Treatment of PPROM with anhydramnion in humans: first experience with different amniotic fluid substitutes for continuous amnioinfusion through a subcutaneously implanted port system
-
Michael Tchirikov
, Gauri Bapayeva
Abstract
Objective: This study aims to treat patients with preterm premature rupture of the membranes (PPROM) and anhydramnion using continuous amnioinfusion through a subcutaneously implanted port system.
Methods: An amniotic fluid replacement port system was implanted in seven patients with PPROM and anhydramnion starting at the 20th week of gestation (range, 14–26 weeks) for long-term amnioinfusion. Saline solutions (2 L/day; Jonosteril®, Sterofundin®, isotonic NaCl 0.9% solution, lactated Ringer’s solution) and a hypotonic aqueous composition with reduced chloride content similar to the electrolyte concentration of human amniotic fluid were used for the continuous amnioinfusion.
Results: The mean duration of the PPROM delivery interval continued for 49 days (range, 9–69 days), with 3 weeks of amnioinfusion via the port system (range, 4–49). The newborns showed no signs of lung hypoplasia.
Conclusion: Long-term lavage of the amniotic cavity via a subcutaneously implanted port system in patients with PPROM and anhydramnion may help prolong the pregnancy and avoid fetal lung hypoplasia. A hypotonic aqueous composition with reduced chloride content similar to human amniotic fluid can be safely used for amnioinfusion. Prospective randomized studies are ongoing.
Introduction
Preterm premature rupture of the membranes (PPROM) is a complication in about 3–5% of all pregnancies and accounts for 45% of preterm deliveries [1, 10, 11]. Mercer et al. [16] reported that ~76% of patients with PPROM delivered within 1 week. The use of antibiotic therapy reduced the number of patients delivering within 1 week to 62%. Early anhydramnion/oligohydramnion, in addition to preterm birth, worsens neonatal outcome because it leads to pulmonary hypoplasia, infection, and restrictive joint deformities [18]. PPROM-induced chorioamnionitis negatively affects neonatal prognosis. With the occurrence of pulmonary hypoplasia, the risk of perinatal mortality increases to 80% [17]. PPROM may also lead to abnormal neurological outcomes [6, 15]. The standard treatment for PPROM in the second trimester is the administration of broad-spectrum antibiotics and antenatal corticosteroids to the mother in order to reduce the risk of respiratory distress syndrome (RDS) in the newborn [16, 17].
Amnioinfusion was first described as a method of preventing or relieving umbilical cord compression during labor [19]. Tranquilli et al. [23] demonstrated in a prospective randomized study that serial transabdominal amnioinfusion significantly prolonged the PPROM-to-delivery period and improved neonatal outcome. Unfortunately, the use of repetitive transabdominal amnioinfusions for the treatment of PPROM showed only a minimal benefit as measured by fluid loss within 6 h [5].
We developed a subcutaneously implanted amniotic fluid replacement port (AFR port) system for long-term amnioinfusion and have successfully implemented its use since 2009 in women with PPROM and anhydramnion [22]. After successfully resolving the technical complications stemming from the dislocation of the intrauterine port catheter (Figure 1), we encountered the additional problem of the long-term effect of the utilized fluid on the fetus (and its intrauterine programming) and on the mother. We have not found any report of a total substitution of amniotic fluid with another solution for an extended period in humans. For example, in the amnioinfusion studies described above, a 250-mL isotonic NaCl solution was administered weekly by amniocentesis [23]. With an expected fetal urine production of 300 mL/kg fetal weight/day [3], combined with leaking due to the PPROM syndrome, the influence of the isotonic saline solution to the fetus continued after the amnioinfusion for only a very short period, which was assumed to be a few hours. A similar situation may be expected after sporadic amnioinfusion, for example, during fetal surgery. The utilized saline solution may be swallowed by the fetus and mixed with its urine and pulmonary fluid within a few hours.
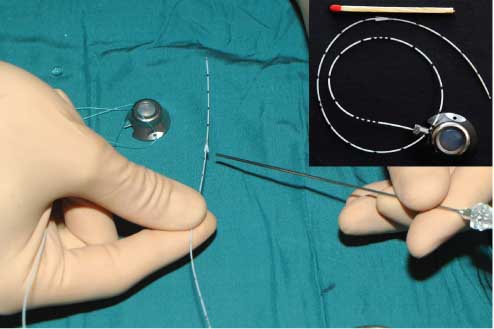
AFT port system.
The needle points at the anchor fixation system that we developed to avoid the dislocation of the catheter. The port capsule is prepared for transplantation.
In our case, after the administration of 2000 mL of the solution into the amniotic cavity, although this fluid may also be swallowed by the fetus throughout the delivery, only very little is likely to be mixed with the fetal urine and pulmonary fluid due to the immediate loss of fluid in severe PPROM. Thus, in 2011, we improved the infused saline solution and made it more similar to human amniotic fluid. In this article, we describe our experience with long-term amnioinfusion through a subcutaneously implanted AFR port system for the treatment of PPROM in humans using different saline solutions and a hypotonic aqueous composition with a reduced chloride content similar to the electrolyte concentration of human amniotic fluid [2, 12].
Materials and methods
The AFR port system was implanted in 7 patients with PPROM starting at the 21st week of gestation (range, 14–26 weeks) for long-term amnioinfusion.
The inclusion criteria were classic PPROM syndrome with anhydramnion in early gestation with a massive loss of amniotic fluid, no sign of a chorioamnionitis, absence of fetal malformation, and normal fetal karyotype. The port implantation protocol was approved by the institutional review board. All procedures were conducted according to the Declaration of Helsinki. Informed consent was obtained from each of the women before intervention.
The AFR port was implanted subcutaneously for long-term saline infusion (100 mL/h) into the amniotic cavity (Figure 1). The first AFR port system was developed in cooperation with Norfolk Medical, USA. We have previously reported problems with the catheter of the port systems [23]. Because of the dislocation of the port catheter in the first 4 patients, a new catheter (PakuMed GmbH, Essen, Germany) for the AFR port system was developed (Figure 2).
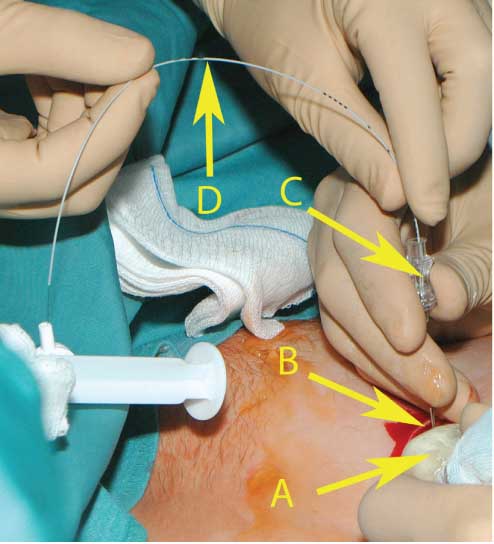
The insertion of the catheter into the amniotic cavity under ultrasound guiding.
(A) Ultrasound probe. (B) The pouch prepared for the port capsule. (C) The amniotic cavity was punctured with an 18-gauge needle through the prepared pouch. (D) The port catheter has been inserted into the amniotic cavity through the 18-gauge needle.
The patients were given Magneven® 2 g/h i.v. (Fresenius Kabi GmbH, Bad Homburg, Germany) and an indomethacin suppository 100 mg p.r. twice per day. Under local anesthesia with 10 mL non-adrenalized 1% Xylocaine, an amnioinfusion with ca. 300 mL saline solution was performed with a 22-gauge needle under ultrasound guidance (Philips iU22; Philips Medical, Hamburg, Germany). A small skin incision was performed with a scalpel under local anesthesia with 20 mL non-adrenalized 1% Xylocaine, and a subcutaneous pouch for the port capsule was prepared using a pair of scissors. The amniotic cavity was punctured with an 18-gauge needle (Echotip® Disposable Trocar Needle; COOK Medical, Spencer, IN, USA) under ultrasound control through the prepared pouch (Figure 3). The catheter was inserted through the needle into the amniotic cavity with a removable 1-French stylette. The thin stylette was pulled out, and the catheter was shortened. The port capsule was connected with the catheter and flushed with the saline solution or prepared amniotic fluid substitute. The port was then inserted into the prepared pouch (Figure 3), where it was fixed with 3-0 Vicryl stitches to the subcutaneous fat tissue, and the skin was closed with Monocryl 4-0 (ETHICON, Cincinnati, OH, USA). The saline solutions [Jonosteril®, n=2 (from Fresenius Kabi GmbH); Sterofundin®, n=1; isotonic NaCl 0.9% solution, n=1 (both from B. Braun AG, Melsungen, Germany), lactated Ringer’s solution, n=1 (Baxter, Germany)] and later a hypotonic aqueous composition with reduced chloride content resembling the electrolyte concentration of human amniotic fluid [based on mean values of amniotic fluid analysis, n=5, during the 22nd to the 30th weeks of gestation, University of Mainz, Germany, and published data [3, 12–14]; Na, 143.8 mmol/L; K, 3.9 mmol/L; Ca, 1.9 mmol/L; Mg, 0.57 mmol/L; Cl– 109.5 mmol/L; P, 3.3 mg/dL; lactate, 9.1 mmol/L; citrate, 66.5 mg/dL; HCO, 16.9 mmol/L; Cu, 16 μg/dL; Se, <13.3 μg/dL; Zn, 10–24 μg/dL; pH, 8.35, osmolality, <271, n=2 (Halle UKH, Germany)] were injected into the port system under color Doppler ultrasound control using atraumatic 25-gauge needle to check for the correct positioning of the catheter [22] (Figure 4).
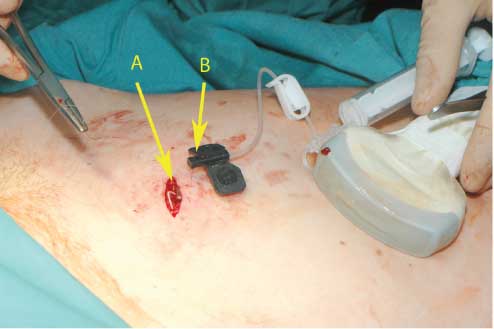
The final control of the system after port implantation.
(A) The prepared pouch with the implanted port system before closing the skin. (B) The amniotic fluid substitute solution is injected into the port system through the atraumatic 25-gauge needle.
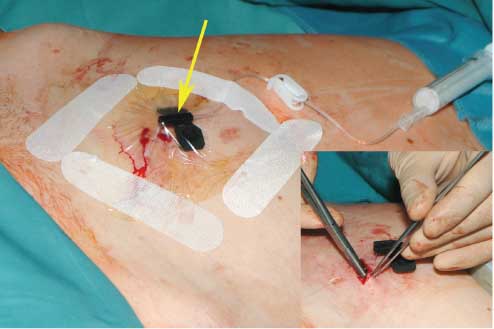
The closure of the operation field.
After the skin has been closed, the port capsule is punctured with a 25-gauge needle (indicated by the arrow). The operation field was covered with the sterile transparent foil for the sufficient control of the wound situation as well as to make a decision before changing the needle.
After the implantation of the port system, the saline solutions were infused intermittently with an infusion rate of 100 mL/h under periodic ultrasound. Bolus tocolysis with Partusisten® (fenoterol hydrobromide; Boehringer Ingelheim Pharma KG, Ingelheim, Germany) was given intravenously at a rate of 4 μg every 3 min for at least 2 days, followed by oral administration of Adalat® Retard (nifedipine; Bayer Vital GmbH, Leverkusen, Germany) 20 mg three times. RDS prophylaxis was performed with 12 μg of betamethasone (Celestan®, Essex Pharma, Munich, Germany) injected intramuscularly, which was repeated 24 h after the first injection. The patients normally received intravenous antibiotics (cefuroxime 2×750 mg and metronidazole 2×500 mg; Fresenius Kabi GmbH) for at least 10 days. In some cases, the therapy was corrected according to the recommendations of the physicians from the Microbiology Department based on culture results (e.g., erythromycin, in case of chlamydia or mycoplasma infections; see Table 1).
Patient data and neonatal outcome.
| Patient | H1 | H2 | H3a | M1 | M2 | M3b | M4 | Mean |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 28 | 39 | 33 | 39 | 27 | 28 | 29 | 32.5 |
| Gestational age at time of PPROM (weeks) | 23 | 26 | 14 | 18 | 20 | 18 | 21 | 20 |
| Gestational age at time of port implantation (weeks) | 25 | 27 | 20 | 22 | 23 | 23 | 24 | 23 |
| Duration of the amnioinfusion (days) | 9 | 8 | 18 | 49 | 35 | 4 | 6 | 21 |
| Cervical cultures | No bacterial growth | Escherichia coli, Mycoplasma, and Ureaplasma | E. coli | E. coli | E. coli, Enterococcus, and Chlamydia | Gardnerella, Enterococcus, and Ureaplasma | E. coli | |
| Antibiotics | C+M | C+M and E+M | C+M | C+M | Ceftriaxon+E | Klacid+M and E+M | Amoxicillin | |
| Gestational age at delivery (weeks) | 34 | 29 | 24 | 29 | 28 | 24 | 29 | 29 |
| PPROM delivery interval (days) | 69 | 9 | 24 | 77 | 56 | 42 | 57 | 49 |
| Apgar 1st mina | 9 | 5 | – | 7 | 8 | – | 7 | 7 |
| Apgar 5th mina | 10 | 8 | – | 8 | 8 | – | 8 | 8 |
| Apgar 10th mina | 10 | 8 | – | 8 | 9 | – | 8 | 9 |
| Arterial pHa | 7.4 | 7.41 | – | 7.32 | 7.42 | – | 7.4 | 7.39 |
| Fetal weight at delivery (g)a | 2055 | 1085 | 374 | 788 | 1360 | – | 950 | 1278 |
aThe deceased fetus during the 24th week of gestation weighing 374 g is not included into the final analysis of the neonatal outcome.
bMissed data, suspected genetic disorder.
C+M=cefuroxime 2×750 mg and metronidazole 2×500 mg i.v. (Fresenius Kabi GmbH), E=erythromycin 3×1 g i.v. (Actavis GmbH & Co., Munich-Riem, Germany).
Results
We successfully implanted the port system in all patients without any complications. Before the port implantation we performed the amniocentesis with an amnioinfusion 300–400 ml with 22 gauge needle. The next steps – the implantation of the port catheter under ultrasound control and its connection with the port capsule – were easily performed. In one case, in addition to PPROM, the patient (23+4 weeks of gestation) had severe symmetric intrauterine growth restriction with zero blood flow in the umbilical artery and brain sparing. Unfortunately, she declined karyotyping due to religion reasons. In this case, we used an isotonic NaCl 0.9% solution (B. Braun AG). The patient developed a hypertonic crisis, with a maximum, blood pressure of 210/110 mm Hg, reversed blood flow in the umbilical artery, and zero flow in the ductus venosus. The fetus died 2 days later. Because a genetic disorder (e.g., trisomy) was suspected, this case was excluded from the final analysis, resulting in a total of six patients in the final analyses (Table 1).
The patients in whom Jonosteril®, Sterofundin®, or lactated Ringer’s solution was used for the continuous amnioinfusion reported significantly increased diuresis. However, without bladder catheterization, we were not able to accurately measure the rate of diuresis, and fluid was continually lost due to the PPROM. After the classic isotonic saline solutions were substituted with the hypotonic amniotic fluid, the problem with increased diuresis was no longer observed.
The mean gestational age of PPROM was 20 weeks. In one case, the patient already exhibited PPROM with an anhydramnion in the 14th week of gestation. The mean gestational age during the AI port implantation was 23 weeks, with 3 weeks’ duration of the amnioinfusion.
The catheter of the first port system (Norfolk Medical) was dislocated within 1 week after port implantation in each case [23]. In two cases, the patients accepted a second port implantation. In one case, the patient did not accept a second intervention. In another case, the increased C-reactive protein and leukocytes rapidly normalized after starting the lavage of cavum uteri due to the port system for amnioinfusion. However, 1 week after ceasing amnioinfusion due to the dislocation of the catheter, the patient displayed signs of chorioamnionitis, and the fetus was delivered per cesarean section at 28+1 weeks of gestation (weight 1085 g, pH 7.41, Apgar 5/8/8).
The use of the improved catheter for amnioinfusion with an anchor fixation system (Figure 2; PakuMed GmbH) resolved the problem. Since the use of this approach, no catheter dislocation has occurred.
After 2 weeks of amnioinfusion, one patient surprisingly developed polyhydramnion. The amnioinfusion was immediately reduced and then ceased. The patient was observed in the clinic for 1 week, but the patient displayed no further signs of PPROM. The port system was explanted under local anesthesia, and the woman was released from the hospital. She delivered spontaneously at 33+4 weeks of gestation (weight, 2055 g; pH, 7.4; Apgar, 9/10/10). One patient who exhibited PPROM since the 14th week of gestation developed symptoms of sepsis after 18 days of amnioinfusion during 23+2 weeks of gestation. The patient was informed about the neonatal outcome of babies between 23 and 24 weeks of gestation. The woman declined to undergo cesarean section or reanimation procedures, and the pregnancy was terminated. The mean duration of the PPROM-to-delivery interval continued for 49 days, with 3 weeks of amnioinfusion via the port system.
Discussion
The preliminary results of the presented retrospective cohort study demonstrate that the use of continuous amnioinfusion with the subcutaneously implanted port system for the treatment of the PPROM with anhydramnion is a safe alternative to amnioinfusion with repetitive amniocentesis. De Santis et al. [5] demonstrated that repetitive transabdominal amnioinfusions for the treatment of PPROM displayed only a minimal benefit in the case of fluid loss within 6 h. In our study, the inclusion criteria for continuous amnioinfusion via a port system were classic PPROM syndrome and anhydramnion in the early gestation with a massive loss of amniotic fluid. Within 1 h, the patients lost the infused fluid. Thus, we directly chose patients with a severe PPROM in which repetitive transabdominal amnioinfusions would not work. However, the mean duration of the PPROM-to-delivery interval (56 days; range, 24–77 days) was more than twice as long (49 vs. 21 days) as that in the randomized trial published by Tranquilli et al. [23] using repetitive transabdominal amnioinfusions by amniocentesis. The Italian team performed at least one amnioinfusion weekly with 250 mL NaCl solution via repetitive amniocentesis. They reported the increase in the duration of the PPROM-to-delivery interval from 8 to 21 days. Porat et al. [24] analyzed four observational studies and three randomized controlled trials with transabdominal amnioinfusion for PPROM. The PPROM-to-delivery interval was 14.4 days (range, 8.2–20.6 days) in the observational studies and 11.41 days (range, 3.4–26.2 days) in the randomized trials. The infused volumes range in these studies from 140 to 350 mL per infusion [24]. The prolongation of the PPROM-to-delivery interval to 49 days in our study could be explained by the 2000- to 2400-mL/day continuous sufficient lavage of the amniotic cavity with saline solutions or, more recently, a hypotonic amniotic fluid substitute. This approach appears to be able to flush out the infected agents, e.g., bacteria, as well as cytokines from the cavum uteri.
The first catheter was dislocated from the cavum uteri because there was no fixation system. The newly developed catheter with an anchor fixation at the distal end solved this problem. Since the use of the anchor fixation, no catheter dislocation occurred.
We were faced with the problem of the long-term effect of the used fluid on the mother. The patients reported significantly increased diuresis when saline solutions, including Sterofundin® (B. Braun AG), Jonosteril® (Fresenius Kabi GmbH), or lactated Ringer’s solution (Baxter, Germany), were used for the continuous amnioinfusion. One patient developed a hypertonic crisis, with a blood pressure of 210/110 mm Hg, under continuous amnioinfusion with isotonic NaCl 0.9% solution (B. Braun AG).
Although both fluids will likely be swallowed by the fetus, in contrast to physiological hypotonic amniotic fluid, the elevated concentration of Na+ and Cl– in the infused iso-osmotic normotonic solutions likely leads to increased plasma saline concentration in the fetus, which will be normalized due to placental transfer to the mother and then extracted by the mother’s kidneys. Hypervolemia can trigger another pathway that provokes a significant increase in atrial natriuretic peptide, human brain natriuretic peptide, and endothelin 1 production and a decrease in vasopressin concentration. These substances could pass through the placenta to the mother and in turn increase urine production. Gilbert and Brace [7] found that fetal swallowing of isotonic saline solution leads to an increase in renal electrolyte excretion and fetal blood volume. However, the authors did not observe any significant deviations in maternal parameters.
Shields et al. [21] investigated the fetal electrolyte and acid-base responses to amnioinfusion with lactated Ringer’s and normal saline solution in fetal sheep at the end of gestation. Continuous amnioinfusion with saline solution significantly increased fetal plasma Na+ and Cl– concentrations. The use of lactated Ringer’s solution also led to an increase in Na+ plasma concentration in fetal sheep.
We tried to measure the rate of diuresis, but we were unable to perform an accurate measurement without the bladder catheterization of the women, and there was also continuous fluid loss because of the PPROM. After the isotonic saline solutions were substituted with the hypotonic solution with reduced chloride, which is more closely related to the electrolyte concentration of the human amniotic fluid, the problem with increased diuresis was no longer observed.
Normally, the amniotic fluid contains nutrients and growth factors that facilitate fetal growth and provide mechanical cushioning and antimicrobial effectors that protect the fetus and allow assessment of fetal maturity and disease [25]. According to Gilbert and Brace [7], the production of the amniotic fluid is predominantly accomplished by the excretion of fetal urine (~300 mL/kg fetal weight/day or 600–1200 mL/day near term) and the secretion of oral, nasal, tracheal, and pulmonary fluids (~60–100 mL/kg fetal weight/day). The fetus swallows ~200–250 mL/kg fetal weight/day, and a significant intramembranous pathway transfers fluids and solutes from the amniotic cavity to the fetal circulation across the amniotic membranes [8]. In the second half of pregnancy, there is a decrease in sodium and chloride concentrations, an increase in urea and creatinine concentrations, and an overall decrease in amniotic fluid osmolality.
We assume that the intrauterine infusion of classical saline solutions can result in an increase in fetal electrolyte concentration, which, during the long period of fetal development, could change the fetal programming and, in the worst case, irreversibly damage fetal organs, including the kidney, skin, eyes, and the bronchiopulmonary system. It could be speculated that epigenetic changes such as altered DNA methylation would lead to an increase in the incidence of chronic diseases such as arterial hypertonia, asthma, and skin eczema. Mulvihill et al. [20] demonstrated that animals receiving amnioinfusion with lactated Ringer’s solution exhibited poor gut development, whereas those infused with bovine amniotic fluid showed more normal gut maturation.
We prepared a hypotonic aqueous composition with reduced chloride content, which is similar to the electrolyte concentration of human amniotic fluid [2, 12], to prevent the occurrence of the medical complications described above and to avoid any possible claims in the future. We are currently working to improve the composition of the amniotic fluid substitute. The amniotic fluid substitute was prepared without glucose or proteins to avoid any substances that could increase the risk of bacterial infection in the amniotic cavity of the PPROM patients. Urea could not be used due to the instability of the solution, and we are still working to address this problem. We also did not use a surfactant [4, 9] in the amniotic fluid substitute because of financial constraints. However, because surfactant is present in the amniotic fluid, its use in amnioinfusion may increase the maturation process of the fetal lungs and improve neonatal outcome. A prospective randomized international study is ongoing.
We express our gratitude to Prof. Dr. Steven A. Johnsen, Institut für Tumorbiologie, University Hamburg-Eppendorf, for his support.
References
[1] Allen SR. Epidemiology of premature rupture of the fetal membranes. Clin Obstet Gynecol. 1988;31:553–841.Search in Google Scholar
[2] Benzie RJ, Doran TA, Harkins JL, Owen VM, Porter CJ. Composition of the amniotic fluid and maternal serum in pregnancy. Am J Obstet Gynecol. 1974;119:798–810.10.1016/0002-9378(74)90093-3Search in Google Scholar
[3] Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol. 1989;161:382–8.10.1016/0002-9378(89)90527-9Search in Google Scholar
[4] Bustos R, Kulovich MV, Gluck L, Gabbe SG, Evertson L, Vargas C, et al. Significance of phosphatidylglycerol in amniotic fluid in complicated pregnancies. Am J Obstet Gynecol. 1979;133:899–903.10.1016/0002-9378(79)90309-0Search in Google Scholar
[5] De Santis M, Scavo M, Noia G, Masini L, Piersigilli F, Romagnoli C, et al. Transabdominal amnioinfusion treatment of severe oligohydramnios in preterm premature rupture of membranes at less than 26 gestational weeks. Fetal Diagn Ther. 2003;18:412–7.10.1159/000073134Search in Google Scholar
[6] Gaudet LM, Flavin M, Islam O, Smith GN. Diffusion MRI brain findings in neonates exposed to chorioamnionitis: a case series. J Obstet Gynaecol Can. 2009;31:497–503.10.1016/S1701-2163(16)34211-6Search in Google Scholar
[7] Gilbert WM, Brace RA. Amniotic fluid volume and normal flows to and from the amniotic cavity. Semin Perinatol. 1993;17:150–7.Search in Google Scholar
[8] Gilbert WM, Newman PS, Eby-Wilkens E, Brace RA. Technetium-99 m rapidly crosses the ovine placenta and intramembranous pathway. Am J Obstet Gynecol. 1996;175:1557–62.10.1016/S0002-9378(96)70106-0Search in Google Scholar
[9] Gluck L, Kulovich MV. Lecithin/sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973;115:539–46.10.1016/0002-9378(73)90404-3Search in Google Scholar
[10] Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84.10.1016/S0140-6736(08)60074-4Search in Google Scholar
[11] Hofmeyr GJ, Essilfie-Appiah G, Lawrie TA. Cochrane Database.Syst Rev. 2011;12:1–34.Search in Google Scholar
[12] Johnell HE, Nilsson BA. Oxygen tension, acid-base status and electrolytes in human amniotic fluid. Acta Obstet Gynecol Scand. 1971;50:183–92.10.3109/00016347109157308Search in Google Scholar PubMed
[13] Lind T, Billewicz WZ, Cheyne GA. Composition of amniotic fluid and maternal blood through pregnancy. J Obstet Gynaecol. 1971;78:505–12.10.1111/j.1471-0528.1971.tb00308.xSearch in Google Scholar PubMed
[14] Lind T, Kendall A, Hytten FE. The role of the fetus in the formation of amniotic fluid. J Obstet Gynaecol Br Commonw. 1972;79:289–98.10.1111/j.1471-0528.1972.tb15799.xSearch in Google Scholar
[15] Locatelli A, Vergani P, Di Pirro G, Doria V, Biffi A, Ghidini A. Role of amnioinfusion in the management of premature rupture of the membranes at <26 weeks’ gestation. Am J Obstet Gynecol. 2000;183:878–82.10.1067/mob.2000.108873Search in Google Scholar
[16] Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271–9.10.1016/S0140-6736(95)91868-XSearch in Google Scholar
[17] Goldberg RL, Miodovnik M, Mapp DC, Meis PJ, Dombrowski MP. The antibiotic treatment of PPROM study: systemic maternal and fetal markers and perinatal outcomes. Am J Obstet Gynecol. 2012;206:145.e1–9.10.1016/j.ajog.2011.08.028Search in Google Scholar
[18] Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–93.Search in Google Scholar
[19] Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of variable or prolonged decelerations. A preliminary report. Am J Obstet Gynecol. 1983;146:670–8.Search in Google Scholar
[20] Mulvihill SJ, Stone MM, Fonkalsrud EW, Debas HT. Trophic effect of amniotic fluid on fetal gastroinestinal development. J Surg Res. 1986;40:291–6.10.1016/0022-4804(86)90189-7Search in Google Scholar
[21] Shields LE, Moore TR, Brace RAJ. Fetal electrolyte and acid-base responses to amnioinfusion: lactated Ringer’s versus normal saline in the ovine fetus. J Soc Gynecol Investig. 1995;2:602–8.10.1177/107155769500200404Search in Google Scholar
[22] Tchirikov M, Steetskamp J, Hohmann M, Koelbl H. Long-term amnioinfusion through a subcutaneously implanted amniotic fluid replacement port system for treatment of PPROM in humans. Eur J Obstet Gynecol Reprod Biol. 2010;152:30–3.10.1016/j.ejogrb.2010.04.023Search in Google Scholar PubMed
[23] Tranquilli AL, Giannubilo SR, Bezzeccheri V, Scagnoli C. Transabdominal amnioinfusion in preterm premature rupture of membranes: a randomised controlled trial. Br J Obstet Gynaecol. 2005;112:759–63.10.1111/j.1471-0528.2005.00544.xSearch in Google Scholar PubMed
[24] Porat S, Amsalem H, Shah PS, Murphy KE. Transabdominal amnioinfusion for preterm premature rupture of membranes: a systematic review and metaanalysis of randomized and observational studies. Am J Obstet Gynecol. 2012;207:393.e1–11.10.1016/j.ajog.2012.08.003Search in Google Scholar PubMed
[25] Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–8.10.1038/sj.jp.7211290Search in Google Scholar PubMed
The authors stated that there are no conflicts of interest regarding the publication of this article.
©2013 by Walter de Gruyter Berlin Boston
This content is open access.
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- Deliberative clinical ethical judgment: an essential component of contemporary obstetrics
- Review article
- Psychosocial stress in pregnancy and preterm birth: associations and mechanisms
- Opinion paper
- Management of prelabour rupture of membranes (PROM) at term
- Replies to Opinion paper
- Reply to “Management of prelabour rupture of membranes (PROM) at term”
- Reply to: Ismail AQT, Lahiri S. Management of prelabor rupture of membranes (PROM) at term. J Perinat Med. 2013
- Original articles – Obstetrics
- Treatment of PPROM with anhydramnion in humans: first experience with different amniotic fluid substitutes for continuous amnioinfusion through a subcutaneously implanted port system
- Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor
- Does carbon monoxide inhibit proinflammatory cytokine production by fetal membranes?
- Mode of delivery at periviable gestational ages: impact on subsequent reproductive outcomes
- Commentary
- Mode of delivery at periviable gestational ages: impact on subsequent reproductive outcomes
- Original articles – Obstetrics
- Is pathologic confirmation of placental abruption more reliable in cases due to chronic etiologies compared with acute etiologies?
- Endouterine hemostatic square suture vs. Bakri balloon tamponade for intractable hemorrhage due to complete placenta previa
- Double exposure to intra-amniotic lipopolysaccharide and maternal betamethasone induces sustained increase of neutrophils in the lungs and disrupts alveolarization in newborn rats
- Nitrous oxide for analgesia in external cephalic version at term: prospective comparative studya
- Original articles – Fetus
- Development and application of an automated extraction algorithm for fetal magnetocardiography – normal data and arrhythmia detection
- Original articles – Newborn
- Growth of very low birth weight infants after increased amino acid and protein administration
- Congress Calendar
- Congress Calendar
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- Deliberative clinical ethical judgment: an essential component of contemporary obstetrics
- Review article
- Psychosocial stress in pregnancy and preterm birth: associations and mechanisms
- Opinion paper
- Management of prelabour rupture of membranes (PROM) at term
- Replies to Opinion paper
- Reply to “Management of prelabour rupture of membranes (PROM) at term”
- Reply to: Ismail AQT, Lahiri S. Management of prelabor rupture of membranes (PROM) at term. J Perinat Med. 2013
- Original articles – Obstetrics
- Treatment of PPROM with anhydramnion in humans: first experience with different amniotic fluid substitutes for continuous amnioinfusion through a subcutaneously implanted port system
- Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor
- Does carbon monoxide inhibit proinflammatory cytokine production by fetal membranes?
- Mode of delivery at periviable gestational ages: impact on subsequent reproductive outcomes
- Commentary
- Mode of delivery at periviable gestational ages: impact on subsequent reproductive outcomes
- Original articles – Obstetrics
- Is pathologic confirmation of placental abruption more reliable in cases due to chronic etiologies compared with acute etiologies?
- Endouterine hemostatic square suture vs. Bakri balloon tamponade for intractable hemorrhage due to complete placenta previa
- Double exposure to intra-amniotic lipopolysaccharide and maternal betamethasone induces sustained increase of neutrophils in the lungs and disrupts alveolarization in newborn rats
- Nitrous oxide for analgesia in external cephalic version at term: prospective comparative studya
- Original articles – Fetus
- Development and application of an automated extraction algorithm for fetal magnetocardiography – normal data and arrhythmia detection
- Original articles – Newborn
- Growth of very low birth weight infants after increased amino acid and protein administration
- Congress Calendar
- Congress Calendar

