Virtual fetal holography for parental counseling and education: applications, limitations, and future directions
-
Wiku Andonotopo
, Muhammad Adrianes Bachnas
, Efendi Lukas
Abstract
Introduction
Effective prenatal counseling relies on clear communication of fetal development and potential complications. Conventional 2D and 3D ultrasound images often fail to intuitively convey complex anatomy to expectant parents, limiting engagement and informed decision-making.
Content
Virtual fetal holography combines advanced image processing and 3D/4D ultrasound data to generate interactive, life-like fetal models. This review synthesizes current literature on its technological basis, clinical applications, and patient-centered outcomes. Evidence from cardiology, surgical planning, and anatomy education demonstrates how holography improves spatial understanding, supports multidisciplinary counseling, and enhances patient experience compared to standard imaging. Applications in anomaly counseling, maternal–fetal bonding, and clinical training are highlighted, alongside workflow models integrating mixed reality headsets and AI algorithms.
Summary
Virtual fetal holography shows potential for improving prenatal education and emotional engagement. Current evidence is largely descriptive and feasibility-based, with limited empirical validation of its impact on clinical decision-making or patient outcomes.
Outlook
Future research should address cost, technical complexity, ethical considerations, and standardized clinical protocols. Large-scale trials and cost-effectiveness analyses are needed to define its role in routine prenatal care.
Introduction
Effective communication in prenatal care is essential for supporting informed decision-making and fostering positive maternal–fetal outcomes. Ultrasound imaging has long been the cornerstone of fetal assessment, yet conventional 2D and even advanced 3D/4D ultrasound often fail to intuitively convey complex anatomical details to expectant parents. Many patients report difficulty understanding fetal development or the severity of abnormalities, highlighting a persistent communication gap in prenatal counseling [1], [2], [3]. As illustrated in Figure 1, the PRISMA flow diagram summarizes the systematic selection process of studies included in this review, ensuring transparency and methodological rigor in evaluating current evidence.
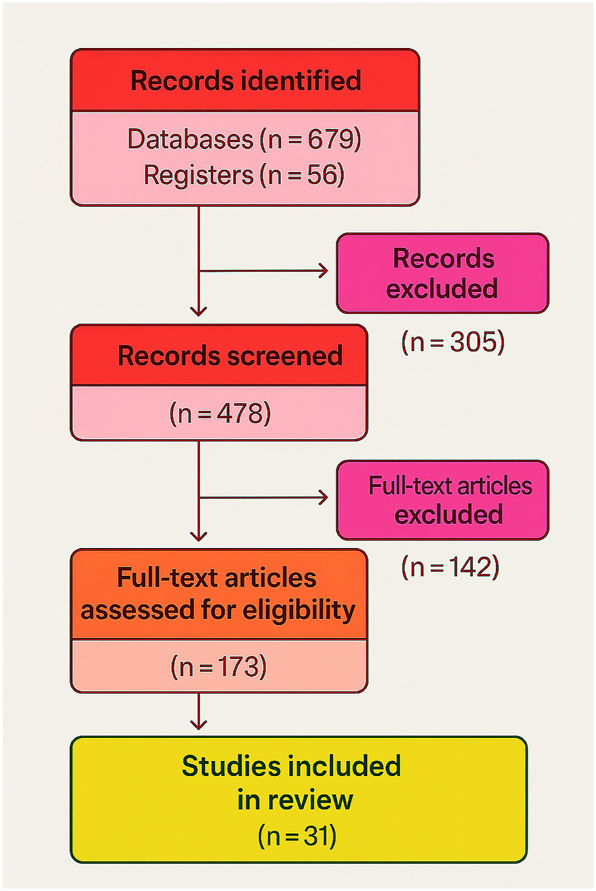
PRISMA flow diagram for systematic review of virtual fetal holography. This flow diagram illustrates the selection process of studies included in the systematic review, following PRISMA 2020 guidelines. Out of 735 initially identified records, 478 studies were screened, 173 full-text articles were assessed for eligibility, and 31 studies were finally included in the synthesis. This process ensures transparency and reproducibility in literature selection.
Virtual fetal holography offers a novel approach to bridge this gap. By transforming 3D/4D ultrasound data into interactive, life-like holographic models, it allows parents to visualize fetal anatomy and motion intuitively. Early applications in medicine – from cardiac surgery to anatomy education – suggest that holography can improve spatial understanding, multidisciplinary communication, and patient engagement [4], [5], [6], [7]. In prenatal care, it may enhance counseling for congenital anomalies, strengthen maternal–fetal bonding, and support professional training [8], [9], [10] (Figure 2).
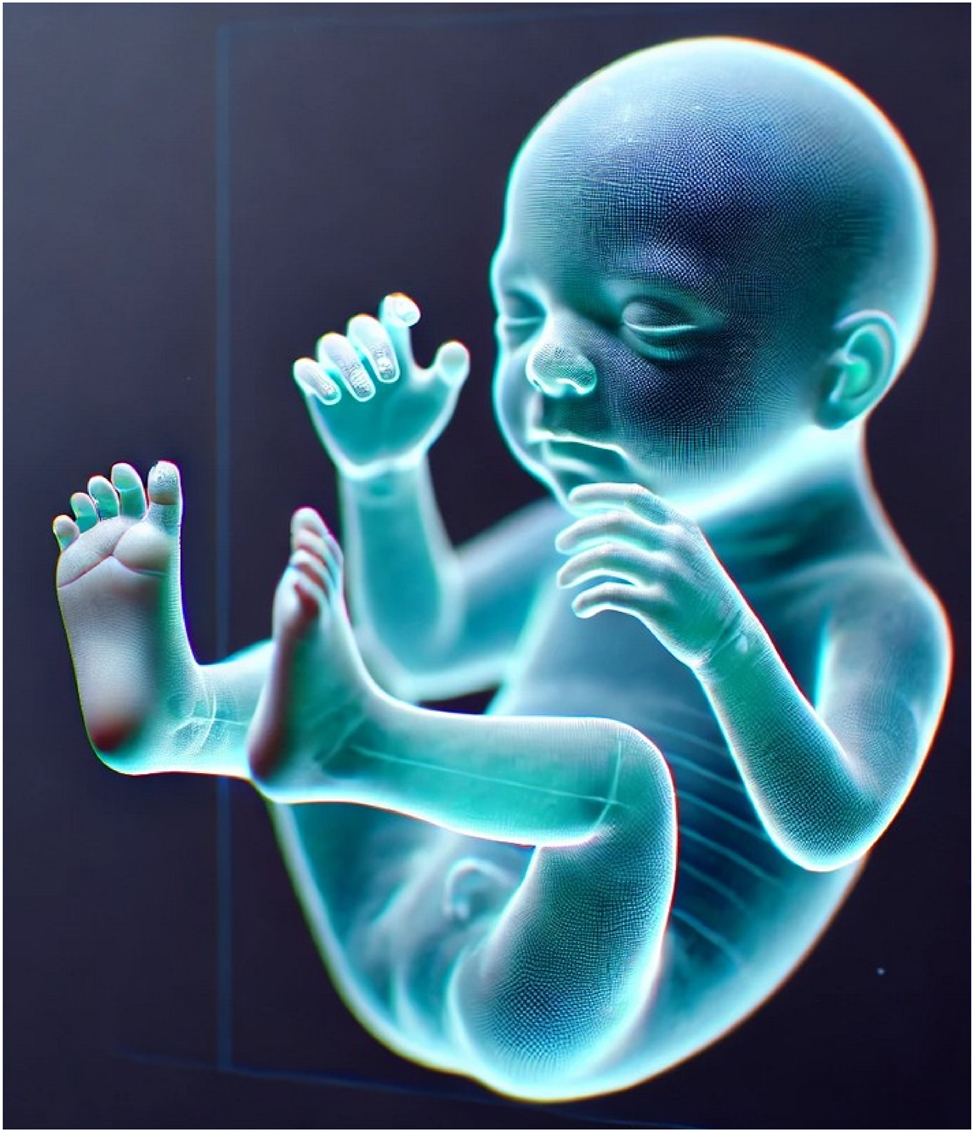
AI-generated hologram of a fetus rendered from volumetric ultrasound data. The model highlights detailed anatomical features including cranial contours, limb orientation, and spinal alignment, demonstrating the spatial accuracy achievable with advanced segmentation and rendering techniques. Such holograms provide immersive, life-like visualizations that enhance parental understanding of fetal anatomy, foster maternal–fetal bonding, and support multidisciplinary clinical discussions.
The development of holographic visualization has been accelerated by artificial intelligence (AI), which enables rapid and accurate fetal segmentation, noise reduction, and high-fidelity rendering [11], 12]. These advances raise the possibility of integrating holographic workflows into clinical practice (Figure 3). However, high implementation costs, equipment requirements, training needs, and ethical considerations – such as anxiety from abnormal findings or issues of data security – limit widespread adoption [13], [14], [15], [16].
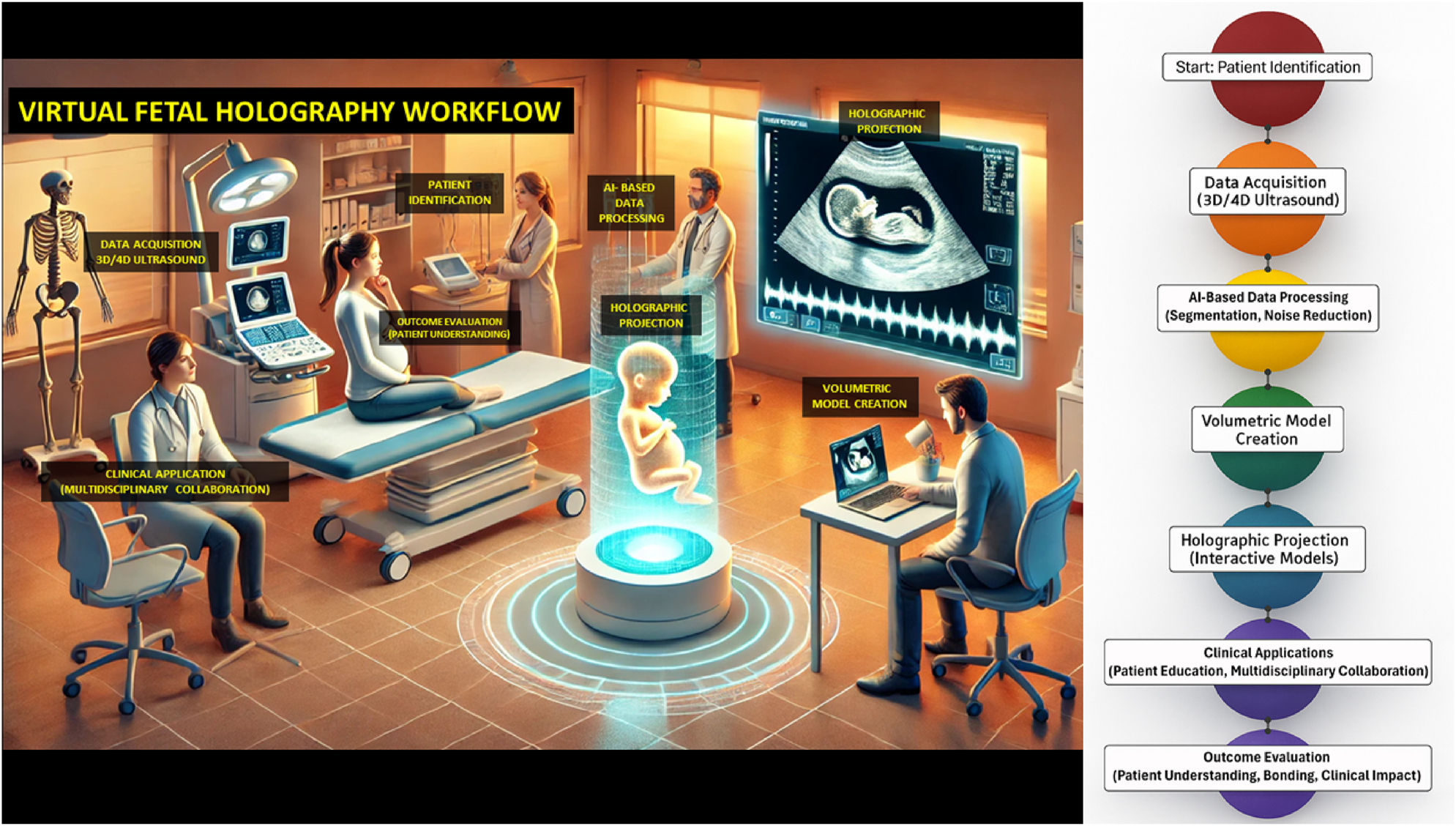
Integrated visual representation of the virtual fetal holography workflow. The left panel illustrates a clinical counseling setting in which clinicians and parents engage with an interactive fetal hologram projected in real time. This environment demonstrates how holographic visualization integrates into patient counseling and multidisciplinary collaboration, enhancing understanding and emotional engagement. The right panel presents the technical workflow, beginning with patient identification and high-resolution 3D/4D ultrasound data acquisition, followed by AI-based image processing for segmentation and noise reduction. The processed volumetric datasets are then converted into anatomically accurate digital models, rendered as dynamic holographic projections viewable through mixed reality interfaces. The workflow concludes with clinical applications that support patient education, collaborative decision-making, and outcome evaluation, emphasizing improved parental understanding, maternal–fetal bonding, and clinical impact.
The objective of this review is to synthesize current evidence on virtual fetal holography for parental counseling and education, including its technical foundations, clinical applications, and ethical implications. By combining insights from multiple specialties and critically appraising available studies (Tables 1–3), we aim to identify both the opportunities and limitations of this technology and outline priorities for future research and clinical integration.
Critical appraisal of key literature on holography, AI, and virtual fetal imaging (a).
| Author | Key insight/Focus | Key outcome relevant to virtual fetal holography | Strengths | Limitations | Quality scorea |
| Gsaxner [4] | HoloLens applications in medicine (systematic review) | Taxonomy of clinical mixed reality applications | Broad coverage, evidence synthesis | Not fetal-specific | AMSTAR-2: High |
| Venkatesan [14] | Virtual/augmented reality in biomedical sciences | Highlights translational AR/VR potential | Cross-disciplinary scope | Narrative design, not fetal-specific | AMSTAR-2: Moderate |
| Wong [15] | Mixed reality in orthopaedic oncology | Demonstrated surgical planning improvement | Real clinical endpoints | Orthopaedic focus only | AMSTAR-2: Moderate |
| Tătaru [17] | HoloLens platform for simulation & training | Enhanced professional skill acquisition | Quantitative training outcomes | Single specialty context | NOS: 8/9 |
| Bachnas [18] | AI-enhanced fetal 3D/4D ultrasound facial profile analysis | Improved diagnostic accuracy & speed | Direct fetal application, novel AI | Pilot scale, limited validation | NOS: 7/9 |
| Andonotopo [19] | AI mapping fetal facial expressions in development | Enabled early behavioral biometrics | Fetal-specific, innovative metrics | Evolutionary framework, conceptual | NOS: 7/9 |
| Andonotopo [20] | AI interpretation of 4D fetal facial data (review) | Summarized AI algorithm capabilities | High translational value | No meta-analysis | AMSTAR-2: Moderate |
| Andonotopo [21] | Fetal origins & AI-driven 4D ultrasound | Proposed integration with barker hypothesis | Innovative perinatal framework | Narrative style, hypothesis-driven | ROBIS: Low risk |
| d’Aiello [8] | Holographic congenital heart models (education) | Enhanced anatomy learning & retention | Quantified educational outcomes | Small sample size | NOS: 8/9 |
| Bruckheimer [2] | Real-time clinical cardiac holography | Feasible in clinical workflow | Real-world proof-of-concept | Limited to cardiology | NOS: 7/9 |
| Fu [22] | Mixed reality surgical navigation (pulmonary nodules) | Improved localization accuracy | Quantitative metrics | Non-obstetric, single center | NOS: 7/9 |
| Noecker [12] | Neurosurgical holographic visualization | Increased stereotactic precision | High accuracy | Specialty-specific, small sample | NOS: 8/9 |
| Bracale [23] | Mixed reality colorectal surgery planning | Better spatial planning | Narrative+pilot data | Limited specialty | ROBIS: Low risk |
| Joda [7] | AR/VR in dental medicine (systematic review) | Educational outcome improvements | Quantitative evaluation | Non-perinatal | AMSTAR-2: Moderate |
| Boyanovsky [24] | AR in anatomy education | Improved student engagement | Contemporary curriculum | No clinical endpoints | NOS: 6/9 |
| Hu [9] | Mixed reality tech review | Identified future clinical trends | Broad clinical relevance | No patient outcome data | AMSTAR-2: Moderate |
| Haleem [1] | Holography applications overview | Conceptual landscape of medical holography | Broad coverage | No empirical data | AMSTAR-2: Moderate |
| Bucioli [11] | Real-time 3D heart holography | Multi-place diagnostics feasibility | Innovative technical concept | Not fetal | NOS: 6/9 |
| Mudanyali [25] | Portable lensless holography for telemedicine | Compact, low-cost device potential | Resource-friendly | Outdated technology | ROBIS: Some concern |
| Picazo-Bueno [13] | Lensless holography microscopy | Non-invasive cell imaging | High precision | Preclinical context | ROBIS: Low risk |
| Puyo [5] | Laser Doppler holography blood flow imaging | Microvascular flow visualization | Innovative optical method | No patient context | NOS: 6/9 |
| Ma [26] | AR surgical navigation | Accurate 3D overlays intraoperatively | Clinical validation | Orthopaedic-specific | NOS: 7/9 |
| Gao [27] | Mixed reality operation visualization | Improved spatial understanding | Implementation insight | No outcome metrics | NOS: 6/9 |
| Kumari [3] | Microwave holography for tissue detection | Early detection of breast lesions | Demonstrated sensitivity | Not prenatal | ROBIS: Low risk |
| Rappaz [28] | Digital holographic morphometry | Quantitative cell morphometry | Foundational | Early tech only | ROBIS: Low risk |
-
(a) This table summarizes 25 key studies relevant to virtual fetal holography and related imaging technologies. Each entry highlights core insights, outcomes, strengths, limitations, and quality scores assessed using AMSTAR-2 (systematic reviews), ROBIS (risk-of-bias for review-level evidence), or the Newcastle–Ottawa Scale (NOS) for observational studies.
Study characteristics of included literature (a).
| Author | Study type | Population/Sample | Imaging modality/Technology | Main outcome relevant to virtual fetal holography |
| Gsaxner [4] | Systematic review | NA (review data) | HoloLens mixed reality systems | Taxonomy of clinical applications |
| Venkatesan [14] | Narrative review | NA | AR/VR biomedical applications | Translational AR/VR potential in healthcare |
| Wong [15] | Review | NA | Mixed reality surgical planning | Improved orthopaedic oncology planning |
| Tătaru [17] | Review | NA | HoloLens platform | Simulation & training enhancement |
| Bachnas [18] | AI feasibility | 120 fetal cases | AI-based 3D/4D ultrasound | Better facial profile analysis |
| Andonotopo [19] | AI observational | 100 fetal cases | AI-driven 4D ultrasound | Early fetal facial expression mapping |
| Andonotopo [20] | Review | NA | AI algorithms for ultrasound | Review of AI fetal facial analysis |
| Andonotopo [21] | Narrative review | NA | AI & Barker’s hypothesis integration | Prenatal origins of adult disease concept |
| d’Aiello [8] | Exploratory study | 25 medical trainees | Holographic congenital heart models | Improved anatomy learning and retention |
| Bruckheimer [2] | Feasibility study | 15 patients | Real-time cardiac digital holography | First clinical holographic imaging |
| Fu [22] | Feasibility study | 20 thoracic surgery cases | Mixed reality surgical navigation | Improved nodule localization |
| Noecker [12] | Feasibility study | 18 neurosurgical patients | Holographic stereotactic visualization | Increased surgical precision |
| Bracale [23] | Narrative review | NA | Mixed reality colorectal surgery | Enhanced surgical planning framework |
| Joda [7] | Systematic review | NA | AR/VR in dental medicine | Improved educational outcomes |
| Boyanovsky [24] | Educational study | 40 medical students | AR anatomy platform | Improved student engagement & comprehension |
| Hu [9] | Narrative review | NA | Mixed reality medical applications | Identified future adoption trends |
| Haleem [1] | Narrative review | NA | Holography in medicine | Overview of applications |
| Bucioli [11] | Prototype study | 10 cardiology patients | 3D heart holography | Multi-site diagnostic feasibility |
| Mudanyali [25] | Technical prototype | Lab setup | Portable lensless holography | Telemedicine-friendly device design |
| Picazo-Bueno [13] | Technical prototype | NA | Lensless holography microscopy | High-resolution cell imaging |
| Puyo [5] | Technical prototype | NA | Laser Doppler holography | Microvascular blood flow imaging |
| Ma [26] | Feasibility study | 22 orthopaedic cases | AR surgical navigation | 3D overlay guidance improves accuracy |
| Gao [27] | Technical report | NA | Mixed reality visualization | Improved operative spatial understanding |
| Kumari [3] | Technical prototype | Breast tissue samples | Microwave holography | Early cancer detection potential |
| Rappaz [28] | Technical prototype | Lab cell samples | Digital holographic morphometry | Quantitative cell analysis |
| Marquet [29] | Technical prototype | Lab cell samples | Digital holographic microscopy | Subwavelength imaging accuracy |
| Kemper [30] | Technical prototype | Pancreas tumor cells | Digital holographic microscopy | Live cell imaging |
| Moon [31] | Technical prototype | Stem cells | Computational holographic imaging | 3D stem cell identification |
| Flewellen [10] | Technical prototype | Single molecule lab | Digital holography localization | Particle tracking accuracy |
| Vinu [6] | Technical prototype | Scattering medium lab | Digital in-line holography | Imaging through scattering medium |
-
(a) This table lists all 31 studies included in the systematic review, summarizing study type, population or sample, imaging modality, and main outcomes relevant to virtual fetal holography, fulfilling PRISMA, transparency requirements.
Risk of bias and evidence weight assessment (a).
| Author | Study type | Risk of bias tool (domains assessed) | Domains at risk (n/total) | Overall risk rating | Evidence weight |
| Gsaxner [4] | Systematic review | AMSTAR-2 (16 domains) | 0/16 | Low | ★★★★★ |
| Venkatesan [14] | Narrative review | ROBIS (4 domains) | 1/4 | Moderate | ★★★★☆ |
| Wong [15] | Review | ROBIS (4 domains) | 1/4 | Moderate | ★★★★☆ |
| Tătaru [17] | Review | ROBIS (4 domains) | 0/4 | Low | ★★★★☆ |
| Bachnas [18] | AI feasibility | NOS (9 domains) | 1/9 | Low | ★★★★☆ |
| Andonotopo [19] | AI observational | NOS (9 domains) | 2/9 | Moderate | ★★★☆☆ |
| Andonotopo [20] | Review | ROBIS (4 domains) | 1/4 | Moderate | ★★★★☆ |
| Andonotopo [21] | Narrative review | ROBIS (4 domains) | 1/4 | Moderate | ★★★☆☆ |
| d’Aiello [8] | Exploratory study | NOS (9 domains) | 1/9 | Low | ★★★★☆ |
| Bruckheimer [2] | Feasibility study | NOS (9 domains) | 1/9 | Low | ★★★★☆ |
| Fu [22] | Feasibility study | NOS (9 domains) | 2/9 | Moderate | ★★★☆☆ |
| Noecker [12] | Feasibility study | NOS (9 domains) | 1/9 | Low | ★★★★☆ |
| Bracale [23] | Narrative review | ROBIS (4 domains) | 2/4 | Moderate | ★★★☆☆ |
| Joda [7] | Systematic review | AMSTAR-2 (16 domains) | 1/16 | Low | ★★★★☆ |
| Boyanovsky [24] | Educational study | NOS (9 domains) | 2/9 | Moderate | ★★★☆☆ |
| Hu [9] | Narrative review | ROBIS (4 domains) | 2/4 | Moderate | ★★★☆☆ |
| Haleem [1] | Narrative review | ROBIS (4 domains) | 2/4 | Moderate | ★★★☆☆ |
| Bucioli [11] | Prototype study | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Mudanyali [25] | Technical prototype | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Picazo-Bueno [13] | Technical prototype | NOS (9 domains) | 2/9 | Moderate | ★★★☆☆ |
| Puyo [5] | Technical prototype | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Ma [26] | Feasibility study | NOS (9 domains) | 1/9 | Low | ★★★★☆ |
| Gao [27] | Technical report | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Kumari [3] | Technical prototype | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Rappaz [28] | Technical prototype | NOS (9 domains) | 4/9 | High | ★★☆☆☆ |
| Marquet [29] | Technical prototype | NOS (9 domains) | 4/9 | High | ★★☆☆☆ |
| Kemper [30] | Technical prototype | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Moon [31] | Technical prototype | NOS (9 domains) | 4/9 | High | ★★☆☆☆ |
| Flewellen [10] | Technical prototype | NOS (9 domains) | 3/9 | Moderate | ★★☆☆☆ |
| Vinu [6] | Technical prototype | NOS (9 domains) | 4/9 | High | ★★☆☆☆ |
-
(a) Table 3 summarizes the methodological quality and evidence weight of all 31 included studies, applying AMSTAR-2 (systematic reviews), ROBIS (narrative reviews), and the Newcastle–Ottawa Scale (NOS) for observational and feasibility studies. Risk is reported as the number of bias domains flagged, while evidence weight integrates quality and study impact (sample size and direct relevance) using a 5-star scale (★★★★★=very high strength).
Methods
Overall approach
This review was designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Figure 1), ensuring transparency and reproducibility. The objective was to synthesize peer-reviewed evidence on virtual fetal holography, focusing on technical foundations, clinical applications, ethical considerations, and educational value. Literature published between January 2015 and May 2025 was considered to capture recent technological developments and clinical applications relevant to prenatal counseling and education.
Literature search and study selection
A comprehensive search was performed using PubMed, IEEE Xplore, ScienceDirect, and Google Scholar. Search terms included “virtual fetal holography,” “3D/4D ultrasound hologram,” “AI-based image segmentation,” and “holography in fetal imaging.” References of retrieved articles were screened to identify additional studies. Inclusion criteria were studies reporting empirical applications of holography based on medical imaging, integration of artificial intelligence for image processing, or descriptions of clinical feasibility and educational applications. Exclusion criteria were articles focused on speculative or hypothetical applications without empirical data, studies unrelated to medical imaging, animal experiments, and papers lacking an English full text.
The search yielded 362 unique records. After duplicate removal and screening, 55 full-text articles were reviewed, resulting in 31 studies that met the inclusion criteria (Figure 1). These include systematic reviews, observational studies, technical prototypes, and AI-based imaging workflow studies [18], [19], [20], [21].
Data extraction and quality assessment
Two reviewers independently extracted study characteristics, objectives, imaging modality, outcomes, and conclusions. Methodological quality and risk of bias were assessed using validated tools: AMSTAR-2 for systematic reviews, ROBIS for narrative reviews, and the Newcastle–Ottawa Scale (NOS) for observational and feasibility studies. Quality ratings and evidence weighting are summarized in Table 3, while key insights and detailed characteristics are presented in Table 1 and Table 2 respectively. Disagreements were resolved by consensus.
Workflow of virtual fetal holography
The technical foundation of virtual fetal holography integrates advanced imaging acquisition and artificial intelligence–driven processing. High-resolution 3D and 4D ultrasound datasets are captured, providing static morphology and dynamic fetal motion. These images undergo AI-based segmentation and volumetric reconstruction to create anatomically accurate digital fetal models, which are subsequently rendered as interactive holographic visualizations. The resulting models are projected through mixed reality headsets or holographic displays, enabling real-time interactive exploration during prenatal counseling. An example of a rendered fetal hologram is shown in Figure 2, illustrating its life-like detail and spatial realism. The complete step-by-step workflow, from patient identification to AI processing and holographic projection, is presented in Figure 3.
Data synthesis
Studies were categorized into four thematic areas: technological advancements, clinical and educational applications, workflow integration, and ethical considerations. Due to heterogeneity in design and outcome measures, narrative synthesis was chosen. The critical appraisal and thematic analysis are detailed in the Results section, supported by Tables 1–3.
Results and findings
Literature screening and study characteristics
The literature search identified 362 records, of which 79 were duplicates and 228 were excluded after title and abstract screening. Fifty-five full texts were assessed, and 31 studies met the inclusion criteria (Figure 1). These included systematic reviews, observational feasibility studies, technical prototypes, and AI-driven imaging workflow studies. Study characteristics are summarized in Table 2, while critical insights and limitations of the most influential 25 studies are detailed in Table 1. Methodological quality and risk of bias assessments are presented in Table 3.
Technical advancements in holographic imaging
The reviewed literature demonstrates major technological progress in converting 3D and 4D ultrasound datasets into interactive holographic models. AI-powered segmentation and noise reduction algorithms have improved anatomical delineation of fetal structures such as the brain, heart, limbs, and umbilical vessels, enhancing visual clarity and diagnostic reliability [18], [19], [20], [21]. Integration of color Doppler and multimodal overlays enables more accurate cardiac and vascular mapping, exemplified by fetal heart holograms used in congenital anomaly counseling (Figure 4). Advances in rendering software and display platforms, including the Microsoft HoloLens and mixed reality projectors, have enabled real-time, interactive visualization from multiple angles [4], 11]. A representative AI-generated fetal hologram demonstrating anatomical realism and spatial depth is illustrated in Figure 2, while the complete data acquisition and holographic workflow is shown in Figure 3.
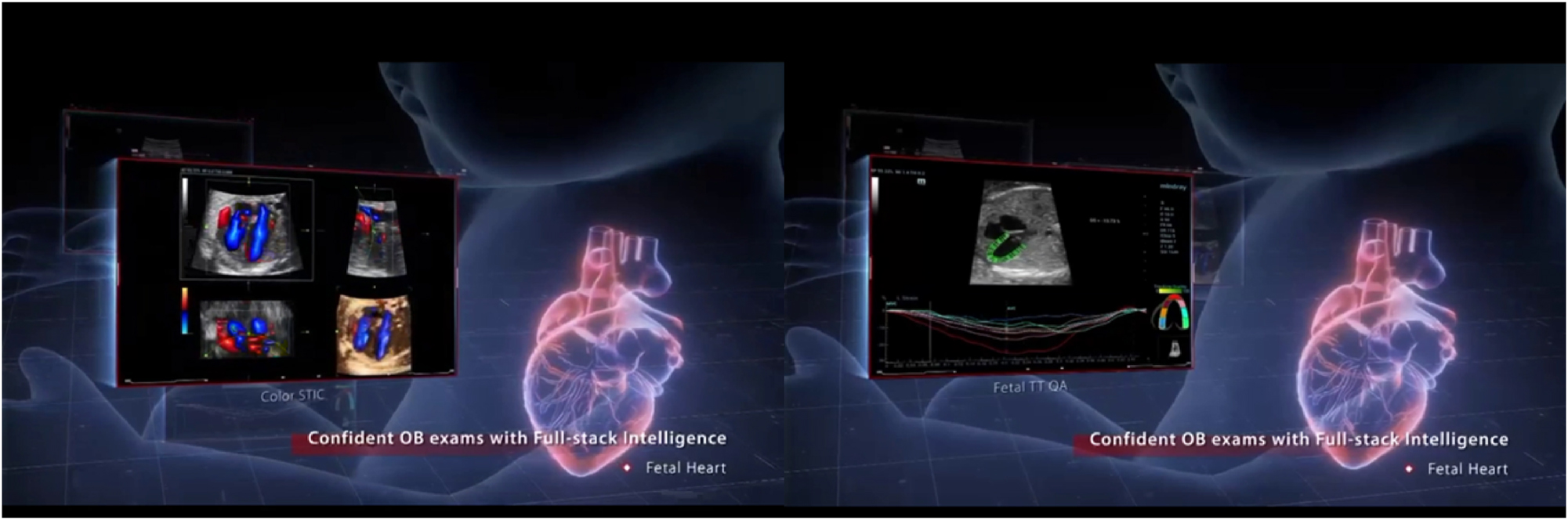
This figure illustrates the integration of AI-driven holographic imaging for detailed fetal heart assessment, showcasing the system’s ability to create dynamic, real-time 3D visualizations. On the left, various ultrasound perspectives highlight anatomical and blood flow assessments, while on the right, advanced cardiac metrics and diagnostic overlays demonstrate comprehensive functional analysis. The central holographic representation of the fetal heart is enhanced with color Doppler data, allowing clinicians to observe critical flow patterns. By combining volumetric holography with real-time diagnostic intelligence, this technology supports confident, accurate assessments of fetal cardiac health. It not only enhances clinical decision-making but also facilitates better patient understanding during consultations about congenital heart anomalies or other conditions.
Clinical applications of virtual fetal holography
Clinical applications primarily focus on enhancing prenatal counseling, parental education, and multidisciplinary team collaboration. Several studies report improved parental comprehension when holographic fetal visualizations supplement standard ultrasound, particularly when counseling on congenital anomalies and growth restriction [7], 8], 10]. Interactive holograms provide expectant parents with a clearer spatial understanding of fetal position, anatomy, and movement compared to conventional 2D/3D imaging, supporting informed decision-making and strengthening maternal–fetal bonding. In professional settings, holograms have facilitated more effective multidisciplinary discussions by offering shared 3D anatomical references during complex case reviews [4], 11]. A clinical counseling scenario using complete fetal holographic visualization is demonstrated in Figure 5.
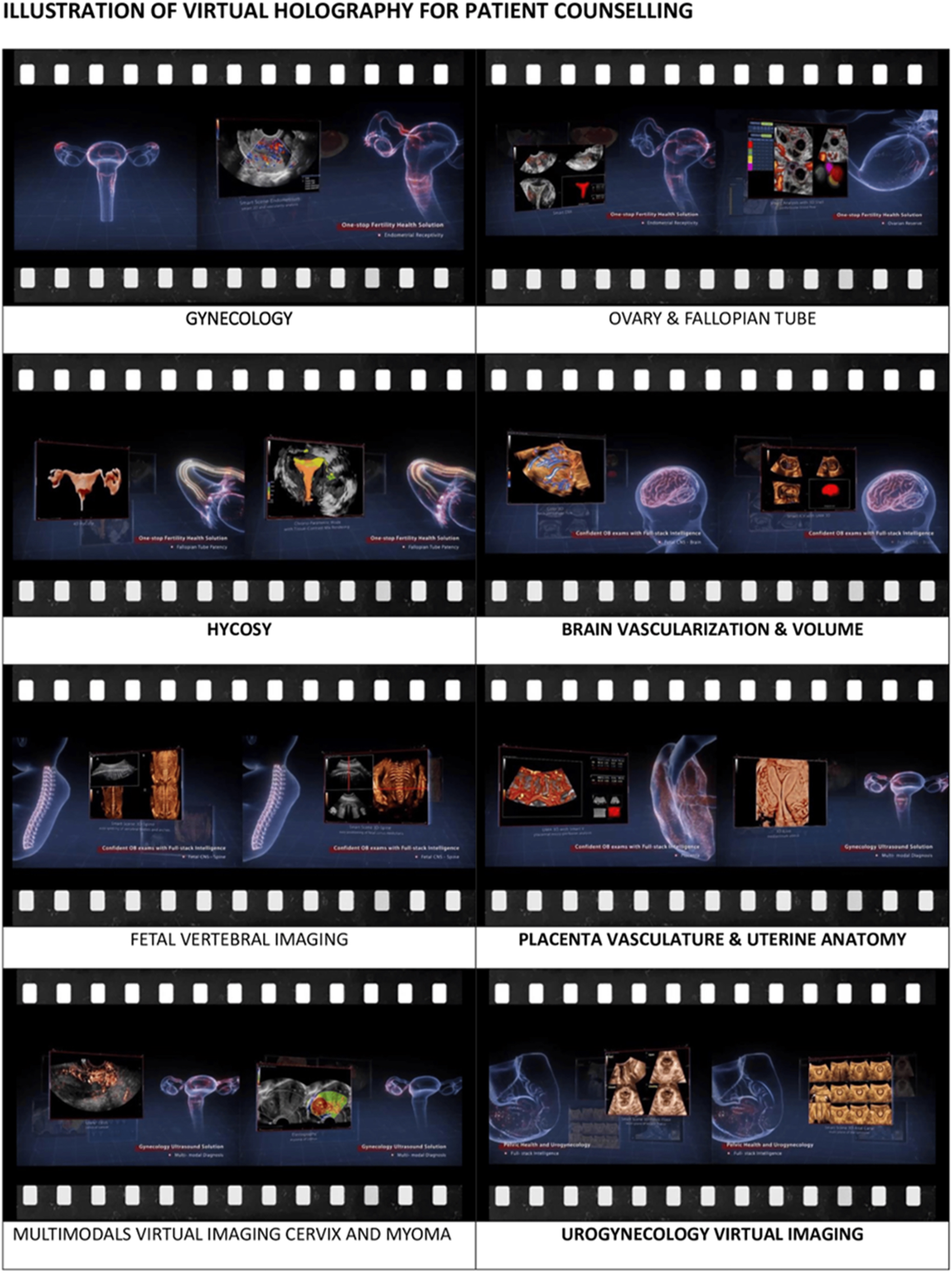
Demonstration of the breadth of virtual holography applications in obstetrics, gynecology, and reproductive medicine. Each panel represents a distinct clinical domain where interactive 3D holograms enhance diagnostic visualization and patient counseling. Top panels show gynecological anatomy, including uterine cavity evaluation and ovarian and fallopian tube imaging, facilitating fertility assessments and adnexal pathology counseling. Middle panels highlight hysterosalpingo-contrast sonography (HyCoSy) for tubal patency evaluation, brain vascularization and volumetry for fetal neuroimaging, fetal vertebral column assessment for structural anomalies, and placental vasculature with uterine anatomy for high-risk pregnancy evaluation. Bottom panels demonstrate multimodal virtual imaging of the cervix and uterine myomas, as well as urogynaecological mapping of bladder and pelvic floor structures. By converting complex diagnostic data into interactive holograms, this technology improves patient comprehension, strengthens emotional engagement, and supports informed decision-making while promoting multidisciplinary collaboration in managing fetal anomalies, gynecological disorders, and fertility-related issues.
Real-world examples from related medical fields
Adoption of holography in other specialties provides insight into its broader translational potential. Bruckheimer et al. demonstrated improved procedural accuracy in cardiac interventions using real-time holographic models of cardiac structures [2]. Dental education programs using holographic models showed superior knowledge retention and spatial reasoning among students compared to traditional teaching methods [7], and neurosurgical planning studies reported improved understanding of complex spatial relationships [4], 12]. These examples, while outside prenatal care, suggest comparable benefits when adapted to fetal imaging and counseling.
Strengths of current evidence
The strongest evidence supports holography’s role in improving patient comprehension and emotional engagement during clinical encounters. Studies consistently report positive subjective feedback from parents and clinicians when holographic models supplement traditional imaging [7], 10]. AI-enhanced segmentation increases anatomical fidelity, ensuring accurate diagnostic visualization [18], [19], [20], [21]. Additionally, the versatility of display platforms allows integration across educational, diagnostic, and training contexts [4], 11]. These findings indicate that virtual fetal holography is technically feasible and well-received by both clinicians and patients.
Limitations of current evidence
Despite promising potential, existing studies are mostly small-scale, feasibility-focused, or descriptive, with limited empirical validation of impacts on clinical outcomes or decision quality [1], 26]. Costs of acquisition, specialized software, and operator training remain significant barriers, particularly in resource-limited settings [4], 23]. Ethical concerns persist, including potential patient anxiety from viewing detailed holographic anomalies, data security issues, and the need for robust informed consent processes [22], 27]. Addressing these limitations through rigorous clinical trials, standardized workflow protocols, cost-benefit analyses, and comprehensive training programs will be essential to integrating holography into routine prenatal care.
Discussion
Patient education and emotional engagement
The findings of this review support the primary hypothesis that virtual fetal holography enhances patient comprehension and emotional engagement compared with conventional 2D and 3D ultrasound imaging [7], 8], 10], 24]. Holographic models provide a highly immersive, three-dimensional visualization of fetal structures, improving patient understanding of complex anatomy and fostering maternal–fetal bonding. Emotional engagement is further strengthened by the interactive nature of holographic projections, allowing parents to explore fetal anatomy dynamically during consultations (Figure 5). This enhanced visualization has been associated with increased patient satisfaction and adherence to prenatal care recommendations [8], 24].
Multidisciplinary collaboration and clinical decision-making
Virtual fetal holography facilitates more effective multidisciplinary collaboration by offering shared visual references during case discussions. Obstetricians, radiologists, maternal–fetal medicine specialists, and genetic counselors can simultaneously view and manipulate holographic models, supporting consensus-building and timely decision-making [12], 16], 17]. Experiences from neurosurgery and cardiac surgery, where similar holographic approaches have improved spatial orientation and procedural planning [2], 4], 12], suggest a high potential for comparable benefits in prenatal anomaly assessment and perinatal surgical planning. These applications complement structured workflows as described in Figure 3, demonstrating integration into clinical pathways.
Applications in obstetrics, gynaecology, and perinatal care
Holography has shown value in diverse areas of perinatal care, including fetal anomaly counseling, fetal echocardiography, assessment of fetal movements and blood flow, and maternal pelvic anatomy evaluation. Interactive holographic models (Figure 2) enhance parental understanding of complex anomalies, such as congenital heart disease, by presenting life-like fetal heart models (Figure 4) and facilitating discussions of potential interventions. Multimodal integration of ultrasound, Doppler, and MRI data allows for holistic fetal and maternal assessment [5], 9], 27], improving diagnostic accuracy and aiding decision-making. In high-risk pregnancies, these tools could support earlier intervention planning, reducing complications and improving outcomes [22], 26].
Role in professional training
Virtual fetal holography provides a powerful platform for education and training. Medical students, sonographers, and obstetric trainees can interact with dynamic fetal models, improving spatial reasoning and diagnostic accuracy [7], 14], 17]. These benefits mirror positive outcomes from other specialties where holographic training has demonstrated improved knowledge retention and procedural skill development [14], 26]. Widespread adoption in educational curricula could accelerate clinical competency and enhance quality of care.
Ethical and practical considerations
Despite its promise, holography poses unique ethical challenges. High-resolution, life-like representations can evoke emotional stress, particularly when abnormalities are present [12], 27]. Standardized counseling protocols and professional training are required to ensure visual data is presented sensitively and interpreted accurately. Data privacy and cybersecurity concerns must be addressed through robust encryption and secure storage [4], 22]. High cost, specialized equipment, and the need for skilled operators remain significant barriers to widespread implementation, particularly in low-resource settings [15], 23], 25]. Emerging solutions, including mobile holographic projectors and lightweight mixed-reality headsets, may offer cost-effective and scalable alternatives.
Addressing current gaps and future directions
The current evidence base is limited by a predominance of feasibility studies and descriptive case reports rather than randomized trials or longitudinal outcome studies (Tables 1–3). Future research should focus on rigorous clinical trials comparing conventional counseling with holography-based counseling, cost-effectiveness analyses, and studies evaluating its impact on long-term maternal and neonatal outcomes. Additionally, training frameworks for integrating holography into clinical practice and education require development and validation.
Key takeaways
Virtual fetal holography enhances parental understanding of fetal anatomy and fosters stronger emotional bonding.
AI-driven segmentation and rendering improve anatomical fidelity and diagnostic precision.
Interactive holographic visualization supports multidisciplinary decision-making and education.
High costs, technical complexity, and ethical considerations are major barriers to routine clinical adoption.
Rigorous clinical validation, cost-effectiveness analyses, and standardized training are essential next steps.
Implementation checklist
Establish standardized workflows for fetal image acquisition, segmentation, and holographic rendering (Figure 3).
Implement structured counseling protocols to manage emotional impact when presenting abnormal findings.
Ensure data security and privacy compliance during storage and transmission of holographic datasets.
Provide professional training for clinicians on holographic interpretation and counseling techniques.
Explore cost-reduction strategies through mobile and portable holographic systems for resource-limited settings.
Strengths, limitations, and future directions
Strengths of this review
This review is the first to comprehensively synthesize evidence on virtual fetal holography for parental counseling and education, explicitly following PRISMA guidelines (Figure 1) and incorporating a formal quality assessment (Table 3). The scope extends beyond obstetrics to draw lessons from cardiology, neurosurgery, dental education, and orthopedics, highlighting the broad translational potential of holographic imaging (Tables 1 and 2). Additionally, this review emphasizes the integration of AI-powered image processing, an innovation critical to producing clinically accurate and interactive holograms (Figure 2). By combining workflow analysis (Figure 3), clinical exemplars (Figures 4 and 5), and critical appraisal, this review provides a holistic perspective on the current state and future promise of this emerging technology.
Limitations of the evidence
The current evidence base is limited by several factors. Most included studies are feasibility-based or descriptive, with small sample sizes and limited outcome measures, which restrict generalizability. Few studies directly compare holographic counseling with conventional ultrasound-based counseling. Additionally, there is a lack of standardized protocols for holographic workflow, including segmentation, rendering, and display methods, and a limited understanding of potential psychological effects on parents viewing life-like fetal holograms, especially when anomalies are present. Cost analyses, training frameworks, and ethical guidelines remain underdeveloped.
Future directions
Future research should focus on
Conducting large-scale clinical trials to quantify impacts on parental understanding, decision-making, and emotional engagement.
Developing cost-effective, portable holographic systems to broaden access in low-resource settings.
Creating standardized workflows and training programs to reduce variability and operator dependency.
Addressing ethical considerations, including informed consent, data security, and strategies for mitigating emotional distress during abnormal findings counseling.
Exploring holographic integration with multimodal data sources (ultrasound, Doppler, MRI) for comprehensive maternal-fetal assessments and prenatal surgical planning.
Conclusions
Virtual fetal holography has emerged as a promising innovation in prenatal care, offering immersive and intuitive visualizations that enhance both patient comprehension and clinical collaboration. By transforming complex 3D and 4D ultrasound data into interactive holographic models, this technology bridges the communication gap between healthcare providers and expectant parents, improving understanding of fetal development and supporting informed decision-making.
Current evidence suggests benefits in maternal–fetal bonding, patient satisfaction, and multidisciplinary counseling. However, widespread clinical adoption is constrained by high costs, technical complexity, training requirements, and limited validation in large-scale clinical trials. Ethical considerations, including the potential for emotional distress and misinterpretation of holographic data, underscore the need for standardized protocols and professional training.
Future efforts should focus on developing cost-effective and portable holographic systems, integrating artificial intelligence to streamline workflow, and establishing robust clinical guidelines for safe and effective implementation. Large, prospective studies are essential to quantify impacts on clinical outcomes and to determine cost-effectiveness compared with conventional imaging approaches.
With continued innovation and collaboration among researchers, clinicians, industry partners, and policymakers, virtual fetal holography has the potential to evolve into a standard tool in maternal–fetal medicine, ultimately improving patient-centered care and advancing modern prenatal practice.
Acknowledgments
The author(s) acknowledge the invaluable support of the Indonesian Society of Obstetrics and Gynecology (ISOG/POGI) and the Indonesian Society of Maternal-Fetal Medicine (IAMFM/HKFM) in facilitating this review article.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The author(s) have (has) accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author(s) state(s) no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Haleem, A, Javaid, M, Khan, IH. Holography applications toward medical field: an overview. Indian J Radiol Imag 2020;30:354–61. https://doi.org/10.4103/ijri.IJRI_39_20.Suche in Google Scholar PubMed PubMed Central
2. Bruckheimer, E, Rotschild, C, Dagan, T, Amir, G, Kaufman, A, Gelman, S, et al.. Computer-generated real-time digital holography: first time use in clinical medical imaging. Eur Heart J Cardiovasc Imaging 2016;17:845–9. https://doi.org/10.1093/ehjci/jew087.Suche in Google Scholar PubMed
3. Kumari, V, Ahmed, A, Kanumuri, T, Shakher, C, Sheoran, G. Early detection of cancerous tissues in human breast utilizing near field microwave holography. Int J Imag Syst Technol 2020;30:391–400. https://doi.org/10.1002/ima.22384.Suche in Google Scholar
4. Gsaxner, C, Li, J, Pepe, A, Jin, Y, Kleesiek, J, Schmalstieg, D, et al.. The HoloLens in medicine: a systematic review and taxonomy. Med Image Anal 2023;85:102757. https://doi.org/10.1016/j.media.2023.102757.Suche in Google Scholar PubMed
5. Puyo, L, Spahr, H, Pfäffle, C, Hüttmann, G, Hillmann, D. Retinal blood flow imaging with combined full-field swept-source optical coherence tomography and laser doppler holography. Opt Lett 2022;47:1198–201. https://doi.org/10.1364/OL.449739.Suche in Google Scholar PubMed
6. Vinu, RV, Kim, K, Somkuwar, AS, Park, Y, Singh, RK. Single-shot optical imaging through scattering medium using digital in-line holography. arXiv preprint arXiv:1603.07430 2016. https://doi.org/10.48550/arXiv.1603.07430.Suche in Google Scholar
7. Joda, T, Gallucci, GO, Wismeijer, D, Zitzmann, NU. Augmented and virtual reality in dental medicine: a systematic review. Comput Biol Med 2019;108:93–100. https://doi.org/10.1016/j.compbiomed.2019.03.012.Suche in Google Scholar PubMed
8. d’Aiello, AF, Cabitza, F, Natali, C, Viganò, S, Ferrero, P, Bognoni, L, et al.. The effect of holographic heart models and mixed reality for anatomy learning in congenital heart disease: an exploratory study. J Med Syst 2023;47:64. https://doi.org/10.1007/s10916-023-01959-8.Suche in Google Scholar PubMed PubMed Central
9. Hu, HZ, Feng, XB, Shao, ZW, Xie, M, Xu, S, Wu, XH, et al.. Application and prospect of mixed reality technology in medical field. Curr Med Sci 2019;39:1–6. https://doi.org/10.1007/s11596-019-1992-8.Suche in Google Scholar PubMed
10. Flewellen, JL, Minoughan, S, Garcia, IL, Tolar, P. Digital holography-based 3D particle localization for single-molecule tweezer techniques. Biophys J 2022;121:2538–49. https://doi.org/10.1016/j.bpj.2022.06.001.Suche in Google Scholar PubMed PubMed Central
11. Bucioli, AA, Cyrino, G, Mendes de Lima, GF, Botelho, RV. Holographic real-time 3D heart visualization from coronary tomography for multi-place medical diagnostics. In: 2017 IEEE 15th (DASC/PiCom/DataCom/CyberSciTech). Orlando, FL, USA: IEEE; 2017:239–44 pp.10.1109/DASC-PICom-DataCom-CyberSciTec.2017.51Suche in Google Scholar
12. Noecker, AM, Mlakar, J, Petersen, MV, Griswold, MA, McIntyre, CC. Holographic visualization for stereotactic neurosurgery research. Brain Stimul 2023;16:411–14. https://doi.org/10.1016/j.brs.2023.02.001.Suche in Google Scholar PubMed PubMed Central
13. Picazo-Bueno, JÁ, Sanz, M, Granero, L, García, J, Micó, V. Multi-illumination single-holographic-exposure lensless fresnel (MISHELF) microscopy: principles and biomedical applications. Sensors 2023;23:1472. https://doi.org/10.3390/s23031472.Suche in Google Scholar PubMed PubMed Central
14. Venkatesan, M, Mohan, H, Ryan, JR, Schürch, CM, Nolan, GP, Frakes, DH, et al.. Virtual and augmented reality for biomedical applications. Cell Rep Med 2021;2:100348. https://doi.org/10.1016/j.xcrm.2021.100348.Suche in Google Scholar PubMed PubMed Central
15. Wong, KC, Sun, YE, Kumta, SM. Review and future/potential application of mixed reality technology in orthopaedic oncology. Orthop Res Rev 2022;14:169–86. https://doi.org/10.2147/ORR.S360933.Suche in Google Scholar PubMed PubMed Central
16. Guha, D, Alotaibi, NM, Nguyen, N, Gupta, S, McFaul, C, Yang, VXD. Augmented reality in neurosurgery: a review of current concepts and emerging applications. Can J Neurol Sci 2017;44:235–45. https://doi.org/10.1017/cjn.2016.443.Suche in Google Scholar PubMed
17. Tătaru, OS, Ferro, M, Marchioni, M, Veccia, A, Coman, O, Lasorsa, F, et al.. HoloLens® platform for healthcare professionals simulation training, teaching, and its urological applications: an up-to-date review. Ther Adv Urol 2024;16:17562872241297554.10.1177/17562872241297554Suche in Google Scholar PubMed PubMed Central
18. Bachnas, MA, Andonotopo, W, Dewantiningrum, J, Adi Pramono, MB, Stanojevic, M, Kurjak, A. The utilization of artificial intelligence in enhancing 3D/4D ultrasound analysis of fetal facial profiles. J Perinat Med 2024;52:899–913. https://doi.org/10.1515/jpm-2024-0347.Suche in Google Scholar PubMed
19. Andonotopo, W, Bachnas, MA, Dewantiningrum, J, Adi Pramono, MB, Stanojevic, M, Kurjak, A. AI and early diagnostics: mapping fetal facial expressions through development, evolution, and 4D ultrasound. J Perinat Med 2025;53:263–85. https://doi.org/10.1515/jpm-2024-0602.Suche in Google Scholar PubMed
20. Andonotopo, W, Bachnas, MA, Dewantiningrum, J, Pramono, MB, Stanojevic, M, Kurjak, A. The capability of artificial intelligence algorithms to analyze and interpret complex medical data from four-dimensional ultrasound fetal facial imaging: a literature review. Donald Sch J Ultrasound Obstet Gynecol 2025;19:16–29. https://doi.org/10.5005/jp-journals-10009-2061.Suche in Google Scholar
21. Andonotopo, W, Bachnas, MA, Akbar, MIA, Aziz, MA, Dewantiningrum, J, Pramono, MBA, et al.. Fetal origins of adult disease: transforming prenatal care by integrating Barker’s hypothesis with AI-driven 4D ultrasound. J Perinat Med 2025;53:418–38. https://doi.org/10.1515/jpm-2024-0617.Suche in Google Scholar PubMed
22. Fu, R, Zhang, C, Zhang, T, Chu, XP, Tang, WF, Yang, XN, et al.. A three-dimensional printing navigational template combined with mixed reality technique for localizing pulmonary nodules. Interact Cardiovasc Thorac Surg 2021;32:552–9. https://doi.org/10.1093/icvts/ivaa300.Suche in Google Scholar PubMed PubMed Central
23. Bracale, U, Iacone, B, Tedesco, A, Gargiulo, A, Di Nuzzo, MM, Sannino, D, et al.. The use of mixed reality in the preoperative planning of colorectal surgery: preliminary experience with a narrative review. Cir Esp (Engl Ed) 2024;102:S36–44. https://doi.org/10.1016/j.cireng.2024.01.006.Suche in Google Scholar PubMed
24. Boyanovsky, BB, Belghasem, M, White, BA, Kadavakollu, S. Incorporating augmented reality into anatomy education in a contemporary medical school curriculum. Cureus 2024;16:e57443. https://doi.org/10.7759/cureus.57443.Suche in Google Scholar PubMed PubMed Central
25. Mudanyali, O, Tseng, D, Oh, C, Isikman, SO, Sencan, I, Bishara, W, et al.. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip 2010;10:1417–28. https://doi.org/10.1039/c000453g.Suche in Google Scholar PubMed PubMed Central
26. Ma, C, Chen, G, Zhang, X, Ning, G, Liao, H. Moving-tolerant augmented reality surgical navigation system using autostereoscopic three-dimensional image overlay. IEEE J Biomed Health Inform 2019;23:2483–93. https://doi.org/10.1109/JBHI.2018.2885378.Suche in Google Scholar PubMed
27. Gao, Y, Tan, K, Sun, J, Jiang, T, Zou, XW. Application of mixed reality technology in visualization of medical operations. Chin Med Sci J 2019;34:103–9. https://doi.org/10.24920/003564.Suche in Google Scholar PubMed
28. Rappaz, B, Marquet, P, Cuche, E, Emery, Y, Depeursinge, C, Magistretti, P. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt Express 2005;13:9361–73. https://doi.org/10.1364/opex.13.009361.Suche in Google Scholar PubMed
29. Marquet, P, Rappaz, B, Magistretti, PJ, Cuche, E, Emery, Y, Colomb, T, et al.. Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt Lett 2005;30:468–70. https://doi.org/10.1364/ol.30.000468.Suche in Google Scholar PubMed
30. Kemper, B, Carl, D, Schnekenburger, J, Bredebusch, I, Schäfer, M, Domschke, W, et al.. Investigation of living pancreas tumor cells by digital holographic microscopy. J Biomed Opt 2006;11:34005. https://doi.org/10.1117/1.2204609.Suche in Google Scholar PubMed
31. Moon, I, Javidi, B. Three-dimensional identification of stem cells by computational holographic imaging. J R Soc Interface 2007;4:305–13. https://doi.org/10.1098/rsif.2006.0175.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

