetal growth restriction: a theoretical computational simulation
- Abnormal fetal genitalia: two- and three-dimensional ultrasound assessment
- Fetal music therapy and AI-driven Doppler ultrasound: a neuromodulation perspective
- Original Article – Neonates
- Impact of low dose nicotine on brain-derived neurotrophic factor after global hypoxia in newborn piglets
- Letters to the Editor
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population: a commentary letter
- Improved visualization of a fetal scalp cyst with B-mode and 3D ultrasound compared to magnetic resonance imaging

