Abstract
With all of our present knowledge, high technology diagnostic equipment, electronic databases and other available supporting resources, detection of fetal syndromes is still a challenge for healthcare providers in prenatal as well as in the postnatal period. Prenatal diagnosis of fetal syndromes is not straightforward, and it is a difficult puzzle that needs to be assembled and solved. Detection of one anomaly should always raise a suspicion of the existence of more anomalies, and can be a trigger to investigate further and raise awareness of possible syndromes. Highly specialized software systems for three- and four-dimensional ultrasound (3D/4D US) enabled detailed depiction of fetal anatomy and assessment of the dynamics of fetal structural and functional development in real time. With recent advances in 3D/4D US technology, antenatal diagnosis of fetal anomalies and syndromes shifted from the 2nd to the 1st trimester of pregnancy. It is questionable what can and should be done after the prenatal diagnosis of fetal syndrome. The 3D and 4D US techniques improved detection accuracy of fetal abnormalities and syndromes from early pregnancy onwards. It is not easy to make prenatal diagnosis of fetal syndromes, so tools which help like online integrated databases are needed to increase diagnostic precision. The aim of this paper is to present the possibilities of different US techniques in the detection of some fetal syndromes prenatally.
Introduction
According to the European Registry of Congenital Malformations (EUROCAT), the prenatal detection rate for 18 selected congenital anomalies excluding genetic conditions ranges from 44.8% for clubfoot (talipes equinovarus) to 98.4% for anencephaly and similar conditions, while all genetic conditions have been prenatally detected in 72.8% of cases, with the range from 66.1% for Down syndrome and 93.6% for Edwards syndrome (Table 1) [1]. The overall detection rate of all abnormalities is reported to be 34.5% in the EUROCAT [1].
Prenatal diagnosis of 18 selected congenital anomaly subgroups for registries with complete EUROCAT data from 2010 to 2014 [1].
| Malformation | Total cases | Cases prenatally diagnosed (% of total cases) |
| Excluding genetic conditions | ||
| All anomalies (excluding genetic conditions) | 32,313 | 11,164 (34.5) |
| Anencephalus and similar (excluding genetic conditions) | 551 | 542 (98.4) |
| Spina bifida (excluding genetic conditions) | 699 | 602 (86.1) |
| Hydrocephalus (excluding genetic conditions) | 731 | 555 (75.9) |
| Transposition of great vessels (excluding genetic conditions) | 450 | 262 (58.2) |
| Hypoplastic left heart (excluding genetic conditions) | 310 | 263 (84.8) |
| Cleft lip with or without palate (excluding genetic conditions) | 1105 | 654 (59.2) |
| Diaphragmatic hernia (excluding genetic conditions) | 387 | 270 (69.8) |
| Gastroschisis (excluding genetic conditions) | 312 | 291 (93.3) |
| Omphalocele (excluding genetic conditions) | 293 | 263 (89.8) |
| Bilateral renal agenesis including Potter syndrome (excluding genetic conditions) | 141 | 128 (90.8) |
| Posterior urethral valve and/or prune belly (excluding genetic conditions) | 173 | 130 (75.1) |
| Limb reduction defects (excluding genetic conditions) | 724 | 367 (50.7) |
| Clubfoot – talipes equinovarus (excluding genetic conditions) | 1621 | 726 (44.8) |
| Chromosomal | ||
| Chromosomal | 5787 | 4211 (72.8) |
| Down syndrome | 3405 | 2252 (66.1) |
| Patau syndrome/trisomy 13 | 257 | 238 (92.6) |
| Edwards syndrome/trisomy 18 | 769 | 720 (93.6) |
Includes the following registries: Antwerp (Belgium), French West Indies (France), Isle de la Reunion (France), Saxony-Anhalt (Germany), Cork and Kerry (Ireland), SE Ireland, Emilia Romagna (Italy), Tuscany (Italy), Malta, N Netherlands (NL), S Portugal, Basque Country (Spain), Valencia Region (Spain), Vaud (Switzerland), Wales (UK), Ukraine.
Data on the prenatal detection rates of some syndromes are missing. A fetal syndrome should always be searched for and considered if at least two congenital malformations have been detected prenatally. Monogenetic syndromes have a low prevalence rate, from 0.02 per 10,000 births for all types of acrocephalopolysyndactyly to 0.96 per 10,000 births for DiGeorge syndrome [1].
With the introduction of ultrasound (US), prenatal detection of congenital malformations became available. Yet, there are still unsatisfactory detection rates in everyday clinical practice. Three-dimensional ultrasound (3D US) has been claimed by some authors to increase the detection rates of all malformations, while others are skeptical about it. Knowledge on the prenatal detection rate of more than 6000 syndromes is still sparse and only a few hundred and counting can be detected prenatally. However, implementing new ideas and knowledge from scientific research along with taking advantage of progress in available diagnostic equipment make prenatal detection much more accurate and precise. With the recent dynamic and quick development of computer technology, like 3D high definition live (3D HDlive) silhouette and flow US technology, there has been an enormous breakthrough in US equipment, with remarkable image quality. Thanks to highly specialized software systems, it is possible to view fetal anatomy in the smallest detail [2] and the dynamics of fetal structural and functional development in real time. Using four-dimensional (4D) technology, one can get an idea of the functionality of some organs and systems, for example, the brain or the eye, introducing new fields of fetal assessment like fetal neurology or fetal sono-ophthalmology [3]. Some new functional tests have even been introduced in everyday clinical practice, like the Kurjak antenatal neurodevelopmental test (KANET), to assess the function of the fetal brain [4], [5], [6], [7], adding some additional valuable input into the diagnosis of fetal syndromes [8]. Many authors reported a shift of prenatal detection of fetal syndromes from the 2nd to the 1st trimester of pregnancy [3], [8], [9], [10], [11], [12], [13].
The aim of this paper is to present and discuss the possibilities of different US techniques to improve the detection rates of some fetal syndromes prenatally.
When to suspect a syndrome prenatally and how to detect it
Common terminology used to describe fetal syndromes can sometimes be confusing. A wide variety of terms and synonyms are used. Sometimes, there is a lack of good definitions of how many major and minor criteria should be present to diagnose each syndrome. The difference in prenatal detection rates for each region or country can be partly explained by differences in screening policies and follow-up practices, as well as the possible variations in practitioners’ skills and available equipment [8].
Clinical dysmorphology is a branch of clinical genetics dedicated to the study of abnormal human development, with emphasis on syndromes expressed mostly as alterations in body morphology [14], [15]. There are many pathophysiological mechanisms for fetal maldevelopment, which can be described as malformation, deformation, disruption or dysplasia [16]. Malformation is commonly defined as a single localized poor formation of tissue initiating a sequence of defects (e.g. anencephaly). The recurrence risk for malformations generally range from 1% to 5%. Deformation is a result of extrinsic mechanical forces on otherwise normal tissue, deforming it (e.g. abnormal faces, pulmonary hypoplasia and limb contractures that result from prolonged oligohydramnios or primary renal agenesis in Potter syndrome with Potter facies). Disruption results from an extrinsic insult that destroys normal tissue, altering the formation of affected structure (e.g. amniotic band syndrome). If the primary defect is absence of normal organization of cells into tissue, then we speak of dysplasia (e.g. achondroplasia) [8], [16].
Any of the mechanisms of fetal maldevelopment can result in altered morphology of fetal organs and systems, which can result in the formation of a fetal syndrome if many organs are involved. The word syndrome originates from ancient Greek meaning “running together” [17], representing a specific pattern of associated signs, symptoms, dysmorphic features and/or behaviors occurring together in the same individual [8], [14], [15].
Some fetal syndromes can be detected prenatally while others cannot; some are expressed prenatally while others are not. In many cases definitive diagnosis can be made posnatally, many years later [8]. The differential diagnosis of fetal syndromes is wide, and there are several available databases online that can be of assistance in the recognition of patterns of anomalies as a syndrome, sequence or association [8]. The most widely used online databases are Online Mendelian Inheritance in Man (OMIM), Orphanet, London Dysmorphology Database, Possumweb and the Phenotip online database. For the sonographer, the most user-friendly database, especially designed to include all antenatal sonographic findings (instead of postnatal findings), is the Phenotip online database, while the London Dysmorphology Database and Possumweb are non-free databases, constructed to aid in differential diagnosis, with the inclusion of postnatal findings. There is quick access to the information, with the possibility to search by ultrasonographic marker, a combination of a few markers or just by the name of the syndrome. The triggers to investigate even more carefully for the syndrome could be known family history, earlier pregnancy with malformed fetus/infant, history of consanguinity, exposure to some teratogenic drug or other agents, traveling to high-risk areas and possible exposure to some infections (Zika virus, TORCH infections) or trauma [8]. There is also the possibility to include parental markers if present. Synonyms of the syndromes are included in the search automatically, which makes it easier and faster.
Clinical application of 3D/4D ultrasound in the prenatal detection of fetal syndromes
An optimized and systematic approach (guidelines) to the evaluation of the fetus by conventional two-dimensional (2D) US should always be followed to avoid mistakes in prenatal assessment [8]. When evaluating structures like the fetal spine or the face, 3D/4D US renders much more accurate images [18]. Magnetic resonance imaging (MRI) has comparable image quality with US, which is the most commonly used modality for pregnancy evaluation. US provides cost-effective real-time imaging, offers high resolution and is considered safe for the mother and the fetus [19]. US is superior to any other imaging technology in pregnancy because of the possibility to be used from the early 1st trimester [18], with the possibility to assess fetal movements in almost real-time [18]. Undoubtedly, one of the best non-invasive diagnostic tools for the detection and visualization of fetal anomalies and syndromes is US, particularly 3D/4D US [9]. With recent advances in 3D/4D technology, antenatal diagnosis of fetal anomalies and syndromes greatly shifted from the 2nd to the 1st trimester of pregnancy [9], [11], [18].
Goncalves et al. [20] reviewed 525 articles on 3D/4D sonography and found that 3D US provides additional diagnostic information for the diagnosis of facial anomalies, especially facial clefts, neural tube defects and skeletal malformations.
Merz and Welter [21] examined a large group of 3472 fetuses evaluated with detailed 2D and 3D US targeted for fetal anomalies. The total number of defects was 1012. Comparing the 2D and 3D techniques, 3D US proved advantageous in 60.8% of the defects, which was related to the favorable demonstration of targeted areas in different views (e.g. multiplanar, surface view) [21], [22].
Only in the last several years have high-frequency transducers and HDlive technology made major improvements in the quality of US imaging. The 3D HDlive rendering method takes advantage of “shadowing effects” to improve the visualization of details on the image [23]. Unlike conventional 3D surface rendering that uses a fixed virtual light source and reflects the light off the skin surface, HDlive rendering calculates the propagation of light through the skin and the tissue [23]. Shadows are created where light has moved through denser tissues. The virtual light source can be changed and directed easily from any angle and can be manipulated to enhance segmentation of tissue structures, define precise outlines and highlight important clinical details [23]. This tool is handy when observing surfaces, particularly of the facial area. Any suspected area or malformation can be investigated and visualized much better than with conventional 2D US. By changing the angle of virtual light, one can adjust it perfectly to emphasize and get depth perception in visualizing a region of interest that may be an anomaly. A translucent effect is gained if the light source is placed behind the object [23]. Enhanced smoothing is obtained by volume-speckle reduction imaging (V-SRI) on quality multi-planar 3D/4D rendered images by applying volume (voxel) vs. traditional single slice (pixel) imaging.
HDlive rendering can be successfully applied during the entire pregnancy [18]. In the 1st trimester, normal and abnormal embryonal and fetal developments can be followed and evaluated in the smallest detail. Early and mid-trimester anomaly scans can be aided with 3D/4D HDlive technology in detecting fetal anomalies and syndromes, as suggested by many studies [10], [18], [24], [25], [26]. Only 2 years ago, new applications in 3D US called HDlive silhouette and HDlive flow were launched. HDlive silhouette found its clinical significance in imaging simultaneously the inner morphology through the outer surface in a transparent fashion. This helps in mapping the exact location and volume of inner structures, which can be hyperechoic, such as bone, or hypoechoic, such as a cyst [12]. HDlive flow adds more spatial resolution to a conventional angiogram. With the simultaneous combination of both techniques (HDlive silhouette and flow), one can visualize the exact location of vascular structures inside the organs and map the direction of the vascular flow (3D HDlive bidirectional power Doppler). These two novel applications enabled the visualization of intracorporeal vascularity, premature forebrain, midbrain and hindbrain, as well as flow in the brain vessels.
The use of a skin-like color tone in HDlive gave an even more realistic impression of a live fetus, with impressive pictorial illustration [3], [7], [8], [9], [10], [11], [12], [18], [24], [25], [26], [27], [28], [29], [30]. Many of the earlier mentioned innovations in 3D/4D US applications are particularly beneficial in the prenatal detection and visualization of anomalies of the fetal face and its discreet details. The fascinating combination of science, research and new technologies is all together implemented in a new upcoming research program called “Give a face to a syndrome” [8], [31]. Facial Dysmorphology Novel Analysis (FDNA®) is a new technology that facilitates detection of facial dysmorphic features and recognizable patterns of human malformations (postnatal/adult life) to present comprehensive and up-to-date neurogenetic references available online [8], [31].
Brain anomalies
An important milestone in the prenatal recognition of normal brain development is the visualisation of corpus callosum (CC) by improved ultrasound imaging; without this prenatal assessment was rather difficult. With 3D surface rendering in the median plane, CC can be visualized with all its segments: genu, body and splenium. Additionally, vascularization of CC with a 3D sonoangiogram of the pericallosal artery (PA) (Figure 1) and the anterior cerebral artery (ACA) became much easier than before [12], [32]. If there is a suspicion or clear prenatal diagnosis of agenesis of CC (ACC), it is important to search for other fetal abnormalities or syndromes [trisomy 18, cerebro-costo-mandibular syndrome, Walker-Warburg syndrome, Pai syndrome, Fryns syndrome and also fetal varicella zoster syndrome, fetal cytomegalovirus (CMV) syndrome and the most recently recognized congenital fetal Zika virus syndrome, etc.] [33]. Despite the fact that we are able to recognize some structural anomalies prenatally, the prediction of the exact extent of the damage and prognosis may still be a challenge [7], [32].
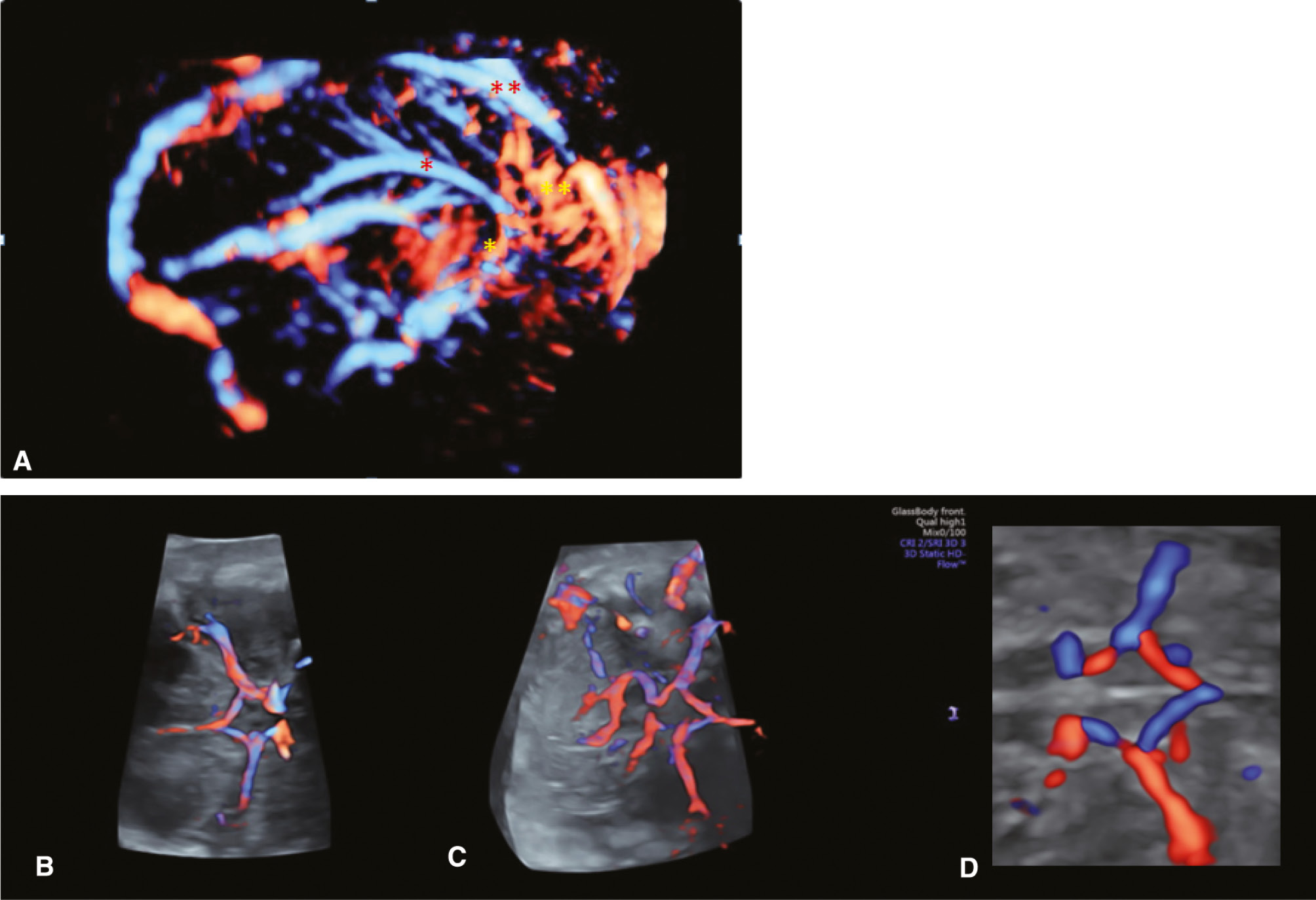
3D angiography (bidirectional power Doppler ultrasound imaging).
(A) Normal fetal intracranial circulation. Notice the pericallosal vascularization.  PA (pericallosal artery),
PA (pericallosal artery),  ACA (anterior cerebral artery),
ACA (anterior cerebral artery), 
 ACA branches,
ACA branches, 
 SSS (superior sagittal sinus). (B–D) The circle of Willis presented in advanced STIC (spatio-temporal image correlation), 3D HD flow, Glass body rendering mode.
SSS (superior sagittal sinus). (B–D) The circle of Willis presented in advanced STIC (spatio-temporal image correlation), 3D HD flow, Glass body rendering mode.
Congenital heart defects
Congenital heart defects (CHD) are the most common congenital anomalies occurring more frequently than chromosomal malformations and spinal defects together. The incidence is estimated to about 4–13 per 1000 live births, representing a significant cause of fetal mortality and morbidity [34].
Prenatal diagnosis of CHD by US is difficult, demanding thorough training and expertise. The detection rate of CHD is variable and it ranges from 35% to 86% in most studies [34]. In the past, many attempts were made to improve the prenatal detection rate of CHD. Four-dimensional US (real-time 3D US) used for fetal cardiac assessment may improve visualization of cardiac anatomy and allow better evaluation of valvular function [20].
Early evaluation of the fetal heart as well as recognition of several major cardiac abnormalities frequently coexisting in many fetal syndromes (Down syndrome, Edwards syndrome, DiGeorge syndrome, tarsal tunnel syndrome, etc.) can be obtained by 3D/4D US and improved by special applications [8], [12], [18], [26]. Two-dimensional US is still the technique of choice for the prenatal diagnosis of CHD; however, the last decade has shown some promising results due to advanced 3D/4D US technology [34], such as advanced spatio-temporal image correlation (STIC), volume contrast imaging (VCI) and Omni view. STIC is a technological development of 3D/4D US developed to assist the detection of CHD. An automated device is incorporated into the ultrasound probe that has the capacity to perform a slow sweep to acquire a single 3D volume [34]. Acquisition of volume data of the fetal heart and connections is done (the intraventricular septum, atrioventricular valves, great vessels’ outflow tracts, aorta and ductal arch) by allowing multiplanar and surface reconstruction of the heart anatomy [34]. The sonographer, even when less experienced in fetal echocardiography, can acquire volume data, which can be digitally stored and analyzed later or sent to a (fetal echocardiography) expert for further analysis. For better evaluation, the acquired data can be also assessed in the cine loop feature [34], played in slow motion and stopped any time to evaluate cardiac or vascular structures of interest. This application is also very helpful in counselling the parents and showing them where the problem is when CHD is found in the fetus.
Detecting the syndrome from the sonographer’s point-of-view: a difficult puzzle to solve
DiGeorge syndrome is the microdeletion of chromosome 22q11.2, the most common human deletion syndrome. This syndrome includes a wide spectrum of abnormalities among them; CHD [conotruncal, ventricular septal defect (VSD), tetralogy of Fallot] in more than 40% of cases, facial dysmorphysm (hyperthelorism, bulbous nasal tip), cleft palate, hypoplasia/aplasia of the thymus, malformation of cortical brain development (polymycrogyria), missing ribs, open spina bifida, polydactyly and clubfoot are the most common (Figure 2) [33]. All the above-mentioned malformations can be detected prenatally by US. Beside the heart anomalies, thymic hypoplasia/aplasia is known to be a typical feature in this condition, and Chaoui et al. [35] suggested that fetal thymic US can be an additional parameter in the assessment of fetuses with CHD. Thymic hypoplasia/aplasia finding appears to be sensitive (90%) in detecting fetuses with 22q11.2 deletion. When defects are found, prenatal cytogenetic evaluation should be offered. A frequently used acronym to remember the features of the syndrome is CATCH-22 (C: cardiac defects, A: abnormal faces, T: thymus aplasia/hypoplasia, C: cleft palate, H: hypocalcemia) [36].

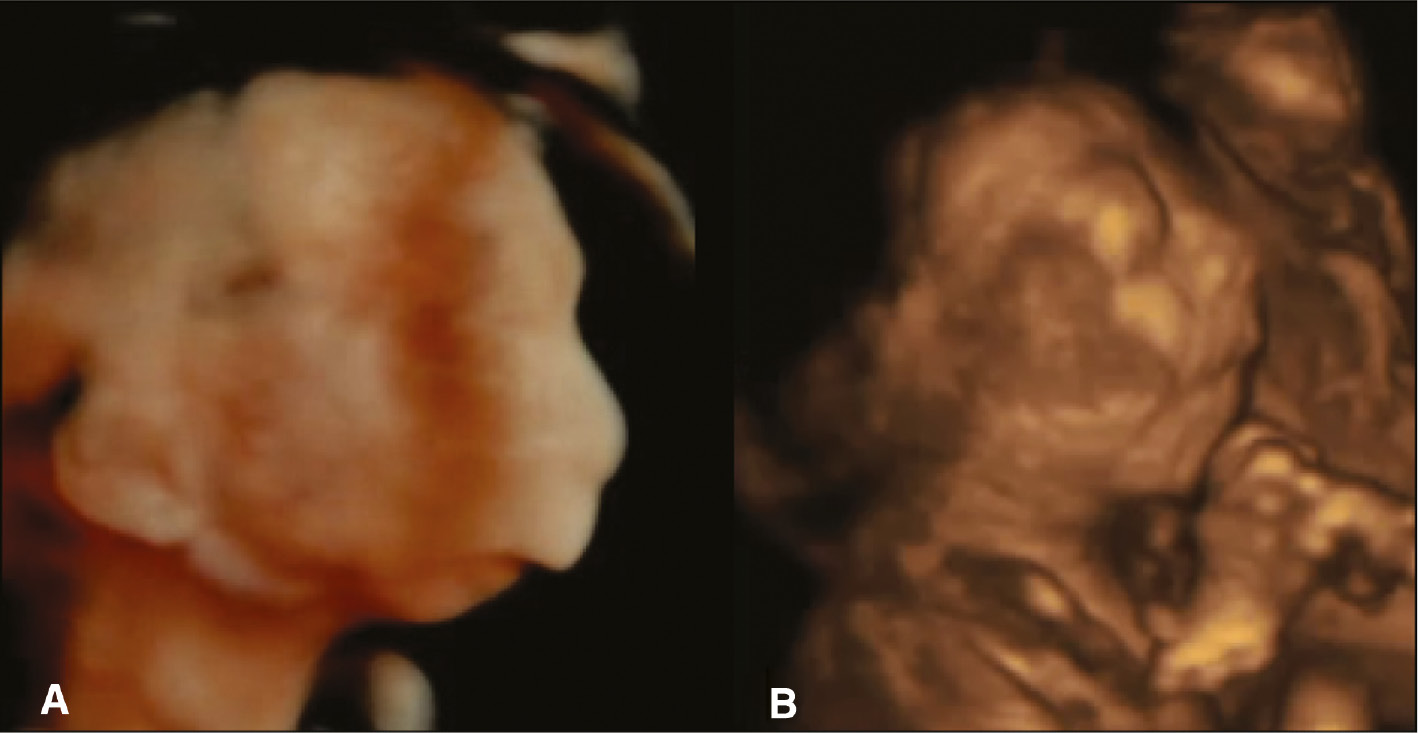
Similarity in visualisation prenatally and postnatally.
(A) Prenatal 3D surface rendering image of clubfeet. (B) Postnatal image of clubfeet.
Prenatally detected one malformatation, should be trigger to search for possible presence of other abnormalities.
While evaluating fetal faces, one must consider ethnic variations and normal differences. For example, an epicanthal fold may be normal for people of Asiatic descent and for some non-Asian infants, but it can be considered as a dysmorphic feature of syndromes such as Down syndrome, Turner syndrome, Noonan syndrome, Williams syndrome, fetal alcohol syndrome, etc. Different shape of the nose in different ethnic groups is another example (Mediterranean, African, Asian, Hispanic and Caucasian). A broad-beaked nose is a feature of Wolf-Hirschhorn syndrome (Greek warrior helmet syndrome) [33] and a short-beaked nose of some craniosynostosis-associated syndromes like Apert syndrome, Pfeiffer syndrome, Crouson syndrome, etc. [37]. A bulbous nose tip is feature of DiGeorge syndrome, as mentioned earlier. However, if there is a syndrome, there will be other associated anomalies too. So even a small deviation from the normal can be a trigger and clue to look further and raise awareness of possible syndromes.
Syndromes featuring primarily craniofacial anomalies
Paramedian cleft lip (CL) or cleft palate (CP) or a combination of the two (Figure 3) are the most common fetal facial anomalies and one of the most common fetal anomalies. They occur between the 8th and 9th gestational week and can be unilateral or bilateral. If this is an isolated finding (in less than 50% of cases), the defect can be surgically repaired with a good postoperative result. Unfortunately, a majority of the fetuses with CL or CP have high incidence of chromosomal abnormalities and other associated anomalies as part of syndromes [37]. Carriers of Van der Woude (VdW) syndrome have facial clefts in 50% of cases. VdW syndrome has an autosomal dominant mode of inheritance, which accounts for approximately 2% of all cases of CL and CP [37]. Incomplete unilateral small CL can easily be missed by conventional 2D US, while 3D HDlive surface rendering is a better method to detect it. Bilateral CL can sometimes also be missed because it does not change the symmetry of facial appearance [13]. Bilateral complete CL and CP, on the other hand, are most likely to be detected because of the protrusion of the inner maxillary segment under the nose, which is an obvious and unusual mass when observing a profile of the face [37]. When checking for CP, 3D HDlive surface and maximum modes are valuable as well as 3D application of tomographic US imaging (TUI), enabling to better determine the extent of the cleft [18].

Paramedian cleft lip (CL) or cleft palate (CP) or a combination of the two (CL/P).
(A) Prenatal detection of unilateral paramedian right CL/P by 3D HDlive surface rendering in fetus with detected Edwards syndrome (trisomy 18). (B) Postnatal presentation of unilateral paramedian right CL/P. (C) Postnatal presentation of unilateral paramedian left only CL. (D) Postnatal presentation of bilateral paramedian CL/P.
Midline clefts are always severe and usually part of some sequence such as holoprosencephaly, with gross facial appearance (cyclopia, midline facial cleft to a diverse extent, cebocephaly, flat nose). It is a common feature of some chromosomal syndromes such as Patau syndrome (trisomy 13) (Figure 4) and Edwards syndrome (trisomy 18). Trisomy 13 is the most common syndrome associated with alobar holoprosencephaly and facial clefts. However, up to 75% of holoprosencephaly cases have normal karyotype [37].

Postnatal findings of anophtalmia, median facial cleft and holoprosencephaly in a fetus with Patau syndrome (trisomy 13).
Mandibular anomalies (agnathia, micrognathia, retrognathia) have been described in various syndromes and seem to be very frequent, either isolated or coexisting as a part of the more heterogeneous syndrome.
Two-dimensional US images first indicate an abnormal profile, while with the different 3D applications (Figure 5), it is possible to explore it in more detail and obtain the complete impression of its appearance and possible coexistence of other orofacial anomalies.
Prenatal detection of micrognathia with low-set ears by 3D HDlive surface rendering.
(A) In a fetus with detected Pierre- Robin sequence (PRS). (B) In a fetus with Meckel-Gruber syndrome (courtesy of S. Panchal).
Pierre-Robin sequence (PRS) is characterized by a triad of orofacial anomalies consisting of retrognathia, glossoptosis and a posterior median soft CP. An osseous defect of the mandible is rarely found. Mandibular hypoplasia is a primary defect that occurs early in gestation between the 7thand 11thweek of gestation and causes the tongue to be maintained high up in the oral cavity, which subsequently prevents fusion of the posterior soft palate [16], [37]. Prenatal diagnosis of micrognathia in PRS by 3D US can be unveiled in the 1st trimester of pregnancy, as reported by several authors [13], [38], [39]. Pooh and Kurjak [13] pictorially presented mandibular hypoplasia and slow jaw development in a case of PRS during pregnancy. Serial 3D scans can be used to clearly reveal improvement and the progress of mandibular growth over several weeks [13], [37]. The catch-up growth of the mandible occurs during the first year of life and the adjusted profile of the child can be expected between the 3rd and 6th year of life [38], [39], [40]. Isolated PRS (without any other associated malformation) occurs in about 50% of cases; however, in the other half of the cases, PRS is part of a malformation syndrome. The clinical expression of the syndrome depends on the existence and severity of associated anomalies [37]. The nature of these anomalies is diverse – most commonly, the first branchial arch anomalies, various chromosomal disorders (DiGeorge syndrome), collagenopathies or syndromes associated with toxic agents such as alcohol (fetal alcohol syndrome), etc. In the review of 115 cases of patient with PRS, as expected, 54% had PRS as an isolated finding. The others included syndromes such as Stickler syndrome (18%), velocardiofacial syndrome (7%), Treacher Collins syndrome (TCS) (5%), facial and hemifacial microsomia (3%) and other defined (3.5%) and undefined disorders (9%) [8], [40]. Facial dysmorphism commonly arises from a combination of migration and inadequate formation of facial mesenchyme (especially when associated with disorders of the first and second branchial arches) [41].
Goldenhar syndrome (GS) or oculo-auriculo-vertebral (OAV) syndrome is the combination of such abnormalities [37]. It is characterized by a wide spectrum of symptoms, facial and associated features that may differ in range and severity from one case to another (Figure 6). A classic feature of GS is asymmetric (mostly unilateral) hypoplasia of the face. Fetuses with GS have major anomalies, such as unilateral mandibular hypoplasia with involvement of the temporomandibular joint and multiple skin tags around the ear, ear hypoplasia/aplasia and/or eye malformations (microphthalmia/anophthalmia) and vertebral anomalies. Typically, these malformations are unilateral (70%) and give an asymmetric appearance of the face. Usually, the right side is more severely affected than the left [42], [43], [44]. There have been some theories about the origin of this condition. Some authors hypothesized that the problem could be unilateral disruption of the blood supply (ischemia) to the 1st and 2ndbrachial arches, which could occur in the timeline between the 4th and 8th weeks of gestation [44]. However, Wang et al. [45] analyzed data from a large congenital birth defects registry in Spain and found a connection between diabetic mothers and increased risk of their infants being born with OAV syndrome. There has been speculation that poorly controlled maternal diabetes interferes with cephalic neural crest cell migration, causing this syndrome [45]. The first sonographic clue for the detection of this syndrome (also with conventional 2D US imaging) can be finding asymmetry of the face due to hemifacial macrosomia or something small and very typical as periauricular skin tags (Figure 7).

Postpartum images of a baby with Goldenhar syndrome.
Note: Hemifacial hypoplasia, external ear deformity, preauricular sinuses and tags.

Prenatal 3D surface rendering image of preauricular tag, postnatal images of external ear deformity, small ipsilateral half of the face and microphtalmia.
By using 3D surface rendering, more can be evaluated. Unilateral craniofacial anomaly underdevelopment of one side of the body can include brain (cerebellar hemisphere hypoplasia) [45], eye (micro/anophtalmia), low-set ears with malformation, face (asymmetry of the soft tissue), kidney (hydronephrosis), etc. With the application of 3D HDlive imaging technology, even small details of the face and other body parts can be visualized in a very realistic way, which can be very helpful while counseling the parents. The combination of micrognathia with low-set ears is a common finding in many syndromes. Detection of bilateral symmetric hypoplasia of the face, preauricular tags in combination with micrognathia may be part of TCS, Nager syndrome or Miller syndrome. TCS is a congenital disorder of craniofacial development caused by mutations in the TCOF1 gene on chromosome 5q32 [33]. TCS is represented by bilateral symmetrical otomandibular dysplasia (Figure 8). TCS is often associated with downward slanting of the palpebral fissures. There could be associated head and neck defects, and abnormalities of the extremities can be also found. The incidence of TCS is estimated at 1:50,000 live births per year. The inheritance is autosomal dominant with variation in expressivity. Cranioskeletal hypoplasia develops due to an insufficient number of neural crest cells as a consequence of neuroepithelial progenitor cell death [46]. The onset of defects occurs very early in embryogenesis between the 4th and 8th weeks of gestation. Prenatal diagnosis so far has been reported mostly in the 2nd trimester of pregnancy [39], [46], [47], but with more powerful 3D applications and HDlive technology, there is a possibility to shift the detection of TCS from the 2nd to the 1sttrimester. With the combination of anomalies, suspicion of a syndrome is very essential for informing the geneticists who can order the gene sequencing added to the usual amniocentesis (AC) panel to confirm the diagnosis, which otherwise would be missed [47]. There have been some animal studies featuring chemical and genetic inhibition of the p53 protein in the attempt to prevent TCS, but so far, no effective method of prevention in humans exists [47].
3D surface rendering image of fetus at 19 gestational weeks with Treacher-Collins syndrome and typical facial dysmorphism: bilateral symmetrical otomandibular dysplasia with hypoplasia of soft tissues is observed in the malar bone, inferior orbital rim and cheek (image courtesy of S. Panchal).
The shape of the skull might sometimes be informative, for example, a strawberry-shaped head seen in Edwards syndrome (trisomy 18), with a flattened occiput due to hypoplasia of the occipital brain lobes, brainstem and cerebellum, along with pointed frontal bones with hypoplasia of the frontal lobes of the brain. A lemon-shaped head can be seen with neural tube defects and as a part of some syndromes. A cloverleaf skull is present in different syndromes characterized by craniosynostosis, which can be found in Crouzon and Pfeiffer syndrome and in skeletal dysplasias such as thanatophoric dysplasia type II [37]. This shape of the fetal head develops due to premature closure of the coronal and lambdoid sutures, which causes bulging of temporal bones and confluence of anterior and posterior fontanelles. So, there are two bulges laterally (temporally) and an expansion superiorly (the anterior cranial fossa). Depending on the involvement of specific fetal skull sutures in premature fusion (craniosynostosis), different shapes of the head may appear.
Apert syndrome is an autosomal dominant disorder with a mutation found in the FGFR2 gene, chromosome 10q26.13 [33]. This syndrome has a few characteristic features that can be depicted while screening for abnormalities. Three signs to remember would be strawberry shaped head, flat face and mitten-like hands [37]. Due to bicoronal craniosynostosis, there is brachycephaly and acrocephaly, resulting in a strawberry-shaped head. In other words, one can detect an abnormal skull with a flat occiput, high forehead, midfacial hypoplasia (flat face), hypertelorism of the eyes and eyelid edema by a combination of conventional 2D imaging with 3D maximum mode (for bony structures), conventional 3D surface and 3D HDlive surface imaging. Mild ventriculomegaly can be detected by the 3D inversion mode and the newest application of HDlive silhouette imaging. ACC can be an accompanying finding best detected with 3D surface rendering in the median plane with additional 3D sonoangiogram visualization (3D HDlive bidirectional power Doppler) of the absent pericallosal artery. Very specific for fetuses with the Apert syndrome is defect on the extremities, called “mitten-like hands/feet”: syndactyly (soft tissue and osseous) of the 2nd, 3rd and 4thfinger in combination with a broad thumb. Pooh and Kurjak [13] published a case of a prenatally detected fetus with Apert syndrome by using 3D sonography. Correlation was made between prenatal 3D US images of anomalies and identical postnatal appearance [13], [18]. Three-dimensional US images can be used to present the parents with the extent of the abnormalities of the face, skull and extremities [48]. Parents should be counseled about prognosis, possibility of different degrees of intellectual impairment and risk of recurrence. When resulting from a de novo mutation, recurrence risk is improbable, but if one of the parents is a carrier, the recurrence risk is 50%. There may be an association between advanced paternal age and higher risk of occurrence of Apert syndrome as a single-gene disorder [49]. To confirm the diagnosis prenatally, the option of AC should be offered to the parents.
Frontal bossing may be a typical finding in achondroplasia (autosomal dominant condition with rhizomelic limb shortening) (Figure 9) and Russell-Silver syndrome (short stature, asymmetric intrauterine growth restriction of the skeleton with normal size of the head). Asymmetry of the fetal skull can also be found in the fetus with amniotic band syndrome (sequence). Due to rupture in the amnion, which initiates the process very early in the 1st trimester of pregnancy, the amniotic band causes a wide variety and severity of destructive fetal malformations, depending on fetal parts that come in contact and get trapped in it. When affecting the skull, asymmetric anencephaly, encephalocele, facial clefting and micrognathia can be detected. Other abnormalities that are also found are limb defects (constriction rings, amputation of the limb or digits, etc.) and anterior abdominal wall defects (gastroschisis, omphalocele) (Figure 10) [16], [37]. Other skull abnormalities detected by US are microcephaly and macrocephaly. Microcephaly indicates a group of disorders characterized by a small head and typically associated with abnormal neurological findings and mental disabilities [37]. Microcephaly usually also implies microencephaly because the head size is commonly determined by the brain size. Fetuses with prenatally suspected microcephaly have head circumference (HC) >3 standard deviations (SDs) below the mean for gestational age [50]. Different associated US features depend greatly on the etiologic factor causing microcephaly. The exact etiology of most microcephaly cases is still unknown. However, it is linked to numerous syndromes associated with chromosomal abnormalities like Cornelia de Lange syndrome, DiGeorge syndrome, Wolf-Hirshhorn syndrome, cri-du-chat syndrome, trisomy 13 and 9, etc., exposure to some toxic agents (alcohol, drugs, chlomiphene, methotrexate, phenylalanine), maternal under-nutrition and certain maternal infections during pregnancy, such as rubella, toxoplasmosis, varicella and cytomegalovirus (CMV). There are reports of a new causation between maternal infection and Zika virus during pregnancy and adverse pregnancy outcomes such as microcephaly, other brain and eye defects and pregnancy loss [51]. Zika congenital syndrome is generally characterized by cerebral atrophy that may interfere in formation and neuronal migration during early cerebral embryogenesis [52]. Other features of this syndrome are the following: severe microcephaly, lissencephaly, cataract of the eye, microophtalmia, clubfoot, contractures and arthrogryposis [51], [52], [53]. Viruses such as CMV or Zika have been shown to invade the brain cells, particularly neural progenitors, infect and destroy the primary stem cells (the radial glial cells) of the brain; therefore, there is a lack of future daughter neurons [54], [55], [56]. The severity of the condition may depend on the timing of infection during pregnancy. Microcephaly is mostly the result of decreased size of the cerebral cortex. In addition, infection may cause scars and calcifications in the brain tissue. Abnormalities such as periventricular and intraparenchymal calcifications, ventriculomegaly secondary to cerebral atrophy, cerebellar hypoplasia and cortical abnormalities are seen and detected much earlier than microcephaly itself. Besides the standard US screening, fetal neurosonography as well as KANET should be performed and repeated in the follow-up period until the delivery. The infection should be confirmed by the real-time reverse transcription polymerase chain reaction (rRT-PCR) [53]. The most recent follow-up studies from Brazil have shown that even though some babies are born with a normal head size, postnatal development of microcephaly can still occur as well as significant neurological sequelae also leading to arthrogryposis, a condition resulting in deformities of joints [52]. In the prenatal assessment of pregnancies at risk, evaluation with 4D US and KANET could be included. Prenatal results can be compared with postnatal results of neonatal neurological evaluation in the follow-up period during the first 2–3 years of life [6], [7], [8].

3D surface rendering.
(A) 3D skeleton. (B) 3D HDlive surface rendering. (C) 3D HDlive surface rendering of the same fetus at 34 gestational weeks with suspected achondroplasia. Notice typical facial features such as frontal bossing and depressed nasal bridge.

Prenatal detection of omphalocele at 13+3 gestational weeks. Different ultrasound modes and rendering applications are used to visualize the omphalocele.
(A) Conventional 2D mode. (B–D and E–G) 3D HDlive surface rendering with different angle of illumination. (H) Postnatal presentation of the baby born at 40 gestational weeks, realistic and same appearance of omphalocele in the prenatal compared to the postnatal period.
During a routine anomaly scan, abnormalities of fetal kidneys can be detected. A wide variety of structural and functional abnormalities can be seen (Figure 11). Dysplastic kidneys with multiple cysts [multcystic dysplastic kidneys (MCDK)] that fluctuate in size can be found in some very severe syndromes. As MCDK are dysfunctional, it could be a lethal condition called Meckel-Gruber syndrome (Figure 12) with autosomal recessive inheritance [57], if found bilaterally. Three pathognomonic anomalies can be found: occipital encephalocele, MCDK (Figure 13) and polydactyly. Neonates die within the first few days of life due to pulmonary hypoplasia and renal failure. Detection of occipital encephalocele in the 1st trimester is easier due to a better overview and normal collection of amniotic fluid. Later in pregnancy, there is progressive oligohydramnios and encephalocele may be missed. Special attention should be paid to evaluate both fetal kidneys because normal sonographic finding of kidneys rules out the lethal Meckel-Gruber syndrome.

Prenatally detected congenital anomalies of the urinary tract.
(A) Unilateral hydronephrosis in fetus at 26 gestational weeks, 3D rendering. (B) Conventional 2D image in fetus at 25 gestational weeks and obstructed urethra with distention of the bladder and hydronephrosis. (C) Conventional 2D image of fetus with massive distention of the bladder in Prune-Belly syndrome at 14 gestational weeks.

Facial appearance of fetus with Meckel-Gruber syndrome.
(A) 2D conventional image of fetal profile (notice the flat face). (B–E) 3D surface rendering of fetal profile.

Multicystic dysplastic kidney (MCDK) found in fetus at 26 gestational weeks.
(A, B) Conventional 2D images (courtesy RM. Nieto). (C, D) 3D HDlive silhouette images, volume extraction (courtesy RK. Pooh).
Conclusion
As soon as it becomes achievable to detect congenital anomalies by prenatal US, there lie questions of what can and should be done. Many ethical issues arise as well. Modern medicine faces some problems when having the possibility to extend the life of the sickest babies with potentially lethal congenital syndromes. The possibility to do so does not always justify the opportunity [8]. Generally, the idea is to find the balance between the benefits and limitations of US imaging. At the same time, one should be able to optimize the recommendations with the expectations of the parents of a severely damaged baby. Given the complexity of the prenatal diagnosis of syndromes, everything involved around it is so complex. That includes the postnatal confirmation of the diagnosis, determination of the prognosis aiming to help the parents to deal with the sick baby and to indicate the necessity of complex, lifelong and costly multidisciplinary care of affected babies.
Taken together, the previously described 3D/4D US techniques (Figure 14) promise to advance clinicians’ accuracy in detecting fetal abnormalities and syndromes as early as possible. So far, there have been many advantages of the prenatal detection of fetal syndromes, but there is also a lot of room for improvement. As the new 3D/4D US technology becomes more available to general use in everyday practice, one should be well informed and keep up with emerging new diagnostic possibilities, so that the number of detected affected fetuses will probably improve over time. Helping tools such as available online databases integrate all necessary information for better diagnostic precision. However, human involvement, knowledge and rational thinking are still irreplaceable in making the right diagnosis of fetal syndromes.

3D HDlive surface rendering of normal appearance of fetal face at 28 gestational weeks.
KANET assessment by 4D. Notice the open eye.
Author’s Statement
Conflict of interest: Authors state no conflict of interest.
Material and Methods: Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human subject use has complied with all the relevant national regulations, and institutional policies, and is in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
References
[1] EUROCAT. Prenatal screening & diagnosis. Prenatal detection (pd) rates. Available at: http://www.eurocatnetwork.eu/prenatalscreeninganddiagnosis/prenatal%20detection(pd)rates. (Accessed on October 11, 2016).Search in Google Scholar
[2] Kurjak A, Pooh RK, Merce LT, Carrera JM, Salihagic-Kadic A, Andonotopo W. Structural and functional early human development assessed by three-dimensional and four-dimensional sonography. Fertil Steril. 2005;84:1285–99.10.1016/j.fertnstert.2005.03.084Search in Google Scholar PubMed
[3] Pooh RK. A New Field of “Fetal Sono-ophthalmology” by 3D HDlive Silhouette and Flow. Donald School J Ultrasound Obstet Gynecol. 2015;9:221–2.10.5005/jp-journals-10009-1407Search in Google Scholar
[4] Kurjak A, Miskovic B, Stanojevic M, Amiel-Tison C, Ahmed B, Azumendi G, et al. New scoring system for fetal neurobehavior assessed by three- and four-dimensional sonography. J Perinat Med. 2008;36:73–81.10.1515/JPM.2008.007Search in Google Scholar PubMed
[5] Kurjak A, Abo-Yaqoub S, Stanojevic M, Yigiter AB, Vasilj O, Lebit D, et al. The potential of 4D sonography in the assessment of fetal neurobehavior-multicentric study in high-risk pregnancies. J Perinat Med. 2010;38:77–82.10.1515/jpm.2010.012Search in Google Scholar PubMed
[6] Stanojevic M, Antsaklis P, Kadic AS, Predojevic M, Vladareanu R, Vladareanu S, et al. Is kurjak antenatal neurodevelopmental test ready for routine clinical application? Bucharest consensus statement. Donald School J Ultrasound Obstet Gynecol. 2015;9:260–5.10.5005/jp-journals-10009-1412Search in Google Scholar
[7] Kurjak A, Barišić LS, Stanojević M, Kadić AS, Porović S. Are we ready to investigate cognitive function of fetal brain? The role of advanced four-dimensional sonography. Donald School J Ultrasound Obstet Gynecol. 2016;10:116–24.10.5005/jp-journals-10009-1453Search in Google Scholar
[8] Barišić LS, Kurjak A, Pooh RK, Delić T, Stanojević M, Porović S. Antenatal detection of fetal syndromes by ultrasound: from a single piece to a complete puzzle. Donald School J Ultrasound Obstet Gynecol. 2016;10:63–77.10.5005/jp-journals-10009-1444Search in Google Scholar
[9] Pooh RK, Kurjak A. Novel application of three-dimensional HDlive imaging in prenatal diagnosis from the first trimester. J Perinat Med. 2015;43:147–58.10.1515/jpm-2014-0157Search in Google Scholar PubMed
[10] Bonilla-Musoles F, Raga F, Castillo JC, Bonilla F Jr, Climent MT, Caballero O. High definition real-time ultrasound (HDlive) of embryonic and fetal malformations before week 16. Donald School J Ultrasound Obstet Gynecol. 2013;7:1–8.10.5005/jp-journals-10009-1266Search in Google Scholar
[11] Pooh RK, Kurjak A. 3D/4D sonography moved prenatal diagnosis of fetal anomalies from the second to the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:433–55.10.3109/14767058.2011.636107Search in Google Scholar PubMed
[12] Pooh RK. Novel application of hdlive Silhouette and hdlive flow: clinical significance of the “see-through fashion” in prenatal diagnosis. Donald School J Ultrasound Obstet Gynecol. 2016;10:90–8.10.5005/jp-journals-10009-1447Search in Google Scholar
[13] Pooh RK, Kurjak A. Three-dimensional ultrasound in detection of fetal anomalies. Donald School J Ultrasound Obstet Gynecol. 2016;10:214–34.10.5005/jp-journals-10009-1471Search in Google Scholar
[14] Lyons KJ, Crandall MJ, del Campo M. Smith’s recognizable patterns of human malformation, 7th ed. Philadelphia, PA: Elsevier Saunders; 2013.Search in Google Scholar
[15] Lanna M, Rustico MA, Pintucci A, Spaccini L, Lalatta F, Nicolini U. Three-dimensional ultrasound and genetic syndromes. Donald School J Ultrasound Obstet Gynecol. 2007;1:54–9.10.5005/jp-journals-10009-1109Search in Google Scholar
[16] Jones KL. Smith’s recognizable patterns of human malformation. 5th ed. Philadelphia, PA: Elsevier Saunders; 1997.Search in Google Scholar
[17] Dorland’s Illustrated Medical Dictionary online. Elsevier. Available at: www.dorlands.com/wsearch.jsp.Search in Google Scholar
[18] Pooh RK, Kurjak A. Donald school atlas of advanced ultrasound in obstetrics and gynecology. 1st ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2015.10.5005/jp/books/12692_2Search in Google Scholar
[19] Reddy UM, Filly RA, Copel JA. Prenatal imaging: ultrasonography and magnetic resonance imaging. Obstet Gynecol. 2008;112:145–57.10.1097/01.AOG.0000318871.95090.d9Search in Google Scholar PubMed PubMed Central
[20] Gonçalves LF, Lee W, Espinoza J, Romero R. Three- and 4-dimensional ultrasound in obstetric practice: does it help? J Ultrasound Med. 2005;24:1599–624.10.7863/jum.2005.24.12.1599Search in Google Scholar PubMed PubMed Central
[21] Merz E, Welter C. Two-dimensional and three-dimensional ultrasound in the evaluation of normal and abnormal fetal anatomy in the second and third trimesters in a level III center. Ultraschall Med. 2005;26:9–16.10.1055/s-2004-813947Search in Google Scholar PubMed
[22] Merz E, Abramowicz JS. Three-dimensional/four-dimensional ultrasound in prenatal diagnosis: is it time for routine use? Clin Obstet Gynecol. 2012;55:336–51.10.1097/GRF.0b013e3182446ef7Search in Google Scholar PubMed
[23] Benoit B, Levaillant JM. Voluson GE healthcare technology. Available at: www.volusonclub.net. Accessed: 10 Dec 2016.Search in Google Scholar
[24] Kagan KO, Pintoffl K, Hoopmann M. First-trimester ultrasound images using HDlive. Ultrasound Obstet Gynecol. 2011;38:607.10.1002/uog.10112Search in Google Scholar PubMed
[25] Hata T. HDlive rendering image at 6 weeks of gestation. J Med Ultrason. 2013;40:495–6.10.1007/s10396-013-0450-7Search in Google Scholar PubMed
[26] Hata T, Mashima M, Ito M, Uketa E, Mori N, Ishimura M. Three-dimensional HDlive rendering images of the fetal heart. Ultrasound Med Biol. 2013;39:1513–7.10.1016/j.ultrasmedbio.2013.03.027Search in Google Scholar PubMed
[27] Hanaoka U, Tanaka H, Koyano K, Uematsu R, Kanenishi K, Hata T. HDlive imaging of the face of fetuses with autosomal trisomies. J Med Ultrasonics. 2014;41:339–42.10.1007/s10396-014-0523-2Search in Google Scholar PubMed
[28] Hata T, Hanaoka U, Mashima M. HDlive rendering image of cyclopia and a proboscis in a fetus with normal chromosomes at 32 weeks of gestation. J Med Ultrason. 2014;41:109–10.10.1007/s10396-013-0466-zSearch in Google Scholar PubMed
[29] Tonni G, Castigliano AP, Grisolia G, Lithuania M, Meagher S, Da Silva Costa F, et al. HDlive in early gestation. J Turk Ger Gynecol Assoc. 2016;17:110–9.10.5152/jtgga.2016.15201Search in Google Scholar PubMed PubMed Central
[30] Pooh RK. Recent advances in 3D ultrasound, silhouette ultrasound, and sonoangiogram in fetal neurology. Donald School J Ultrasound Obstet Gynecol. 2016;10:193–200.10.5005/jp-journals-10009-1468Search in Google Scholar
[31] Basel-Vanagaite L, Wolf L, Orin M, Larizza L, Gervasini C, Krantz ID, et al. Recognition of the Cornelia de Lange syndrome phenotype with facial dysmorphology novel analysis. Clin Genet. 2016;89:557–63.10.1111/cge.12716Search in Google Scholar PubMed
[32] Merz E, Pashaj S. What is known about corpus callosum prenatally? Donald School J Ultrasound Obstet Gynecol. 2016;10:163–9.10.5005/jp-journals-10009-1461Search in Google Scholar
[33] The Phenotip Team. Phenotip tutorial. Available at: http://phenotip.com/possible-syndromes/. (Accessed on September 21, 2016).Search in Google Scholar
[34] Ahmed BI. The new 3D/4D based spatio-temporal imaging correlation (STIC) in fetal echocardiography: a promising tool for the future. J Matern Fetal Neonatal Med. 2014;27:1163–8.10.3109/14767058.2013.847423Search in Google Scholar PubMed
[35] Chaoui R, Kalache D, Heling KS, Tennstedt C, Bommer CC, Korner H. Absent or hypoplastic thymus on ultrasound: a marker for deletion 22q11.2 in fetal cardiac defect. Ultrasound Obstet Gynecol. 2002;20:546–52.10.1046/j.1469-0705.2002.00864.xSearch in Google Scholar PubMed
[36] Wilson DI, Burn J, Scambler P, Goodship J. DiGeorge syndrome: part of CATCH 22. J Med Genet. 1993;30:852–6.10.1136/jmg.30.10.852Search in Google Scholar PubMed PubMed Central
[37] Benacerraf BR. Ultrasound of fetal syndromes. 2nd ed. London: Churchill Livingstone; 2008.Search in Google Scholar
[38] Teoh M, Meagher S. First-trimester diagnosis of micrognathia as a presentation of Pierre Robin syndrome. UltrasoundObstet Gynecol. 2003;21:616–8.10.1002/uog.141Search in Google Scholar
[39] Tsai MY, Lan KC, Ou CY, Chen JH, Chang SY, Hsu TY. Assessment of the facial features and chin development of fetuses with use of serial three-dimensional sonography and the mandibular size monogram in a Chinese population. Am J Obstet Gynecol. 2004;190:541–6.10.1016/j.ajog.2003.07.031Search in Google Scholar
[40] Evans AK, Rahbar R, Rogers GF, Mulliken JB, Volk MS. Robinsequence: a retrospective review of 115 patients. Int J Pediatr Otorhinolaryngol. 2006;70:973–80.10.1016/j.ijporl.2005.10.016Search in Google Scholar
[41] Johnson JM, Moonis G, Green GE, Carmody R, Burbank HN. Syndromes of the first and second branchial arches, part 2: syndromes. Am J Neuroradiol. 2011;32:230–7.10.3174/ajnr.A2073Search in Google Scholar
[42] Castori M, Brancati F, Rinaldi R, Adami L, Mingarelli R, Grammatico P, et al. Antenatal presentation of the oculo-auriculo-vertebral spectrum (OAVS). Am J Med Genet A. 2006;140:1573–79.10.1002/ajmg.a.31290Search in Google Scholar
[43] Miller TD, Metry D. Multiple accessory tragi as a clue to the diagnosis of the oculo-auriculo-vertebral (Goldenhar) syndrome. J Am Acad Dermatol. 2004;50(2 Suppl):S11–13.10.1016/S0190-9622(03)00479-1Search in Google Scholar
[44] Martinelli P, Maurotti GM, Agangi A, Mazzarelli LL, Bifulco G, Paladini D. Prenatal diagnosis of hemifacial microsomia and ipsilateral cerebellar hypoplasia in a fetus with oculoauriculovertebral spectrum. Ultrasound Obstet Gynecol. 2004;24:199–201.10.1002/uog.1118Search in Google Scholar PubMed
[45] Wang R, Martinez-Frias ML, Graham JM Jr. Infants of diabetic mothers are at increased risk for the oculo-auriculo-vertebral sequence: a case-based and case-control approach. J Pediatr. 2002;141:611–7.10.1067/mpd.2002.128891Search in Google Scholar PubMed
[46] Paul A, Trainor PA, Dixon J, Dixon MJ. Treacher Collins syndrome: etiology, pathogenesis and prevention. Eur J Human Genetics. 2009;17:275–83.10.1038/ejhg.2008.221Search in Google Scholar PubMed PubMed Central
[47] Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–33.10.1038/nm1725Search in Google Scholar PubMed PubMed Central
[48] David AL, Turnbull C, Scott R, Freeman J, Bilardo CM, van Maarle M, et al. Diagnosis of Apert syndrome in the second-trimester using 2D and 3D ultrasound. Prenat Diagn. 2007;27:629–32.10.1002/pd.1758Search in Google Scholar
[49] Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–60.10.1097/GIM.0b013e318176fabbSearch in Google Scholar
[50] Chervenak FA, Rosenberg J, Brightman RC, Chitkara U, Jeanty P. A prospective study of the accuracy of ultrasound in predicting fetal microcephaly. Obstet Gynecol. 1987;69:908–1043.Search in Google Scholar
[51] De Araújo TV, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda- Filho D, Montarroyos UR, de Melo AP, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;12:1356–63.10.1016/S1473-3099(16)30318-8Search in Google Scholar
[52] van der Linden V, Pessoa A, Dobyns W, Barkovich AJ, van der Linden HJ, Rolim Filho EL, et al. Description of 13 infants born during October 2015–January 2016 with congenital zika virus infection without microcephaly at birth, Brazil. MMWR Morb Mortal Wkly Rep. 2016;65;1343–8.10.15585/mmwr.mm6547e2Search in Google Scholar PubMed
[53] Melo AS de O, Aguiar RS, Amorim MMR, Tanuri A, Melo FO, Ribeiro ST, et al. Congenital Zika Virus Infection. JAMA Neurol. Published online October 3, 2016. Corrected on October 24, 2016.Search in Google Scholar
[54] Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18:591–6.10.1016/j.stem.2016.03.012Search in Google Scholar PubMed PubMed Central
[55] Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–6.10.1016/j.stem.2016.04.017Search in Google Scholar PubMed
[56] Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement. Ultrasound screening for fetal microcephaly following zika virus exposure. Am J Obstet Gynecol. 2016;214:B2–B4.10.1016/j.ajog.2016.02.043Search in Google Scholar PubMed
[57] Barišić I, Odak LJ, Loane M, Garne E, Wellesley D, Calzolari E, et al. Prevalence, prenatal diagnosis and clinical features of oculo-auriculo-vertebral spectrum: a registry-based study in Europe. Eur J Human Genetics. 2014;22:1026–33.10.1038/ejhg.2013.287Search in Google Scholar PubMed PubMed Central
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- 3D/4D Sonography
- Advantages of 3D ultrasound in the assessment of fetal abnormalities
- Diagnosis of fetal syndromes by three- and four-dimensional ultrasound: is there any improvement?
- Three-dimensional ultrasound for prenatal assessment of conjoined twins: additional advantages?
- Three dimensional power Doppler of the placenta and its clinical applications
- 3D power Doppler in the evaluation of abnormally invasive placenta
- 4D assessment of fetal brain function in diabetic patients
- Multicentric studies of the fetal neurobehavior by KANET test
- Fetal face as important indicator of fetal brain function
- 4D ultrasound study of fetal movement early in the second trimester of pregnancy
- Preimplantation 3D ultrasound: current uses and challenges
- 3D and 4D studies from human reproduction to perinatal medicine
- Congress Calendar
- Congress Calendar
Articles in the same Issue
- Frontmatter
- Editorial
- 3D/4D Sonography
- Advantages of 3D ultrasound in the assessment of fetal abnormalities
- Diagnosis of fetal syndromes by three- and four-dimensional ultrasound: is there any improvement?
- Three-dimensional ultrasound for prenatal assessment of conjoined twins: additional advantages?
- Three dimensional power Doppler of the placenta and its clinical applications
- 3D power Doppler in the evaluation of abnormally invasive placenta
- 4D assessment of fetal brain function in diabetic patients
- Multicentric studies of the fetal neurobehavior by KANET test
- Fetal face as important indicator of fetal brain function
- 4D ultrasound study of fetal movement early in the second trimester of pregnancy
- Preimplantation 3D ultrasound: current uses and challenges
- 3D and 4D studies from human reproduction to perinatal medicine
- Congress Calendar
- Congress Calendar

